Tratamiento no quirúrgico versus tratamiento quirúrgico para el cáncer esofágico
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised controlled trial (RCT) | |
| Participants | Country: India Number randomised: 99 Postrandomisation exclusions: 12 (12%) Number analysed: 87 Average age: 52 years Females: 26 (26%) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups Further details: 50 gray of external radiation in 28 fractions followed by an external boost of 15 gray in 8 fractions or intraluminal radiotherapy of 15 gray with a 200 centigray/hour dose rate at 1 cm off axis Group 2: oesophagectomy (N = 44) Further details: standard Ivor‐Lewis procedure | |
| Outcomes | Outcomes reported were short‐term mortality and long‐term mortality | |
| Cancer stage/histology | Not reported/squamous cell carcinoma | |
| Tumour location | Infra‐aortic | |
| American Society of Anaesthesiologists (ASA) physical status score | Not reported | |
| Notes | We attempted to contact the study authors in February 2015 Reasons for postrandomisation exclusions: 2 participants from the surgery arm due to direct spread to the bronchus, 10 participants from the radiotherapy arm as 7 received radiotherapy at other treatment centres and 3 did not take any treatment Participants were followed‐up for a maximum of 3 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised by closed envelope method". Comment: further details were unavailable. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomised by closed envelope method". Comment: further details were unavailable. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: it was impossible to blind the participants and healthcare providers. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was unavailable. |
| Incomplete outcome data (attrition bias) | High risk | Comment: there was an imbalance in postrandomisation exclusions. |
| Selective reporting (reporting bias) | High risk | Comment: treatment‐related complications were not reported. |
| Source of funding | Unclear risk | Comment: this information was unavailable. |
| Other bias | Low risk | Comment: there was no other source of bias. |
| Methods | RCT | |
| Participants | Country: France Number randomised: 259 Postrandomisation exclusions: 0 (0%) Number analysed: 259 Average age: 58 years Females: 17 (6.7%) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups Group 1: chemoradiotherapy (N = 130) Further details: external radiotherapy of 15 gray over 4 days and fluorouracil (FU) 800 mg/m² daily and cisplatin 15mg/m² daily for 5 days (x 3 cycles with a 2 week interval between cycles) Group 2: oesophagectomy (N = 129) Further details: different methods of oesophagectomy as required. Preoperative chemoradiotherapy was administered | |
| Outcomes | Outcomes reported were short‐term mortality, long‐term mortality, recurrence, length of hospital stay, short‐term health‐related quality of life, and medium‐term health‐related quality of life. | |
| Cancer stage/histology | T3N0‐1M0/squamous cell carcinoma or adenocarcinoma | |
| Tumour location | Thoracic oesophagus | |
| American Society of Anaesthesiologists (ASA) physical status score | Not reported | |
| Notes | We attempted to contact the study authors in February 2015 Both groups received external radiotherapy (30 gray over 10 days with a 2 week interval between day 5 and day 6) and fluorouracil (FU) 800 mg/m² daily and cisplatin 15 mg/m² daily for 5 days (x 2 cycles with a 2 week interval between cycles) prior to randomisation Participants were followed up starting at 4 months after starting the treatment (in the surgical arm, 2 months after resection, in the non‐surgical arm, 3 weeks after the end of chemotherapy). Follow‐up was carried out every 3 months for 2 years and then every 6 months thereafter Median follow‐up: 4 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomly assigned by telephone at the FFCD Data Center through a minimization program". |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomly assigned by telephone at the FFCD Data Center through a minimization program". |
| Blinding of participants and personnel (performance bias) | High risk | Comment: it was impossible to blind the participants and healthcare providers. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was unavailable. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no postrandomisation exclusions. |
| Selective reporting (reporting bias) | High risk | Comment: treatment‐related complications were not reported. |
| Source of funding | Low risk | Quote: "Supported by grants from the Ligue Nationale Contre le Cancer, the Fonds de Recherche de la Société Nationale Francaise de Gastroentérologie, the Programme Hospitalier pour la Recher‐ che Clinique, and the Association pour la Recherche Contre le Cancer". |
| Other bias | Low risk | Comment: there was no other source of bias. |
| Methods | RCT | |
| Participants | Country: UK Sample size: 5 Postrandomisation exclusions: 0 (0%) Number analysed: 5 Average age: not reported Females: not reported Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups Group 1: chemoradiotherapy (N = 2) Further details: chemotherapy was given for a total of 84 days (including induction therapy) of 21 days cycles of either cisplatin 80 mg/m² by intravenous infusion on days 1 and 5 followed by fluorouracil 1 g/m² per day intravenous infusion for 4 days or cisplatin 80 mg/m² by intravenous infusion on day 1 and capecitabine 625 mg/m² orally twice daily continuously and radiotherapy total 50 gray in 25 fractions Group 2: oesophagectomy (N = 3) Further details: different methods of oesophagectomy as required | |
| Outcomes | The trial did not report any of the outcomes of interest | |
| Cancer stage/histology | T2‐4N0‐1M0/Squamous cell carcinoma | |
| Tumour location | Any part of the oesophagus up to 2 cm away from the cricopharyngeus | |
| American Society of Anaesthesiologists (ASA) physical status score | Not reported | |
| Notes | We attempted to contact the study authors in February 2015 Both groups received induction chemotherapy given as 21 days of either cisplatin 80 mg/m² by intravenous infusion on days 1 and 5 followed by fluorouracil 1 g/m² per day intravenous infusion for 4 days or cisplatin 80 mg/m² by intravenous infusion on day 1 and capecitabine 625 mg/m² orally twice daily continuously | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "When eligible patients consented to randomisation, treatment allocation was determined by an automated randomisation web‐based system". |
| Allocation concealment (selection bias) | Low risk | Quote: "When eligible patients consented to randomisation, treatment allocation was determined by an automated randomisation web‐based system". |
| Blinding of participants and personnel (performance bias) | High risk | Comment: it was impossible to blind the participants and healthcare providers. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was unavailable. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no postrandomisation exclusions. |
| Selective reporting (reporting bias) | High risk | Comment: mortality and complications were not reported. |
| Source of funding | Low risk | Quote: "This article summarises independent research funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Program (Grant reference PB‐PG‐0807– 14131)". |
| Other bias | Low risk | Comment: there was no other source of bias. |
| Methods | RCT | |
| Participants | Country: Norway and Sweden Number randomised: 91 Postrandomisation exclusions: 0 (0%) Number analysed: 91 Average age: not reported Females: not reported Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups Group 1: chemoradiotherapy (N = 46) Further details: external radiotherapy total of 64 gray given in 32 fractions over 9 weeks and three 3‐weekly cycles of cisplatin 100 mg/m² on day 1 and 5‐fluorouracil chemotherapy 750 mg/m²/day from day 1 to 5 Group 2: oesophagectomy (N = 45) Further details: Ivor‐Lewis procedure | |
| Outcomes | Outcomes reported were: short‐term mortality and long‐term mortality | |
| Cancer stage/histology | Not reported/squamous cell carcinoma or adenocarcinoma | |
| Tumour location | Not specified | |
| American Society of Anaesthesiologists (ASA) physical status score | Not reported | |
| Notes | We attempted to contact the study authors in February 2015, and the authors replied in March 2015 The trial authors did not specify the follow‐up period but reported 4 year follow‐up results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was unavailable. |
| Allocation concealment (selection bias) | Low risk | Comment: participants were centrally allocated (information retrieved directly from the trial author). |
| Blinding of participants and personnel (performance bias) | High risk | Comment: it was impossible to blind the participants and healthcare providers. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was unavailable. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was unavailable. |
| Selective reporting (reporting bias) | High risk | Comment: treatment‐related complications were not reported. |
| Source of funding | Unclear risk | Comment: this information was unavailable. |
| Other bias | Low risk | Comment: there was no other source of bias. |
| Methods | RCT | |
| Participants | Country: China Number randomised: 81 Postrandomisation exclusions: 1 (1.2%) Number analysed: 80 Average age: 62 years Females: 14 (17.3%) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups Group 1: chemoradiotherapy (N = 36) Further details: external radiotherapy total of 50 to 60 gray given in 25 to 30 fractions over 5 to 6 weeks and two 3‐weekly cycles of cisplatin 60 mg/m² on day 1 and day 22 and 5‐fluorouracil chemotherapy 200 mg/m²/day from day 1 to day 42 Group 2: oesophagectomy (N = 44) Further details: 2‐ or 3‐stage oesophagectomy | |
| Outcomes | Outcomes reported were short‐term mortality, long‐term mortality, short‐term adverse events, short‐term serious adverse events, long‐term recurrence, and length of hospital stay | |
| Cancer stage/histology | Not reported (must be deemed resectable and have no metastases)/squamous cell carcinoma | |
| Tumour location | Mid‐ or lower‐thorax | |
| American Society of Anaesthesiologists (ASA) physical status score | Not reported | |
| Notes | We attempted to contact the study authors in February 2015 Reason for postrandomisation drop‐out: initially deemed resectable but later considered unresectable Patients were followed up at 6 to 8 week intervals in the 1st year and at 3‐month intervals thereafter Median follow‐up: 1.5 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was unavailable. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was unavailable. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: it was impossible to blind the participants and healthcare providers. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was unavailable. |
| Incomplete outcome data (attrition bias) | High risk | Comment: there was an imbalance in postrandomisation exclusions. |
| Selective reporting (reporting bias) | Low risk | Comment: the trial reported all important outcomes. |
| Source of funding | Low risk | Quote: "This study was supported by the Research Grant Council (RGC) of Hong Kong Special Administrative Region, China". |
| Other bias | Low risk | Comment: there was no other source of bias. |
| Methods | RCT | |
| Participants | Country: China Number randomised: 156 Postrandomisation exclusions: 0 (0%) Number analysed: 156 Average age: 55 years Females: not reported Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 4 groups Group 1: radiotherapy (N = 35): Further details: 43 to 53 gray over 4 to 5 weeks Group 2: oesophagectomy with neoadjuvant radiotherapy (N = 40) Group 3: oesophagectomy with adjuvant radiotherapy (N = 42) Group 4: oesophagectomy without radiotherapy (N = 39) | |
| Outcomes | Outcomes reported were long‐term mortality only | |
| Cancer stage/histology | Not reported ('potentially curable')/squamous cell carcinoma | |
| Tumour location | Middle third of the oesophagus | |
| American Society of Anaesthesiologists (ASA) physical status score | Not reported | |
| Notes | We attempted to contact the study authors in February 2015 Long‐term follow‐up with partial data were available at 10 years, however details were not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was unavailable. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was unavailable. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: it was impossible to blind the participants and healthcare providers. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was unavailable. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no postrandomisation exclusions. |
| Selective reporting (reporting bias) | High risk | Comment: treatment‐related complications were not reported. |
| Source of funding | Unclear risk | Comment: this information was unavailable. |
| Other bias | Low risk | Comment: there was no other source of bias. |
| Methods | RCT | |
| Participants | Country: Germany Number randomised: 172 Postrandomisation exclusions: 0 (0%) Number analysed: 172 Average age: 57 years Females: 34 (19.8%) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups Group 1: chemoradiotherapy (N = 86) Further details: additional external radiotherapy with 2 x 1.5 gray/day for a total dose of at least 20 gray Group 2: oesophagectomy (N = 86) | |
| Outcomes | Outcomes reported were short‐term mortality and long‐term mortality | |
| Cancer stage/histology | T3‐4 N0‐1 M0/squamous cell carcinoma | |
| Tumour location | Upper and mid third of the oesophagus | |
| American Society of Anaesthesiologists (ASA) physical status score | Not reported (WHO performance status grade of 0 to 1) | |
| Notes | We attempted to contact the study authors in February 2015 Both groups received external radiotherapy with 2 x 1.5 gray/day for a total dose of 40 gray and chemotherapy (bolus fluorouracil 500 mg/m², leucovorin 300 mg/m², etoposide 100 mg/m², and cisplatin 30 mg/m² on days 1 to 3 every 3 weeks) and cisplatin 50 mg/m² on days 2 to 8 and etoposide 80 mg/m² on days 3 to 5 concomitant with radiotherapy Participants were seen for the first follow‐up 8 to 12 weeks after the end of treatment and, thereafter, every 3 months up to 2 years. Afterwards, follow‐up was planned every 6 months up to 5 years Median follow‐up: 6 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Allocation to treatment groups was performed...using a computerized randomization program". |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation to treatment groups was performed...using a computerized randomization program". |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "This was an unblinded, prospectively randomized phase III trial". |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "This was an unblinded, prospectively randomized phase III trial". |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no postrandomisation exclusions. |
| Selective reporting (reporting bias) | High risk | Comment: treatment related complications were not reported. |
| Source of funding | Low risk | Quote: "Supported by the Stiftung Deutsche Krebshilfe (German Cancer Aid)". |
| Other bias | Low risk | Comment: there was no other source of bias. |
| Methods | RCT | |
| Participants | Country: China Number randomised: 269 Postrandomisation exclusions: 0 (0%) Number analysed: 269 Average age: 56 years Females: 67 (24.9%) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups Group 1: radiotherapy (N = 134) Further details: external radiotherapy conventionally fractionated at 1.8 to 2.0 Gray/day for the 1st two thirds of treatment course to a dose of about 50 to 50.4 gray followed by late course accelerated hyperfractionated radiotherapy, twice daily at 1.5 gray per fraction (with a minimal interval of 6 hours between fractions) to a dose of 18 to 21 gray. The total dose whole radiotherapy was 68.4 to 71.0 gray Group 2: oesophagectomy (N = 135) Further details: abdominothoracic approach | |
| Outcomes | Outcomes reported were long‐term mortality and long‐term recurrence | |
| Cancer stage/histology | Not reported | |
| Tumour location | Upper, middle, and lower oesophagus | |
| American Society of Anaesthesiologists (ASA) physical status score | Not reported | |
| Notes | We attempted to contact the study authors in February 2015 Median follow‐up: 4.8 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was unavailable. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was unavailable. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: it was impossible to blind the participants and healthcare providers. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was unavailable. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was unavailable. |
| Selective reporting (reporting bias) | High risk | Comment: treatment‐related complications were not reported. |
| Source of funding | Unclear risk | Comment: this information was unavailable. |
| Other bias | Low risk | Comment: there was no other source of bias. |
Abbreviations: RCT: randomised controlled trial; TNM = tumour stage, nodal stage, and metastasis; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Participants were not randomised. | |
| The trial was stopped due to poor recruitment in May 1988 16 months after the start because only 31 participants had been entered. No randomised data was generated. | |
| The protocol for this trial was changed so allocation was not randomised. | |
| This was not a randomised controlled trial. However, we retrieved and screened the full‐text article as this was unclear based on the title alone. | |
| This trial is expected to be completed in 2018 and the current report (a conference abstract) includes details of the safety of surgery in participants randomised to the surgical arm. The report included non‐randomised participants undergoing surgery as well. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term mortality Show forest plot | 5 | 689 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.11, 1.35] |
| Analysis 1.1  Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 1 Short‐term mortality. | ||||
| 2 Long‐term mortality (binary) Show forest plot | 3 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.92, 1.14] |
| Analysis 1.2  Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 2 Long‐term mortality (binary). | ||||
| 3 Long‐term mortality (time‐to‐event) Show forest plot | 7 | 1114 | Hazard Ratio (Fixed, 95% CI) | 1.09 [0.97, 1.22] |
| Analysis 1.3  Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 3 Long‐term mortality (time‐to‐event). | ||||
| 4 Proportion with a serious adverse event within 3 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 4 Proportion with a serious adverse event within 3 months. | ||||
| 5 Short‐term health‐related quality of life Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 5 Short‐term health‐related quality of life. | ||||
| 6 Medium‐term health‐related quality of life Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.6  Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 6 Medium‐term health‐related quality of life. | ||||
| 7 Long‐term recurrence (binary) Show forest plot | 2 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.87, 1.28] |
| Analysis 1.7  Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 7 Long‐term recurrence (binary). | ||||
| 8 Long‐term recurrence (time‐to‐event) Show forest plot | 2 | 349 | Hazard Ratio (Fixed, 95% CI) | 0.96 [0.80, 1.16] |
| Analysis 1.8  Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 8 Long‐term recurrence (time‐to‐event). | ||||
| 9 Local recurrence (binary) Show forest plot | 3 | 449 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.70, 1.12] |
| Analysis 1.9  Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 9 Local recurrence (binary). | ||||
| 10 Proportion with any adverse event within 3 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.10  Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 10 Proportion with any adverse event within 3 months. | ||||
| 11 Length of hospital stay (days) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.11  Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 11 Length of hospital stay (days). | ||||
| 12 Dysphagia at maximal follow‐up Show forest plot | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.01, 2.19] |
| Analysis 1.12  Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 12 Dysphagia at maximal follow‐up. | ||||
| 13 Long‐term mortality (time‐to‐event): stratified by treatment Show forest plot | 7 | 1114 | Hazard Ratio (Fixed, 95% CI) | 1.09 [0.97, 1.22] |
| Analysis 1.13  Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 13 Long‐term mortality (time‐to‐event): stratified by treatment. | ||||
| 13.1 Chemoradiotherapy | 4 | 602 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.76, 1.03] |
| 13.2 Radiotherapy | 3 | 512 | Hazard Ratio (Fixed, 95% CI) | 1.39 [1.18, 1.64] |
| 14 Long‐term mortality (binary): definitive chemoradiotherapy versus surgery with neoadjuvant chemotherapy Show forest plot | 2 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.93, 1.16] |
| Analysis 1.14  Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 14 Long‐term mortality (binary): definitive chemoradiotherapy versus surgery with neoadjuvant chemotherapy. | ||||
| 15 Long‐term mortality (time‐to‐event): definitive chemoradiotherapy versus surgery with neoadjuvant chemotherapy or chemoradiotherapy Show forest plot | 2 | 431 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.78, 1.26] |
| Analysis 1.15  Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 15 Long‐term mortality (time‐to‐event): definitive chemoradiotherapy versus surgery with neoadjuvant chemotherapy or chemoradiotherapy. | ||||
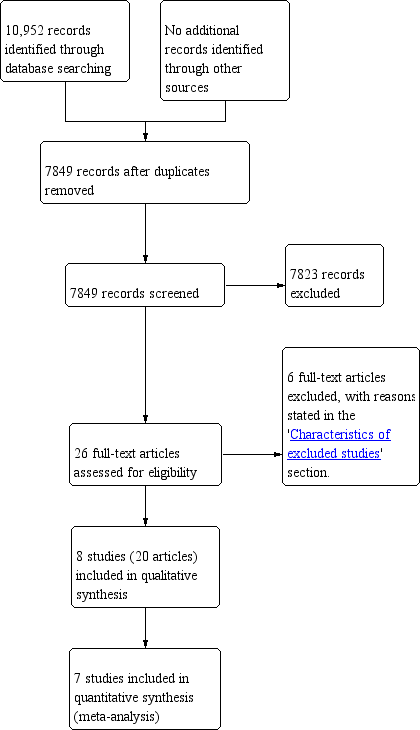
Study flow diagram.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.

Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 1 Short‐term mortality.

Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 2 Long‐term mortality (binary).
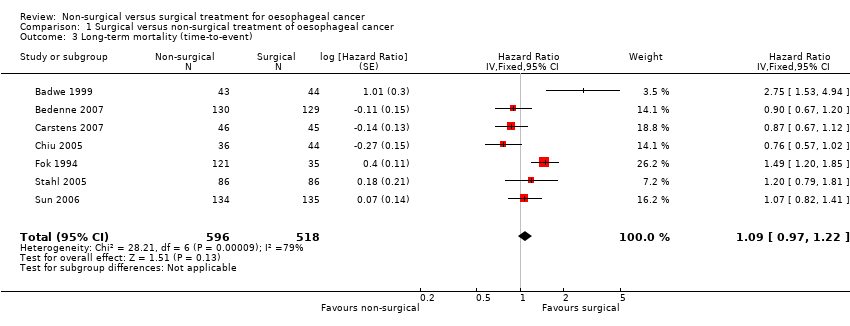
Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 3 Long‐term mortality (time‐to‐event).

Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 4 Proportion with a serious adverse event within 3 months.

Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 5 Short‐term health‐related quality of life.

Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 6 Medium‐term health‐related quality of life.

Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 7 Long‐term recurrence (binary).
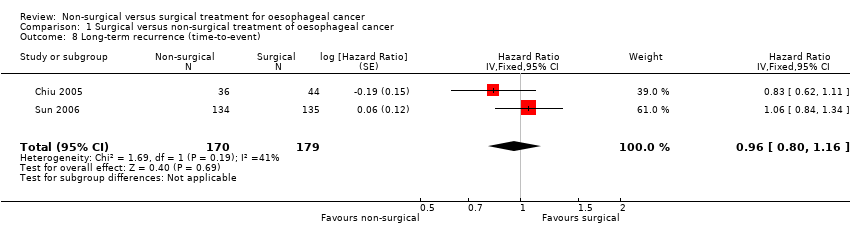
Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 8 Long‐term recurrence (time‐to‐event).
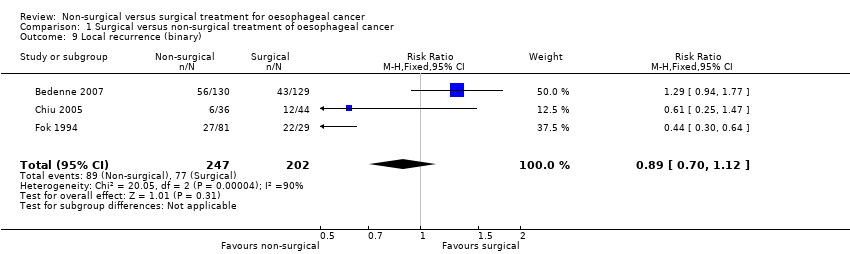
Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 9 Local recurrence (binary).

Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 10 Proportion with any adverse event within 3 months.

Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 11 Length of hospital stay (days).

Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 12 Dysphagia at maximal follow‐up.

Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 13 Long‐term mortality (time‐to‐event): stratified by treatment.
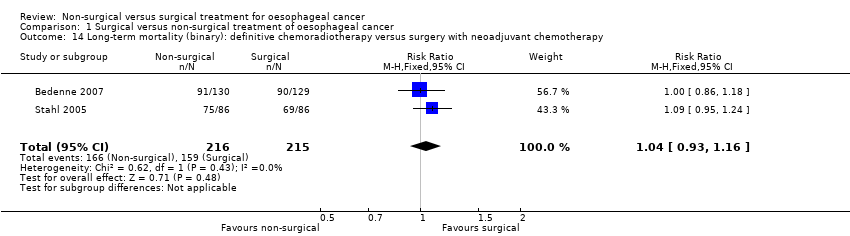
Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 14 Long‐term mortality (binary): definitive chemoradiotherapy versus surgery with neoadjuvant chemotherapy.

Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 15 Long‐term mortality (time‐to‐event): definitive chemoradiotherapy versus surgery with neoadjuvant chemotherapy or chemoradiotherapy.
| Non‐surgical versus surgical treatment of oesophageal cancer | |||||
| Patient or population: people with oesophageal cancer | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Surgical treatment | Non‐surgical treatment | ||||
| Short‐term mortality | 78 per 10001 | 30 per 1000 | RR 0.39 | 689 | ⊕⊝⊝⊝ |
| Long‐term mortality (binary outcome) | 691 per 10001 | 712 per 1000 | RR 1.03 | 511 | ⊕⊕⊝⊝ |
| Long‐term mortality (time‐to‐event outcome): chemoradiotherapy versus surgery | 349 per 10001 | 314 per 1000 | HR 0.88 | 602 | ⊕⊕⊝⊝ |
| Long‐term mortality (time‐to‐event outcome): radiotherapy versus surgery | 350 per 10001 | 451 per 1000 | HR 1.39 | 512 | ⊕⊝⊝⊝ |
| Long‐term mortality (binary): definitive chemoradiotherapy versus surgery with neoadjuvant chemotherapy All‐cause mortality for the duration of follow‐up | 740 per 1000 | 769 per 1000 | RR 1.04 | 431 | ⊕⊝⊝⊝ |
| Long‐term mortality (time‐to‐event): definitive chemoradiotherapy versus surgery with neoadjuvant chemotherapy or chemoradiotherapy All‐cause mortality for the duration of follow‐up | 349 per 1000 | 346 per 1000 | HR 0.99 | 431 | ⊕⊝⊝⊝ |
| Proportion with a serious adverse event within 3 months | 273 per 10001 | 166 per 1000 | RR 0.61 | 80 | ⊕⊝⊝⊝ |
| Short‐term health‐related quality of life | The mean short‐term health‐related quality of life in the control groups was | The mean short‐term health‐related quality of life in the intervention groups was | ‐ | 165 | ⊕⊝⊝⊝ |
| Medium‐term health‐related quality of life | The mean medium‐term health‐related quality of life in the control groups was | The mean medium‐term health‐related quality of life in the intervention groups was | ‐ | 62 | ⊕⊝⊝⊝ |
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1The basis for control risk is the event rate across all studies (i.e. the sum of all events in the surgical group across all studies reporting the outcome divided by the sum of all people in the surgical group in the trials reporting the outcome) for all outcomes except long‐term mortality (time‐to‐event) where a control group risk of 0.35 was used (based on similar control group risks at 2 years in a number of trials included in this analysis) and long‐term recurrence (time‐to‐event) where a control group risk of 0.4 was used (based on similar control group risk at 2 year in a trial included for this analysis). | |||||
| Non‐surgical versus surgical treatment of oesophageal cancer | ||||||
| Patient or population: people with oesophageal cancer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Surgical treatment | Non‐surgical treatment | |||||
| Long‐term recurrence (binary outcome) | 526 per 10001 | 552 per 1000 | RR 1.05 | 339 | ⊕⊝⊝⊝ | ‐ |
| Long‐term recurrence (time‐to‐event outcome) | 508 per 10001 | 494 per 1000 | HR 0.96 | 349 | ⊕⊕⊝⊝ | ‐ |
| Local recurrence (binary) | 381 per 1000 | 339 per 1000 | RR 0.89 | 449 | ⊕⊝⊝⊝ | ‐ |
| Proportion with any adverse event within 3 months | 386 per 10001 | 668 per 1000 | RR 1.73 | 80 | ⊕⊝⊝⊝ | ‐ |
| Length of hospital stay (days) | See comment | See comment | Not estimable | 342 | ⊕⊝⊝⊝ | Significant heterogeneity present (I² statistic = 93%, P = 0.0001) making meta‐analysis inappropriate. The mean hospital stay was 16 days shorter (3 days shorter to 29 days shorter) in non‐surgical treatment than surgical treatment in 1 trial (Bedenne 2007) and 14 days longer (5 days longer to 23 days longer) in non‐surgical treatment than surgical treatment in another trial (Chiu 2005). |
| Dysphagia at maximal follow‐up | 367 per 1000 | 543 per 1000 | RR 1.48 | 139 | ⊕⊝⊝⊝ | ‐ |
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The basis for control risk is the event rate across all studies (i.e. the sum of all events in the surgical group across all studies reporting the outcome divided by the sum of all people in the surgical group in the trials reporting the outcome). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term mortality Show forest plot | 5 | 689 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.11, 1.35] |
| 2 Long‐term mortality (binary) Show forest plot | 3 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.92, 1.14] |
| 3 Long‐term mortality (time‐to‐event) Show forest plot | 7 | 1114 | Hazard Ratio (Fixed, 95% CI) | 1.09 [0.97, 1.22] |
| 4 Proportion with a serious adverse event within 3 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Short‐term health‐related quality of life Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Medium‐term health‐related quality of life Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Long‐term recurrence (binary) Show forest plot | 2 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.87, 1.28] |
| 8 Long‐term recurrence (time‐to‐event) Show forest plot | 2 | 349 | Hazard Ratio (Fixed, 95% CI) | 0.96 [0.80, 1.16] |
| 9 Local recurrence (binary) Show forest plot | 3 | 449 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.70, 1.12] |
| 10 Proportion with any adverse event within 3 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Length of hospital stay (days) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12 Dysphagia at maximal follow‐up Show forest plot | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.01, 2.19] |
| 13 Long‐term mortality (time‐to‐event): stratified by treatment Show forest plot | 7 | 1114 | Hazard Ratio (Fixed, 95% CI) | 1.09 [0.97, 1.22] |
| 13.1 Chemoradiotherapy | 4 | 602 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.76, 1.03] |
| 13.2 Radiotherapy | 3 | 512 | Hazard Ratio (Fixed, 95% CI) | 1.39 [1.18, 1.64] |
| 14 Long‐term mortality (binary): definitive chemoradiotherapy versus surgery with neoadjuvant chemotherapy Show forest plot | 2 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.93, 1.16] |
| 15 Long‐term mortality (time‐to‐event): definitive chemoradiotherapy versus surgery with neoadjuvant chemotherapy or chemoradiotherapy Show forest plot | 2 | 431 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.78, 1.26] |

