Administración prenatal de suplementos dietéticos con mioinositol en pacientes embarazadas para prevenir la diabetes gestacional
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011507.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 17 diciembre 2015see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Embarazo y parto
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Tineke Crawford is guarantor for this review.
Tineke Crawford screened search results, retrieved relevant papers, screened retrieved papers against eligibility criteria, appraised quality of papers, extracted data from papers, wrote to authors of papers for additional information, entered data into RevMan, analysed and interpreted data, wrote the first draft of the review, incorporated feedback into subsequent versions of the review.
Julie Brown conceived the review, screened retrieved papers against eligibility criteria, appraised quality of papers, extracted data from papers, checked data in RevMan, analysed and interpreted data, providing a methodological perspective, contributed to subsequent versions of the review.
Jane Alsweiler appraised quality of papers, extracted data from papers, analysed and interpreted data, providing a neonatal clinical perspective, and contributed to subsequent versions of the review.
Caroline Crowther provided a maternal clinical and methodological perspective.
Sources of support
Internal sources
-
None, Other.
External sources
-
The Cochrane Pregnancy and Childbirth Review Group editorial team, Liverpool, UK.
-
The Australasian Satellite of the Cochrane Pregnancy and Childbirth Review Group (Funded by NHMRC), Adelaide, Australia.
(incorporating the New Zealand branch)
-
The Liggins Institute, The University of Auckland, New Zealand.
Declarations of interest
Professor Caroline Crowther, Dr Julie Brown and Dr Jane Aslweiler are investigators on a planned trial of myo‐inositol supplements in pregnancy for the prevention of gestational diabetes. If this trial is eligible for inclusion in this review, Professor Caroline Crowher, Dr Julie Brown and Dr Jane Aslweiler will not be involved in any aspect of data extraction or risk of bias relating to this trial. Tineke Crawford and another researcher not involved in the trial will deal with the handling of these data.
Acknowledgements
Dahineswari Rajamanickam assisted the review authors in preparing the first draft of the protocol.
Portions of the methods section of this protocol are based on a standard template used by the Cochrane Pregnancy and Childbirth Review Group. Outcomes may be similar to other Cochrane reviews for preventing gestational diabetes due to the attempt to have consistent outcomes for reviews on this condition.
We acknowledge the support from the Cochrane Pregnancy and Childbirth Review Group editorial team in Liverpool, the Australian and New Zealand Satellite of the Cochrane Pregnancy and Childbirth Review Group (funded by NHMRC) and the Liggins Institute, University of Auckland, New Zealand.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team) and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2023 Feb 15 | Antenatal dietary supplementation with myo‐inositol for preventing gestational diabetes | Review | Soana K Motuhifonua, Luling Lin, Jane Alsweiler, Tineke J Crawford, Caroline A Crowther | |
| 2015 Dec 17 | Antenatal dietary supplementation with myo‐inositol in women during pregnancy for preventing gestational diabetes | Review | Tineke J Crawford, Caroline A Crowther, Jane Alsweiler, Julie Brown | |
| 2015 Feb 04 | Myo‐inositol for preventing gestational diabetes | Protocol | Julie Brown, Tineke J Crawford, Jane Alsweiler, Caroline A Crowther | |
Differences between protocol and review
There are some differences between our published protocol (Brown 2015) and the full review.
The title was listed as Myo‐inositol for preventing gestational diabetes in our published protocol but we have edited this to Antenatal dietary supplementation with myo‐inositol in women during pregnancy for preventing gestational diabetes in order to allow more clarity around the intervention, population and outcome.
Methods/Criteria for considering studies for this review
Types of interventions: we have expanded this section to include myo‐inositol in a combination preparation; this is also reflected in our list of planned subgroup analyses.
Types of participants: we have clarified that participants will be pregnant women rather than pregnant women at risk of gestational diabetes mellitus (GDM).
We have incorporated the use of GRADE to assess the quality of the body of evidence and have included 'Summary of findings' tables; this was not pre‐specified in our published protocol.
We have reported on the outcome 'need for supplementary insulin therapy' ‐ whilst this is not listed in our methods/outcomes section (and was not pre‐specified in our published protocol), we report on this outcome for interest.
Following a consultative process with Professor Caroline Crowther, Dr Julie Brown, Dr Philippa Middleton, Emily Bain and Tineke Crawford, a core set of primary and secondary outcomes for GDM systematic reviews and core outcomes for GRADE assessment for GDM systematic reviews were drawn up. This has resulted in a number of changes detailed below. These core outcomes were agreed upon after this review had been submitted for peer review.
Additionally, as this is a review on the use of a dietary supplement as an intervention, adverse effects of the intervention has been added as an outcome.
Previous maternal primary outcomes listed in protocol
-
Incidence of gestational diabetes mellitus (GDM) (diagnostic criteria as defined in individual trials)
-
Pre‐eclampsia
-
Caesarean section
Updated maternal primary outcomes used in review
-
Gestational diabetes mellitus (GDM)
-
Hypertensive disorders of pregnancy (including pre‐eclampsia, eclampsia, pregnancy‐induced hypertension)
Previous neonatal primary outcomes listed in protocol
-
Large‐for‐gestational age (birthweight greater than the 90th centile; or as defined by individual trial)
-
Perinatal mortality
-
Death or morbidity composite (variously defined by trials, e.g. infant death, shoulder dystocia, bone fracture or nerve palsy)
Updated neonatal primary outcomes used in review
-
Large‐for‐gestational age (birthweight greater than the 90th centile; or as defined by individual trial)
-
Perinatal mortality (stillbirth and neonatal mortality)
-
Mortality or morbidity composite (variously defined by trials, e.g. infant death, shoulder dystocia, bone fracture or nerve palsy)
Previous maternal secondary outcomes listed in protocol
-
Postnatal weight retention
-
Body mass index (BMI)
-
Development of type 2 diabetes mellitus
-
Development of type 1 diabetes mellitus
-
Impaired glucose tolerance (as defined in individual trials)
-
Insulin sensitivity (as defined in individual trials)
-
Incidence of pregnancy hyperglycaemia not meeting GDM diagnostic criteria (diagnostic criteria as defined in individual trials)
-
Induction of labour
-
Perineal trauma
-
Weight gain during pregnancy
-
Adiponectin levels
-
Gestational age at screening for GDM
-
Postpartum haemorrhage
-
Postpartum infection
-
Placental abruption
-
Polyhydramnios
-
Compliance with treatment
-
Breastfeeding at discharge, six weeks' postpartum
-
Women’s sense of well‐being and quality of life (as defined in individual trials)
-
Women’s view of intervention
Updated maternal secondary outcomes used in review
-
Caesarean section
-
Placental abruption
-
Induction of labour
-
Perineal trauma
-
Postpartum haemorrhage
-
Postpartum infection
-
Weight gain during pregnancy
-
Adherence to the intervention (as defined by trialists)
-
Behaviour changes associated with the intervention (as defined by trialists)
-
Relevant biomarker changes associated with the intervention (e.g. adiponectin, free fatty acids, triglycerides, high density lipoproteins, low density lipoproteins, insulin)
-
Sense of well‐being and quality of life
-
Views of the intervention
-
Breastfeeding (e.g. at discharge, six weeks postpartum)
-
Adverse effects of intervention
Long‐term maternal outcomes
-
Postnatal depression
-
Postnatal weight retention or return to pre‐pregnancy weight
-
Body mass index (BMI)
-
Gestational diabetes mellitus in a subsequent pregnancy
-
Type I diabetes mellitus
-
Type II diabetes mellitus
-
Impaired glucose tolerance
-
Cardiovascular health (as defined by trialists, including blood pressure (BP), hypertension, cardiovascular disease, metabolic syndrome)
Previous neonatal secondary outcomes listed in protocol
-
Macrosomia (as defined in individual trials)
-
Birthweight and z‐score
-
Head circumference and z‐score
-
Length and z‐score
-
Small‐for‐gestational age (as defined in individual trials)
-
Neonatal hypoglycaemia requiring treatment (as defined in individual trials)
-
Gestational age at birth
-
Preterm birth (less than 37 weeks’ gestational age)
-
Shoulder dystocia
-
Bone fracture
-
Nerve palsy
-
Respiratory distress syndrome
-
Hyperbilirubinaemia requiring treatment (as defined in individual trials)
-
Apgar scores (less than seven at five minutes)
-
Ponderal index
-
Fetal adiposity (as defined in individual trials)
-
Neonatal glucose concentration
-
Infant mortality (fetal, neonatal, perinatal)
Updated secondary outcomes used in review
-
Stillbirth
-
Neonatal mortality
-
Gestational age at birth
-
Preterm birth (less than 37 weeks' gestation and less than 32 weeks' gestation)
-
Apgar score (less than seven at five minutes)
-
Macrosomia
-
Small‐for‐gestational age
-
Birthweight and z‐score
-
Head circumference and z‐score
-
Length and z‐score
-
Ponderal index
-
Adiposity
-
Shoulder dystocia
-
Bone fracture
-
Nerve palsy
-
Respiratory distress syndrome
-
Hypoglycaemia (variously defined)
-
Hyperbilirubinaemia
Previous childhood outcomes listed in protocol
-
Weight
-
Height
-
Head circumference
-
Body mass index
-
Adiposity (fat mass/fat free mass (variously measured))
-
Blood pressure
-
Impaired glucose tolerance (as defined in individual trials)
-
Development of type 1 diabetes mellitus
-
Development of type 2 diabetes mellitus
-
Insulin sensitivity
-
Dyslipidaemia or metabolic syndrome
-
Neurodisability
-
Educational achievement
Updated childhood outcomes used in review
-
Weight and z scores
-
Height and z scores
-
Head circumference and z scores
-
Adiposity (e.g. as measured by BMI, skinfold thickness)
-
Blood pressure
-
Type I diabetes mellitus
-
Type II diabetes mellitus
-
Impaired glucose tolerance
-
Dyslipidaemia or metabolic syndrome
-
Neurodisability
-
Educational achievement
Previous adulthood outcomes listed in protocol
-
Weight
-
Height
-
BMI
-
Adiposity (fat mass/fat‐free mass (variously measured))
-
Blood pressure
-
Impaired glucose tolerance (as defined in individual trials)
-
Development of type 1 diabetes
-
Development of type 2 diabetes
-
Insulin sensitivity (as defined in individual trials)
-
Dyslipidaemia or metabolic syndrome
-
Educational achievement
Updated adulthood outcomes used in review
-
Weight
-
Height
-
Adiposity (e.g. as measured by BMI, skinfold thickness)
-
Cardiovascular health (as defined by trialists, including BP, hypertension, cardiovascular disease, metabolic syndrome)
-
Type I diabetes mellitus
-
Type II diabetes mellitus
-
Impaired glucose tolerance
-
Dyslipidaemia or metabolic syndrome
-
Employment, education and social status/achievement
Previous health services cost outcomes listed in protocol
-
Number of hospital visits or health professional visits (e.g. midwife, obstetrician, physician, dietitian)
-
Antenatal visits for mother
-
Direct costs to families in relation to the management provided
-
Length of postnatal stay (mother)
-
Admission to neonatal ward/ neonatal intensive care unit
-
Length of postnatal stay (baby)
-
Cost of maternal care
-
Cost of offspring care
Updated health services cost outcomes used in review
-
Number of hospital or health professional visits (e.g. midwife, obstetrician, physician, dietitian, diabetic nurse)
-
Number of antenatal visits or admissions
-
Length of antenatal stay
-
Neonatal intensive care unit admission
-
Length of postnatal stay (mother)
-
Length of postnatal stay (baby)
-
Costs to families associated with the management provided
-
Costs associated with the intervention
-
Cost of maternal care
-
Cost of offspring care
Previous GRADE outcomes listed in protocol
-
Incidence of GDM (diagnostic criteria as defined in individual trials)
-
Pre‐eclampsia
-
Mode of birth
-
Large‐for‐gestational age (birthweight greater than the 90th centile; or as defined by individual trial)
-
Perinatal mortality
-
Fetal adiposity
-
Impaired glucose tolerance as child/adult
Updated GRADE outcomes used in review
Maternal
-
Diagnosis of GDM
-
Gestational weight gain
-
Hypertensive disorders of pregnancy (including pre‐eclampsia, eclampsia, pregnancy‐induced hypertension)
-
Caesarean Section
-
Perineal trauma
-
Postnatal depression
-
Development of subsequent type II diabetes mellitus
Offspring (infant, child, adult)
-
Large‐for‐gestational age
-
Perinatal mortality (stillbirth and neonatal mortality)
-
Composite of serious neonatal outcomes
-
Neonatal hypoglycaemia (variously defined)
-
Offspring adiposity (e.g. as measured by BMI, skinfold thickness)
-
Offspring diabetes
-
Neurosensory disability
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Female; Humans; Pregnancy;
PICO

‐Study flow diagram.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
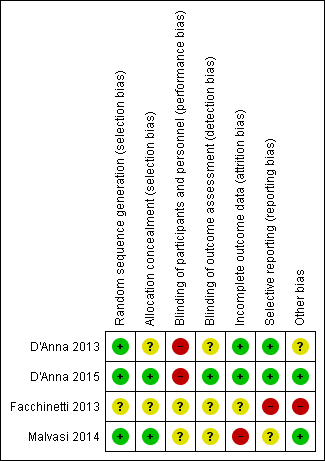
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Myo‐inositol versus control, Outcome 1 Gestational diabetes mellitus.

Comparison 1 Myo‐inositol versus control, Outcome 2 Fasting OGTT.
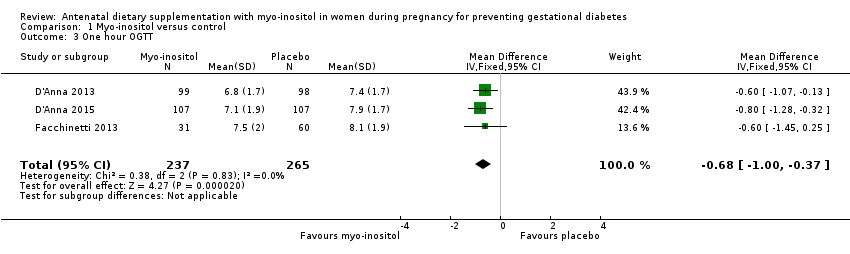
Comparison 1 Myo‐inositol versus control, Outcome 3 One hour OGTT.
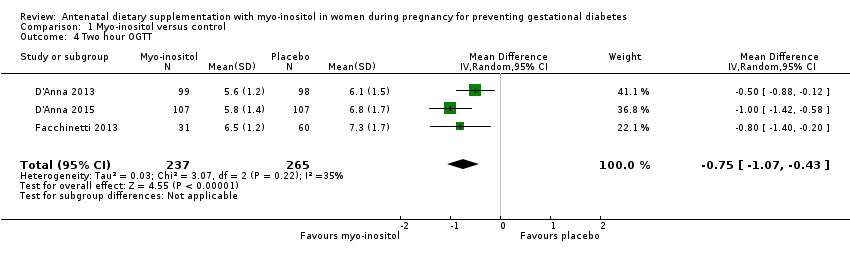
Comparison 1 Myo‐inositol versus control, Outcome 4 Two hour OGTT.

Comparison 1 Myo‐inositol versus control, Outcome 5 Hypertensive disorders of pregnancy.

Comparison 1 Myo‐inositol versus control, Outcome 6 Caesarean section.
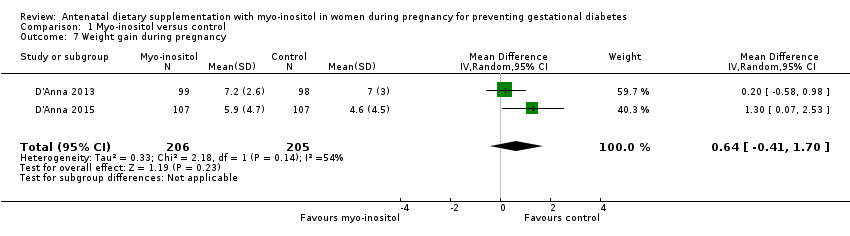
Comparison 1 Myo‐inositol versus control, Outcome 7 Weight gain during pregnancy.
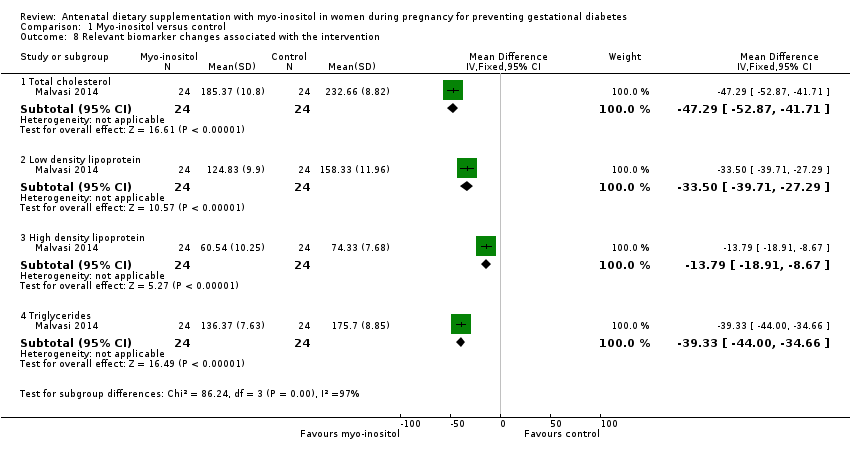
Comparison 1 Myo‐inositol versus control, Outcome 8 Relevant biomarker changes associated with the intervention.
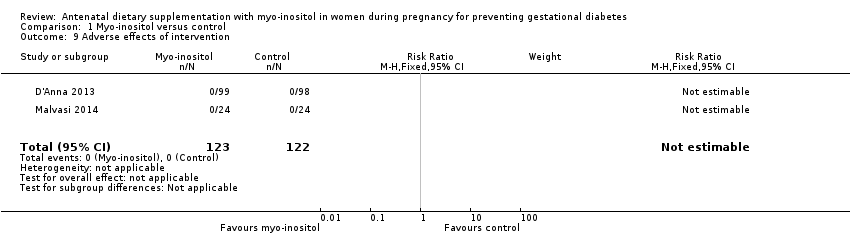
Comparison 1 Myo‐inositol versus control, Outcome 9 Adverse effects of intervention.
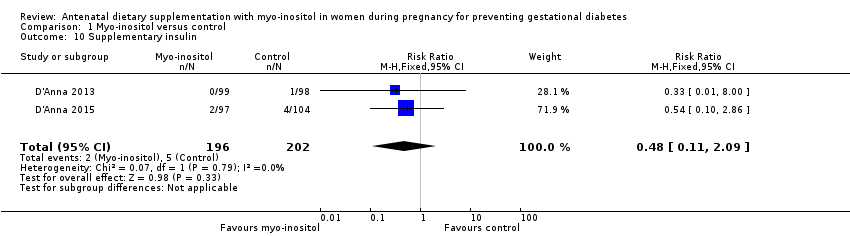
Comparison 1 Myo‐inositol versus control, Outcome 10 Supplementary insulin.

Comparison 1 Myo‐inositol versus control, Outcome 11 Gestational age at birth.

Comparison 1 Myo‐inositol versus control, Outcome 12 Preterm birth (less than 37 weeks' gestation).

Comparison 1 Myo‐inositol versus control, Outcome 13 Macrosomia.
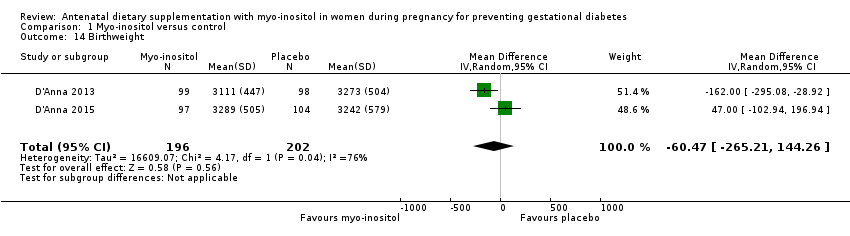
Comparison 1 Myo‐inositol versus control, Outcome 14 Birthweight.

Comparison 1 Myo‐inositol versus control, Outcome 15 Shoulder dystocia.

Comparison 1 Myo‐inositol versus control, Outcome 16 Respiratory distress syndrome.

Comparison 1 Myo‐inositol versus control, Outcome 17 Neonatal hypoglycaemia.
| Antenatal supplementation with myo‐inositol for preventing gestational diabetes | ||||||
| Patient or population: pregnant women (women with pre‐existing type 1 or type 2 diabetes are NOT included) Setting: Italy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with Myo‐inositol | |||||
| Gestational diabetes mellitus | Study population | RR 0.43 | 502 | ⊕⊕⊝⊝ | GDM diagnosed using IADPSG 2010 criteria | |
| 28 per 100 | 12 per 100 | |||||
| Weight gain during pregnancy | The mean weight gain during pregnancy was 0 | The mean weight gain during pregnancy in the intervention group was 0.64 more (0.41 fewer to 1.7 more) | ‐ | 411 | ⊕⊝⊝⊝ | D'Anna 2015 included obese pregnant women and D'Anna 2013 included non‐obese women with a family history of type 2 diabetes Random‐effects model |
| Hypertensive disorders of pregnancy | Study population | RR 0.43 | 398 | ⊕⊝⊝⊝ | Random‐effects model | |
| 4 per 100 | 2 per 100 | |||||
| Caesarean section | Study population | RR 0.95 | 398 | ⊕⊕⊝⊝ | ||
| 45 per 100 | 43 per 100 | |||||
| Perineal trauma | Not estimable | (0 studies) | No data reported for perineal trauma in any of the included studies | |||
| Postnatal depression | Not estimable | (0 studies) | No data reported for postnatal depression in any of the included studies | |||
| Type 2 diabetes | Not estimable | (0 studies) | No data reported for type 2 diabetes in any of the included studies | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded (‐1) due to unclear risk of bias for allocation concealment in two of the included trials (one trial did not provide sufficient detail to determine allocation concealment and one trial (reported as a conference abstract) had no details of random sequence generation, allocation concealment or blinding) and for high risk of performance bias for lack of blinding (two trials were open‐label trials with no blinding of participants or researchers, however one trial explicitly described blinding of outcome assessors and was assessed as low risk of detection bias). 2 Studies were conducted in Italy with Caucasian women and generalisability of findings is limited, downgraded (‐1). 3 Evidence of imprecision with wide confidence intervals crossing the line of no effect, downgraded (‐1). 4 Heterogeneity high with I2 = 54% (indirectness) probably due to different study populations, downgraded (‐1). 5 Wide confidence intervals with very low event rates and a small sample size suggest evidence of imprecision, downgraded (‐1). 6 Downgraded (‐1) due to insufficient evidence to judge allocation concealment in one trial and subsequent judgement of unclear risk of bias. The other trial had a low risk of bias for allocation concealment. Both trials were open‐label with no blinding of participants or researchers, although one trial explicitly stated that outcome assessors were blinded to treatment allocation. | ||||||
| Antenatal supplementation with myo‐inositol for preventing gestational diabetes | ||||||
| Patient or population: pregnant women who were at risk of GDM Setting: Italy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with Myo‐inositol | |||||
| Large‐for‐gestational age | not estimable | (0 studies) | No data reported for large‐for‐gestational age in any of the included studies | |||
| Perinatal mortality | not estimable | (0 studies) | No data reported for perinatal mortality in any of the included studies | |||
| Composite of serious neonatal outcomes | not estimable | (0 studies) | No data reported for composite of serious neonatal outcomes in any of the included studies | |||
| Neonatal hypoglycaemia | Study population | RR 0.36 | 398 | ⊕⊝⊝⊝ | ||
| 0 per 100 | 0 per 100 | |||||
| Adiposity | not estimable | (0 studies) | No data reported for adiposity in any of the included studies | |||
| Diabetes | not estimable | (0 studies) | No data reported for diabetes in any of the included studies | |||
| Neurosensory disability | not estimable | (0 studies) | No data reported for neurosensory disability in any of the included studies | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 No blinding in either study and reporting of allocation concealment was unclear in one of the studies, downgraded (‐1). 2 Both studies were conducted in Italy with Caucasian women and may not be generalisable to other settings, downgraded (‐1). 3 Wide confidence intervals with very low event rates suggest evidence of imprecision, downgraded (‐1). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gestational diabetes mellitus Show forest plot | 3 | 502 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.29, 0.64] |
| 2 Fasting OGTT Show forest plot | 3 | 502 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.28, ‐0.12] |
| 3 One hour OGTT Show forest plot | 3 | 502 | Mean Difference (IV, Fixed, 95% CI) | ‐0.68 [1.00, ‐0.37] |
| 4 Two hour OGTT Show forest plot | 3 | 502 | Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.07, ‐0.43] |
| 5 Hypertensive disorders of pregnancy Show forest plot | 2 | 398 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.02, 8.41] |
| 6 Caesarean section Show forest plot | 2 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.76, 1.19] |
| 7 Weight gain during pregnancy Show forest plot | 2 | 411 | Mean Difference (IV, Random, 95% CI) | 0.64 [‐0.41, 1.70] |
| 8 Relevant biomarker changes associated with the intervention Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Total cholesterol | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐47.29 [‐52.87, ‐41.71] |
| 8.2 Low density lipoprotein | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐33.50 [‐39.71, ‐27.29] |
| 8.3 High density lipoprotein | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐13.79 [‐18.91, ‐8.67] |
| 8.4 Triglycerides | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐39.33 [‐44.00, ‐34.66] |
| 9 Adverse effects of intervention Show forest plot | 2 | 245 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Supplementary insulin Show forest plot | 2 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.11, 2.09] |
| 11 Gestational age at birth Show forest plot | 2 | 398 | Mean Difference (IV, Random, 95% CI) | 5.50 [‐7.24, 18.24] |
| 12 Preterm birth (less than 37 weeks' gestation) Show forest plot | 2 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.17, 1.14] |
| 13 Macrosomia Show forest plot | 2 | 398 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.02, 6.37] |
| 14 Birthweight Show forest plot | 2 | 398 | Mean Difference (IV, Random, 95% CI) | ‐60.47 [‐265.21, 144.26] |
| 15 Shoulder dystocia Show forest plot | 2 | 398 | Risk Ratio (M‐H, Random, 95% CI) | 2.33 [0.12, 44.30] |
| 16 Respiratory distress syndrome Show forest plot | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.60] |
| 17 Neonatal hypoglycaemia Show forest plot | 2 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 8.66] |

