Terlipresina versus otros fármacos vasoactivos para el síndrome hepatorrenal
Resumen
Antecedentes
El síndrome hepatorrenal se define como la insuficiencia renal grave que se presenta en pacientes con cirrosis y ascitis. Las revisiones sistemáticas de los ensayos clínicos aleatorios encontraron que, en comparación con placebo, la terlipresina puede reducir la mortalidad y mejorar la función renal en los pacientes con síndrome hepatorrenal, aunque se necesitan pruebas actuales provenientes de revisiones sistemáticas sobre los efectos beneficiosos y perjudiciales de la terlipresina versus otros fármacos vasoactivos.
Objetivos
Evaluar los efectos beneficiosos y perjudiciales de la terlipresina versus otros fármacos vasoactivos para los pacientes con síndrome hepatorrenal.
Métodos de búsqueda
Se hicieron búsquedas en el Registro de Ensayos Controlados del Grupo Cochrane Hepatobiliar (Cochrane Hepato‐Biliary Group Controlled Trials Register), CENTRAL, MEDLINE, Embase y en Science Citation Index Expanded; se realizaron búsquedas manuales de referencias en la literatura relevante; y se escribió a expertos y a compañías farmacéuticas (fecha de la última búsqueda noviembre 2016).
Criterios de selección
Ensayos clínicos aleatorios que compararan terlipresina versus cualquier otro tipo de fármaco vasoactivo para el síndrome hepatorrenal. Se permitió la administración de albúmina y otras cointervenciones cuando se proporcionaban por igual en los grupos de comparación.
Obtención y análisis de los datos
Tres autores de la revisión extrajeron los datos de forma independiente. Los resultados primarios fueron la mortalidad, el síndrome hepatorrenal (síndrome hepatorrenal persistente a pesar del tratamiento) y los eventos adversos graves. Se realizaron los metanálisis y se presentaron los resultados como cocientes de riesgos (CR) con intervalos de confianza (IC) del 95%. Se realizaron análisis de sensibilidad, de subgrupos y Análisis Secuenciales de Ensayos, y se evaluó el control del sesgo basado en los dominios del Grupo Cochrane Hepatobiliar.
Resultados principales
Se incluyeron 10 ensayos clínicos aleatorios con 474 participantes. Los ensayos compararon terlipresina versus noradrenalina (siete ensayos), octreotida (un ensayo), midodrina y octreotida (un ensayo), o dopamina (un ensayo). Todos los participantes de ambos grupos recibieron albúmina como cointervención. Dos ensayos se clasificaron en riesgo bajo de sesgo y ocho ensayos en alto riesgo de sesgo en la evaluación de la mortalidad y todos los ensayos en alto riesgo de sesgo para los resultados restantes. En cinco ensayos, los investigadores declararon específicamente que no recibieron financiamiento de organizaciones con fines de lucro. No hubo información acerca de la fuente de financiamiento de los cinco ensayos restantes.
La terlipresina no fue superior ni inferior en comparación con otros fármacos vasoactivos en cuanto a la mortalidad al incluir los dos ensayos en riesgo bajo de sesgo (CR 0,92; IC del 95%: 0,63 a 1,36; 94 participantes, evidencia de muy baja calidad) o al incluir los diez ensayos (CR 0,96; IC del 95%: 0,88 a 1,06; 474 participantes; I² = 0%; evidencia de muy baja calidad). Un metanálisis que incluyó los nueve ensayos indicó un efecto beneficioso de la terlipresina sobre el síndrome hepatorrenal (CR 0,79; IC del 95%: 0,63 a 0,99; 394 participantes; I² = 26%; evidencia de muy baja calidad). Debido a la mortalidad alta del síndrome hepatorrenal, el registro de otros eventos adversos graves es incierto, aunque al comparar la terlipresina y otros fármacos vasoactivos no se encontró ninguna diferencia significativa (CR 0,96; IC del 95%: 0,88 a 1,06; 474 participantes; I² = 0%; evidencia de muy baja calidad). Varios ensayos no informaron sistemáticamente los eventos adversos, aunque la terlipresina pareció aumentar los riesgos de diarrea o dolor abdominal, o ambos (CR 3,50; IC del 95%: 1,19 a 10,27; 221 participantes; cinco ensayos, I² = 0%). Sin embargo, los Análisis Secuenciales de Ensayos encontraron pruebas insuficientes para apoyar o refutar cualquier diferencia entre las intervenciones para todos los resultados. Al considerar la reversión del síndrome hepatorrenal, los análisis de subgrupos sobre los otros tipos de fármacos vasoactivos encontraron que la terlipresina fue superior en comparación con midodrina y octreotida (CR 0,47; IC del 95%: 0,30 a 0,72) u octreotida sola (CR 0,56; IC del 95%: 0,33 a 0,96), aunque cada subgrupo sólo incluyó un ensayo pequeño. Ninguno de los análisis de subgrupos ni de sensibilidad restantes encontró diferencias entre la terlipresina y otros fármacos vasoactivos. La calidad de la evidencia se disminuyó a muy baja debido al alto riesgo de sesgo, la imprecisión y los resultados de los Análisis Secuenciales de Ensayos.
Conclusiones de los autores
Esta revisión halló evidencia insuficiente para apoyar o refutar los efectos beneficiosos o perjudiciales de la terlipresina y la albúmina versus otros fármacos vasoactivos y albúmina. Se necesita investigación adicional para evaluar si existen diferencias clínicamente significativas entre las intervenciones.
PICO
Resumen en términos sencillos
Terlipresina versus otros fármacos vasoactivos para el síndrome hepatorrenal
Antecedentes
El síndrome hepatorrenal es un tipo de insuficiencia renal (relacionada con los riñones) que se presenta en pacientes con enfermedad hepática grave y líquido en el abdomen (ascitis). No se comprende completamente por qué algunos pacientes con enfermedades hepáticas desarrollan el síndrome hepatorrenal, aunque por lo general se cree que la presión arterial baja y el suministro de sangre reducido a los riñones es una de las razones principales. En teoría, los fármacos que aumentan la presión arterial pueden ser beneficiosos. El fármaco terlipresina combinado con la infusión de albúmina (una proteína) es el tratamiento recomendado para los pacientes con síndrome hepatorrenal según las guías. Algunos países (p.ej. los EE.UU.) no han aprobado la administración de terlipresina y los investigadores han sugerido que pueden usarse otros fármacos vasoactivos en su lugar.
Pregunta de la revisión
¿La terlipresina es más beneficiosa o segura que otros fármacos vasoactivos para el tratamiento del síndrome hepatorrenal?
Fecha de la búsqueda
Noviembre 2016.
Características de los estudios
Se incluyeron 10 ensayos clínicos con 474 participantes. Siete ensayos compararon terlipresina y albúmina versus noradrenalina y albúmina. Los tres ensayos restantes compararon terlipresina y albúmina versus midodrina y octreotida, u octreotida sola, o dopamina. En total, 241 participantes recibieron terlipresina y 233 participantes recibieron otros fármacos vasoactivos (fármacos que cambian la presión arterial; noradrenalina, octreotida, midodrina, o dopamina).
Fuentes de financiación de los estudios
En cinco ensayos, los investigadores declararon específicamente que no recibieron financiamiento por parte de organizaciones que podrían beneficiarse con los resultados del ensayo. No se tenía información sobre la fuente de financiación de los cinco ensayos restantes.
Resultados clave
Los análisis encontraron evidencia incierta para apoyar o refutar la terlipresina versus otros fármacos vasoactivos en el tratamiento del síndrome hepatorrenal al evaluar la mortalidad o los efectos secundarios graves. Los análisis indicaron que el tratamiento con terlipresina puede tener un efecto beneficioso sobre el síndrome hepatorrenal al reducir el número de participantes con síndrome hepatorrenal persistente. Los análisis adicionales demostraron que el número de participantes de los ensayos fue demasiado pequeño para tener seguridad acerca de lo anterior. En consecuencia, se encontró que pueden haberse omitido diferencias importantes entre la terlipresina y otros fármacos vasoactivos.
Calidad de la evidencia
Se encontró que la evidencia fue de muy baja calidad debido al alto riesgo de sesgo y al número pequeño de participantes.
Conclusiones de los autores
Se necesitan ensayos adicionales amplios de alta calidad para evaluar si la terlipresina es más beneficiosa o más segura que otros fármacos vasoactivos.
Authors' conclusions
Summary of findings
| Terlipressin compared to other vasoactive drugs for hepatorenal syndrome | ||||||
| Patient or population: people with cirrhosis and hepatorenal syndrome | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with other vasoactive drugs | Risk with terlipressin | |||||
| Mortality (All‐cause) | Study population | RR 0.96 | 474 | ⊕⊝⊝⊝ | Downgraded because of clinical heterogeneity, 8/10 randomised clinical trials were at high risk of bias and, the results of Trial Sequential Analysis. | |
| 601 per 1000 | 577 per 1000 | |||||
| Hepatorenal syndrome (Number of participants who did not achieve reversal of hepatorenal syndrome) | Study population | RR 0.79 | 394 | ⊕⊝⊝⊝ | Downgraded because of clinical heterogeneity, all trials were judged as high risk of bias, and results of Trial Sequential Analysis. | |
| 560 per 1000 | 442 per 1000 | |||||
| Serious adverse events | Study population | RR 0.96 | 474 | ⊕⊝⊝⊝ | Downgraded because of clinical heterogeneity, all trials were judged as high risk of bias, and results of Trial Sequential Analysis. | |
| 609 per 1000 | 585 per 1000 | |||||
| Non‐serious adverse events: diarrhoea or abdominal pain, or both | Study population | RR 3.50 | 221 | ⊕⊝⊝⊝ | Downgraded because of clinical heterogeneity, all trials were judged as high risk of bias, and results of the Trial Sequential Analysis. | |
| 19 per 1000 | 65 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aIn the assessment of mortality, we classified two randomised clinical trials at low risk of bias and eight at high risk of bias. bThe randomised clinical trials were not designed for equivalence or inferiority analysis. The Trial Sequential Analysis showed that sample size did not reach the required information size for equivalence/inferiority meta‐analysis. cClinical heterogeneity. dWe classified all randomised clinical trials at high risk of bias in all non‐mortality outcomes. | ||||||
Background
Description of the condition
Hepatorenal syndrome is a potentially reversible acute kidney injury associated with severe liver disease (Arroyo 1996). The diagnosis hepatorenal syndrome includes cirrhosis, ascites, and impaired renal function as well as exclusion of parenchymal renal disease and factors that may precipitate renal insufficiency (Salerno 2007). Among people hospitalised with cirrhosis and ascites, 25% develop acute renal insufficiency (Fede 2012). Among people with cirrhosis and acute renal insufficiency, 25% have hepatorenal syndrome (Fede 2012). In one cohort study of 234 non‐azotaemic participants with cirrhosis and ascites, 18% developed hepatorenal syndrome after one year (Gines 1993).
Hepatorenal syndrome is divided into two types. Type 1 has the most rapid course. Factors such as infection, alcoholic hepatitis, and bleeding may precipitate type 1 hepatorenal syndrome (Israelsen 2015). Without treatment, the median survival is about two weeks. Type 2 hepatorenal syndrome is often associated with refractory ascites and has a more protracted course with a median survival of about six months (Arroyo 1996; Gines 2003; Salerno 2007).
Description of the intervention
Main factors in the pathophysiology of hepatorenal syndrome are splanchnic vasodilation and increased cardiac output. Hepatorenal syndrome develops when the arterial pressure drops as the underlying condition progresses or due to factors leading to low blood pressure. Therefore, administration of vasoconstrictors may be beneficial. Terlipressin, an analogue of vasopressin, induces vasoconstriction mediated by type 1 vasopressin receptors in the smooth muscle cells of the blood vessel wall (Krag 2008). Other vasoactive drugs such as noradrenaline, octreotide, and midodrine may be equally effective. Noradrenaline is a catecholamine with high affinity for the alpha‐adrenergic receptors that mediate vasoconstriction in the venous and arterial system (Duvoux 2002). Octreotide is an octapeptide that mimics somatostatin. Octreotide is a potent vasoconstrictor and is used in bleeding oesophageal varices. The active metabolite of midodrine is an alpha‐1‐receptor agonist which increases the vascular tone (Angeli 1999).
Albumin infusions are recommended in combination with vasoconstrictors for type 1 hepatorenal syndrome (EASL 2010; Runyon 2013). By increasing the cardiac preload and cardiac output, albumin improves the effective arterial blood volume (Sort 1999). Furthermore, albumin infusions decrease the risk of developing hepatorenal syndrome, and, since 2007, plasma expansion with albumin for 48 hours is mandatory prior to diagnose hepatorenal syndrome (Salerno 2007; Angeli 2015). Consequently, almost all randomised clinical trials assessing vasoactive drugs for hepatorenal syndrome used standardised doses of cotreatment with albumin.
How the intervention might work
Hepatorenal syndrome is associated with the circulatory changes seen in cirrhosis including portal hypertension leading to splanchnic vasodilation; effective underfilling of the renal arteries; and activation of the endogenous vasoconstrictors; renin‐angiotensin‐aldosterone, the arginine‐vasopressin, and the sympathetic nervous systems (Pasqualetti 1998; Cardenas 2003; Moller 2004; Ruiz‐del‐Arbol 2005). Activation of the the endogenous vasoconstrictors may result in severe vasoconstriction of the renal arteries leading to hepatorenal syndrome (Cardenas 2003). Vasoactive drugs that increase splanchnic arterial tone may reverse the process.
Why it is important to do this review
Six randomised clinical trials compared terlipressin versus placebo for hepatorenal syndrome (Hadengue 1998; Solanki 2003; Martín‐Llahí 2008; Neri 2008; Sanyal 2008; Boyer 2016). One meta‐analysis including participants with type 1 hepatorenal syndrome found a beneficial effect of terlipressin versus placebo on reversal of hepatorenal syndrome (Fabrizi 2009). Our previous Cochrane systematic review including participants with type 1 or type 2 hepatorenal syndrome also found that terlipressin versus placebo or no intervention may reduce mortality and increase the proportion of participants with improved renal function (Gluud 2010; Gluud 2012). At present, guidelines recommend terlipressin as the treatment of choice for people with hepatorenal syndrome (EASL 2010). The drug is not available in the USA and other countries (Runyon 2013). Randomised clinical trials have compared terlipressin versus noradrenaline (Duvoux 2002; Alessandria 2007; Sharma 2008). The results suggested that the interventions were equally effective. However, due to the size of the trials, the results may have been inconclusive. Our previous Cochrane Review and one subsequent meta‐analysis found no significant differences between terlipressin and other vasoactive drugs (Gluud 2010; Gluud 2012; Nassar 2014). The Cochrane Review included two randomised clinical trials comparing terlipressin versus noradrenaline and the meta‐analysis included four randomised clinical trials. Both reviews found inconclusive evidence. Therefore, we conducted this updated review.
Objectives
To evaluate the beneficial and harmful effects of terlipressin versus other vasoactive drugs for people with hepatorenal syndrome.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised clinical trials regardless of blinding, publication status, or language in analyses of benefits and harms. We planned to include quasi‐randomised and observational studies in the assessment of harms identified in the searches. If, during the selection of trials, we had identified observational studies (i.e. quasi‐randomised studies, cohort studies, or patient reports) reporting adverse events caused by, or associated with, the interventions in our review, we planned to include these studies for a review of the adverse events only. We did not specifically search for observational studies for inclusion, which is a known limitation of our systematic review.
Types of participants
People with cirrhosis and type 1 or type 2 hepatorenal syndrome according to current or earlier diagnostic criteria (Arroyo 1996; Salerno 2007; Angeli 2015).
Types of interventions
Comparisons of terlipressin versus other vasoactive drugs (including noradrenaline, octreotide, midodrine, or dopamine) regardless of dose or duration of interventions. We accepted cointerventions including albumin.
Types of outcome measures
We assessed all outcomes at the maximum duration of follow‐up (Gluud 2017).
Primary outcomes
-
Mortality (all‐cause).
-
Hepatorenal syndrome (persistent hepatorenal syndrome despite treatment).
-
Serious adverse events: defined as any untoward medical occurrence that led to death, was life‐threatening, or required hospitalisation or prolongation of hospitalisation (ICH‐GCP 1997). We assessed serious adverse events as a composite outcome and conducted analyses of individual serious adverse events.
Secondary outcomes
-
Health‐related quality of life.
-
Non‐serious adverse events defined as adverse events that did not fulfil the criteria for serious adverse events.
Search methods for identification of studies
Electronic searches
We searched The Cochrane Hepato‐Biliary Group Controlled Trials Register (Gluud 2017; November 2016), Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (2016, Issue 11), MEDLINE Ovid (1946 to November 2016), Embase Ovid (1974 to November 2016), and Science Citation Index Expanded (Web of Science; 1900 to November 2016) (Royle 2003), using the search strategies described in Appendix 1.
Searching other resources
We scanned the reference lists of relevant articles, and proceedings from meetings of the British Society for Gastroenterology, the British Association for the Study of the Liver, the European Association for the Study of the Liver, the United European Gastroenterology Week, the American Gastroenterological Association, and the American Association for the Study of Liver Diseases. We wrote to the principal authors of randomised clinical trials and the pharmaceutical companies involved in the production of vasoactive drugs for additional information about completed randomised clinical trials and for information about any ongoing randomised clinical trials, and searched the database ClinicalTrials.gov (clinicaltrials.gov) and the World Health Organization (WHO) online trial meta‐register (apps.who.int/trialsearch/).
Data collection and analysis
We performed the review following the recommendations of the Cochrane Hepato‐Biliary Group (Gluud 2017).
Selection of studies
All authors participated independently in the literature searches, the identification of potentially eligible trials and studies, and in the decision regarding inclusion or exclusion of trials. We reached the final selection through consensus and resolved disagreements through discussion. We listed details of all included randomised clinical trials in summary tables and listed all excluded studies with the reasons for their exclusion.
Data extraction and management
Three authors (MI, AA, and LG) independently collected data using pilot‐tested data extraction sheets and resolved contrary opinions through discussion. We requested missing data and other information from authors of included randomised clinical trials.
We collected the following data:
-
general study information:
-
year, country, and language of publication (if published);
-
funding;
-
design;
-
-
intervention:
-
type, dose and duration;
-
cointerventions;
-
-
participants:
-
characteristics (age, proportion with cirrhosis, proportion of men/women, aetiology of liver disease);
-
criteria used to diagnose hepatorenal syndrome;
-
withdrawals and losses to follow‐up;
-
-
outcomes:
-
outcomes assessed and duration of follow‐up.
-
Assessment of risk of bias in included studies
We assessed bias control using the domains described in the Cochrane Hepato‐Biliary Group Module (Gluud 2017), and classified the risk of bias for separate domains as high, unclear, or low (Higgins 2011). We also combined the bias domains in an overall assessment as described below.
Allocation sequence generation
-
Low risk of bias: the study authors performed sequence generation using computer random number generation or a random number table. We considered drawing lots, tossing a coin, shuffling cards, and throwing dice as adequate if an independent person not otherwise involved in the study performed them.
-
Unclear risk of bias: the study authors did not specify the method of sequence generation.
-
High risk of bias: the sequence generation method was not random. We only considered such studies for assessment of harms.
Allocation concealment
-
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. A central and independent randomisation unit controlled allocation. The investigators were unaware of the allocation sequence (e.g. if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
-
Unclear risk of bias: the study authors did not describe the method used to conceal the allocation so the intervention allocations may have been foreseen in advance of, or during, enrolment.
-
High risk of bias: it was likely that the investigators who assigned the participants knew the allocation sequence. We only considered such studies for assessment of harms.
Blinding of participants and personnel
-
Low risk of bias: any of the following: no blinding or incomplete blinding, but the outcome was not likely to be influenced by lack of blinding (e.g. mortality) (Wood 2008; Savović 2012); or blinding of participants and key study personnel ensured, and it was unlikely that the blinding could have been broken.
-
Unclear risk of bias: insufficient information to permit judgement of 'low risk' or 'high risk.'
-
High risk of bias: any of the following: no blinding or incomplete blinding, and the outcome was likely to be influenced by lack of blinding (non‐mortality outcomes).
Blinded outcome assessment
-
Low risk of bias: any of the following: no blinding of outcome assessment, but outcome measurement was not likely to be influenced by lack of blinding (mortality) (Wood 2008; Savović 2012); or blinding of outcome assessment ensured, and unlikely that the blinding could have been broken.
-
Unclear risk of bias: insufficient information to permit judgement of 'low risk' or 'high risk.'
-
High risk of bias: any of the following: no blinding of outcome assessment, and the outcome measurement was likely to be influenced by lack of blinding (non‐mortality outcomes); or blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement was likely to be influenced by lack of blinding (non‐mortality outcomes).
Incomplete outcome data
-
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. The study used sufficient methods, such as multiple imputations, to handle missing data.
-
Unclear risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
-
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
-
Low risk of bias: the trial reported the following predefined outcomes: all‐cause mortality, hepatorenal syndrome, and serious adverse events. If the original trial protocol was available, the outcomes should have been those called for in that protocol. We only considered information from trial registries (e.g. www.clinicaltrials.gov) if the protocol was registered at the time that the trial was begun.
-
Unclear risk of bias: the study authors did not report all predefined outcomes fully, or it was unclear whether the study authors recorded data on these outcomes or not.
-
High risk of bias: the study authors did not report one or more predefined outcomes.
For‐profit bias
-
Low risk of bias: the trial appeared free of industry sponsorship or other type of for‐profit support.
-
Unclear risk of bias: the trial did not provide any information on clinical trial support or sponsorship.
-
High risk of bias: the trial was sponsored by industry or received other type of for‐profit support.
Other bias
-
Low risk of bias: the trial appeared free of other factors that could put it at risk of bias (e.g. different follow‐up or administration of an inappropriate doses).
-
Unclear risk of bias: the trial may or may not have been free of other factors that could put it at risk of bias.
-
High risk of bias: there were other factors in the trial that could put it at risk of bias.
Overall bias assessment
-
Low risk of bias: all domains were low risk of bias using the definitions described above.
-
High risk of bias: one or more of the bias domains were of unclear or high risk of bias.
Measures of treatment effect
We used risk ratios (RR) with 95% confidence intervals (CI).
Unit of analysis issues
We planned to include the first period from cross‐over trials due to the severe prognosis associated with the condition. None of the identified randomised clinical trials used a cross‐over design.
Dealing with missing data
We extracted data on all participants randomised to allow intention‐to‐treat analyses, the number of participants with missing outcome, and reasons for missing data. To evaluate the importance of missing data, we planned to conduct a worst‐case scenario analysis and a best‐worst case scenario analysis (Gluud 2017). We did not conduct the analyses because we were unable to identify the number of participants who had missing data.
Assessment of heterogeneity
We visually inspected forest plots and expressed heterogeneity as I² values using the following thresholds: 0% to 40% (unimportant), 41% to 60% (moderate), 61% to 80% (substantial), and greater than 80% (considerable).
Assessment of reporting biases
For meta‐analyses with at least 10 randomised clinical trials, we planned to assess reporting biases through regression analyses using the Harbord test (Harbord 2006).
Data synthesis
We performed the analyses in Review Manager 5 (RevMan 2014), STATA version 14 (Stata 2014), and Trial Sequential Analysis (TSA 2011), and used the GRADEpro software (GRADEpro) to prepare a 'Summary of findings' table.
Meta‐analysis
In our primary analyses, we stratified randomised clinical trials based on the type of control intervention. We compared the fixed‐effect and random‐effects estimates of the intervention effect. The estimates were similar. Accordingly, we assumed that any small‐study effects had little influence on the intervention effect estimate. If the random‐effects estimate had been more beneficial, we planned to re‐evaluate whether it was reasonable to conclude that the intervention was more effective in the smaller studies. If the larger studies tended to be those conducted with greater methodological rigour, or conducted in circumstances more typical of the use of the intervention in practice, then we planned to report the results of meta‐analyses restricted to the larger, more rigorous studies. Based on the expected clinical heterogeneity, we expected that a number of analyses would display statistical between‐trial heterogeneity (I² greater than 0%). For random‐effects models, precision decreased with increasing heterogeneity and CIs widened correspondingly. Therefore, we expected that the random‐effects model would give the most conservative (and a more correct) estimate of the intervention effect. Accordingly, we reported the results of our analyses based on random‐effects meta‐analyses.
Trial Sequential Analysis
We performed Trial Sequential Analyses for our primary outcomes (Higgins 2008; Wetterslev 2008; Thorlund 2011; Wetterslev 2017). We defined the required information size (also known as the heterogeneity‐adjusted required information size) as the number of participants needed to detect or reject an intervention effect based on the relative risk reduction (RRR) and the control group risk (CGR). The analyses show firm evidence if the Z‐curve crosses the monitoring boundary (also known as the trial sequential monitoring boundary) before reaching the required information size. We constructed futility boundaries to evaluate the uncertainty of obtaining a chance negative finding and performed the analyses with alpha set to 3% and power to 90% model‐based diversity. We originally planned to limit the analyses to randomised clinical trials with a low risk of bias, but we only identified two such trials. Therefore, we included all randomised clinical trials in our analyses. We reduced the RRR based on the recommendations from the Hepato‐Biliary Group and based the CGR on the proportions of events in the terlipressin group in the meta‐analysis in Allegretti 2017.
-
Mortality: CGR 52%, RRR 20%, heterogeneity correction 30%.
-
Hepatorenal syndrome (persistent hepatorenal syndrome despite treatment): CGR 63%, RRR 25%, heterogeneity correction 50%.
-
Serious adverse events: CGR 11%, RRR 25%, heterogeneity correction 20%.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses comparing types of other vasoactive drug and participants with type 1 or type 2 hepatorenal syndrome. We conducted subgroup analyses on mortality based on our assessment of bias control.
Sensitivity analysis
We performed a sensitivity analysis excluding randomised clinical trials published in abstract form. We planned to conduct a worse‐case‐scenario analysis as described above, but we did not identify randomised clinical trials describing the number of participants with missing outcome data in the two groups.
'Summary of findings' table
We used the GRADE system to evaluate the quality of the evidence for outcomes reported in the review considering the within‐study risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimate, and risk of publication bias (GRADEpro). Two authors (MI and LG) created the 'Summary of findings' table, which included the primary outcomes and the most common adverse events; that is, diarrhoea and abdominal pain.
Results
Description of studies
We included 10 randomised clinical trials (see Characteristics of included studies table). Our searches did not identify eligible non‐randomised studies. We excluded 11 randomised clinical trials, one quasi‐randomised trial, and one observational study (see Characteristics of excluded studies table).
Results of the search
We identified 619 potentially relevant references in electronic databases and four additional records through manual searches (Figure 1). After removing duplicates and clearly irrelevant references, 427 references remained. After screening these 427 references, we retrieved 26 references for further assessment. In total, we excluded 14 references referring to 13 randomised clinical trials because they did not compare terlipressin with other vasoactive drugs. The remaining 12 references referred to 10 randomised clinical trials fulfilling all of our inclusion criteria and none of our exclusion criteria. Eight of these were published in full‐paper articles (Alessandria 2007; Sharma 2008; Singh 2012; Badawy 2013; Ghosh 2013; Srivastava 2015; Cavallin 2016; Goyal 2016), and two in abstracts (Copaci 2013; Indrabi 2013).

Study flow diagram.
We wrote to the authors of included randomised clinical trials to ask for additional information about included participants. However, we received no additional data.
Included studies
Participants
The countries of origin were India (Sharma 2008; Singh 2012; Ghosh 2013; Indrabi 2013; Srivastava 2015; Goyal 2016), Italy (Alessandria 2007; Cavallin 2016), Egypt (Badawy 2013), and Romania (Copaci 2013). The trials included 474 participants. The mean age ranged from 39 to 65 years and the proportion of men from 52% to 93%. The proportion of participants with alcoholic liver disease ranged from 20% to 94%.
Four randomised clinical trials (Alessandria 2007; Sharma 2008; Badawy 2013; Ghosh 2013) used the 1996 criteria (Arroyo 1996; Appendix 2) to diagnose hepatorenal syndrome and four randomised clinical trials (Singh 2012; Srivastava 2015; Cavallin 2016; Goyal 2016) used the 2007 criteria (Salerno 2007; Appendix 2). The remaining two randomised clinical trials did not specify the criteria used to diagnose hepatorenal syndrome (Copaci 2013; Indrabi 2013). Seventy‐seven per cent of the included participants had type 1 hepatorenal syndrome and the remaining 23% had type 2 hepatorenal syndrome. Two papers did not provide separate outcome data for participants with type 1 and type 2 hepatorenal syndrome (Copaci 2013; Cavallin 2016). The vast majority (80/88 participants) in these two randomised clinical trials had type 1 hepatorenal syndrome, and consequently due to the lack of separate outcome data, we included all 88 participants in our subgroup analyses of type 1 hepatorenal syndrome.
Interventions
All randomised clinical trials compared terlipressin versus other vasoactive drugs. All participants received cointervention with albumin. Seven randomised clinical trials used a treatment duration protocol running until reversal of hepatorenal syndrome, death, liver transplantation, or a maximum of two weeks (Alessandria 2007; Sharma 2008; Singh 2012; Badawy 2013; Ghosh 2013; Cavallin 2016; Goyal 2016). One randomised clinical trial used five days of treatment (Srivastava 2015), and two randomised clinical trials did not describe the treatment duration (Copaci 2013; Indrabi 2013).
Terlipressin
Six randomised clinical trials used an intravenous bolus injection (Alessandria 2007; Sharma 2008; Singh 2012; Ghosh 2013; Srivastava 2015; Goyal 2016), and two randomised clinical trials used continuous infusions (Badawy 2013; Cavallin 2016). We were unable to gather information of the administration form (bolus or continuous infusion) from two trials (Copaci 2013; Indrabi 2013). Eight randomised clinical trials used a dose titration regimen for terlipressin (Alessandria 2007; Sharma 2008; Singh 2012; Badawy 2013; Copaci 2013; Ghosh 2013; Cavallin 2016; Goyal 2016). The initial daily dose ranged from 2 mg to 6 mg and was increased in a stepwise manner to a maximum dose of 6 mg to 12 mg until reaching an absolute reduction in serum creatinine of less than 1 mg/dL or less than 25% from baseline after 48 to 72 hours. One randomised clinical trial used a fixed dose of 0.5 mg per six hours (Srivastava 2015). One randomised clinical trial did not provide information about the dose (Indrabi 2013).
Other vasoactive drugs
Seven randomised clinical trials compared terlipressin versus noradrenaline (Alessandria 2007; Sharma 2008; Singh 2012; Badawy 2013; Ghosh 2013; Indrabi 2013; Goyal 2016), one randomised clinical trial compared terlipressin versus midodrine and octreotide (Cavallin 2016), one randomised clinical trial compared terlipressin versus octreotide (Copaci 2013), and one randomised clinical trial compared terlipressin versus dopamine (Srivastava 2015). Noradrenaline and dopamine were administrated as continuous intravenous infusions. Octreotide was given as subcutaneous bolus injections and midodrine as oral tablets. The dose depended on the drug.
-
Noradrenaline: in six randomised clinical trials, the initial dose of 0.5 mg/hour was increased in a stepwise manner to a maximum dose of 3 mg/hour (Sharma 2008; Singh 2012; Badawy 2013; Ghosh 2013; Indrabi 2013; Goyal 2016). One randomised clinical trial used an initial dose of 0.1 μg/kg/minute increased stepwise to a maximum of 0.7 μg/kg/minute in lack of response (Alessandria 2007). All randomised clinical trials adjusted the dose based on the mean arterial pressure and urine output.
-
Dopamine: 2 μg/kg/minute (Srivastava 2015).
-
Midodrine: the initial dose of 7.5 mg was increased to 12.5 mg if the change in serum creatinine was less than 25% within 48 hours (Cavallin 2016).
-
Octreotide: the initial dose of 100 μg was increased to 200 μg if the change in serum creatinine was less than 25% within 48 hours (Copaci 2013; Cavallin 2016).
Cointerventions
All included randomised clinical trials treated both intervention groups using equal doses of intravenous albumin infusion in combination with the vasoactive drugs. Overall, the mean dose of albumin ranged from 20 g/day to 56 g/day. Four trials used 20 g/day to 40 g/day (Sharma 2008; Singh 2012; Ghosh 2013; Srivastava 2015). Two randomised clinical trials used 1 g/kg bodyweight at the inclusion day, followed by 20 g/day to 40 g/day (Copaci 2013; Cavallin 2016). Two randomised clinical trials titrated the dose to maintain a central venous pressure between 10 cmH2O and 15 cmH2O (Alessandria 2007; Badawy 2013). One randomised clinical trial did not report the albumin dose (Indrabi 2013).
Outcome measures
All 10 randomised clinical trials described mortality and serious adverse events using follow‐up between 15 and 90 days. Nine randomised clinical trials reported reversal of hepatorenal syndrome (Alessandria 2007; Sharma 2008; Singh 2012; Badawy 2013; Copaci 2013; Ghosh 2013; Indrabi 2013; Cavallin 2016; Goyal 2016) and six randomised clinical trials reported non‐serious adverse events (Sharma 2008; Singh 2012; Ghosh 2013; Srivastava 2015; Goyal 2016; Cavallin 2016). None reported health‐related quality of life.
Excluded studies
In total, we excluded 13 trials evaluating vasoactive drugs for hepatorenal syndrome (see Characteristics of excluded studies table). We excluded eight randomised clinical trials evaluating terlipressin versus placebo (Hadengue 1998; Yang 2001; Solanki 2003; Martín‐Llahí 2008; Neri 2008; Pulvirenti 2008; Sanyal 2008; Boyer 2016), and one randomised clinical trial comparing noradrenaline versus midodrine and octreotide (Tavakkoli 2012). In addition, we excluded two randomised clinical trials comparing the administration form (Cavallin 2015) or dose of terlipressin (Wan 2014). Finally, we excluded two non‐randomised studies without information about harms (Silawat 2011 (quasi‐randomised trial); Nguyen‐Tat 2015 (observational study)).
Risk of bias in included studies
For 'Risk of bias' summary, see Figure 2 and Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

"Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
'+' = low risk of bias;
'‐' = high risk of bias;
'?' = unclear risk of bias.
Allocation
Three randomised clinical trials generated the allocation sequence using a computer‐generated list of random numbers and concealed the allocation by using serially numbered opaque sealed envelopes (Singh 2012; Ghosh 2013; Cavallin 2016). We classified these three randomised clinical trials as low risk of selection bias. The remaining seven randomised clinical trials did not provide adequate descriptions of both randomisation and concealment and were classified at unclear risk of bias in at least one of the domains (Alessandria 2007; Sharma 2008; Badawy 2013; Copaci 2013; Indrabi 2013; Srivastava 2015; Goyal 2016).
Blinding
All randomised clinical trials were open and none used blinded outcome assessment. We classified all randomised clinical trials as high risk of bias for these two domains in the evaluation of non‐mortality outcomes (Alessandria 2007; Sharma 2008; Singh 2012; Badawy 2013; Copaci 2013; Ghosh 2013; Indrabi 2013; Cavallin 2016; Srivastava 2015; Goyal 2016).
Incomplete outcome data
Eight randomised clinical trials had no losses to follow‐up or withdrawals, and included all participants in their analyses (Alessandria 2007; Sharma 2008; Singh 2012; Copaci 2013; Indrabi 2013; Srivastava 2015; Goyal 2016; Cavallin 2016). Two randomised clinical trials excluded participants after randomisation in their analyses, but they did not describe the number of participants with missing outcomes (Badawy 2013; Ghosh 2013). Consequently, we classified eight randomised clinical trials at low risk and two at high risk of attrition bias.
Selective reporting
Nine randomised clinical trials defined and described clinically relevant outcomes (Alessandria 2007; Sharma 2008; Singh 2012; Copaci 2013; Badawy 2013; Ghosh 2013; Srivastava 2015; Goyal 2016; Cavallin 2016). One randomised clinical trial reported mortality and serious adverse events but not hepatorenal syndrome (Srivastava 2015). We classified nine randomised clinical trials at low risk and one at high risk of reporting bias.
For‐profit bias
Five randomised clinical trials described competing interests (Alessandria 2007; Sharma 2008; Singh 2012; Srivastava 2015; Cavallin 2016). None received funding or other support from for‐profit companies. The remaining five randomised clinical trials did not describe funding (Badawy 2013; Copaci 2013; Ghosh 2013; Indrabi 2013; Goyal 2016).
Overall bias assessment
In the assessment of mortality, we classified two randomised clinical trials at low risk of bias (Singh 2012; Cavallin 2016), and eight at high risk of bias (Alessandria 2007; Sharma 2008; Badawy 2013; Copaci 2013; Ghosh 2013; Indrabi 2013; Srivastava 2015; Goyal 2016).
All randomised clinical trials were high risk of bias in all remaining outcomes (Alessandria 2007; Sharma 2008; Singh 2012; Badawy 2013; Copaci 2013; Ghosh 2013; Indrabi 2013; Srivastava 2015; Goyal 2016; Cavallin 2016).
Effects of interventions
We evaluated the effects of interventions on the primary outcomes; mortality, hepatorenal syndrome (persistent despite treatment), and serious adverse events.
Mortality (all‐cause)
We retrieved mortality data from all 10 randomised clinical trials (Analysis 1.1). The analysis showed no significant difference between terlipressin and other vasoactive drugs when including all trials (RR 0.96, 95% CI 0.88 to 1.06; 474 participants; I² = 0%) or when including the two trials with a low risk of bias in the overall assessment (RR 0.92, 95% CI 0.63 to 1.36; 94 participants). In Trial Sequential Analysis including all trials regardless of bias control (Figure 4), the cumulative Z‐curve did not cross the monitoring boundaries for benefit, harm, or futility. Subgroup analyses found lack of evidence supporting different effects between terlipressin and noradrenaline (RR 0.98, 95% CI 0.88 to 1.08; 306 participants; 7 trials; I² = 0%); midodrine and octreotide (RR 0.71, 95% CI 0.40 to 1.28; 48 participants; 1 trial); octreotide alone (RR 0.75, 95% CI 0.32 to 1.77; 40 participants; 1 trial); or dopamine and furosemide (RR 0.97, 95% CI 0.77 to 1.22; 80 participants; 1 trial) (Analysis 1.2), in participants with type 1 or type 2 hepatorenal syndrome (Analysis 1.3), or trials published as full paper articles or abstracts (Analysis 1.4).

Trial Sequential Analysis of 10 randomised clinical trials (474 participants) evaluating terlipressin versus other vasoactive drugs for people with hepatorenal syndrome on mortality. The analysis was made with power 90%, alpha 3%, a relative risk reduction (RRR) of 20%, a control group risk (CGR) of mortality of 52%, and a model variance ‐ based heterogeneity correction of 30%. The risk ratio was 0.96 (97% confidence interval 0.79 to 1.18). The cumulative Z‐curve (blue line) did not cross the diversity‐adjusted trial monitoring boundary for benefit.
Hepatorenal syndrome
One trial did not report the number of participants with persistent hepatorenal syndrome despite treatment (Srivastava 2015). The meta‐analysis of remaining nine trials found a significant beneficial effect of terlipressin versus other vasoactive drugs (RR 0.79, 95% CI 0.63 to 0.99; 394 participants; I² = 26%; Analysis 1.5). The analysis did not include trials with a low risk of bias. In Trial Sequential Analysis, including the nine trials (Figure 5), the cumulative Z‐curve did not cross the monitoring boundaries for benefit, harm, or futility. Subgroup analyses showed that terlipressin was superior to octreotide alone (RR 0.56, 95 % CI 0.33 to 0.96) and midodrine combined with octreotide (RR 0.47, 95 % CI 0.30 to 0.72), but each analysis was only based on one trial (Analysis 1.5). There were no differences between trials comparing terlipressin and noradrenaline (Analysis 1.5), participants with type 1 or type 2 hepatorenal syndrome (Analysis 1.6), or trials published as full‐paper articles or abstracts (Analysis 1.7).
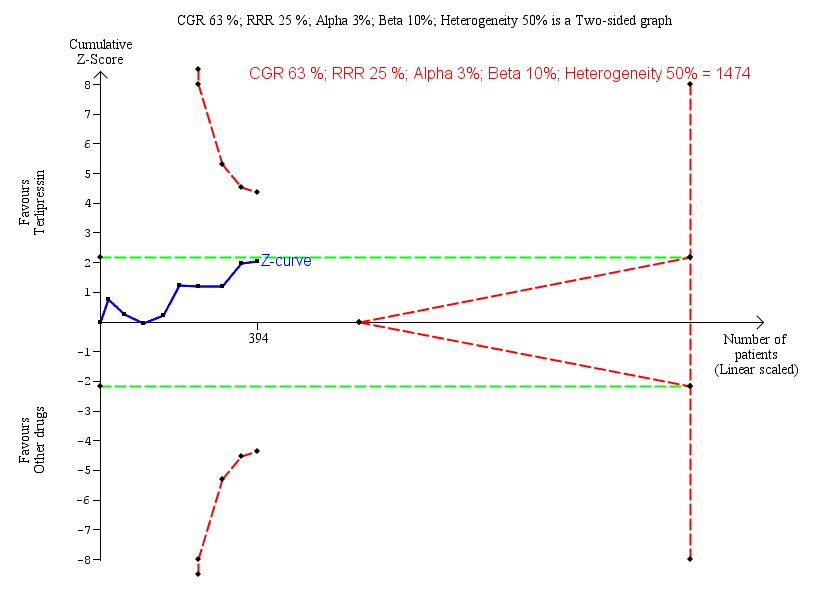
Trial Sequential Analysis of nine randomised clinical trials (394 participants) evaluating terlipressin versus other vasoactive drugs for people with hepatorenal syndrome on lack of reversal of hepatorenal syndrome. The analysis was made with power 90%, alpha 3%, a relative risk reduction (RRR) of 25%, a control group risk (CGR) of lack of reversal of hepatorenal syndrome of 63%, and a heterogeneity correction of 50%. The risk ratio was 0.79 (97% confidence interval 0.48 to 1.31). The cumulative Z‐curve (blue line) does not cross the diversity‐adjusted trial monitoring boundary for benefit.
Serious adverse events
We extracted data of serious adverse events as a composite outcome from all trials (Analysis 1.8). None had a low risk of bias in the overall assessment. Overall, there was no significant difference between terlipressin and other vasoactive drugs (RR 0.96, 95% CI 0.88 to 1.06; I² = 0%). In subgroup analysis, terlipressin did not increase the risk of major cardiovascular events (RR 0.88, 95% CI 0.13 to 5.98; Analysis 1.9). Trial Sequential Analysis showed insufficient evidence to support or refute a beneficial or detrimental effect of terlipressin versus other vasoactive drugs (Figure 6).

Trial Sequential Analysis of two randomised clinical trials (88 participants) evaluating terlipressin versus other vasoactive drugs for people with hepatorenal syndrome on cardiovascular adverse events. The analysis was made with power 90%, alpha 3%, a relative risk reduction (RRR) of 25%, a control group risk (CGR) of cardiovascular adverse events of 15%, and a heterogeneity correction of 20%. The diversity‐adjusted trial monitoring boundary for harm was not included in the figure due to insufficient information. The estimated required information size was 4831 participants. Accordingly, with an accrued number of participants of 88, the required number of participants was not achieved.
Secondary outcomes
Health‐related quality of life
None of the included trials evaluated the effect of the interventions on health‐related quality of life.
Non‐serious adverse events
We were able to gather data on non‐serious adverse events from six trials (Analysis 1.10). Overall, we found no evidence supporting differences between terlipressin and other vasoactive drugs regarding the overall risk of non‐serious adverse events (RR 1.82, 95 % CI 1.00 to 3.31; 301 participants; I² = 0%), but that terlipressin increased the risk of diarrhoea or abdominal pain, or both (RR 3.50, 95% CI 1.19 to 10.27; 221 participants; 5 trials; I² = 0%; Analysis 1.11). We were unable to evaluate these two non‐serious adverse events (diarrhoea or abdominal pain) separately.
'Summary of findings' tables
We downgraded the evidence to very low quality (summary of findings Table for the main comparison). The main reasons for downgrading the evidence were risk of bias, imprecision, and the results from the Trial Sequential Analyses.
Discussion
Summary of main results
This systematic review included a small number of randomised clinical trials comparing terlipressin versus other vasoactive drugs. The statistical strength of the evidence was weak and the risk of bias considerable. The analyses found no evidence supporting differences between terlipressin and other vasoactive drugs regarding mortality and serious adverse events, but we found a beneficial effect of terlipressin on hepatorenal syndrome based on the proportion of participants without reversal. Subgroup analysis of the outcome hepatorenal syndrome showed a potential benefit of terlipressin compared to octreotide alone or octreotide combined with midodrine, but that analysis only included two small trials. Terlipressin is associated with an increased risk of abdominal pain or diarrhoea, or both. Trial Sequential Analyses confirmed that our review found insufficient evidence to support or refute a beneficial or harmful effect of terlipressin and that additional randomised clinical trials are needed. In this review, we both conducted overall analyses (to increase power and precision) and subgroup analyses comparing terlipressin versus the individual different comparators. The overall analysis was difficult to interpret, but apparently we found no heterogeneity.
Overall completeness and applicability of evidence
We included randomised clinical trials with participants diagnosed with hepatorenal syndrome. The initial diagnostic recommendations from 1996 included several major and minor criteria (Arroyo 1996). The subsequent revised diagnostic criteria now focus on evidence of severe liver disease, ascites, and exclusion of other causes of renal failure (Salerno 2007). We originally assumed that using the different diagnostic criteria would result in some degree of clinical heterogeneity. The potential influence of the diagnostic criteria did not lead to statistical heterogeneity. However, the lack of heterogeneity may also reflect the small number of trials and participants. Recommendations have suggested a revision based on the accepted criteria for diagnosing acute renal failure (Angeli 2015; Appendix 4). This suggestion was based on the criteria of acute kidney injury defined by the Acute Kidney Injury Network (Mehta 2007). In the previous criteria, a fixed value of serum creatinine greater than 133 mmol/L was used in diagnostic assessment. The revised criteria suggest that we should use an increase of serum creatinine of 0.3 mg/dL or greater (26.5 μmol/L or greater) or greater than 50% from baseline within 48 hours. The reasoning is that changes in serum creatinine are more sensitive to an acute reduction of renal function. The aim of these criteria is to allow earlier detection and intervention to improve the overall outcome for people diagnosed with cirrhosis and acute kidney injury or hepatorenal syndrome. The consensus recommendation suggests that people meeting the new criteria should be treated with vasoconstrictors and albumin. Additional studies are needed to evaluate if this is correct. In addition, the potential importance of albumin should be addressed in future randomised clinical trials. One meta‐analysis comprising 19 studies found a dose‐response association between survival and increased cumulative doses of albumin (Salerno 2015). We were unable to address the question in our review and were, therefore, unable to evaluate if the results reflect bias.
One study used three months of follow‐up and had one of the highest response rates (83%) and the lowest mortality (32%) (Alessandria 2007). During the follow‐up, all survivors except one had reversal of hepatorenal syndrome and underwent liver transplantations after the reversal of hepatorenal syndrome. This may suggest that some populations, such as candidates for liver transplantation, are more likely to benefit from treatment with vasoactive drugs.
Quality of the evidence
The lack of large, high‐quality randomised clinical trials is the main limitation of this review. We only identified two randomised clinical trials with a low risk of bias in the assessment of mortality. For the remaining outcomes, we classified all trials at high risk of bias. Lack of blinding was a concern as was an unclear control of selection bias. In addition, two trials had a high risk of attrition bias (Badawy 2013; Ghosh 2013). These trials excluded more than 15% of the participants after randomisation. Unfortunately, we were unable to gather data that allowed a worst‐case or an extreme worst‐case scenario analysis and we were, therefore, unable to evaluate the influence of losses to follow‐up. We contacted the authors, but we were unable to gather additional data. Another major concern is that several trials did not report systematically adverse events. Consequently, our results may underestimate the actual risk of adverse events. Similarly to this present meta‐analysis none of the included trials were powered for equivalence or inferiority analysis. We increased the risk of clinical heterogeneity by pooling type 1 and type 2 hepatorenal syndrome, two diagnostic criteria of hepatorenal syndrome and all types of other vasoactive drugs than terlipressin. The trials were not designed for equivalence or inferiority analysis. The Trial Sequential Analysis showed that the sample size did not reach the required information size for equivalence/inferiority meta‐analysis. Due to the lack of power, increased risk of clinical heterogeneity combined with the large proportion of trials classified at high risk of bias, we must classify the overall quality of evidence as very low in this present review.
Potential biases in the review process
One of the main limitations in this present review was the small sample size. Another limitation was the predominance of randomised clinical trials at high risk of bias.
According to the results of our Trial Sequential Analyses, none of our findings reached the required information size for superiority, equivalence, or inferiority meta‐analyses. This means that our significant findings of terlipressin versus other vasoactive drugs were inconclusive. In the same way, our non‐significant findings were inconclusive and may cover efficacy differences between the type of other vasoactive drugs.
Methodological concerns are highlighted in the Risk of bias in included studies section. However, it must be emphasised in particular, that the majority of trials in this present review had unsystematic reporting of adverse events, which may compromise the validity of our results on safety. Treatment with terlipressin is associated with an increased risk of ischaemia including cardiovascular events and with less serious events such as abdominal pain or diarrhoea, or both (Krag 2008). However, the risk of ischaemia is not unique to terlipressin as a vasoactive drug. One large‐scale randomised clinical trial including 778 participants tested noradrenaline versus vasopressin in septic shock and found equal risk of ischaemia, of which the majority of adverse events included cardiovascular and intestinal ischaemia (Russell 2008). The profile of adverse events of terlipressin and noradrenaline are closely linked to the mode of action of vasoconstrictors. From our results, we cannot suggest which vasoactive drug is safest. However, we must emphasise that treatment with all types of vasoactive drugs require close monitoring to balance between improving renal perfusion and prevent ischaemia.
We were unable to assess long‐term benefits and harms of terlipressin versus other vasoactive drugs because the majority of trials had 30 days' follow‐up and the longest follow‐up was three months.
We contacted the pharmaceutical companies producing vasoactive drugs, but we did not search for files of regulatory authorities.
Agreements and disagreements with other studies or reviews
Two meta‐analyses comparing terlipressin versus noradrenaline for hepatorenal syndrome included four randomised clinical trials (Nassar 2014; Mattos 2016). In agreement with our findings, the meta‐analyses found no clear difference between terlipressin and noradrenaline. Two additional meta‐analyses evaluated vasoactive drugs for type 1 hepatorenal syndrome and found results similar to ours (Facciorusso 2017; Gilford 2017). One of the meta‐analyses had a positive evaluation of the quality of the evidence (Facciorusso 2017). In agreement with the second meta‐analysis, we found no convincing evidence and that the majority of randomised clinical trials were too small and entailed a high risk of bias (Gilford 2017). The guidelines of management of hepatorenal type 1 by the European Association of Studying the Liver suggest that the first‐line treatment should be terlipressin combined with albumin (EASL 2010). The guidelines of management of hepatorenal syndrome type 1 by the American Association for Study of Liver Diseases recommends vasoactive drugs in combination with albumin (Runyon 2013). Terlipressin is not available in the USA and other countries; consequently the recommended vasoactive drugs include midodrine and octreotide, whereas noradrenaline should be considered in intensive care units.

Study flow diagram.
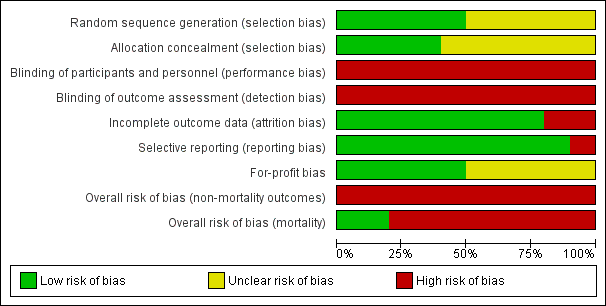
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

"Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
'+' = low risk of bias;
'‐' = high risk of bias;
'?' = unclear risk of bias.

Trial Sequential Analysis of 10 randomised clinical trials (474 participants) evaluating terlipressin versus other vasoactive drugs for people with hepatorenal syndrome on mortality. The analysis was made with power 90%, alpha 3%, a relative risk reduction (RRR) of 20%, a control group risk (CGR) of mortality of 52%, and a model variance ‐ based heterogeneity correction of 30%. The risk ratio was 0.96 (97% confidence interval 0.79 to 1.18). The cumulative Z‐curve (blue line) did not cross the diversity‐adjusted trial monitoring boundary for benefit.
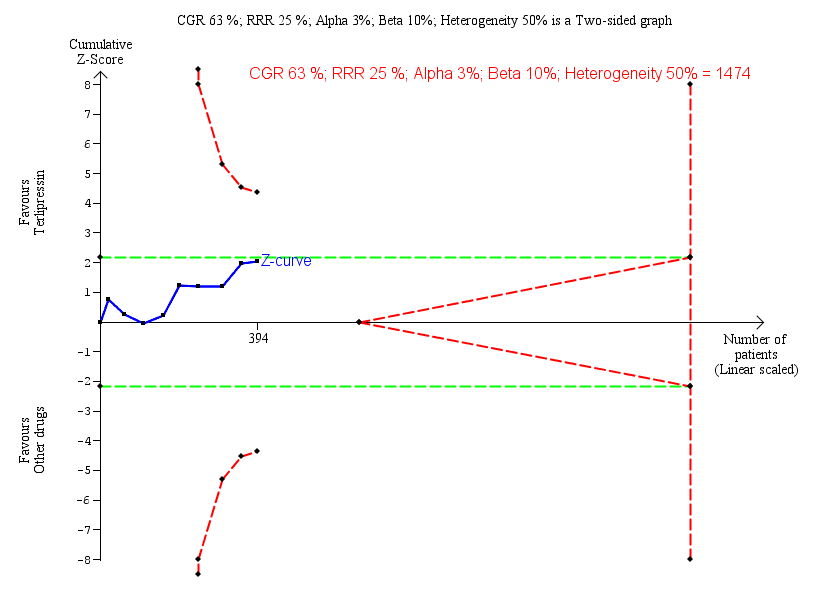
Trial Sequential Analysis of nine randomised clinical trials (394 participants) evaluating terlipressin versus other vasoactive drugs for people with hepatorenal syndrome on lack of reversal of hepatorenal syndrome. The analysis was made with power 90%, alpha 3%, a relative risk reduction (RRR) of 25%, a control group risk (CGR) of lack of reversal of hepatorenal syndrome of 63%, and a heterogeneity correction of 50%. The risk ratio was 0.79 (97% confidence interval 0.48 to 1.31). The cumulative Z‐curve (blue line) does not cross the diversity‐adjusted trial monitoring boundary for benefit.

Trial Sequential Analysis of two randomised clinical trials (88 participants) evaluating terlipressin versus other vasoactive drugs for people with hepatorenal syndrome on cardiovascular adverse events. The analysis was made with power 90%, alpha 3%, a relative risk reduction (RRR) of 25%, a control group risk (CGR) of cardiovascular adverse events of 15%, and a heterogeneity correction of 20%. The diversity‐adjusted trial monitoring boundary for harm was not included in the figure due to insufficient information. The estimated required information size was 4831 participants. Accordingly, with an accrued number of participants of 88, the required number of participants was not achieved.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 1 Mortality: bias control.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 2 Mortality: type of vasoactive drug.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 3 Mortality: type of hepatorenal syndrome.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 4 Mortality: publication status.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 5 Hepatorenal syndrome: type of vasoactive drug.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 6 Hepatorenal syndrome: type hepatorenal syndrome.
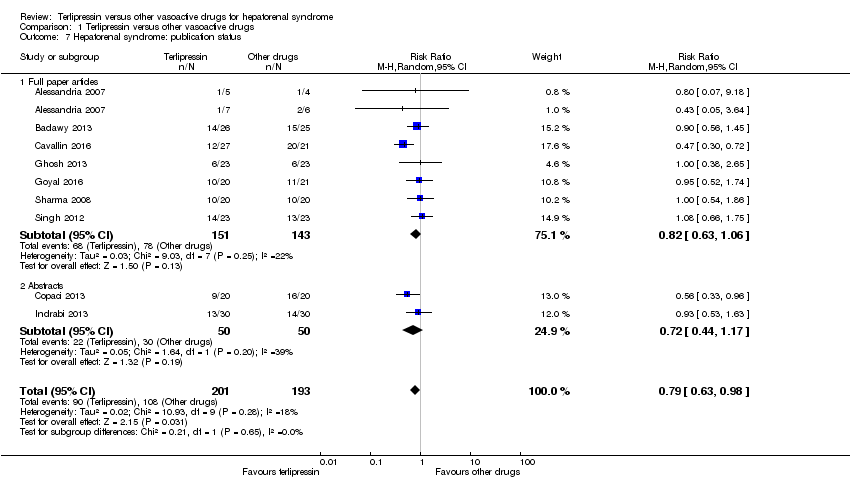
Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 7 Hepatorenal syndrome: publication status.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 8 Serious adverse events, type of vasoactive drug.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 9 Serious adverse events, type of event.
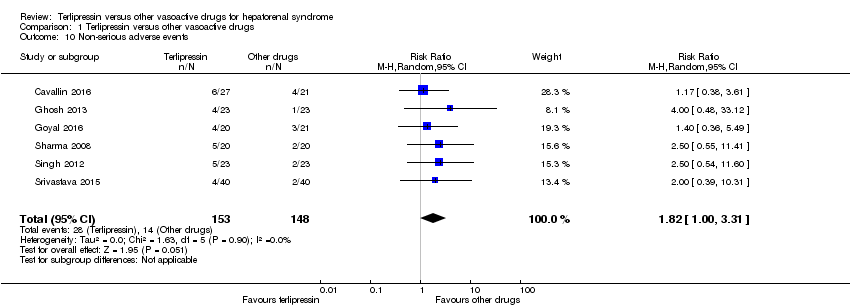
Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 10 Non‐serious adverse events.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 11 Non‐serious adverse event: types.
| Terlipressin compared to other vasoactive drugs for hepatorenal syndrome | ||||||
| Patient or population: people with cirrhosis and hepatorenal syndrome | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with other vasoactive drugs | Risk with terlipressin | |||||
| Mortality (All‐cause) | Study population | RR 0.96 | 474 | ⊕⊝⊝⊝ | Downgraded because of clinical heterogeneity, 8/10 randomised clinical trials were at high risk of bias and, the results of Trial Sequential Analysis. | |
| 601 per 1000 | 577 per 1000 | |||||
| Hepatorenal syndrome (Number of participants who did not achieve reversal of hepatorenal syndrome) | Study population | RR 0.79 | 394 | ⊕⊝⊝⊝ | Downgraded because of clinical heterogeneity, all trials were judged as high risk of bias, and results of Trial Sequential Analysis. | |
| 560 per 1000 | 442 per 1000 | |||||
| Serious adverse events | Study population | RR 0.96 | 474 | ⊕⊝⊝⊝ | Downgraded because of clinical heterogeneity, all trials were judged as high risk of bias, and results of Trial Sequential Analysis. | |
| 609 per 1000 | 585 per 1000 | |||||
| Non‐serious adverse events: diarrhoea or abdominal pain, or both | Study population | RR 3.50 | 221 | ⊕⊝⊝⊝ | Downgraded because of clinical heterogeneity, all trials were judged as high risk of bias, and results of the Trial Sequential Analysis. | |
| 19 per 1000 | 65 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aIn the assessment of mortality, we classified two randomised clinical trials at low risk of bias and eight at high risk of bias. bThe randomised clinical trials were not designed for equivalence or inferiority analysis. The Trial Sequential Analysis showed that sample size did not reach the required information size for equivalence/inferiority meta‐analysis. cClinical heterogeneity. dWe classified all randomised clinical trials at high risk of bias in all non‐mortality outcomes. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality: bias control Show forest plot | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 1.1 Low risk of bias | 2 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.63, 1.36] |
| 1.2 High risk of bias | 8 | 380 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.88, 1.07] |
| 2 Mortality: type of vasoactive drug Show forest plot | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 2.1 Noradrenaline | 7 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.08] |
| 2.2 Midodrine/octreotide | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.40, 1.28] |
| 2.3 Octreotide | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.32, 1.77] |
| 2.4 Dopamine/furosemide | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.22] |
| 3 Mortality: type of hepatorenal syndrome Show forest plot | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 3.1 Type 1 hepatorenal syndrome | 9 | 375 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.87, 1.06] |
| 3.2 Type 2 hepatorenal syndrome | 3 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.68, 1.33] |
| 4 Mortality: publication status Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Full paper | 8 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.83, 1.14] |
| 4.2 Abstract | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.86, 1.08] |
| 5 Hepatorenal syndrome: type of vasoactive drug Show forest plot | 9 | 394 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 0.99] |
| 5.1 Noradrenaline | 7 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.76, 1.21] |
| 5.2 Midodrine/octreotide | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.30, 0.72] |
| 5.3 Octreotide | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.33, 0.96] |
| 6 Hepatorenal syndrome: type hepatorenal syndrome Show forest plot | 9 | 394 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 0.98] |
| 6.1 Type 1 hepatorenal syndrome | 8 | 335 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.62, 1.01] |
| 6.2 Type 2 hepatorenal syndrome | 2 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.36, 2.10] |
| 7 Hepatorenal syndrome: publication status Show forest plot | 9 | 394 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 0.98] |
| 7.1 Full paper articles | 7 | 294 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.63, 1.06] |
| 7.2 Abstracts | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.44, 1.17] |
| 8 Serious adverse events, type of vasoactive drug Show forest plot | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 8.1 Noradrenaline | 7 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.08] |
| 8.2 Midodrine/octreotide | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.42, 1.23] |
| 8.3 Octreotide | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.32, 1.77] |
| 8.4 Dopamine/furosemide | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.22] |
| 9 Serious adverse events, type of event Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Death | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 9.2 Major cardiovascular events | 7 | 323 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.13, 5.98] |
| 10 Non‐serious adverse events Show forest plot | 6 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [1.00, 3.31] |
| 11 Non‐serious adverse event: types Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Diarrhoea or abdominal pain, or both | 5 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 3.50 [1.19, 10.27] |
| 11.2 Peripheral cyanosis | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.32, 27.83] |
| 11.3 Minor cardiovascular events | 6 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.37, 1.93] |

