Administración prenatal de suplementos alimentarios con mioinositol para prevenir la diabetes gestacional
Resumen
Antecedentes
La diabetes gestacional, con inicio o primera detección durante el embarazo, es un problema creciente en todo el mundo. El mioinositol, un isómero del inositol, es un azúcar natural que se encuentra habitualmente en los cereales, el maíz, las legumbres y la carne. Es uno de los mediadores intracelulares de la señal de insulina y se correlaciona con la sensibilidad a la insulina en la diabetes tipo 2. El posible efecto beneficioso sobre la mejoría en la sensibilidad a la insulina indica que el mioinositol podría ser útil en las mujeres para prevenir la diabetes gestacional. Esta es una actualización de una revisión publicada por primera vez en 2015.
Objetivos
Evaluar si la administración prenatal de suplementos alimentarios con mioinositol es segura y eficaz, para la madre y el feto, en la prevención de la diabetes gestacional.
Métodos de búsqueda
Se realizaron búsquedas en el Registro de ensayos del Grupo Cochrane de Embarazo y parto (Cochrane Pregnancy and Childbirth), ClinicalTrials.gov, ICTRP de la OMS (17 de marzo de 2022) y en las listas de referencias de los estudios identificados.
Criterios de selección
Se incluyeron ensayos controlados aleatorizados (ECA) publicados y no publicados, incluidos ECA por conglomerados y resúmenes de congresos, que evaluaran los efectos del mioinositol para la prevención de la diabetes gestacional en mujeres embarazadas. Se incluyeron los estudios que compararan cualquier dosis de mioinositol, solo o en un preparado farmacéutico combinado, con ningún tratamiento, placebo u otra intervención. No fueron elegibles para inclusión los ensayos cuasialeatorizados ni los ensayos cruzados (cross‐over). Se excluyeron las mujeres con diabetes tipo 1 o tipo 2 preexistente.
Obtención y análisis de los datos
Dos autores de la revisión evaluaron de forma independiente los estudios para inclusión, evaluaron el riesgo de sesgo y extrajeron los datos. Se verificó la exactitud de los datos. La certeza de la evidencia se evaluó mediante el método GRADE.
Resultados principales
Se incluyeron siete ECA (uno realizado en Irlanda, seis realizados en Italia) que informaron sobre 1319 mujeres que tenían entre 10 y 24 semanas de embarazo al inicio de los estudios. Los estudios tenían tamaños muestrales relativamente pequeños y el riesgo general de sesgo fue bajo.
Para los desenlaces principales maternos, el metanálisis mostró que el mioinositol podría reducir la incidencia de diabetes gestacional (razón de riesgos [RR] 0,53; intervalo de confianza [IC] del 95%: 0,31 a 0,90; seis estudios, 1140 mujeres) y los trastornos hipertensivos del embarazo (RR 0,34; IC del 95%: 0,19 a 0,61; cinco estudios, 1052 mujeres). Sin embargo, la calidad de la evidencia fue baja a muy baja. Para los desenlaces principales neonatales, solo un estudio midió el riesgo de tener un neonato grande para la edad gestacional y encontró que el mioinositol se asoció tanto con efectos beneficiosos como perjudiciales importantes (RR 1,40; IC del 95%: 0,65 a 3,02; un estudio, 234 lactantes; evidencia de certeza baja). Ninguno de los estudios incluidos informó sobre los otros desenlaces neonatales principales (mortalidad perinatal, mortalidad o morbilidad compuesta).
Para los desenlaces secundarios maternos, no está claro el efecto del mioinositol sobre el aumento de peso durante el embarazo (diferencia de medias [DM] ‐0,25 kilogramos [kg]; IC del 95%: ‐1,26 a 0,75 kg; cuatro estudios, 831 mujeres) ni el traumatismo perineal (RR 4,0; IC del 95%: 0,45 a 35,25; un estudio, 234 mujeres) porque la evidencia se consideró de certeza muy baja. Además, el mioinositol podría dar lugar a poca o ninguna diferencia en la cesárea (RR 0,91; IC del 95%: 0,77 a 1,07; cuatro estudios, 829 mujeres; evidencia de certeza baja). Ninguno de los estudios incluidos informó sobre los otros desenlaces maternos secundarios (depresión posparto y aparición posterior de diabetes mellitus tipo 2). Para los desenlaces neonatales secundarios, el metanálisis no mostró hipoglucemia neonatal (RR 3,07; IC del 95%: 0,90 a 10,52; cuatro estudios; 671 lactantes; evidencia de certeza muy baja). Sin embargo, el mioinositol se podría asociar con una reducción en la incidencia de partos prematuros (RR 0,35; IC del 95%: 0,17 a 0,70; cuatro estudios; 829 lactantes). No hubo datos suficientes de un gran número de desenlaces secundarios maternos y neonatales, como tampoco hubo datos de ninguno de los desenlaces de la infancia o de la edad adulta a largo plazo, ni de los desenlaces del coste de los servicios sanitarios.
Conclusiones de los autores
Siete estudios muestran que la administración prenatal de suplementos con mioinositol durante el embarazo podría reducir la incidencia de diabetes gestacional, trastornos hipertensivos del embarazo y parto prematuro. Datos limitados indican que la administración de suplementos con mioinositol podría no reducir el riesgo de un neonato grande para la edad gestacional.
La evidencia actual se basa en estudios pequeños que no tenía potencia estadística suficiente para detectar diferencias en desenlaces como la mortalidad perinatal y la morbilidad infantil grave. Seis de los estudios incluidos se realizaron en Italia y uno en Irlanda, lo que suscita dudas sobre la falta de generalizabilidad a otros contextos. Existe evidencia de incoherencias entre las dosis de mioinositol, el momento de administración y la población de estudio. Como resultado, la certeza de la evidencia para muchos desenlaces se disminuyó a certeza baja o muy baja.
Se recomienda realizar más estudios sobre esta prometedora intervención prenatal para prevenir la diabetes gestacional, que deberían incluir a embarazadas de diferentes etnias y con distintos factores de riesgo. El mioinositol, en diferentes dosis, frecuencia y momento de administración, se debe comparar con el placebo, la dieta y el ejercicio, así como las intervenciones farmacológicas. Se debe considerar un seguimiento a largo plazo y los desenlaces deben incluir los posibles daños, incluidos los efectos adversos.
PICO
Resumen en términos sencillos
Mioinositol como suplemento alimentario durante el embarazo para prevenir la diabetes gestacional
Mensajes clave
Las mujeres que desarrollan diabetes gestacional tienen un riesgo mayor de presentar complicaciones durante el embarazo y el parto, así como de desarrollar diabetes posteriormente. Los recién nacidos de madres con diabetes gestacional pueden ser más grandes de lo debido y sufrir lesiones al nacer. Estos recién nacidos tienen riesgo de presentar diabetes cuando son niños pequeños o adultos jóvenes. El número de embarazadas diagnosticadas de diabetes gestacional ha aumentado en todo el mundo, por lo que es importante encontrar formas sencillas y rentables para evitar que las mujeres desarrollen esta enfermedad.
El mioinositol es un azúcar natural que se encuentra en los cereales, el maíz, las verduras y la carne, y que interviene en la sensibilidad del cuerpo a la insulina.
¿Qué se quería averiguar?
Esta revisión intentó investigar si el mioinositol es un suplemento alimentario prenatal eficaz para prevenir la diabetes gestacional en embarazadas.
¿Qué se hizo?
Se buscaron estudios que compararan el mioinositol (administrado solo o en combinación con otro tratamiento) con ningún tratamiento u otro tratamiento. Se compararon y resumieron los resultados de los estudios y la confianza en la evidencia se evaluó sobre la base de factores como la metodología y el tamaño de los estudios.
¿Qué se encontró?
Se encontraron siete estudios con 1319 embarazadas de entre 10 y 24 semanas.
Resultados principales
No está claro si la administración de suplementos con mioinositol se asocia con una reducción de la tasa de diabetes gestacional. Sin embargo, el mioinositol se podría asociar con una reducción de los trastornos hipertensivos del embarazo. No está claro si la administración de suplementos con mioinositol disminuye el número de recién nacidos grandes para la edad gestacional.
Los estudios no proporcionaron información sobre el número de bebés que murieron (antes de nacer o poco después), la depresión o la diabetes tipo 2 posterior al parto. No hubo efectos adversos maternos del tratamiento en los cinco estudios que informaron sobre este desenlace; los otros dos estudios no lo mencionaron.
No está claro el efecto de la administración de suplementos con mioinositol sobre el aumento de peso durante el embarazo ni sobre los recién nacidos con niveles bajos de glucosa en sangre. Esta revisión no encontró un efecto sobre otros desenlaces, como el riesgo de realizar una cesárea o de un recién nacido grande. Lo anterior se podría deber a que los ensayos fueron demasiado pequeños para detectar diferencias en estos desenlaces y a que no todos los estudios informaron sobre los desenlaces. Sin embargo, el mioinositol se podría asociar con una reducción en la tasa de partos prematuros en comparación con el grupo control.
Los estudios incluidos no informaron sobre muchos otros desenlaces relevantes para la madre y el recién nacido, no proporcionaron datos relacionados con los desenlaces a más largo plazo para la madre o el recién nacido, ni sobre el coste para los servicios sanitarios.
No hay evidencia suficiente para apoyar que la administración de mioinositol como suplemento alimentario durante el embarazo prevenga la diabetes gestacional. Sin embargo, el mioinositol podría prevenir los trastornos hipertensivos (tensión alta) del embarazo y el parto prematuro. Se necesitan ensayos controlados aleatorizados grandes y bien diseñados adicionales para evaluar la efectividad del mioinositol en la prevención de la diabetes gestacional y la mejoría de otros desenlaces de salud de las madres y los recién nacidos.
¿Cuáles son las limitaciones de estas evidencias?
Se tiene poca confianza en la evidencia porque no hubo suficientes estudios para tener seguridad en los desenlaces y muchos de los desenlaces de esta revisión no se informaron en los estudios identificados. Los estudios también se limitaron a poblaciones de contextos de ingresos altos, por lo que los resultados podrían no ser aplicables a otras poblaciones. Los estudios también presentaban algunas limitaciones en cuanto a la forma en que se describieron los métodos.
¿Cuál es el grado de actualización de esta evidencia?
La evidencia está actualizada hasta diciembre de 2022.
Authors' conclusions
Summary of findings
| Antenatal supplementation with myo‐inositol for preventing gestational diabetes | ||||||
| Patient or population: pregnant women (women with pre‐existing type 1 or type 2 diabetes are NOT included) Setting: hospital | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with control | Risk with myo‐inositol | |||||
| Gestational diabetes mellitus | Study population | RR 0.53 | 1140 | ⊕⊝⊝⊝ Very lowa,b,c | GDM diagnosed using IADPSG 2010 criteria
Random‐effects model | |
| 217 per 1000 | 115 per 1,000 (67 to 196) | |||||
| Weight gain during pregnancy | Comparator | The mean weight gain during pregnancy in the intervention group was 0.25 kg lower (1.26 kg fewer to 0.76 kg more) | ‐ | 831 | ⊕⊝⊝⊝ |
Random‐effects model |
| Hypertensive disorders of pregnancy | Study population | RR 0.34 | 1052 | ⊕⊕⊝⊝ Lowc,f | Random‐effects model | |
| 86 per 1,000 | 29 per 1,000 | |||||
| Caesarean section | Study population | RR 0.91 | 829 | ⊕⊕⊝⊝ |
| |
| 430 per 1,000 | 391 per 1,000 | |||||
| Perineal trauma | Study population | RR 4.00 | 234 (1 RCT) | ⊕⊝⊝⊝ |
| |
| 9 per 1,000 | 34 per 1,000 (4 to 301) | |||||
| Postnatal depression | See comments | Not estimable | (0 studies) |
| No data reported this outcome in any of the included studies | |
|
|
| |||||
| Development of subsequent type 2 diabetes mellitus | See comments | Not estimable | (0 studies) |
| No data reported this outcome in any of the included studies | |
|
|
| |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a. Downgraded (‐1) for serious limitations in study design: due to unclear risk of selection bias in two of the six included studies; five of the six included studies were at high risk of performance bias; two of the six included studies were at high risk of detection bias; one study was at high risk of attrition bias. b. Downgraded (‐1) for serious inconsistency; considerable heterogeneity, possible due to different study populations. c. Downgraded (‐1) for serious indirectness; only one of the included studies was conducted outside Italy, and the Italian studies only included white women, the generalisability of findings is limited. d. Downgraded (‐1) for serious limitations in study design: all studies were at high risk of performance bias; one study was at high risk of detection bias. e. Downgraded (‐1) for serious imprecision; evidence of imprecision with wide confidence intervals crossing the line of no effect. f. Downgraded (‐1) for serious limitations in study design: all studies were at high risk of performance bias; two studies were at high risk of detection bias. g. Downgraded (‐1) for serious limitations in study design: all studies were at high risk of performance bias. One study was at high risk of detection bias, and insufficient evidence to judge detection bias and subsequent judgement of unclear risk of bias. Due to insufficient evidence to judge allocation concealment in two studies and subsequent judgement of unclear risk of bias. Due to insufficient evidence to judge attrition bias in two studies and subsequent judgement of unclear risk of bias. h. Downgraded (‐1) for serious limitations in study design: the study was at high risk of performance bias and detection bias for lack of blinding. i. Downgraded (‐1) for serious Indirectness: only one study conducted in Ireland reported this outcome. j. Downgraded (‐1) for serious imprecision: wide confidence intervals with very low event rates. | ||||||
| Antenatal supplementation with myo‐inositol for preventing gestational diabetes | ||||||
| Patient or population: infants of pregnant women Setting: hospital | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with control | Risk with myo‐inositol | |||||
| Large‐for‐gestational age | Study population | RR 1.40 | 234 | ⊕⊕⊝⊝ |
| |
| 85 per 1000 | 120 per 1000 (56 to 258) | |||||
| Perinatal mortality (stillbirth and neonatal mortality) | See comments | Not estimable | (0 studies) |
| No data reported this outcome in any of the included studies | |
|
|
| |||||
| Composite of serious neonatal outcomes | See comments | not estimable | (0 studies) |
| No data reported this outcome in any of the included studies | |
|
|
| |||||
| Neonatal hypoglycaemia | Study population | RR 3.07 | 671 | ⊕⊝⊝⊝ |
| |
| 9 per 1,000 | 27 per 1000 | |||||
| Adiposity | See comments | not estimable | (0 studies) |
| No data reported this outcome in any of the included studies | |
|
|
| |||||
| Diabetes | See comments | not estimable | (0 studies) |
| No data reported this outcome in any of the included studies | |
|
|
| |||||
| Neurosensory disability | See comments | not estimable | (0 studies) |
| No data reported this outcome in any of the included studies | |
|
|
| |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a. Downgraded (‐1) for serious limitations in study design: the study was at high risk of performance bias and detection bias for lack of blinding. b. Downgraded (‐1) for serious indirectness: only one study conducted in Ireland reported this outcome. c. Downgraded (‐1) for serious limitations in study design: all studies were at high risk of performance bias; one study was at high risk of detection bias. d. Downgraded (‐1) for serious indirectness: only one of the included studies was conducted outside Italy, and the Italian studies only included Caucasian women. Thus, the generalisability of findings is limited. e. Downgraded (‐1) for serious imprecision: evidence of imprecision with wide confidence intervals crossing the line of no effect. | ||||||
Background
Description of the condition
Gestational diabetes is defined as any degree of glucose intolerance with onset or first recognition during pregnancy (Alberti 1998). Gestational diabetes can lead to complications for affected women and their babies, making it crucial that effective strategies for its prevention are found.
Screening for, and diagnosis of gestational diabetes, is usually undertaken between 24 and 28 weeks' of pregnancy. However, screening regimes vary from country to country, with some countries selectively screening based on risk factors (NICE 2015), and other countries using universal screening of all pregnant women (Nankervis 2013). If thresholds for the oral glucose challenge test (OGCT) are exceeded, a diagnostic oral glucose tolerance test (OGTT) is used to confirm diagnosis, or a diagnostic OGTT can be used without screening by OGCT (MoH 2014).
A number of risk factors are associated with developing gestational diabetes (Nankervis 2013):
-
previous gestational diabetes;
-
previously elevated blood glucose level;
-
ethnicity: south and southeast Asian, Aboriginal, Pacific Islander, Māori, Middle Eastern, African;
-
age 40 years or over;
-
family history of diabetes mellitus (first‐degree relative with diabetes mellitus or a sister with gestational diabetes);
-
obesity, especially body mass index (BMI) greater than 35 kg/m2;
-
previous macrosomia (baby with birthweight greater than 4500 g or greater than 90th percentile);
-
polycystic ovarian syndrome;
-
medications: corticosteroids, antipsychotics;
-
pregnancy weight gain.
Some studies have reported an increasing prevalence of gestational diabetes (Ferrara 2007; Zhu 2016). As many as 50% of women with gestational diabetes will develop type 2 diabetes within five years of the index pregnancy (Kim 2002; Vounzoulaki 2020). Gestational diabetes increases the risk of serious injury at birth, the likelihood of caesarean delivery, and the incidence of newborn intensive care unit (NICU) admission (Ali 2011). Infants of women with gestational diabetes are at increased risk of developing obesity, impaired glucose tolerance, and diabetes as children or young adults (Boney 2005; Pettitt 1983; Pettitt 1988; Silverman 1998).
Description of the intervention
Both non‐pharmacological and pharmacological interventions have been used to try to prevent gestational diabetes
Metformin, an oral anti‐diabetic drug in the biguanide class, is the first‐line drug of choice for the treatment of type 2 diabetes (Nankervis 2013). Metformin has been used to prevent gestational diabetes in pregnant women with a history of polycystic ovary syndrome (PCOS) with contrasting results (Glueck 2008; Tang 2012). A randomised trial on the effect of metformin on obese pregnant women found that while fasting glucose and insulin were lower at 28 weeks' gestation in the metformin group, there was no difference in the risk of developing gestational diabetes, by either International Association of Diabetes and Pregnancy Study Groups (IADPSG) or World Health Organization (WHO) criteria, between those women who received metformin and those who received placebo (Chiswick 2015).
Myo‐inositol, an isomer of inositol, is commonly found in cereals, legumes and nuts (Croze 2013). It is a nutrient the body requires for cell membrane formation and cellular reactions to environmental messages (Croze 2013). Myo‐inositol is one of the intracellular mediators of the insulin signal and is correlated with insulin sensitivity in type 2 diabetes (Kennington 1990; Suzuki 1994). Due to its role as a second messenger, myo‐inositol has many benefits. When used as a co‐treatment in people with subclinical hypothyroidism and autoimmune thyroiditis, myo‐inositol aided maintenance of euthyroidism (normal production of thyroid hormone; Nordio 2013). Myo‐inositol has been associated with an improvement in a range of conditions. These include: premenstrual dysphoric disorder (PMDD), a mood disorder disrupting the social or occupational life, or both, of affected women (Carlomagno 2011); symptoms of PCOS, a medical condition characterised by insulin resistance (Papaleo 2007); insulin sensitivity and ovulatory function in young women affected by PCOS (Genazzani 2008; Nestler 1999); hyperandrogenism in women with PCOS (Minozzi 2008); and increased number and quality of oocytes in women undergoing in vitro fertilisation (IVF) treatment for a previous history of infertility (Unfer 2011).
Antenatal supplementation with myo‐inositol for the prevention of gestational diabetes is novel, and whether myo‐inositol is viewed as a nutritional supplement or as a medicine requiring prescription, seems to vary in different parts of the world.
How the intervention might work
Given these beneficial effects on improving insulin sensitivity, myo‐inositol may be useful for women with gestational diabetes. A retrospective review of 46 pregnant women treated with myo‐inositol compared with 37 controls described it as safe during the pre‐pregnancy and early pregnancy period when used in insulin‐resistant conditions (D'Anna 2012). No women reported any side effects of treatment.
Why it is important to do this review
Gestational diabetes is an increasing problem worldwide. To date, three Cochrane Reviews on the prevention of gestational diabetes have been conducted. In Dietary advice in pregnancy for preventing gestational diabetes mellitus, Tieu 2017 concluded that while a low glycaemic index (GI) diet was beneficial for some outcomes for the mother (lower maternal fasting glucose concentration) and child (reduction in large‐for‐gestational‐age infants, lower ponderal index), the evidence is limited. Similarly, in Exercise for pregnant women for preventing gestational diabetes mellitus, Han and colleagues concluded that there is limited evidence to support exercise during pregnancy for the prevention of glucose intolerance or gestational diabetes (Han 2012). Bain and colleagues assessed the effects of physical exercise in combination with dietary advice for pregnant women for preventing gestational diabetes, and health consequences for the mother and her infant/child (Bain 2015). They found no clear differences in outcomes between women receiving diet and exercise interventions compared with those receiving no intervention. Thus, identification of effective preventive measures for gestational diabetes remains of great importance. This is an update of a review first published in 2015, that found that myo‐inositol taken during pregnancy may prevent the development of gestational diabetes, but further trials were required (Crawford 2015). Since then further trials have been published that may be eligible for inclusion in this review.
Objectives
To assess if antenatal dietary supplementation with myo‐inositol is safe and effective, for the mother and fetus, in preventing gestational diabetes.
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished randomised controlled trials (RCTs) including conference abstracts assessing the effects of myo‐inositol for the prevention of gestational diabetes. We planned to include cluster‐RCTs, but we did not identify any. We excluded quasi‐randomised trials and cross‐over trials.
Types of participants
We included pregnant women but excluded women with pre‐existing type 1 or type 2 diabetes.
Types of interventions
Any dose of myo‐inositol in pregnancy, alone or in a combination preparation, for the purpose of preventing gestational diabetes. We included studies where such intervention was compared with no treatment, placebo or another intervention.
Types of outcome measures
Studies that met the above inclusion criteria were included regardless of whether they reported on the following outcomes for the review.
Primary outcomes
Maternal outcomes
-
Gestational diabetes (diagnostic criteria as defined in individual studies)
-
Hypertensive disorders of pregnancy (including pre‐eclampsia, eclampsia, pregnancy‐induced hypertension)
Neonatal outcomes
-
Large‐for‐gestational age (birthweight greater than the 90th centile; or as defined by individual study)
-
Perinatal mortality (stillbirth and neonatal mortality)
-
Mortality or morbidity composite (variously defined by trials, e.g. infant death, shoulder dystocia, bone fracture or nerve palsy)
Secondary outcomes
Maternal outcomes
-
Caesarean section
-
Placental abruption
-
Induction of labour
-
Perineal trauma
-
Postpartum hemorrhage
-
Postpartum infection
-
Weight gain during pregnancy
-
Adherence to the intervention (as defined by study authors)
-
Behaviour changes associated with the intervention (as defined by study authors)
-
Relevant biomarker changes associated with the intervention (e.g. adiponectin, free fatty acids, triglycerides, high‐density lipoproteins (HDL), low‐density lipoproteins (LDL), insulin)
-
Sense of well‐being and quality of life
-
Views of the intervention
-
Breastfeeding (e.g. at discharge, six weeks postpartum)
-
Adverse effects of intervention
Long‐term maternal outcomes
-
Postnatal depression
-
Postnatal weight retention or return to pre‐pregnancy weight
-
Body mass index (BMI)
-
Gestational diabetes in a subsequent pregnancy
-
Type 1 diabetes mellitus
-
Type 2 diabetes mellitus
-
Impaired glucose tolerance
-
Cardiovascular health (as defined by trialists, including blood pressure (BP), hypertension, cardiovascular disease, metabolic syndrome)
Infant outcomes
-
Stillbirth
-
Neonatal mortality
-
Gestational age at birth
-
Preterm birth (less than 37 weeks' gestation and less than 32 weeks' gestation)
-
Apgar score (less than seven at five minutes)
-
Macrosomia
-
Small‐for‐gestational age
-
Birthweight and birthweight z‐score
-
Head circumference and head circumference z‐score
-
Length and length z‐score
-
Ponderal index
-
Adiposity
-
Shoulder dystocia
-
Bone fracture
-
Nerve palsy
-
Respiratory distress syndrome
-
Hypoglycaemia (variously defined)
-
Hyperbilirubinaemia
Childhood outcomes
-
Weight and weight z‐score
-
Height and height z‐score
-
Head circumference and head circumference z‐score
-
Adiposity (e.g. as measured by BMI, skinfold thickness)
-
Blood pressure
-
Type 1 diabetes mellitus
-
Type 2 diabetes mellitus
-
Impaired glucose tolerance
-
Dyslipidaemia or metabolic syndrome
-
Neurodisability
-
Educational achievement
Adulthood outcomes
-
Weight
-
Height
-
Adiposity (e.g. as measured by BMI, skinfold thickness)
-
Cardiovascular health (as defined by study authors, including BP, hypertension, cardiovascular disease, metabolic syndrome)
-
Type 1 diabetes mellitus
-
Type 2 diabetes mellitus
-
Impaired glucose tolerance
-
Dyslipidaemia or metabolic syndrome
-
Employment, education and social status/achievement
Health services cost
-
Number of hospital or health professional visits (e.g. midwife, obstetrician, physician, dietitian, diabetic nurse)
-
Number of antenatal visits or admissions
-
Length of antenatal stay
-
Neonatal intensive care unit (NICU) admission
-
Length of postnatal stay (mother)
-
Length of postnatal stay (baby)
-
Costs to families associated with the management provided
-
Costs associated with the intervention
-
Cost of maternal care
-
Cost of offspring care
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (17 March 2022).
The Register is a database containing over 34,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register, including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of hand searched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) which includes centralised searches of the WHO International Clinical Trials Registry Platform (ICTRP);
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
monthly searches of CINAHL (EBSCO);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
These search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above are reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth Group review topic (or topics) and is then added to the Register.
The Information Specialist searched the Register for this review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included, Excluded, Awaiting Classification or Ongoing).
In addition, we searched ClinicalTrials.gov and the WHO ICTRP for unpublished, planned and ongoing trial reports (17 March 2022). The search terms used are given in Appendix 1.
Searching other resources
We searched reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
Selection of studies
Three review authors (SM, LL and CC) independently assessed all potential studies identified from the search strategy for inclusion. We resolved any disagreement through discussion. We created a study flow diagram to map out the number of records identified, included and excluded (Figure 1).

Study flow diagram for updated review
Screening eligible studies for scientific integrity or trustworthiness
Two review authors evaluated all studies that initially met our inclusion criteria against predefined criteria to determine which studies, based on available information, were deemed to be sufficiently untrustworthy to be excluded. We used the following criteria.
Research governance
-
No prospective trial registration for studies published after 2010 without plausible explanation.
-
When requested, study authors refused to share the protocol and or ethics approval letter.
-
Study authors refused to engage in communication with the Cochrane Review authors.
-
Study authors refused to provide individual patient data (IPD) upon request with no justifiable reason.
Baseline characteristics
Characteristics of the study participants being too similar (distribution of mean (SD) excessively narrow or excessively wide, as noted by Carlisle 2017).
Feasibility
-
Implausible numbers (e.g. 500 women with severe cholestasis of pregnancy recruited in 12 months).
-
(Close to) zero losses to follow‐up without plausible explanation.
Results
-
Implausible results (e.g. massive risk reduction for main outcomes with small sample size).
-
Unexpectedly even numbers of women ‘randomised’ including a mismatch between the numbers and the methods (e.g. if they say no blocking was used but still end up with equal numbers, or they say they used blocks of four, but the final numbers differ by six).
We excluded studies assessed as being potentially high risk. Where a study was classified as high risk for one or more of the above criteria, we attempted to contact the study authors to address any possible lack of information or concerns. If adequate information remained unavailable, we kept the study in Studies awaiting classification and we reported the reasons and communications with the study author (or lack of), in detail.
The process used is described in Figure 2.
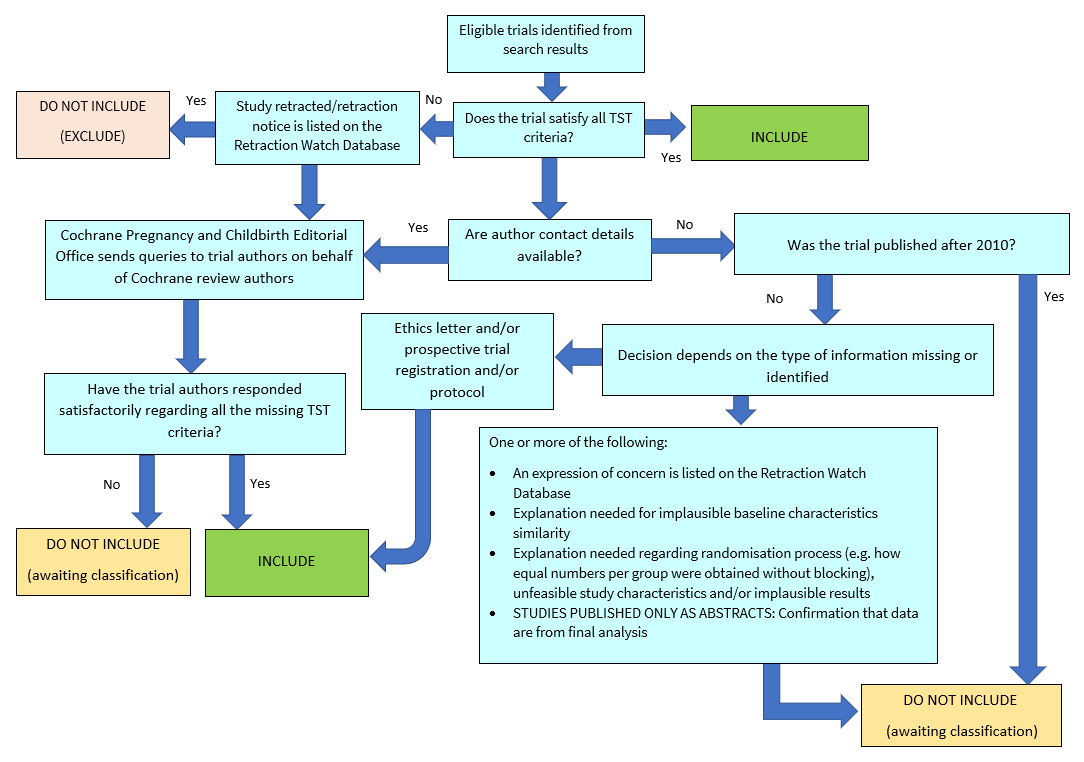
Applying the trustworthiness screening tool criteria. TST: Trustworthiness Screening Tool.
Abstracts
We only included data from abstracts if, in addition to the trustworthiness assessment, the study authors confirmed in writing that the data to be included in the review had come from the final analysis and would not change. If such information was not available or provided, the study remained in Studies awaiting classification (as above).
Data extraction and management
We designed a form to extract data based on the Cochrane Pregnancy and Childbirth Group's data extraction form. For eligible studies, two review authors (SM, LL, JA and CC) independently extracted the data using the agreed form. We resolved discrepancies through discussion. We entered data into Review Manager software (Review Manager 2020) and checked for accuracy. When information regarding any of the above was unclear, we attempted to contact study authors of the original reports to provide further details.
Assessment of risk of bias in included studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). We resolved any disagreement by discussion.
Random sequence generation (checking for possible selection bias)
For each included study we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
Allocation concealment (checking for possible selection bias)
For each included study we described the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias
Blinding of participants and personnel (checking for possible performance bias)
For each included study we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
Blinding of outcome assessment (checking for possible detection bias)
For each included study we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For each outcome or class of outcomes in each included study, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the study authors, we re‐included missing data in the analyses which we undertook.
We assessed the methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomization);
-
unclear risk of bias.
Selective reporting (checking for reporting bias)
For each included study we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it is clear that all the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
Other bias (checking for bias due to problems not covered by the domains above)
For each included study we described any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
-
low risk of other bias;
-
high risk of other bias;
-
unclear whether there is risk of other bias.
Overall risk of bias
We made explicit judgments about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). With reference to the domains above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias by undertaking sensitivity analyses (see Sensitivity analysis).
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratios (RR) with 95% confidence intervals (CIs).
Continuous data
For continuous data where outcomes were measured on the same scale, we presented the mean difference (MD) with 95% CIs. For studies that measured the same outcome on different scales, we planned to report the standardised mean difference (SMD) and 95% CIs.
Unit of analysis issues
Cluster‐RCTs
We did not identify any cluster‐RCTs for inclusion in this review. If we identify cluster‐RCTs for inclusion in future updates of this review, we will include them in the analyses along with individually‐randomised studies. We will make adjustments using the methods described in sections 16.3.4 and 16.3.6 in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021), using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar study or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. We will consider it reasonable to combine the results from both cluster‐RCTs and individually‐randomised studies if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomization unit is considered to be unlikely.
Multiple pregnancy
There may be unit of analysis issues that arise when women randomised have a multiple pregnancy. We present maternal data as per woman randomised and neonatal data as per infant.
Multiple arm studies
In future updates of this review, where a study has multiple intervention arms, we will avoid 'double counting' of participants by combining groups to create a single pair‐wise comparison if possible. Where this is not possible, we will split the 'shared' group into two or more groups with smaller sample size and include two or more (reasonably independent) comparisons.
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat (ITT) basis; that is, we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether they received the allocated intervention. The denominator for each outcome in each study was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and either Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
We did not undertake investigation of reporting biases because we included only seven studies. In future updates of this review, if 10 or more studies are included in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager (RevMan) software (Review Manager 2020). We used fixed‐effect meta‐analyses for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect (i.e. where studies were examining the same intervention, and the studies’ populations and methods were judged sufficiently similar). If there was sufficient clinical heterogeneity to suggest that the underlying treatment effects differed between studies, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across studies was considered clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects, and we discussed the clinical implications of treatment effects differing between studies. If the average treatment effect was not clinically meaningful, we did not combine studies.
Where we used random‐effects analyses, we present the results as the average treatment effect with 95% CIs, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses where data were available. We considered whether an overall summary was meaningful, and if it was, used random‐effects analysis to produce it.
We planned to conduct the following subgroup analyses:
-
women with polycystic ovary syndrome (PCOS) versus women without PCOS;
-
obese women versus non‐obese women;
-
dosage: high versus low dose;
-
myo‐inositol alone or in combination versus non myo‐inositol combination;
-
commencement of myo‐inositol supplementation: pre‐pregnancy versus first trimester.
However, we were unable to split the participant data into subgroups, and none of the included studies commenced supplementation with myo‐inositol pre‐pregnancy.
We planned to restrict subgroup analysis to this review's primary outcomes.
In future versions of this review, we will assess subgroup differences by interaction tests available within RevMan (Review Manager 2020). We will report the results of subgroup analyses quoting the Chi2 statistic and P value, and the interaction test I² value.
Sensitivity analysis
We had insufficient studies to conduct sensitivity analysis for this review. If in future updates there are sufficient studies for analysis, and there is evidence of significant heterogeneity for primary outcomes, we will explore heterogeneity by using the quality of the included studies. We will compare studies that have low risk of bias for allocation concealment with those judged to be of unclear or high risk of bias.
Summary of findings and assessment of the certainty of the evidence
We assessed the certainty of the evidence using the GRADE approach, as outlined in the GRADE handbook, in order to assess the certainty of the body of evidence relating to the following outcomes for the main comparisons. We produced two summary of findings tables for seven maternal outcomes and seven neonatal, child and adult outcomes.
Maternal
-
Gestational diabetes
-
Weight gain during pregnancy
-
Hypertensive disorders of pregnancy (including pre‐eclampsia, eclampsia, and pregnancy‐induced hypertension)
-
Caesarean section
-
Perineal trauma
-
Postnatal depression
-
Development of subsequent type 2 diabetes mellitus
Neonatal, child, adult outcomes
-
Large‐for‐gestational age
-
Perinatal mortality (stillbirth and neonatal mortality)
-
Composite of serious neonatal outcomes
-
Neonatal hypoglycaemia (variously defined)
-
Adiposity (e.g. as measured by BMI, skinfold thickness)
-
Diabetes
-
Neurosensory disability
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (Review Manager 2020) in order to create summary of findings tables. We produced a summary of the intervention effect and a measure of certainty for each of the above outcomes using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence for each outcome. The evidence can be downgraded from 'high certainty' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Results
Description of studies
See Characteristics of included studies.
Results of the search
See Figure 1.
In the previous version of the review, we included four studies (seven reports) and excluded two studies. The updated search (March 2022) retrieved 28 new study reports after we removed duplicates. We deemed five of these records not relevant based on title and abstract. We assessed the remaining 23 records, plus one report of an ongoing study (Farren 2017) in the previous review. We classified seven studies (12 reports) as ongoing trials (Amaefule 2018; Asimakopoulos 2020; CTRI/2018/06/014477; Ibrahim 2022; IRCT20120826010664N4; NCT04801485; NL7799). We excluded two studies as the participants did not meet the inclusion criteria of the review (Celentano 2020; Godfrey 2017). We considered four studies (8 records) as eligible for inclusion in the updated review (Farren 2017; Malvasi 2017; Santamaria 2016; Vitale 2019). Facchinetti 2013 was included in the previous version of this review, but we confirmed with the authors that Facchinetti 2013 is an interim report of D'Anna 2015. We classified one study (two reports) (Esmaeilzadeh 2021) as awaiting classification whilst awaiting further details. Therefore, we included seven studies in the updated review.
Screening eligible studies for trustworthiness
From the seven eligible studies identified from the search, we judged that all studies met our criteria for trustworthiness.
Included studies
See Characteristics of included studies.
Study design
We included seven RCTs (D'Anna 2013; D'Anna 2015; Farren 2017; Malvasi 2014; Malvasi 2017; Santamaria 2016; Vitale 2019).
Setting
Six studies were conducted in Italy (D'Anna 2013; D'Anna 2015; Malvasi 2014; Malvasi 2017; Santamaria 2016; Vitale 2019) and one study was conducted in Ireland (Farren 2017). The included studies were conducted between 2010 and 2018.
Participants
All studies were conducted in pregnant women. Gestational age at study entry was 10 to 16 weeks in one study (Farren 2017); 12 to 13 weeks in four studies (D'Anna 2013; D'Anna 2015; Santamaria 2016; Vitale 2019); 13 to 24 weeks in one study (Malvasi 2014); and 24 to 28 weeks in another study (Malvasi 2017). Six studies were on women with a BMI less than 30 kg/m2 (D'Anna 2013; Malvasi 2014; Malvasi 2017; Farren 2017; Santamaria 2016; Vitale 2019) while one study was on obese women with a BMI greater 30 kg/m2 (D'Anna 2015). Three studies included women exclusively of white ethnicity (D'Anna 2013; Santamaria 2016; Vitale 2019). An inclusion criterion in D'Anna 2013 was a first‐degree relative with type 2 diabetes. Women with pre‐existing diabetes mellitus were excluded from all included studies
Five studies (D'Anna 2013; D'Anna 2015; Farren 2017; Santamaria 2016; Vitale 2019) used the International Association of Diabetes and Pregnancy Study Groups (IADPSG 2010) to diagnose GDM while the two studies (Malvasi 2014; Malvasi 2017) used the Italian Society of Diabetology criteria.
Groups were comparable at baseline for age, parity and BMI in Malvasi 2014 and Malvasi 2017. In D'Anna 2015; D'Anna 2013; Santamaria 2016; Farren 2017, the participants were comparable between groups at baseline for maternal age, BMI and gestational age at the commencement of treatment. In Vitale 2019, groups were comparable at baseline for age and haematological parameters. D'Anna 2013, Santamaria 2016, and Vitale 2019 included women exclusively of Caucasian ethnicity. An inclusion criterion in D'Anna 2013 was a first‐degree relative with type 2 diabetes. Women with pre‐existing diabetes mellitus were excluded from all included studies.
Intervention
The following doses of myo‐inositol were reported.
-
4 g myo‐inositol, 400 mcg folic acid daily in divided doses (2 g myo‐inositol plus 200 mcg folic acid twice a day) (D'Anna 2013; D'Anna 2015; Santamaria 2016; Vitale 2019)
-
1100 mg myo‐inositol, 27.6 mg C‐chiro‐inositol, 400 mcg folic acid per day (Farren 2017)
-
2 g myo‐inositol, 400 mg D‐chiro‐inositol, 400 mcg folic acid and 10 mg manganese per day in one dose (Malvasi 2014)
-
200 mg myo‐inositol, 500 mg D‐chiro‐inositol, 80 mg of Revifast (Malvasi 2017)
Comparison
The following comparisons were reported.
-
200 mcg folic acid (D'Anna 2013; D'Anna 2015; Santamaria 2016; Vitale 2019)
-
400 mcg folic acid (Farren 2017)
-
The authors stated women received placebo but did not state what the placebo was (Malvasi 2014; Malvasi 2017).
One study provided nutritional and lifestyle counselling to women in both the treatment and control groups (D'Anna 2015). None of the other included studies reported this.
Diagnostic criteria used to diagnose GDM
International Association of Diabetes and Pregnancy Study Groups (IADPSG 2010): D'Anna 2013; D'Anna 2015; Farren 2017; Santamaria 2016; Vitale 2019.
Italian Society of Diabetology: Malvasi 2014; Malvasi 2017.
Outcomes
Five studies reported on gestational diabetes and provided fasting, one‐ and two‐hour blood glucose results (D'Anna 2013; D'Anna 2015; Farren 2017; Santamaria 2016; Vitale 2019). One study reported on gestational diabetes (Malvasi 2017) but did not provide blood glucose results. Five studies reported hypertensive disorders of pregnancy (D'Anna 2013; D'Anna 2015; Santamaria 2016; Vitale 2019; Farren 2017). Five studies reported on adverse effects of intervention (D'Anna 2013; Farren 2017; Malvasi 2014; Malvasi 2017; Santamaria 2016). Four studies reported a number of maternal and infant outcomes such as caesarean section, gestational age at birth, preterm birth, macrosomia, birthweight, neonatal hypoglycaemia, and shoulder dystocia (D'Anna 2013; D'Anna 2015; Farren 2017; Santamaria 2016). Four trials reported on weight gain during pregnancy (D'Anna 2013; D'Anna 2015; Santamaria 2016; Vitale 2019). Three studies reported on the use of insulin (D'Anna 2015; Santamaria 2016; Vitale 2019). Two studies reported on respiratory distress syndrome (D'Anna 2013; Farren 2017). Two studies reported on relevant biomarker changes associated with the intervention (Malvasi 2014; Malvasi 2017). Only one study reported on the following maternal and neonatal outcomes: postpartum hemorrhage, adherence to the intervention, perineal trauma, large for gestation age, small for gestational age, nerve palsy, neonatal hyperbilirubinemia and admission to neonatal intensive care unit or special care baby unit (Farren 2017).
Funding sources
Two studies reported no funding source, with participants buying their own supplements (D'Anna 2013; Santamaria 2016). Farren 2017 reported that they did not receive any specific grant. Two studies did not state the source of funding (Malvasi 2014; Vitale 2019). D'Anna 2015 was funded by a grant from Messina University, Italy. Farren 2017 was supported by the Coombe Women & Infants University Hospital, Ireland and the food supplement was provided at no cost from Lo.Li. Pharma. Malvasi 2017 reported the research did not receive a specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors. All seven studies reported that none of the authors had any potential financial conflicts of interest (D'Anna 2013; D'Anna 2015; Farren 2017; Malvasi 2014; Malvasi 2017; Santamaria 2016; Vitale 2019).
Ongoing studies
See Characteristics of ongoing studies.
One record of an ongoing study from the previous version has now been added to the included studies (Farren 2013). In this update, seven ongoing studies (12 records) have been identified for potential inclusion when published (Amaefule 2018; Asimakopoulos 2020; CTRI/2018/06/014477; Ibrahim 2022; IRCT20120826010664N4; NCT04801485; NL7799).
Amaefule 2018 will compare outcomes of participants who take 2 g of myo‐inositol twice daily from 12 + 0 to 15 + 6 weeks’ gestational age until delivery with those who take an identical placebo at the same dose and duration. Their participants will be pregnant women with a singleton pregnancy recruited from 15 + 6 weeks of gestation. Asimakopoulos 2020 aims to compare outcomes between participants who take 4 g of myo‐inositol and 400 mcg of folic acid daily from 11 to 13 + 6 to 26 to 28 weeks of gestation with those who take 400 mcg of folic acid daily for the same duration. Their participants will not have pre‐existing impaired glucose tolerance and will have a singleton pregnancy. CTRI/2018/06/014477 aims to compare outcomes between participants who take myo‐d‐chiro inositol and vitamin D3 sachets twice daily in water and those who take placebo and vitamin D3 sachets twice daily. Their participants will have a BMI ≤ 35. Ibrahim 2022 aims to compare outcomes in pregnant women who will either take myo‐inositol supplementation or a placebo, with all participants completing at least 12 weeks of supplementation prior to undertaking the OGTT at 24 to 28 weeks. IRCT20120826010664N4 will analyse differences in outcomes of their participants who take 2 g of myo‐inositol and 200 mcg of folic acid twice daily from 14 to 28 weeks gestation with those who take only 200 mcg folic acid twice daily. They will recruit participants they define to have a high risk of developing gestational diabetes. NCT04801485 will examine outcomes in women considered at high risk of gestational diabetes who will either take myo‐inositol 1 gram per day as well as health guidance about diet and exercise or a placebo and the same health guidance. NL7799 will examine outcomes in pregnant women with confirmed PCOS who take 4 g myo‐inositol in addition to folic acid supplement, twice daily throughout pregnancy and those with PCOS who take the standard dose of folic acid without the myo‐inositol supplement.
(See Ongoing studies).
Excluded studies
See Characteristics of excluded studies.
In the previous version of this review, two studies were excluded (Corrado 2011; Matarrelli 2013). These studies were ineligible as they used myo‐inositol as a treatment for women already diagnosed with gestational diabetes rather than as a preventative intervention.
In this update, we excluded two studies (Celentano 2020; Godfrey 2017) as their participants did not meet the inclusion criteria for this review. Celentano 2020 recruited pregnant women with elevated fasting glucose levels (> 92 mg/dL and < 126 mg/dL) which may include women with pre‐gestational diabetes. Godfrey 2017 did not recruit pregnant women to their study but recruited women prior to conception.
Risk of bias in included studies
The overall risk of bias for most domains were low. See Figure 3 and Figure 4.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
We judged all seven included studies to be at low risk of selection bias for random allocation. Four studies used a computer‐generated random sequence (D'Anna 2015; D'Anna 2013; Santamaria 2016; Vitale 2019), two used a random number table (Malvasi 2014; Malvasi 2017), and randomisation was carried out by an independent statistician in the other study (Farren 2017).
We judged five studies to be at low risk of selection bias for allocation concealment (D'Anna 2015; Farren 2017; Malvasi 2014; Malvasi 2017; Santamaria 2016). In three studies, allocation was assigned by a centralised contact who was independent of the recruitment process (D'Anna 2015; Malvasi 2014; Malvasi 2017). Two studies used sealed opaque and sequentially numbered envelopes (Farren 2017; Santamaria 2016). We judged two studies to be at unclear risk of bias as allocation concealment was not reported (D'Anna 2013; Vitale 2019).
Blinding
Performance bias
We deemed two studies to be at unclear risk of performance bias as although participants were blinded, the clinicians involved were aware of the treatment allocation (Malvasi 2014; Malvasi 2017). The remaining five studies were open‐label, and we therefore judged them to be at high risk of performance bias (D'Anna 2013; D'Anna 2015; Farren 2017; Santamaria 2016; Vitale 2019).
Detection bias
We judged three studies to be at low risk of detection bias as outcome assessors were blinded to allocation group (D'Anna 2015; Malvasi 2014; Malvasi 2017). We judged D'Anna 2013 and Santamaria 2016 as unclear risk due to inadequate reporting of blinding of outcome assessors. In D'Anna 2013, whilst the outcome of incidence of gestational diabetes was diagnosed by a blood test and unlikely to be affected by blinding, other outcomes such as neonatal respiratory distress syndrome are more subjective and may be impacted by knowledge of treatment group. We judged the remaining two studies to be at high risk of detection bias as they were open‐label (Farren 2017; Vitale 2019).
Incomplete outcome data
We judged four studies to be at low risk of attrition bias as losses to follow up were low (D'Anna 2013; D'Anna 2015; Farren 2017; Santamaria 2016). In Santamaria 2016, there was a 10.5% overall loss to follow‐up but study authors provided a detailed explanation. We judged two studies to be at unclear risk of attrition bias (Malvasi 2017; Vitale 2019). In Malvasi 2017 randomisation and allocation were conducted after excluding six participants, but the three participants who left the study after the first visit were not treated as lost to follow‐up. Analysis was conducted on the remaining 104 women. Vitale 2019 reported a 10.8% overall loss but did not provide a detailed consort flow diagram. Finally, we judged Malvasi 2014 to be at high risk of attrition bias due to 26% overall attrition (17 women excluded from final analysis). Seven women left the study spontaneously but their group allocation or reasons for withdrawing were not stated.
Selective reporting
We judged five studies to be at low risk of reporting bias as all pre‐specified outcome measures were reported (D'Anna 2015; D'Anna 2013; Malvasi 2014; Malvasi 2017; Santamaria 2016). We judged Farren 2017 to be at low risk of reporting bias as all assessed outcome were reported, one outcome was pre‐specified but was not assessed. We judged Vitale 2019 to be at unclear risk of reporting bias as not all outcomes specified in the methodology section were reported.
Other potential sources of bias
We judged all included studies as being at low risk of other bias. The authors of all included studies declared no potential conflicts of interest.
Effects of interventions
See: Summary of findings 1 Myo‐inositol for preventing gestational diabetes: maternal outcomes; Summary of findings 2 Myo‐inositol for preventing gestational diabetes: infant, child and adult outcomes
The certainty of the evidence of the included studies is summarised in the summary of findings Table 1 and summary of findings Table 2 for the pre‐specified outcomes of this review.
Myo‐inositol versus placebo
All seven included studies compared myo‐inositol and placebo (D'Anna 2015; D'Anna 2013; Farren 2017; Malvasi 2014; Malvasi 2017; Santamaria 2016; Vitale 2019).
Maternal primary outcomes
Gestational diabetes
Six studies reported this outcome (D'Anna 2013: D'Anna 2015; Farren 2017; Malvasi 2017; Santamaria 2016; Vitale 2019). Meta‐analysis showed that supplementation of myo‐inositol may reduce the incidence of gestational diabetes compared with placebo (risk ratio (RR) 0.53, 95% confidence interval (CI) 0.31 to 0.90; 1140 women; very low‐certainty evidence; Analysis 1.1). Caution is required when interpreting the data due to significant heterogeneity (I2 = 71%). The difference is most likely due to differences in the study populations. D'Anna 2015 included only obese pregnant women while Malvasi 2017, Santamaria 2016 and Vitale 2019 recruited overweight women. D'Anna 2013 and Farren 2017 recruited women with a family history of type 1 or type 2 diabetes in a first‐degree relative. Nutritional and lifestyle counselling was provided to both the intervention and control groups in D'Anna 2015, but was not reported as being provided in the other studies.
Five studies reported on blood glucose concentrations at the time of the diagnostic 75 g OGCT for GDM at 24 to 28 weeks' gestation. Myo‐inositol may be associated with a reduction in blood glucose concentrations compared to placebo.
-
Fasting: mean difference (MD) ‐0.14 mmol/L, 95% CI ‐0.21 to ‐0.07; 1071 women; Analysis 1.2).
-
One hour: MD ‐0.34 mmol/L, 95% CI ‐0.55 to ‐0.14; 1071 women; Analysis 1.3.
-
Two hours: MD ‐0.38mmol/L, 95% CI ‐0.77 to 0.01; 1071 women; Analysis 1.4.
Hypertensive disorders of pregnancy (including pre‐eclampsia, eclampsia, pregnancy‐induced hypertension)
Five studies reported this outcome (D'Anna 2013; D'Anna 2015; Malvasi 2017; Santamaria 2016; Vitale 2019). Meta‐analysis showed that myo‐inositol may reduce the incidence of gestational hypertension (RR 0.34, 95% CI 0.19 to 0.61; 1052 women; low‐certainty evidence; Analysis 1.5).
Infant primary outcomes
Large‐for‐gestational‐age
Farren 2017 reported data on the primary neonatal outcome of large‐for‐gestational‐age and showed no difference between myo‐inositol and placebo (RR 1.40, 95% CI 0.65 to 3.02; 234 infants; low‐certainty evidence; Analysis 1.6).
Perinatal mortality
None of the included studies reported data on this outcome.
Mortality or morbidity composite (variously defined by trials, e.g. infant death, shoulder dystocia, bone fracture or nerve palsy)
None of the included studies reported data on this outcome.
Maternal secondary outcomes
Caesarean section
Four studies reported this outcome (D'Anna 2013; D'Anna 2015; Farren 2017; Santamaria 2016). Meta‐analysis showed that myo‐inositol resulted in little to no effect in caesarean section rate (RR 0.91, 95% CI 0.77 to 1.07; 829 women; low‐certainty evidence; Analysis 1.7).
Weight gain during pregnancy
Four studies reported this outcome (D'Anna 2013; D'Anna 2015; Santamaria 2016; Vitale 2019). Meta‐analysis showed that myo‐inositol resulted in little to no effect on weight gain during pregnancy compared to placebo (MD ‐0.25kg, 95% CI ‐1.26 to 0.76; I2 = 81%, 831 women, very low‐certainty evidence; Analysis 1.8). Caution is required when interpreting the data due to significant heterogeneity (I2 = 81%). The difference is most likely due to differences in the study populations.
Relevant biomarker changes associated with the intervention
Three studies on a total of 340 women reported this outcome (Malvasi 2014; Malvasi 2017; Vitale 2019). Meta‐analysis showed that myo‐inositol may reduce total cholesterol (MD ‐29.57 mg/dL, 95% CI ‐32.80 to ‐26.33), low‐density lipoproteins (LDL) (MD ‐22.43 mg/dL, 95% CI ‐25.86 to ‐19.00), high‐density lipoproteins (HDL) (MD ‐1.46 mg/dL, 95% CI ‐2.72 to ‐0.20), and triglycerides (MD ‐24.92 mg/dL, 95% CI ‐27.82 to ‐22.02), compared with the control group (Analysis 1.9).
Adverse effects of intervention
Five studies measured this outcome and reported no adverse effects of therapy (D'Anna 2013; Farren 2017; Malvasi 2014; Malvasi 2017; Santamaria 2016). The remaining two studies did not report on this outcome (D'Anna 2015; Vitale 2019).
Perineal trauma
One study reported data on perineal trauma (Farren 2017). The evidence is very uncertain about the effect of myo‐inositol on perineal trauma (RR 4.00, 95% CI 0.45 to 35.25; 234 women; Analysis 1.10).
Postpartum hemorrhage
One study reported data on postpartum hemorrhage (Farren 2017) and found no difference in the risk of postpartum haemorrhage between myo‐inositol and placebo (RR 0.67, 95% CI 0.31 to 1.42; 234 women; Analysis 1.11).
Adherence to the intervention
One study reported data on adherence to the intervention (Farren 2017). There was no difference in the risk of adherence to the intervention between myo‐inositol and placebo (RR 0.99, 95% CI 0.84 to 1.16; 240 women; Analysis 1.12).
Other secondary outcomes
No data were reported for any of the other pre‐specified maternal secondary outcomes for this systematic review (placental abruption, induction of labour, postpartum infection, behaviour changes associated with the intervention (as defined by study authors), sense of well‐being and quality of life, views of the intervention, breastfeeding (e.g. at discharge, six weeks postpartum), postnatal depression, postnatal weight retention or return to pre‐pregnancy weight, body mass index (BMI), GDM in a subsequent pregnancy, type I diabetes, type 2 diabetes, impaired glucose tolerance or cardiovascular health (as defined by trialists, including blood pressure (BP), hypertension, cardiovascular disease, metabolic syndrome)).
Other outcomes not pre‐specified
Although the main aim of the included studies was the prevention of GDM, three of the included studies that continued the intervention until the end of pregnancy (D'Anna 2013; D'Anna 2015; Santamaria 2016), reported on the need for additional pharmacological therapy to treat gestational diabetes For interest, we include a summary of these data. There was no difference between myo‐inositol and placebo for the need for use of insulin therapy (RR 0.50, 95% CI 0.17 to 1.52; 595 women; Analysis 1.13).
Infant secondary outcomes (infant, child and adult)
There were no differences in secondary infant outcomes between infants of mothers supplemented with myo‐inositol and placebo
Gestational age at birth
Four studies reported this outcome (D'Anna 2013; D'Anna 2015; Farren 2017; Santamaria 2016). Meta‐analysis showed no difference in the gestational age at birth between myo‐inositol and placebo (MD 3.69 days, 95%CI ‐1.48 to 8.86; 829 infants; Analysis 1.14). Caution is required when interpreting the data due to significant heterogeneity (I2 = 91%). The difference is most likely due to differences in the populations. D'Anna 2015 included only obese pregnant women while Malvasi 2017, Santamaria 2016 and Vitale 2019 recruited overweight women. D'Anna 2013 and Farren 2017 recruited women with a family history of type 1 or type 2 diabetes in a first‐degree relative. Nutritional and lifestyle counselling was provided to both the intervention and control groups in D'Anna 2015, but was not reported as being provided in the other studies.
Preterm birth
Four studies reported this outcome (D'Anna 2013; D'Anna 2015; Farren 2017; Santamaria 2016). Meta‐analysis showed that myo‐inositol may be associated with a reduction in the incidence of preterm birth compared with placebo (RR 0.35, 95% CI 0.17 to 0.70; 829 infants; Analysis 1.15).
Macrosomia
Four studies reported this outcome (D'Anna 2013; D'Anna 2015; Farren 2017; Santamaria 2016). Meta‐analysis showed no difference between myo‐inositol and placebo for the risk of macrosomia (RR 0.55, 95% CI 0.16 to 1.96; 829 infants; Analysis 1.16). Caution is required when interpreting the data due to significant heterogeneity (I2 = 46%). The difference is most likely due to differences in the populations. D'Anna 2015 included only obese pregnant women while Malvasi 2017, Santamaria 2016 and Vitale 2019 recruited overweight women. D'Anna 2013 and Farren 2017 recruited women with a family history of type 1 or type 2 diabetes in a first‐degree relative. Nutritional and lifestyle counselling was provided to both the intervention and control groups in D'Anna 2015, but was not reported as being provided in the other studies.
Birthweight
Four studies reported this outcome (D'Anna 2013; D'Anna 2015; Farren 2017; Santamaria 2016). Meta‐analysis showed no difference between myo‐inositol and placebo for birthweight (MD ‐8.65 g, 95% CI ‐140.36 to 123.07; 829 infants; Analysis 1.17) ). No data were reported for birthweight z‐scores. Caution is required when interpreting the data due to significant heterogeneity (I2 = 72%). The difference is most likely due to differences in the populations. D'Anna 2015 included only obese pregnant women while Malvasi 2017, Santamaria 2016 and Vitale 2019 recruited overweight women. D'Anna 2013 and Farren 2017 recruited women with a family history of type 1 or type 2 diabetes in a first‐degree relative. Nutritional and lifestyle counselling was provided to both the intervention and control groups in D'Anna 2015, but was not reported as being provided in the other studies.
Shoulder dystocia
Four studies reported this outcome (D'Anna 2013; D'Anna 2015; Farren 2017; Santamaria 2016). Meta‐analysis showed no difference between myo‐inositol and placebo on the risk of shoulder dystocia (RR 1.43, 95% CI 0.15 to 13.54; 829 infants; very low‐certainty evidence; Analysis 1.18). Caution is required when interpreting the data due to significant heterogeneity (I2 = 59%). The difference is most likely due to differences in the populations. D'Anna 2015 included only obese pregnant women while Malvasi 2017, Santamaria 2016 and Vitale 2019 recruited overweight women. D'Anna 2013 and Farren 2017 recruited women with a family history of type 1 or type 2 diabetes in a first‐degree relative. Nutritional and lifestyle counselling was provided to both the intervention and control groups in D'Anna 2015, but was not reported as being provided in the other studies.
Respiratory distress syndrome
Two studies reported this outcome (D'Anna 2013; Farren 2017), and showed no benefit of myo‐inositol on the risk of respiratory distress syndrome (RR 1.49, 95% CI 0.25 to 8.85; 2 studies; 431 infants; very low‐certainty evidence; Analysis 1.19)
Neonatal hypoglycaemia
Four studies reported this outcome (D'Anna 2013; D'Anna 2015; Farren 2017; Santamaria 2016). Meta‐analysis showed no difference between myo‐inositol and placebo on neonatal hypoglycaemia (RR 3.07, 95% CI 0.90 to 10.52; 671 infants; very low‐certainty evidence; Analysis 1.20) . For infants of women who received myo‐inositol, the risk of neonatal hypoglycaemia ranged from 0.8% to 9.1%; for infants of women given a placebo, the risk of neonatal hypoglycaemia was 0.9%.
Small‐for‐gestational‐age
Farren 2017 reported data on small‐for‐gestational‐age infants and found no difference between myo‐inositol and placebo (RR 2.33, 95% CI 0.62 to 8.80; 234 infants; Analysis 1.21).
Nerve palsy
Farren 2017 measured nerve palsy but reported no cases.
Neonatal hyperbilirubinemia
Farren 2017 reported data on neonatal hyperbilirubinemia and found no difference between myo‐inositol and placebo (RR 0.25, 95% CI 0.05 to 1.15; 234 infants; Analysis 1.22).
Other secondary outcomes
No studies reported the other secondary neonatal (infant, child, adult) outcomes of this systematic review were reported (stillbirth, neonatal mortality, Apgar score < five at seven minutes, head circumference and z score, length and z score, ponderal index, adiposity, bone fracture). For the infant as a child and adult, no data were reported for any of the pre‐specified outcomes (weight, height, adiposity (e.g. as measured by BMI, skinfold thickness), cardiovascular health (as defined by trialists, including blood pressure, hypertension, cardiovascular disease, metabolic syndrome), type I diabetes, type 2 diabetes mellitus, impaired glucose tolerance, dyslipidaemia or metabolic syndrome, employment, education and social status/achievement).
Health service outcomes
D'Anna 2015 and Farren 2017 reported on admission to the neonatal intensive care unit (NICU) and found no difference between myo‐inositol and placebo (RR 0.40, 95% CI 0.14 to 1.18; 435 infants; very low‐certainty evidence; Analysis 1.23).
None of the included studies reported any of the other health service outcomes (number of hospital or health professional visits (e.g. midwife, obstetrician, physician, dietitian, diabetic nurse), number of antenatal visits or admissions, length of antenatal stay, length of postnatal stay (mother), length of postnatal stay (baby), costs to families associated with the management provided, costs associated with the intervention, cost of maternal care, and cost of offspring care).
Myo‐inositol versus no treatment
None of the included studies assessed myo‐inositol versus no treatment.
Myo‐inositol versus another intervention
None of the included studies assessed myo‐inositol versus another intervention.
Discussion
Summary of main results
In this updated review that included seven RCTs involving 1319 women, we found that the evidence is very uncertain about the effect of supplementation with myo‐inositol on the incidence of gestational diabetes, weight gain during pregnancy or perineal trauma. However, supplementation with myo‐inositol may result in a large reduction in hypertensive disorders of pregnancy but little to no difference in the risk of caesarean section. For infants, the evidence is also very uncertain about the effect of myo‐inositol on the risk of a large‐for‐gestational‐age infant or neonatal hypoglycaemia, but myo‐inositol may be associated with a reduction in the incidence of preterm birth. None of the current trials reported on postnatal depression, development of subsequent type 2 diabetes mellitus, perinatal mortality or serious neonatal outcomes.
Overall completeness and applicability of evidence
The included studies were conducted in healthy women and those considered at high risk of developing gestational diabetes, including obese and non‐obese women, and those with a family history of type 2 diabetes mellitus. However, applicability is limited by six studies being conducted in Italy and only one being conducted elsewhere, in Ireland, and participants were predominantly white women. Further studies in diverse settings, including participants of different ethnicities and varying risk factors, would improve the applicability of the evidence. Not all the outcomes of interest for this review were addressed in the included studies, including pre‐eclampsia, perinatal mortality, and longer‐term maternal and infant health outcomes. Furthermore, we were unable to conduct sensitivity analysis due to the small number of included studies reporting few outcomes. Several factors may influence the outcome effects, including the differences in myo‐inositol doses (that varied from 200 mg to 4 g in the included trials), women at different risks (both obese and non‐obese populations) and different gestational age at study entry.
Quality of the evidence
The current evidence is based on seven RCTs that included a total of 1319 women and their infants. The overall risk of bias was judged to be low. Where studies had a high risk of performance bias and detection bias this was due to their open‐label study design. Where there was an unclear risk of bias this was because of insufficient information provided to enable a judgment of risk to be made.
Using the GRADE method, we assessed the certainty of the body of evidence for the maternal outcomes of gestational diabetes, weight gain during pregnancy, hypertensive disorders of pregnancy, caesarean section, perineal trauma, postnatal depression, and type 2 diabetes, and the neonatal outcomes of large‐for‐gestational age, perinatal mortality, composite of serious neonatal outcomes, neonatal hypoglycaemia, adiposity, diabetes, and neurosensory disability. No data were reported for the maternal outcomes of postnatal depression and type 2 diabetes. No data were reported for the neonatal outcomes of perinatal mortality, composite of serious neonatal outcomes, adiposity, diabetes, and neurosensory disability. The certainty of evidence was downgraded in the summary of findings Table 1 and summary of findings Table 2 to low or very low, due to limitation in study design, indirectness and imprecision.
Potential biases in the review process
The Trials Search Co‐ordinator of the Cochrane Pregnancy and Childbirth Group searched multiple databases, without language or date restrictions in an attempt to limit bias by identifying all relevant trials. Where necessary, we contacted trial authors to seek clarification or further information. As per the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021), at least two review authors appraised studies for inclusion and extracted the data in order to minimise bias.
Agreements and disagreements with other studies or reviews
The increasing prevalence of gestational diabetes worldwide has led to greater interest in findings ways to prevent and treat gestational diabetes. The body of evidence for the use of antenatal myo‐inositol supplementation for the prevention of gestational diabetes continues to grow. Other literature (Di Benedetto 2013), a meta‐analysis (Zheng 2015), and systematic reviews (Rogozinska 2015; Guo 2018; Noventa 2016; Vitagliano 2019; Zhang 2019), cited most of the studies included in this updated review, and draw similar conclusions that myo‐inositol shows potential in preventing gestational diabetes. All are unanimous in their call for large, high‐quality RCTs in more diverse populations to further assess this potential treatment. We await the publication of ongoing studies that can be incorporated into future updates of this review.

Study flow diagram for updated review

Applying the trustworthiness screening tool criteria. TST: Trustworthiness Screening Tool.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Comparison 1: Myo‐inositol versus control, Outcome 1: Gestational diabetes mellitus

Comparison 1: Myo‐inositol versus control, Outcome 2: Fasting OGTT

Comparison 1: Myo‐inositol versus control, Outcome 3: One hour OGTT

Comparison 1: Myo‐inositol versus control, Outcome 4: Two hour OGTT

Comparison 1: Myo‐inositol versus control, Outcome 5: Hypertensive disorders of pregnancy

Comparison 1: Myo‐inositol versus control, Outcome 6: Large‐for‐gestational‐age

Comparison 1: Myo‐inositol versus control, Outcome 7: Caesarean section

Comparison 1: Myo‐inositol versus control, Outcome 8: Weight gain during pregnancy

Comparison 1: Myo‐inositol versus control, Outcome 9: Relevant biomarker changes associated with the intervention

Comparison 1: Myo‐inositol versus control, Outcome 10: Perineal trauma

Comparison 1: Myo‐inositol versus control, Outcome 11: Postpartum haemorrhage

Comparison 1: Myo‐inositol versus control, Outcome 12: Adherence to intervention

Comparison 1: Myo‐inositol versus control, Outcome 13: Supplementary insulin

Comparison 1: Myo‐inositol versus control, Outcome 14: Gestational age at birth

Comparison 1: Myo‐inositol versus control, Outcome 15: Preterm birth (less than 37 weeks' gestation)

Comparison 1: Myo‐inositol versus control, Outcome 16: Macrosomia

Comparison 1: Myo‐inositol versus control, Outcome 17: Birthweight

Comparison 1: Myo‐inositol versus control, Outcome 18: Shoulder dystocia

Comparison 1: Myo‐inositol versus control, Outcome 19: Respiratory distress syndrome

Comparison 1: Myo‐inositol versus control, Outcome 20: Neonatal hypoglycaemia

Comparison 1: Myo‐inositol versus control, Outcome 21: Small‐for‐gestational‐age

Comparison 1: Myo‐inositol versus control, Outcome 22: Neonatal hyperbilirubinaemia

Comparison 1: Myo‐inositol versus control, Outcome 23: Admission to neonatal intensive care unit or special care baby unit
| Antenatal supplementation with myo‐inositol for preventing gestational diabetes | ||||||
| Patient or population: pregnant women (women with pre‐existing type 1 or type 2 diabetes are NOT included) Setting: hospital | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with control | Risk with myo‐inositol | |||||
| Gestational diabetes mellitus | Study population | RR 0.53 | 1140 | ⊕⊝⊝⊝ Very lowa,b,c | GDM diagnosed using IADPSG 2010 criteria
Random‐effects model | |
| 217 per 1000 | 115 per 1,000 (67 to 196) | |||||
| Weight gain during pregnancy | Comparator | The mean weight gain during pregnancy in the intervention group was 0.25 kg lower (1.26 kg fewer to 0.76 kg more) | ‐ | 831 | ⊕⊝⊝⊝ |
Random‐effects model |
| Hypertensive disorders of pregnancy | Study population | RR 0.34 | 1052 | ⊕⊕⊝⊝ Lowc,f | Random‐effects model | |
| 86 per 1,000 | 29 per 1,000 | |||||
| Caesarean section | Study population | RR 0.91 | 829 | ⊕⊕⊝⊝ |
| |
| 430 per 1,000 | 391 per 1,000 | |||||
| Perineal trauma | Study population | RR 4.00 | 234 (1 RCT) | ⊕⊝⊝⊝ |
| |
| 9 per 1,000 | 34 per 1,000 (4 to 301) | |||||
| Postnatal depression | See comments | Not estimable | (0 studies) |
| No data reported this outcome in any of the included studies | |
|
|
| |||||
| Development of subsequent type 2 diabetes mellitus | See comments | Not estimable | (0 studies) |
| No data reported this outcome in any of the included studies | |
|
|
| |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a. Downgraded (‐1) for serious limitations in study design: due to unclear risk of selection bias in two of the six included studies; five of the six included studies were at high risk of performance bias; two of the six included studies were at high risk of detection bias; one study was at high risk of attrition bias. b. Downgraded (‐1) for serious inconsistency; considerable heterogeneity, possible due to different study populations. c. Downgraded (‐1) for serious indirectness; only one of the included studies was conducted outside Italy, and the Italian studies only included white women, the generalisability of findings is limited. d. Downgraded (‐1) for serious limitations in study design: all studies were at high risk of performance bias; one study was at high risk of detection bias. e. Downgraded (‐1) for serious imprecision; evidence of imprecision with wide confidence intervals crossing the line of no effect. f. Downgraded (‐1) for serious limitations in study design: all studies were at high risk of performance bias; two studies were at high risk of detection bias. g. Downgraded (‐1) for serious limitations in study design: all studies were at high risk of performance bias. One study was at high risk of detection bias, and insufficient evidence to judge detection bias and subsequent judgement of unclear risk of bias. Due to insufficient evidence to judge allocation concealment in two studies and subsequent judgement of unclear risk of bias. Due to insufficient evidence to judge attrition bias in two studies and subsequent judgement of unclear risk of bias. h. Downgraded (‐1) for serious limitations in study design: the study was at high risk of performance bias and detection bias for lack of blinding. i. Downgraded (‐1) for serious Indirectness: only one study conducted in Ireland reported this outcome. j. Downgraded (‐1) for serious imprecision: wide confidence intervals with very low event rates. | ||||||
| Antenatal supplementation with myo‐inositol for preventing gestational diabetes | ||||||
| Patient or population: infants of pregnant women Setting: hospital | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with control | Risk with myo‐inositol | |||||
| Large‐for‐gestational age | Study population | RR 1.40 | 234 | ⊕⊕⊝⊝ |
| |
| 85 per 1000 | 120 per 1000 (56 to 258) | |||||
| Perinatal mortality (stillbirth and neonatal mortality) | See comments | Not estimable | (0 studies) |
| No data reported this outcome in any of the included studies | |
|
|
| |||||
| Composite of serious neonatal outcomes | See comments | not estimable | (0 studies) |
| No data reported this outcome in any of the included studies | |
|
|
| |||||
| Neonatal hypoglycaemia | Study population | RR 3.07 | 671 | ⊕⊝⊝⊝ |
| |
| 9 per 1,000 | 27 per 1000 | |||||
| Adiposity | See comments | not estimable | (0 studies) |
| No data reported this outcome in any of the included studies | |
|
|
| |||||
| Diabetes | See comments | not estimable | (0 studies) |
| No data reported this outcome in any of the included studies | |
|
|
| |||||
| Neurosensory disability | See comments | not estimable | (0 studies) |
| No data reported this outcome in any of the included studies | |
|
|
| |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a. Downgraded (‐1) for serious limitations in study design: the study was at high risk of performance bias and detection bias for lack of blinding. b. Downgraded (‐1) for serious indirectness: only one study conducted in Ireland reported this outcome. c. Downgraded (‐1) for serious limitations in study design: all studies were at high risk of performance bias; one study was at high risk of detection bias. d. Downgraded (‐1) for serious indirectness: only one of the included studies was conducted outside Italy, and the Italian studies only included Caucasian women. Thus, the generalisability of findings is limited. e. Downgraded (‐1) for serious imprecision: evidence of imprecision with wide confidence intervals crossing the line of no effect. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Gestational diabetes mellitus Show forest plot | 6 | 1140 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.31, 0.90] |
| 1.2 Fasting OGTT Show forest plot | 5 | 1071 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.21, ‐0.07] |
| 1.3 One hour OGTT Show forest plot | 5 | 1071 | Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.55, ‐0.14] |
| 1.4 Two hour OGTT Show forest plot | 5 | 1071 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.77, 0.01] |
| 1.5 Hypertensive disorders of pregnancy Show forest plot | 5 | 1052 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.19, 0.61] |
| 1.6 Large‐for‐gestational‐age Show forest plot | 1 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.65, 3.02] |
| 1.7 Caesarean section Show forest plot | 4 | 829 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.77, 1.07] |
| 1.8 Weight gain during pregnancy Show forest plot | 4 | 831 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐1.26, 0.76] |
| 1.9 Relevant biomarker changes associated with the intervention Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.9.1 Total cholesterol | 3 | 340 | Mean Difference (IV, Fixed, 95% CI) | ‐29.57 [‐32.80, ‐26.33] |
| 1.9.2 Low density lipoprotein | 3 | 340 | Mean Difference (IV, Fixed, 95% CI) | ‐22.43 [‐25.86, ‐19.00] |
| 1.9.3 High density lipoprotein | 3 | 340 | Mean Difference (IV, Fixed, 95% CI) | ‐1.46 [‐2.72, ‐0.20] |
| 1.9.4 Triglycerides | 3 | 340 | Mean Difference (IV, Fixed, 95% CI) | ‐24.92 [‐27.82, ‐22.02] |
| 1.10 Perineal trauma Show forest plot | 1 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.00 [0.45, 35.25] |
| 1.11 Postpartum haemorrhage Show forest plot | 1 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.31, 1.42] |
| 1.12 Adherence to intervention Show forest plot | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.84, 1.16] |
| 1.13 Supplementary insulin Show forest plot | 3 | 595 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.17, 1.52] |
| 1.14 Gestational age at birth Show forest plot | 4 | 829 | Mean Difference (IV, Random, 95% CI) | 3.69 [‐1.48, 8.86] |
| 1.15 Preterm birth (less than 37 weeks' gestation) Show forest plot | 4 | 829 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.17, 0.70] |
| 1.16 Macrosomia Show forest plot | 4 | 829 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.16, 1.96] |
| 1.17 Birthweight Show forest plot | 4 | 829 | Mean Difference (IV, Random, 95% CI) | ‐8.65 [‐140.36, 123.07] |
| 1.18 Shoulder dystocia Show forest plot | 4 | 829 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.15, 13.54] |
| 1.19 Respiratory distress syndrome Show forest plot | 2 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.25, 8.85] |
| 1.20 Neonatal hypoglycaemia Show forest plot | 4 | 671 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.07 [0.90, 10.52] |
| 1.21 Small‐for‐gestational‐age Show forest plot | 1 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.62, 8.80] |
| 1.22 Neonatal hyperbilirubinaemia Show forest plot | 1 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.05, 1.15] |
| 1.23 Admission to neonatal intensive care unit or special care baby unit Show forest plot | 2 | 435 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.14, 1.18] |

