Terlipresina versus otros fármacos vasoactivos para el síndrome hepatorrenal
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011532.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 27 septiembre 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Hepatobiliar
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
MI and LG collaborated on the initial draft.
All authors participated in the revision of the review and approved of the final version.
Sources of support
Internal sources
-
None, Other.
External sources
-
None, Other.
Declarations of interest
MI, MJ, AHG, RWW: no conflicts of interest.
ASA: served on a Scientific Advisory Board for Ferring Pharmaceuticals (makers of terlipressin in Europe) after the submission of this manuscript.
AK: served on a Scientific Advisory Board for Norgine, planned scientific meetings for Norgine and Intercept, and received funding for research from Norgine.
LG: acted as investigator in studies funded by AbbVie, Intercept, Merck and Norgine, received funding for travel expenses and consultancy from Novo Nordisk, and for scientific presentations at meetings funded by Norgine and Eli Lily.
Acknowledgements
We would like to thank Dimitrinka Nikolova and Sarah Klingenberg from the Cochrane Hepato‐Biliary Group for their assistance with the preparation of our review protocol and search strategy.
Cochrane Review Group funding acknowledgement: The Danish State is the largest single funder of the Cochrane Hepato‐Biliary Group through its investment in The Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark. Disclaimer: the views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State or The Copenhagen Trial Unit.
Peer reviewers: Fiona J Gifford, UK; Jonel Trebicka, Germany.
Contact editor: Janus Christian Jakobsen, Denmark.
Sign‐off editor: Christian Gluud, Denmark.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Sep 27 | Terlipressin versus other vasoactive drugs for hepatorenal syndrome | Review | Mads Israelsen, Aleksander Krag, Andrew S Allegretti, Manol Jovani, Alison H Goldin, Rachel W Winter, Lise Lotte Gluud | |
| 2015 Feb 11 | Terlipressin versus other vasoactive drugs for hepatorenal syndrome | Protocol | Mads Israelsen, Aleksander Krag, Lise Lotte Gluud | |
Differences between protocol and review
-
The review author's team expanded with four more people.
-
We changed the inclusion criteria from including "participants with hepatorenal syndrome" to including "people with cirrhosis and type 1 or type 2 hepatorenal syndrome." Cirrhosis has been mandatory in the diagnostic criteria of hepatorenal syndrome since Salerno 2007. All randomised clinical trials included in this review that used the former diagnostic criteria by Arroyo 1996 included participants only diagnosed with cirrhosis.
-
Regarding all outcome assessments, we changed the follow‐up from "end of treatment and at maximum follow‐up" to "maximum duration of follow‐up". Due to the severe prognosis of hepatorenal syndrome, the treatment is usually ended at reversal of hepatorenal syndrome or death. We choose to leave out the assessments at end of treatment because our primary outcomes included reversal of hepatorenal syndrome and all‐cause mortality.
-
We did not assess "death from renal failure," a secondary outcome in the protocol. Hepatorenal syndrome occurs in people with end‐stage liver disease and it is usually not possible to point to a single cause that leads to death. This is reflected in randomised clinical trials on hepatorenal syndrome that do not usually report the cause of death.
-
We updated the parameters for the Trial Sequential Analyses according to latest findings (see Data synthesis).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Antihypertensive Agents [adverse effects, *therapeutic use];
- Dopamine [therapeutic use];
- Hepatorenal Syndrome [*drug therapy, mortality];
- Lypressin [adverse effects, *analogs & derivatives, therapeutic use];
- Midodrine [therapeutic use];
- Norepinephrine [therapeutic use];
- Octreotide [therapeutic use];
- Randomized Controlled Trials as Topic;
- Terlipressin;
- Vasoconstrictor Agents [adverse effects, *therapeutic use];
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram.
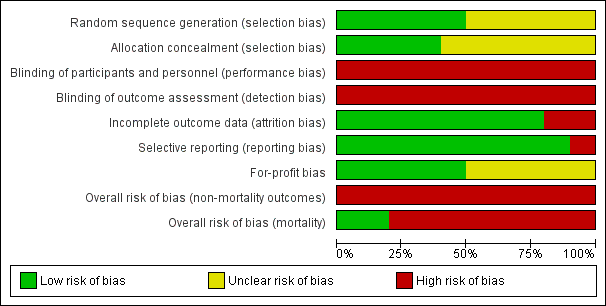
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

"Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
'+' = low risk of bias;
'‐' = high risk of bias;
'?' = unclear risk of bias.

Trial Sequential Analysis of 10 randomised clinical trials (474 participants) evaluating terlipressin versus other vasoactive drugs for people with hepatorenal syndrome on mortality. The analysis was made with power 90%, alpha 3%, a relative risk reduction (RRR) of 20%, a control group risk (CGR) of mortality of 52%, and a model variance ‐ based heterogeneity correction of 30%. The risk ratio was 0.96 (97% confidence interval 0.79 to 1.18). The cumulative Z‐curve (blue line) did not cross the diversity‐adjusted trial monitoring boundary for benefit.
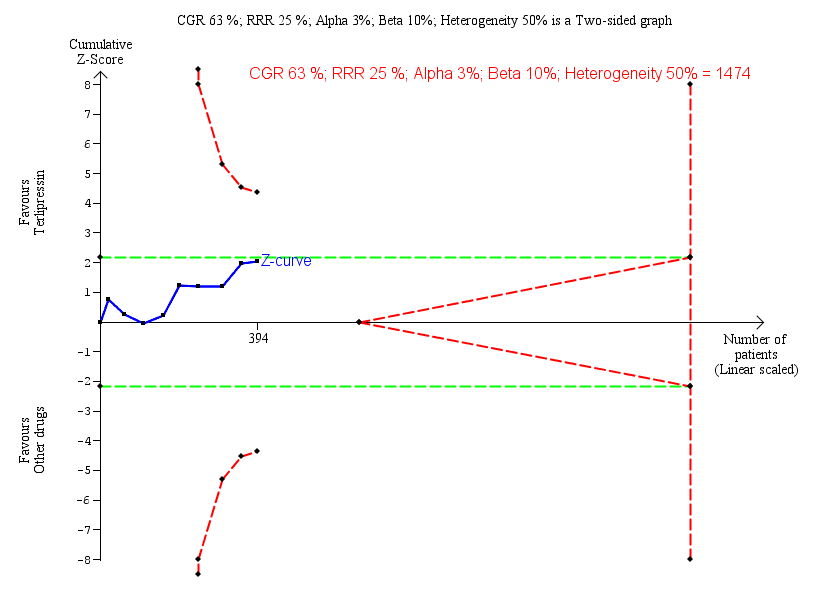
Trial Sequential Analysis of nine randomised clinical trials (394 participants) evaluating terlipressin versus other vasoactive drugs for people with hepatorenal syndrome on lack of reversal of hepatorenal syndrome. The analysis was made with power 90%, alpha 3%, a relative risk reduction (RRR) of 25%, a control group risk (CGR) of lack of reversal of hepatorenal syndrome of 63%, and a heterogeneity correction of 50%. The risk ratio was 0.79 (97% confidence interval 0.48 to 1.31). The cumulative Z‐curve (blue line) does not cross the diversity‐adjusted trial monitoring boundary for benefit.

Trial Sequential Analysis of two randomised clinical trials (88 participants) evaluating terlipressin versus other vasoactive drugs for people with hepatorenal syndrome on cardiovascular adverse events. The analysis was made with power 90%, alpha 3%, a relative risk reduction (RRR) of 25%, a control group risk (CGR) of cardiovascular adverse events of 15%, and a heterogeneity correction of 20%. The diversity‐adjusted trial monitoring boundary for harm was not included in the figure due to insufficient information. The estimated required information size was 4831 participants. Accordingly, with an accrued number of participants of 88, the required number of participants was not achieved.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 1 Mortality: bias control.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 2 Mortality: type of vasoactive drug.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 3 Mortality: type of hepatorenal syndrome.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 4 Mortality: publication status.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 5 Hepatorenal syndrome: type of vasoactive drug.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 6 Hepatorenal syndrome: type hepatorenal syndrome.
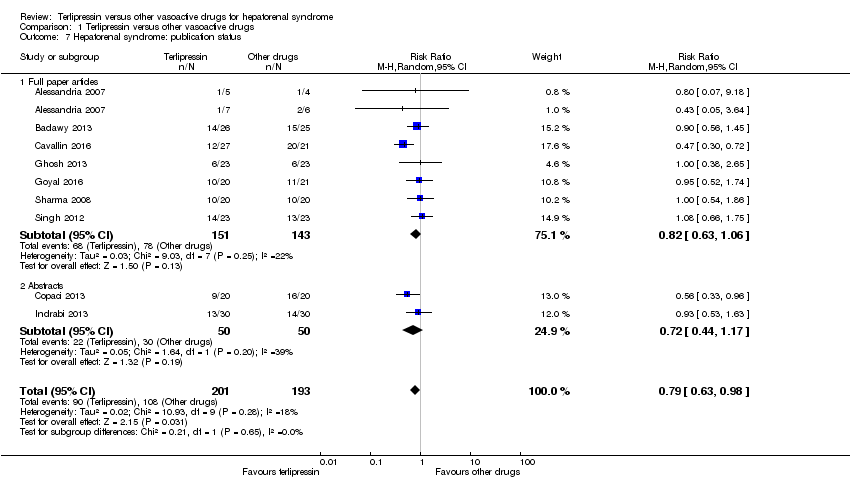
Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 7 Hepatorenal syndrome: publication status.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 8 Serious adverse events, type of vasoactive drug.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 9 Serious adverse events, type of event.
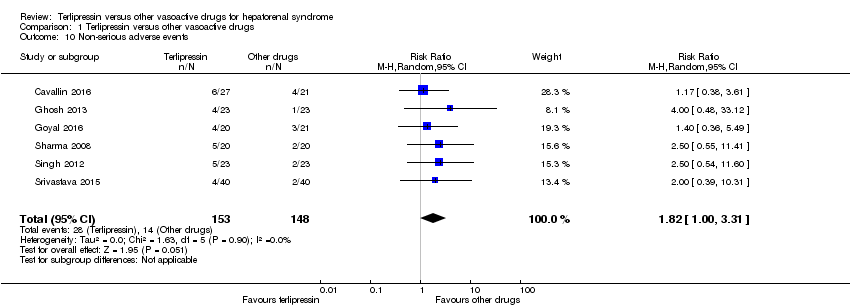
Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 10 Non‐serious adverse events.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 11 Non‐serious adverse event: types.
| Terlipressin compared to other vasoactive drugs for hepatorenal syndrome | ||||||
| Patient or population: people with cirrhosis and hepatorenal syndrome | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with other vasoactive drugs | Risk with terlipressin | |||||
| Mortality (All‐cause) | Study population | RR 0.96 | 474 | ⊕⊝⊝⊝ | Downgraded because of clinical heterogeneity, 8/10 randomised clinical trials were at high risk of bias and, the results of Trial Sequential Analysis. | |
| 601 per 1000 | 577 per 1000 | |||||
| Hepatorenal syndrome (Number of participants who did not achieve reversal of hepatorenal syndrome) | Study population | RR 0.79 | 394 | ⊕⊝⊝⊝ | Downgraded because of clinical heterogeneity, all trials were judged as high risk of bias, and results of Trial Sequential Analysis. | |
| 560 per 1000 | 442 per 1000 | |||||
| Serious adverse events | Study population | RR 0.96 | 474 | ⊕⊝⊝⊝ | Downgraded because of clinical heterogeneity, all trials were judged as high risk of bias, and results of Trial Sequential Analysis. | |
| 609 per 1000 | 585 per 1000 | |||||
| Non‐serious adverse events: diarrhoea or abdominal pain, or both | Study population | RR 3.50 | 221 | ⊕⊝⊝⊝ | Downgraded because of clinical heterogeneity, all trials were judged as high risk of bias, and results of the Trial Sequential Analysis. | |
| 19 per 1000 | 65 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aIn the assessment of mortality, we classified two randomised clinical trials at low risk of bias and eight at high risk of bias. bThe randomised clinical trials were not designed for equivalence or inferiority analysis. The Trial Sequential Analysis showed that sample size did not reach the required information size for equivalence/inferiority meta‐analysis. cClinical heterogeneity. dWe classified all randomised clinical trials at high risk of bias in all non‐mortality outcomes. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality: bias control Show forest plot | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 1.1 Low risk of bias | 2 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.63, 1.36] |
| 1.2 High risk of bias | 8 | 380 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.88, 1.07] |
| 2 Mortality: type of vasoactive drug Show forest plot | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 2.1 Noradrenaline | 7 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.08] |
| 2.2 Midodrine/octreotide | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.40, 1.28] |
| 2.3 Octreotide | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.32, 1.77] |
| 2.4 Dopamine/furosemide | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.22] |
| 3 Mortality: type of hepatorenal syndrome Show forest plot | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 3.1 Type 1 hepatorenal syndrome | 9 | 375 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.87, 1.06] |
| 3.2 Type 2 hepatorenal syndrome | 3 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.68, 1.33] |
| 4 Mortality: publication status Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Full paper | 8 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.83, 1.14] |
| 4.2 Abstract | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.86, 1.08] |
| 5 Hepatorenal syndrome: type of vasoactive drug Show forest plot | 9 | 394 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 0.99] |
| 5.1 Noradrenaline | 7 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.76, 1.21] |
| 5.2 Midodrine/octreotide | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.30, 0.72] |
| 5.3 Octreotide | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.33, 0.96] |
| 6 Hepatorenal syndrome: type hepatorenal syndrome Show forest plot | 9 | 394 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 0.98] |
| 6.1 Type 1 hepatorenal syndrome | 8 | 335 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.62, 1.01] |
| 6.2 Type 2 hepatorenal syndrome | 2 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.36, 2.10] |
| 7 Hepatorenal syndrome: publication status Show forest plot | 9 | 394 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 0.98] |
| 7.1 Full paper articles | 7 | 294 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.63, 1.06] |
| 7.2 Abstracts | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.44, 1.17] |
| 8 Serious adverse events, type of vasoactive drug Show forest plot | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 8.1 Noradrenaline | 7 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.08] |
| 8.2 Midodrine/octreotide | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.42, 1.23] |
| 8.3 Octreotide | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.32, 1.77] |
| 8.4 Dopamine/furosemide | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.22] |
| 9 Serious adverse events, type of event Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Death | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 9.2 Major cardiovascular events | 7 | 323 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.13, 5.98] |
| 10 Non‐serious adverse events Show forest plot | 6 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [1.00, 3.31] |
| 11 Non‐serious adverse event: types Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Diarrhoea or abdominal pain, or both | 5 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 3.50 [1.19, 10.27] |
| 11.2 Peripheral cyanosis | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.32, 27.83] |
| 11.3 Minor cardiovascular events | 6 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.37, 1.93] |

