Administración prenatal de suplementos dietéticos con mioinositol en pacientes embarazadas para prevenir la diabetes gestacional
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Type of study: parallel, randomised controlled trial. | |
| Participants | 220 women from Italy. Eligibility criteria: first‐degree relative (mother, father or both) affected by type 2 diabetes, prepregnancy BMI < 30 kg/m2, fasting plasma glucose < 126 mg/dL and random glycaemia < 200 mg/dL, singleton pregnancy, Caucasian. Women were 12‐13 weeks' gestation at trial entry. Exclusion criteria: pre‐pregnancy BMI ≥ 30 kg/m2, previous GDM, pre‐gestational diabetes, first trimester glycosuria, first‐degree relative (mother or father) not affected by type 2 diabetes, fasting plasma glucose ≥ 126 mg/dL or random glycaemia ≥ 200 mg/dL, twin pregnancy, associated therapy with corticosteroids, polycystic ovarian syndrome. Location: Department of Gynecology and Obstetrics, University of Messina, Messina, Italy. Timeframe: 2010‐2012. | |
| Interventions | Intervention: 4 g myo‐inositol plus 400 mcg folic acid daily (2 g myo‐inositol plus 200 mcg folic acid twice a day) (n = 110). Duration of myo‐inositol supplementation: from trial entry until the end of pregnancy. Comparison: 400 mcg folic acid daily (200 mcg folic acid twice a day) as 'placebo' (n = 110). | |
| Outcomes | Maternal: incidence of GDM, gestational hypertension, caesarean section. Criteria used to diagnose GDM: IADPSG. Infant: fetal macrosomia (> 4000 g), preterm birth, shoulder dystocia, neonatal hypoglycaemia, respiratory distress syndrome. | |
| Notes | Sample size calculation: not stated. Intention‐to‐treat analysis: yes (carried out but not reported). Losses to follow‐up: 11 women in the intervention group, and 12 in the comparison group. Funding: source of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer randomization was used." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial. Blinding not carried out. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Primary outcome of incidence of GDM diagnosed by blood test so blinding unlikely to impact assessment of this outcome. However, other secondary outcomes are more subjective. |
| Incomplete outcome data (attrition bias) | Low risk | Overall 10% loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcome measures were reported on. |
| Other bias | Unclear risk | Intention‐to‐ treat analysis was carried out on the available data, but was not reported in the manuscript. |
| Methods | Type of study: parallel, randomised controlled trial. | |
| Participants | 220 obese pregnant women from Italy. Eligibility criteria: pre‐pregnancy BMI ≥ 30 kg/m2, singleton gestation. Women were 12‐13 weeks' gestation at trial entry. Exclusion criteria: previous GDM, pre‐gestational diabetes, first trimester glycosuria (urine glucose value 10 mg/dL or greater), first trimester fasting plasma glucose 126 mg/dL or greater, or random plasma glucose 200 mg/dL or greater, concomitant treatment with corticosteroids, hypertension or renal or hepatic disease. Location: obstetric departments of 2 university hospitals located in Messina and Modena, Italy. Timeframe: January 2011 ‐ April 2014. | |
| Interventions | Intervention: 4 g myo‐inositol plus 400 mg folic acid daily (2 g myo‐inositol + 200 mg folic acid orally twice a day), and nutritional and lifestyle counselling (n = 110). Duration of myo‐inositol supplementation: from trial entry until the end of pregnancy. Comparison: 400 mg folic acid daily (200 mg folic acid orally twice a day), and nutritional and lifestyle counselling (n = 110). | |
| Outcomes | Maternal: occurrence of GDM, changes of insulin resistance from the first trimester to the performance of the OGTT performed at 24‐28 weeks as measured by the homeostasis model assessment of insulin resistance, caesarean section, gestational hypertensive disorders. Criteria used to diagnose GDM: IADPSG. Infant: preterm delivery, shoulder dystocia, macrosomia (birthweight > 4000 g), neonatal hypoglycaemia, neonatal transfer to intensive care unit. | |
| Notes | Sample size calculation was conducted. Intention‐to‐treat analysis. Funded by a grant from Messina University. The authors did not report any potential financial conflicts of interest. ClinicalTrials.gov trial registration NCT01047982. Further information was received following email contact with the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated random number list prepared by an investigator with no clinical involvement with the trial." |
| Allocation concealment (selection bias) | Low risk | "Allocation concealment was ensured by central randomization." "After the research investigator had obtained the patients consent, he telephoned a contact who was independent of the recruitment process for allocation assignment." |
| Blinding of participants and personnel (performance bias) | High risk | Trial was open label so blinding of participants and clinicians was not possible. |
| Blinding of outcome assessment (detection bias) | Low risk | "Data collectors were blinded to treatment allocation and the data came from the patients record." "objective measurements of primary laboratory outcomes." |
| Incomplete outcome data (attrition bias) | Low risk | 9% loss to follow‐up overall. More participants chose to drop out of the myo‐inositol group (n = 8) than the 'placebo' group (n = 0). |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes are reported on. |
| Other bias | Low risk | Appears free of other bias. The authors do not report any potential conflicts of interest. |
| Methods | Type of study: randomised controlled trial, parallel, 1:2 ratio. | |
| Participants | 91 women from Italy. Eligibility criteria: pregnant women, BMI > 27 kg/m2, normal glucose and HbA1c. Women were < 11 weeks' gestation at trial entry. Exclusion criteria: previous GDM, chronic disorder (not specified). Location: Messina, Italy. Timeframe: not stated. This is an interim report at 50% recruitment. | |
| Interventions | Intervention: 4 g myo‐inositol plus 400 mg folic acid daily (2 g myo‐inositol + 200 mg folic acid orally twice a day), and diet counselling (n = 31). Duration of myo‐inositol supplementation not stated. Comparison: 400 mg folic acid daily (200 mg folic acid orally twice a day), and diet counselling (n = 60). | |
| Outcomes | Maternal: 75 g 2 hour OGTT result at 24 to 26 weeks, diagnosis of GDM. Criteria used to diagnose GDM: not stated. Infant: not stated. | |
| Notes | Sample size calculation: not stated. Intention‐to‐treat analysis: not stated. Losses to follow‐up: not stated. Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomized" but no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | "Randomization was done at each centre." Unclear by whom. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details provided, but unlikely due to the nature of the therapy (myo‐inositol + folic acid versus folic acid alone). |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) | Unclear risk | Interim analysis at 50% recruitment conducted on 31 participants in the intervention group, and 60 participants in the control group. Unclear how many will be recruited as target denominator is not stated |
| Selective reporting (reporting bias) | High risk | Primary and secondary outcomes are not stated, only reports on OGTT and GDM results. |
| Other bias | High risk | Conference abstract only, at high risk of publication bias. Groups appeared comparable at baseline. |
| Methods | Parallel, randomised controlled trial. | |
| Participants | 65 pregnant women from Italy. Eligibility criteria: healthy pregnant women, aged between 30‐40, between 13 and 24 weeks' gestation, BMI between 25‐30 kg/m2. Exclusion criteria: diabetes mellitus, cardiovascular disease, chronic hypertension, autoimmune disease, dysthyroidism. Location: Bari, Italy. Timeframe: January to December 2012. | |
| Interventions | Intervention: a combination of 2000 mg myo‐inositol, 400 mg d‐chiro‐inositol, 400 mcg folic acid, 10 mg manganese. Duration of myo‐inositol supplementation: 60 days. Comparison: not stated. | |
| Outcomes | Maternal: total cholesterol, LDL, HDL, blood glucose. Criteria used to diagnose GDM: not stated. Infant: not stated. | |
| Notes | Sample size calculation not stated. Funding not stated. The authors did not report any potential financial conflicts of interest. Authors were contacted and provided further information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence was generated by a random number table. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by an independent statistician who assigned numbered patients to groups using sealed numbered containers. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants were blinded. Clinicians were aware of treatment allocation. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | High risk | 65 women were initially enrolled, 17 of which were excluded – 6 did not meet inclusion criteria, 4 refused to participate, 7 left the study spontaneously. Analysis was conducted on the remaining 48 women. |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes are reported on (total cholesterol, LDL, HDL, blood glucose). No other maternal, pregnancy or neonatal outcomes are specified or reported. |
| Other bias | Low risk | Appears free of other bias. The authors do not report any potential conflicts of interest. |
BMI: body mass index
GDM: gestational diabetes mellitus
g: grams
HbA1c: Glycated haemoglobin
HDL: high density lipoprotein
IADPSG: International Association of Diabetes and Pregnancy Study Groups
kg/m2: kilograms per metre squared
LDL: low density lipoprotein
mcg: micrograms
mg/dL: milligrams per decilitre
OGTT: oral glucose tolerance test
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Used myo‐inositol as a treatment intervention in women diagnosed with gestational diabetes, not preventative. | |
| Used myo‐inositol as a treatment intervention in women diagnosed with gestational diabetes, not a preventative. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A randomised controlled trial to investigate the role of the food supplement inositol in the general health of those at risk of developing gestational diabetes mellitus. |
| Methods | Single‐blind randomised controlled trial. |
| Participants | Any woman aged over 18 booking before 14 weeks' gestation with a first‐degree relative with diabetes mellitus. |
| Interventions | 2 intervention arms: myo‐inositol 4 g + 400 mcg folic acid; per day; myo‐inositol 550 mg + 13.8 mg D‐chiro‐inositol + 400 mcg folic acid per day. Placebo group: folic acid 400 mcg per day. |
| Outcomes | Development of gestational diabetes mellitus, measured at 26 weeks' gestation. |
| Starting date | 01/11/2013. |
| Contact information | Dr Maria Farren, [email protected] |
| Notes | Expected completion 01/06/2015. Alternative primary investigator: Sean Daly ISRCTN92466608 |
mcg: micrograms
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gestational diabetes mellitus Show forest plot | 3 | 502 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.29, 0.64] |
| Analysis 1.1 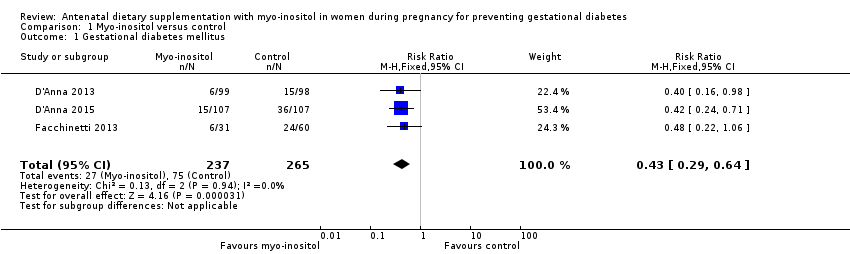 Comparison 1 Myo‐inositol versus control, Outcome 1 Gestational diabetes mellitus. | ||||
| 2 Fasting OGTT Show forest plot | 3 | 502 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.28, ‐0.12] |
| Analysis 1.2 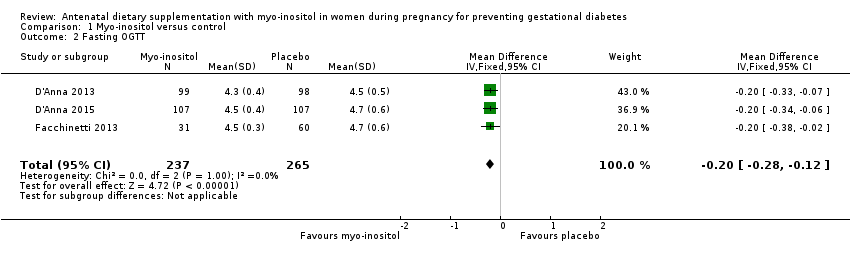 Comparison 1 Myo‐inositol versus control, Outcome 2 Fasting OGTT. | ||||
| 3 One hour OGTT Show forest plot | 3 | 502 | Mean Difference (IV, Fixed, 95% CI) | ‐0.68 [1.00, ‐0.37] |
| Analysis 1.3 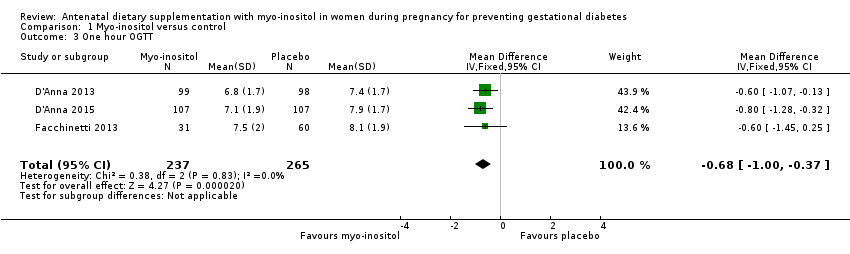 Comparison 1 Myo‐inositol versus control, Outcome 3 One hour OGTT. | ||||
| 4 Two hour OGTT Show forest plot | 3 | 502 | Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.07, ‐0.43] |
| Analysis 1.4  Comparison 1 Myo‐inositol versus control, Outcome 4 Two hour OGTT. | ||||
| 5 Hypertensive disorders of pregnancy Show forest plot | 2 | 398 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.02, 8.41] |
| Analysis 1.5  Comparison 1 Myo‐inositol versus control, Outcome 5 Hypertensive disorders of pregnancy. | ||||
| 6 Caesarean section Show forest plot | 2 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.76, 1.19] |
| Analysis 1.6  Comparison 1 Myo‐inositol versus control, Outcome 6 Caesarean section. | ||||
| 7 Weight gain during pregnancy Show forest plot | 2 | 411 | Mean Difference (IV, Random, 95% CI) | 0.64 [‐0.41, 1.70] |
| Analysis 1.7 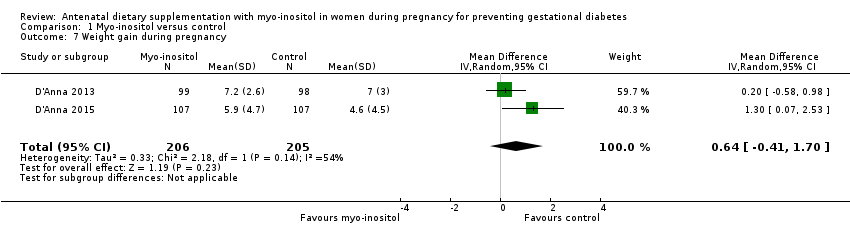 Comparison 1 Myo‐inositol versus control, Outcome 7 Weight gain during pregnancy. | ||||
| 8 Relevant biomarker changes associated with the intervention Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.8 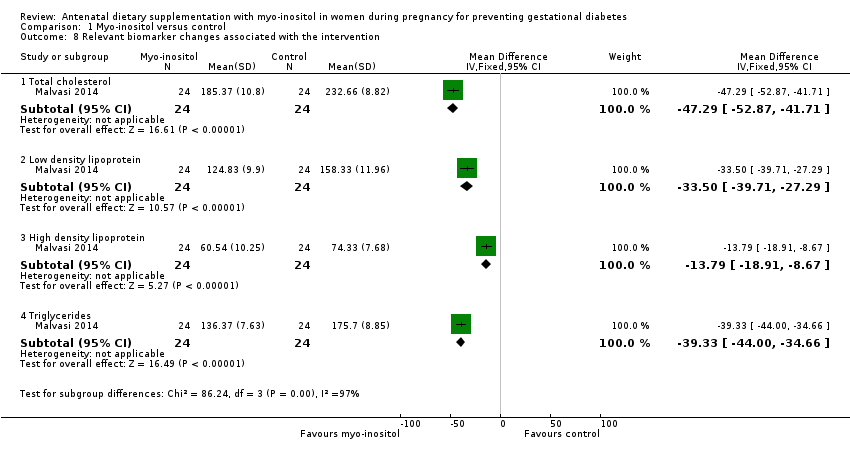 Comparison 1 Myo‐inositol versus control, Outcome 8 Relevant biomarker changes associated with the intervention. | ||||
| 8.1 Total cholesterol | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐47.29 [‐52.87, ‐41.71] |
| 8.2 Low density lipoprotein | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐33.50 [‐39.71, ‐27.29] |
| 8.3 High density lipoprotein | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐13.79 [‐18.91, ‐8.67] |
| 8.4 Triglycerides | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐39.33 [‐44.00, ‐34.66] |
| 9 Adverse effects of intervention Show forest plot | 2 | 245 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.9  Comparison 1 Myo‐inositol versus control, Outcome 9 Adverse effects of intervention. | ||||
| 10 Supplementary insulin Show forest plot | 2 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.11, 2.09] |
| Analysis 1.10 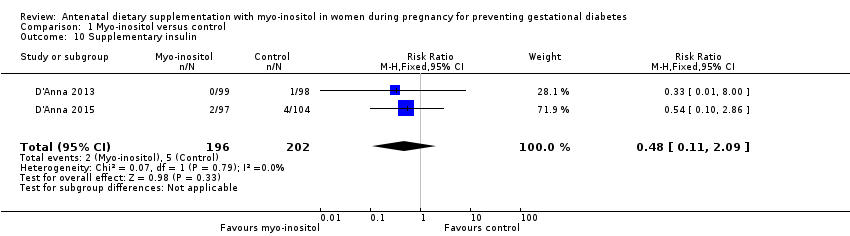 Comparison 1 Myo‐inositol versus control, Outcome 10 Supplementary insulin. | ||||
| 11 Gestational age at birth Show forest plot | 2 | 398 | Mean Difference (IV, Random, 95% CI) | 5.50 [‐7.24, 18.24] |
| Analysis 1.11 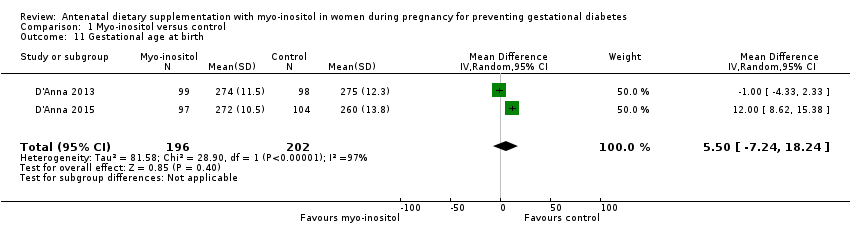 Comparison 1 Myo‐inositol versus control, Outcome 11 Gestational age at birth. | ||||
| 12 Preterm birth (less than 37 weeks' gestation) Show forest plot | 2 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.17, 1.14] |
| Analysis 1.12 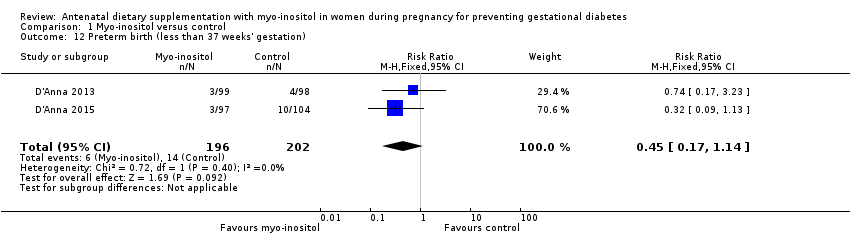 Comparison 1 Myo‐inositol versus control, Outcome 12 Preterm birth (less than 37 weeks' gestation). | ||||
| 13 Macrosomia Show forest plot | 2 | 398 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.02, 6.37] |
| Analysis 1.13  Comparison 1 Myo‐inositol versus control, Outcome 13 Macrosomia. | ||||
| 14 Birthweight Show forest plot | 2 | 398 | Mean Difference (IV, Random, 95% CI) | ‐60.47 [‐265.21, 144.26] |
| Analysis 1.14  Comparison 1 Myo‐inositol versus control, Outcome 14 Birthweight. | ||||
| 15 Shoulder dystocia Show forest plot | 2 | 398 | Risk Ratio (M‐H, Random, 95% CI) | 2.33 [0.12, 44.30] |
| Analysis 1.15  Comparison 1 Myo‐inositol versus control, Outcome 15 Shoulder dystocia. | ||||
| 16 Respiratory distress syndrome Show forest plot | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.60] |
| Analysis 1.16  Comparison 1 Myo‐inositol versus control, Outcome 16 Respiratory distress syndrome. | ||||
| 17 Neonatal hypoglycaemia Show forest plot | 2 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 8.66] |
| Analysis 1.17 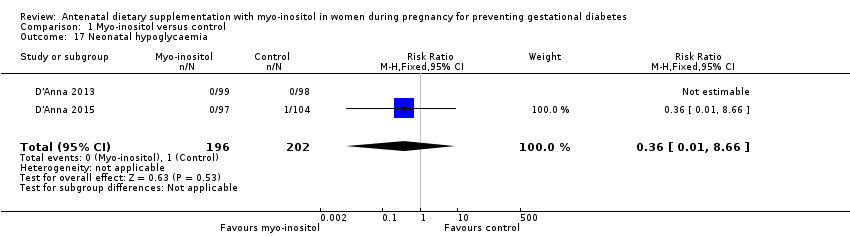 Comparison 1 Myo‐inositol versus control, Outcome 17 Neonatal hypoglycaemia. | ||||

‐Study flow diagram.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
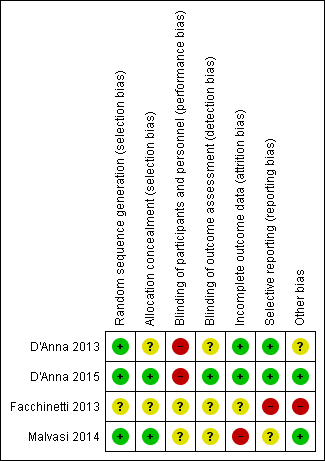
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Myo‐inositol versus control, Outcome 1 Gestational diabetes mellitus.

Comparison 1 Myo‐inositol versus control, Outcome 2 Fasting OGTT.
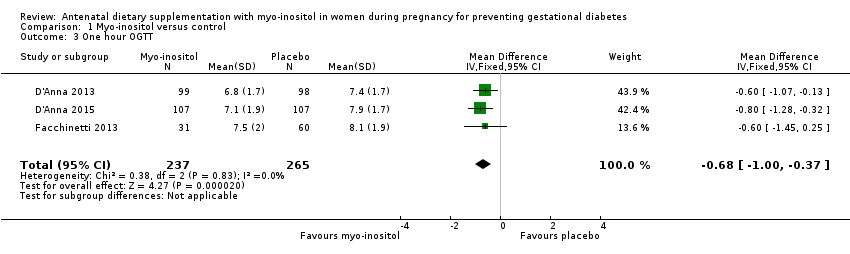
Comparison 1 Myo‐inositol versus control, Outcome 3 One hour OGTT.
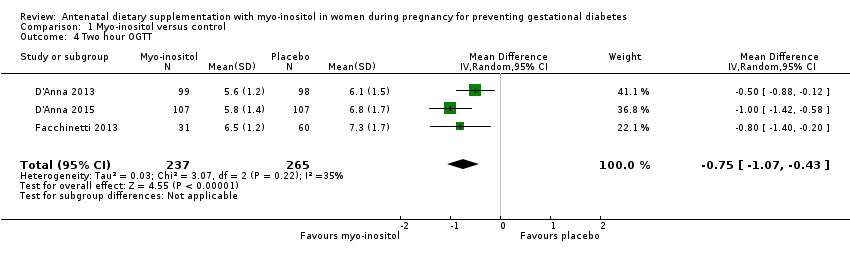
Comparison 1 Myo‐inositol versus control, Outcome 4 Two hour OGTT.

Comparison 1 Myo‐inositol versus control, Outcome 5 Hypertensive disorders of pregnancy.

Comparison 1 Myo‐inositol versus control, Outcome 6 Caesarean section.
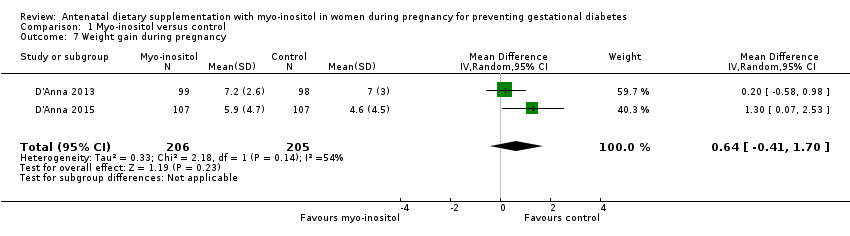
Comparison 1 Myo‐inositol versus control, Outcome 7 Weight gain during pregnancy.
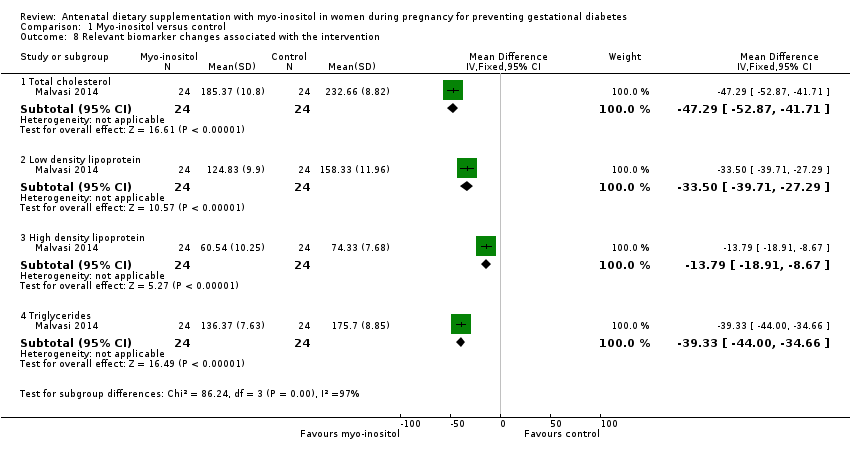
Comparison 1 Myo‐inositol versus control, Outcome 8 Relevant biomarker changes associated with the intervention.
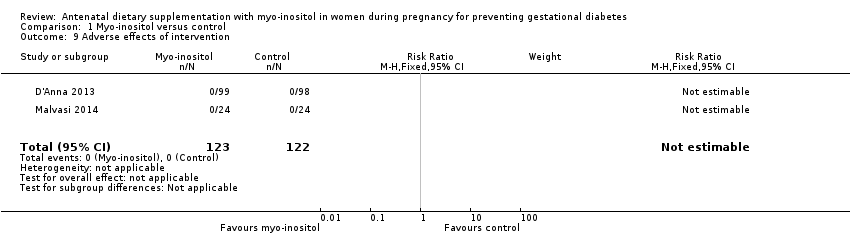
Comparison 1 Myo‐inositol versus control, Outcome 9 Adverse effects of intervention.
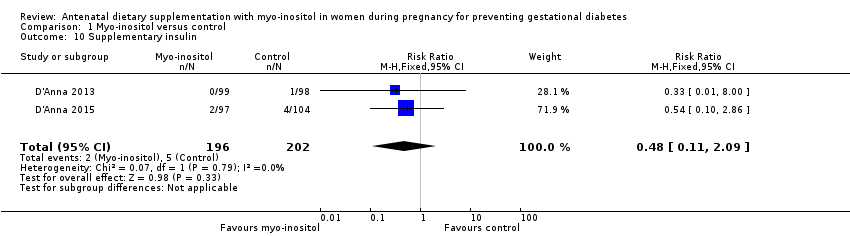
Comparison 1 Myo‐inositol versus control, Outcome 10 Supplementary insulin.

Comparison 1 Myo‐inositol versus control, Outcome 11 Gestational age at birth.

Comparison 1 Myo‐inositol versus control, Outcome 12 Preterm birth (less than 37 weeks' gestation).

Comparison 1 Myo‐inositol versus control, Outcome 13 Macrosomia.
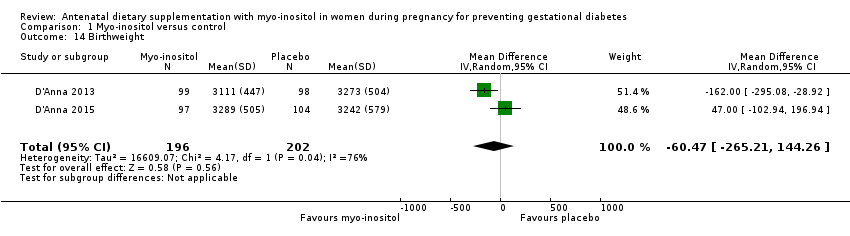
Comparison 1 Myo‐inositol versus control, Outcome 14 Birthweight.

Comparison 1 Myo‐inositol versus control, Outcome 15 Shoulder dystocia.

Comparison 1 Myo‐inositol versus control, Outcome 16 Respiratory distress syndrome.

Comparison 1 Myo‐inositol versus control, Outcome 17 Neonatal hypoglycaemia.
| Antenatal supplementation with myo‐inositol for preventing gestational diabetes | ||||||
| Patient or population: pregnant women (women with pre‐existing type 1 or type 2 diabetes are NOT included) Setting: Italy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with Myo‐inositol | |||||
| Gestational diabetes mellitus | Study population | RR 0.43 | 502 | ⊕⊕⊝⊝ | GDM diagnosed using IADPSG 2010 criteria | |
| 28 per 100 | 12 per 100 | |||||
| Weight gain during pregnancy | The mean weight gain during pregnancy was 0 | The mean weight gain during pregnancy in the intervention group was 0.64 more (0.41 fewer to 1.7 more) | ‐ | 411 | ⊕⊝⊝⊝ | D'Anna 2015 included obese pregnant women and D'Anna 2013 included non‐obese women with a family history of type 2 diabetes Random‐effects model |
| Hypertensive disorders of pregnancy | Study population | RR 0.43 | 398 | ⊕⊝⊝⊝ | Random‐effects model | |
| 4 per 100 | 2 per 100 | |||||
| Caesarean section | Study population | RR 0.95 | 398 | ⊕⊕⊝⊝ | ||
| 45 per 100 | 43 per 100 | |||||
| Perineal trauma | Not estimable | (0 studies) | No data reported for perineal trauma in any of the included studies | |||
| Postnatal depression | Not estimable | (0 studies) | No data reported for postnatal depression in any of the included studies | |||
| Type 2 diabetes | Not estimable | (0 studies) | No data reported for type 2 diabetes in any of the included studies | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded (‐1) due to unclear risk of bias for allocation concealment in two of the included trials (one trial did not provide sufficient detail to determine allocation concealment and one trial (reported as a conference abstract) had no details of random sequence generation, allocation concealment or blinding) and for high risk of performance bias for lack of blinding (two trials were open‐label trials with no blinding of participants or researchers, however one trial explicitly described blinding of outcome assessors and was assessed as low risk of detection bias). 2 Studies were conducted in Italy with Caucasian women and generalisability of findings is limited, downgraded (‐1). 3 Evidence of imprecision with wide confidence intervals crossing the line of no effect, downgraded (‐1). 4 Heterogeneity high with I2 = 54% (indirectness) probably due to different study populations, downgraded (‐1). 5 Wide confidence intervals with very low event rates and a small sample size suggest evidence of imprecision, downgraded (‐1). 6 Downgraded (‐1) due to insufficient evidence to judge allocation concealment in one trial and subsequent judgement of unclear risk of bias. The other trial had a low risk of bias for allocation concealment. Both trials were open‐label with no blinding of participants or researchers, although one trial explicitly stated that outcome assessors were blinded to treatment allocation. | ||||||
| Antenatal supplementation with myo‐inositol for preventing gestational diabetes | ||||||
| Patient or population: pregnant women who were at risk of GDM Setting: Italy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with Myo‐inositol | |||||
| Large‐for‐gestational age | not estimable | (0 studies) | No data reported for large‐for‐gestational age in any of the included studies | |||
| Perinatal mortality | not estimable | (0 studies) | No data reported for perinatal mortality in any of the included studies | |||
| Composite of serious neonatal outcomes | not estimable | (0 studies) | No data reported for composite of serious neonatal outcomes in any of the included studies | |||
| Neonatal hypoglycaemia | Study population | RR 0.36 | 398 | ⊕⊝⊝⊝ | ||
| 0 per 100 | 0 per 100 | |||||
| Adiposity | not estimable | (0 studies) | No data reported for adiposity in any of the included studies | |||
| Diabetes | not estimable | (0 studies) | No data reported for diabetes in any of the included studies | |||
| Neurosensory disability | not estimable | (0 studies) | No data reported for neurosensory disability in any of the included studies | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 No blinding in either study and reporting of allocation concealment was unclear in one of the studies, downgraded (‐1). 2 Both studies were conducted in Italy with Caucasian women and may not be generalisable to other settings, downgraded (‐1). 3 Wide confidence intervals with very low event rates suggest evidence of imprecision, downgraded (‐1). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gestational diabetes mellitus Show forest plot | 3 | 502 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.29, 0.64] |
| 2 Fasting OGTT Show forest plot | 3 | 502 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.28, ‐0.12] |
| 3 One hour OGTT Show forest plot | 3 | 502 | Mean Difference (IV, Fixed, 95% CI) | ‐0.68 [1.00, ‐0.37] |
| 4 Two hour OGTT Show forest plot | 3 | 502 | Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.07, ‐0.43] |
| 5 Hypertensive disorders of pregnancy Show forest plot | 2 | 398 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.02, 8.41] |
| 6 Caesarean section Show forest plot | 2 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.76, 1.19] |
| 7 Weight gain during pregnancy Show forest plot | 2 | 411 | Mean Difference (IV, Random, 95% CI) | 0.64 [‐0.41, 1.70] |
| 8 Relevant biomarker changes associated with the intervention Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Total cholesterol | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐47.29 [‐52.87, ‐41.71] |
| 8.2 Low density lipoprotein | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐33.50 [‐39.71, ‐27.29] |
| 8.3 High density lipoprotein | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐13.79 [‐18.91, ‐8.67] |
| 8.4 Triglycerides | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐39.33 [‐44.00, ‐34.66] |
| 9 Adverse effects of intervention Show forest plot | 2 | 245 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Supplementary insulin Show forest plot | 2 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.11, 2.09] |
| 11 Gestational age at birth Show forest plot | 2 | 398 | Mean Difference (IV, Random, 95% CI) | 5.50 [‐7.24, 18.24] |
| 12 Preterm birth (less than 37 weeks' gestation) Show forest plot | 2 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.17, 1.14] |
| 13 Macrosomia Show forest plot | 2 | 398 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.02, 6.37] |
| 14 Birthweight Show forest plot | 2 | 398 | Mean Difference (IV, Random, 95% CI) | ‐60.47 [‐265.21, 144.26] |
| 15 Shoulder dystocia Show forest plot | 2 | 398 | Risk Ratio (M‐H, Random, 95% CI) | 2.33 [0.12, 44.30] |
| 16 Respiratory distress syndrome Show forest plot | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.60] |
| 17 Neonatal hypoglycaemia Show forest plot | 2 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 8.66] |

