Tratamiento con hierro para la anemia preoperatoria
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011588.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 22 diciembre 2015see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Lesiones
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
AM, BK, AS: Main contribution to the study concepts and study design.
ON, BK: Main contribution to the data acquisition, analysis, interpretation and manuscript preparation.
KN: Main contribution to statistical support.
MB, AA: Main contribution to supervision and revision of the manuscript.
Declarations of interest
Ng O: I have received honoraria and travel support for consulting from Pharmacosmos A/S.
Keeler B: None known.
Mishra A: None known.
Simpson A: None known.
Neal K: None known.
Brookes MJ: My research department has received grant support from Syner‐Med and Vifor Pharma. I have received honoraria and travel support for consulting or lecturing from Vifor Pharma and Merck Sharp.
Acheson AG: My research department has received grant support from Syner‐Med, Vifor Pharma and Pharmacosmos A/S. I have received honoraria and travel support for consulting or lecturing from Ethicon Endosurgery, Johnson and Johnson Ltd, Olympus and Vifor Pharma.
Acknowledgements
The authors would like to thank Dr George Dowswell and Dr Shu Li Ng for their support and guidance in the protocol design.
This project was supported by the UK National Institute for Health Research, through Cochrane Infrastructure funding to the Cochrane Injuries Group. The views and opinions expressed are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2019 Dec 07 | Iron therapy for preoperative anaemia | Review | Oliver Ng, Barrie D Keeler, Amitabh Mishra, J A Simpson, Keith Neal, Hafid Omar Al‐Hassi, Matthew J Brookes, Austin G Acheson | |
| 2015 Dec 22 | Iron therapy for pre‐operative anaemia | Review | Oliver Ng, Barrie D Keeler, Amitabh Mishra, Alastair Simpson, Keith Neal, Matthew J Brookes, Austin G Acheson | |
| 2015 Apr 02 | Iron therapy for pre‐operative anaemia | Protocol | Oliver Ng, Barrie Keeler, Amitabh Mishra, Alastair Simpson, Keith Neal, Matthew J Brookes, Austin G Acheson | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Three studies are included in this review.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Iron therapy versus placebo or no iron therapy, Outcome 1 Proportion of patients who received a blood transfusion.

Comparison 1 Iron therapy versus placebo or no iron therapy, Outcome 2 Haemoglobin levels pre‐treatment (g/dL).

Comparison 1 Iron therapy versus placebo or no iron therapy, Outcome 3 Haemoglobin levels at end of treatment pre‐op (g/dL).

Comparison 1 Iron therapy versus placebo or no iron therapy, Outcome 4 Haemoglobin levels after treatment post‐op (g/dL).
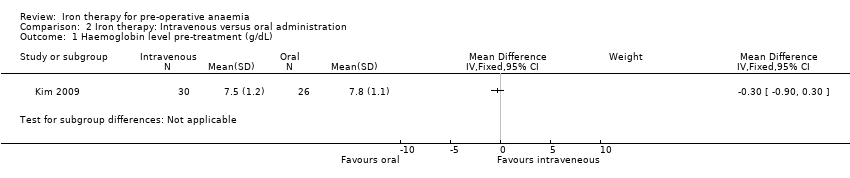
Comparison 2 Iron therapy: Intravenous versus oral administration, Outcome 1 Haemoglobin level pre‐treatment (g/dL).

Comparison 2 Iron therapy: Intravenous versus oral administration, Outcome 2 Haemoglobin level post‐treatment pre‐op (g/dL).

Comparison 2 Iron therapy: Intravenous versus oral administration, Outcome 3 Ferritin level pre‐treatment (ɥg/L).

Comparison 2 Iron therapy: Intravenous versus oral administration, Outcome 4 Ferritin level post‐treatment (ɥg/L).
| Iron therapy versus placebo or no iron therapy for pre‐operative anaemia | ||||||
| Patient or population: Patients with pre‐operative anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Iron therapy | |||||
| Proportion of patients who received a blood transfusion | 635 per 1000 | 356 per 1000 | RR 0.56 | 38 | ⊕⊕⊝⊝ | |
| Amount of blood transfused per patient (in units) | Data from two small studies could not be combined as they were skewed and reported as medians and ranges. One RCT in 18 people reported a difference in medians of 0 (interquartile range: 1) with iron therapy. Another RCT in 20 people reported a median difference of 1 unit with iron therapy (range 0 to 2). | ‐ | 38 | ⊕⊕⊝⊝ | It is not possible to combine the data because they are skewed. These are the raw data. | |
| Post‐operative mortality | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in either of the two studies available. |
| Post‐operative morbidity | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in either of the two studies available. |
| Any validated measure of quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in either of the two studies available. |
| Haemoglobin levels at end of treatment pre‐op (g/dL) | mean 11.9 g/dL (SD 2.6) | mean 11.2 g/d L(SD 1.95) | The mean haemoglobin levels at end of treatment pre‐op (g/dl) in the intervention groups was 0.7 g/dL lower | 18 | ⊕⊕⊝⊝ | Data from one study; the raw data are presented. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Only two small randomised control trials and a subset of anaemic patients resulting in a very small number of participants. | ||||||
| Iron therapy: Intravenous versus oral administration for pre‐operative anaemia | ||||||
| Patient or population: Patients with pre‐operative anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral iron therapy | Intravenous iron therapy | |||||
| Proportion of patients who received a blood transfusion | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the one study available. |
| Amount of blood transfused per patient (in units) | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the one study available. |
| Post‐operative mortality | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the one study available. |
| Post‐operative morbidity | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the one study available. |
| Any validated measure of quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the one study available. |
| Haemoglobin levels at end of treatment pre‐op (g/dL) | mean 8.6 g/dL (SD 1.4) | mean 10.5 g/dL (SD 1.4) | The mean haemoglobin levels at end of treatment pre‐op (g/dl) in the intravenous group was | 56 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study excluded those with less than 80% compliance with therapy and compliance was lower in the oral administration group. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of patients who received a blood transfusion Show forest plot | 2 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.27, 1.18] |
| 2 Haemoglobin levels pre‐treatment (g/dL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Haemoglobin levels at end of treatment pre‐op (g/dL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Haemoglobin levels after treatment post‐op (g/dL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Haemoglobin level pre‐treatment (g/dL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 Haemoglobin level post‐treatment pre‐op (g/dL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 Ferritin level pre‐treatment (ɥg/L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Ferritin level post‐treatment (ɥg/L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |

