Tratamiento no quirúrgico versus tratamiento quirúrgico para el cáncer esofágico
Resumen
Antecedentes
El cáncer esofágico es la sexta causa más frecuente de mortalidad relacionada con el cáncer en el mundo. Actualmente la cirugía es la forma de tratamiento recomendada cuando es posible. Sin embargo, no está claro si las opciones de tratamiento no quirúrgicas son equivalentes a la esofagectomía con respecto a la supervivencia.
Objetivos
Evaluar los efectos beneficiosos y perjudiciales del tratamiento no quirúrgico versus la esofagectomía en los pacientes con cáncer esofágico.
Métodos de búsqueda
Se hicieron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL), MEDLINE, EMBASE, Science Citation Index, ClinicalTrials.gov, y en la World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) hasta el 4 marzo 2016. También se examinaron las listas de referencias de los estudios incluidos.
Criterios de selección
Dos autores revisan, de forma independiente, examinaron todos los títulos y resúmenes de los artículos obtenidos a partir de las búsquedas bibliográficas y seleccionaron las referencias para una evaluación adicional. Para estas referencias seleccionadas, la inclusión de los ensayos se basó en la evaluación del texto completo de los artículos.
Obtención y análisis de los datos
Dos autores de la revisión extrajeron los datos de forma independiente. Para los resultados binarios se calculó el riesgo relativo (RR) con el intervalo de confianza (IC) del 95%, para los resultados continuos se calculó la diferencia de medias (DM) o la diferencia de medias estandarizada (DME) con IC del 95%, y para los resultados de tiempo hasta el evento se calculó el cociente de riesgos instantáneos (CRI). Cuando fue significativo se realizaron metanálisis.
Resultados principales
Ocho ensayos, que incluyeron 1132 participantes en total, cumplieron los criterios de inclusión de esta revisión Cochrane. Estos ensayos tuvieron alto riesgo de sesgo. Un ensayo (que incluyó cinco participantes) no contribuyó con datos a esta revisión Cochrane y se excluyeron 13 participantes en los ensayos restantes después de la asignación al azar; lo anterior dejó un total de 1114 participantes para el análisis, 510 asignados al azar a tratamiento no quirúrgico y 604 a tratamiento quirúrgico. El tratamiento no quirúrgico fue quimiorradioterapia definitiva en cinco ensayos y radioterapia definitiva en tres ensayos. Todos los participantes eran aptos para cirugía mayor. La mayoría de los datos se obtuvo de los ensayos que compararon quimiorradioterapia con cirugía. No hubo diferencias en la mortalidad a largo plazo entre la quimiorradioterapia y la cirugía (CRI 0,88; IC del 95%: 0,76 a 1,03; 602 participantes; cuatro estudios; evidencia de baja calidad). La mortalidad a largo plazo fue mayor en la radioterapia que en la cirugía (CRI 1,39; IC del 95%: 1,18 a 1,64; 512 participantes; tres estudios; evidencia de muy baja calidad). No hubo diferencias en la recidiva a largo plazo entre el tratamiento no quirúrgico y la cirugía (CRI 0,96; IC del 95%: 0,80 a 1,16; 349 participantes; dos estudios;evidencia de baja calidad). La diferencia entre los tratamientos no quirúrgicos y quirúrgicos no fue precisa para la mortalidad a corto plazo (RR 0,39; IC del 95%: 0,11 a 1,35; 689 participantes; cinco estudios; evidencia de calidad muy baja), la proporción de participantes con eventos adversos graves en el transcurso de tres meses (RR 0,61; IC del 95%: 0,25 a 1,47; 80 participantes; un estudio; evidencia de calidad muy baja) y la proporción de pacientes con recidiva local al seguimiento máximo (RR 0,89; IC del 95%: 0,70 a 1,12; 449 participantes; tres estudios; evidencia de muy baja calidad). La calidad de vida relacionada con la salud fue mayor en el tratamiento no quirúrgico entre las cuatro semanas y los tres meses después del tratamiento (Spitzer Quality of Life Index; DM 0,93; IC del 95%: 0,24 a 1,62; 165 participantes;un estudio; evidencia de muy baja calidad). La diferencia entre los tratamientos no quirúrgicos y quirúrgicos no fue precisa para la calidad de vida relacionada con la salud a medio plazo (tres meses a dos años después del tratamiento) (Spitzer Quality of Life Index; DM ‐0,95; IC del 95%: ‐2,10 a 0,20; 62 participantes; un estudio; evidencia de muy baja calidad). La proporción de pacientes con disfagia en la última visita de seguimiento antes de la muerte fue mayor con la quimiorradioterapia definitiva en comparación con el tratamiento quirúrgico (RR 1,48; IC del 95%: 1,01 a 2,19; 139 participantes; un estudio; evidencia de muy baja calidad).
Conclusiones de los autores
Según evidencia de calidad muy baja, la quimiorradioterapia parece ser al menos equivalente a la cirugía en cuanto a la supervivencia a corto y a largo plazo en los pacientes con cáncer esofágico (tipo carcinoma escamocelular) aptos para cirugía y que responden a la quimiorradioterapia de inducción. Sin embargo, hay incertidumbre en la comparación de quimiorradioterapia definitiva versus cirugía por cáncer esofágico (tipo adenocarcinoma) y no es posible descartar efectos beneficiosos o perjudiciales significativos de la quimiorradioterapia definitiva versus la cirugía. Según evidencia de calidad muy baja, la proporción de pacientes con disfagia en la última visita de seguimiento antes de la muerte fue mayor con la quimiorradioterapia definitiva en comparación con la cirugía. Según evidencia de calidad muy baja, la radioterapia da lugar a una menor supervivencia a largo plazo que la cirugía en los pacientes con cáncer esofágico aptos para cirugía. Sin embargo, hay un riesgo de sesgo y de errores aleatorios en estos resultados, aunque el riesgo de sesgo en los estudios incluidos en esta revisión sistemática es probable que sea inferior que en los estudios no aleatorizados.
Se necesitan ensayos adicionales con bajo riesgo de sesgo. Dichos ensayos necesitan comparar el tratamiento endoscópico con el tratamiento quirúrgico en el cáncer esofágico en estadio inicial (carcinoma in situ y estadio Ia) y la quimiorradioterapia definitiva con tratamientos quirúrgicos en otros estadios del cáncer esofágico y deben medir e informar resultados orientados al paciente. La identificación temprana de los pacientes que responden a la quimiorradioterapia y del mejor tratamiento de segunda línea para los pacientes que no responden también aumentará la necesidad y la aceptabilidad de ensayos que comparen la quimiorradioterapia definitiva con la cirugía.
PICOs
Resumen en términos sencillos
Tratamiento no quirúrgico versus tratamiento quirúrgico para el cáncer de esófago
Pregunta de la revisión
¿El tratamiento no quirúrgico es equivalente al tratamiento quirúrgico para el tratamiento de los pacientes con cáncer esofágico (cáncer del conducto de alimentación)?
Antecedentes
El cáncer esofágico es la sexta causa más frecuente de muerte relacionada con el cáncer en el mundo y se ha vuelto cada vez más habitual. El tratamiento y la supervivencia dependen de la extensión del cáncer. Cuando el cáncer está limitado al esófago y el paciente es apto para someterse a cirugía mayor, la extracción quirúrgica del esófago (esofagectomía) es el tratamiento actualmente recomendado. En algunos pacientes con cáncer esofágico se pueden administrar además de la cirugía la quimioterapia adicional (uso de productos químicos para destruir selectivamente el cáncer) y la radioterapia (uso de rayos X para destruir el cáncer). Sin embargo, la quimioterapia, la radioterapia o una combinación de las dos (quimiorradioterapia) se pueden utilizar solas sin la cirugía, pero actualmente sólo se recomiendan en los pacientes que no son aptos para cirugía mayor debido a su estado general. La quimiorradioterapia por sí sola puede causar efectos secundarios como daño renal grave, infección y vómitos, pero es menos invasiva que la esofagectomía y puede dar lugar a una estancia hospitalaria más corta y reducir el riesgo de muerte. La esofagectomía puede tener posibles efectos secundarios significativos que incluyen infección del sitio quirúrgico, estrechamiento y rotura del tejido cuando el extremo cortado del esófago se une al intestino, neumonía y dificultad para tragar. La tasa de mortalidad también puede ser mayor, en particular cuando se realiza en centros más pequeños. No está claro si el tratamiento no quirúrgico puede ser tan eficaz como la cirugía en la curación del cáncer.
Características de los estudios
Ocho estudios cumplieron los criterios de inclusión de esta revisión Cochrane, y siete estudios proporcionaron información para la revisión. El tratamiento no quirúrgico fue quimiorradioterapia solamente en cinco estudios y radioterapia solamente en tres estudios. Se incluyeron 1114 participantes que recibieron tratamiento no quirúrgico (510 participantes) o tratamiento quirúrgico (604 participantes) en los diversos análisis de los siete estudios que proporcionaron información. Se utilizaron métodos similares al lanzamiento de una moneda para decidir si un participante recibía tratamiento no quirúrgico o tratamiento quirúrgico y para asegurar que los participantes de los dos grupos fueran similares. La mayoría de los ensayos incluyó pacientes sanos en relación con aspectos diferentes de la afección que requería cirugía. La evidencia está actualizadas hasta el 4 de marzo 2016.
Resultados clave
La mayor parte de la información era de ensayos que compararon la quimiorradioterapia con la cirugía. No hubo diferencias en las muertes a largo plazo entre la quimiorradioterapia y la cirugía en los pacientes con cáncer esofágico aptos para cirugía. Más pacientes murieron con radioterapia que con cirugía entre los pacientes con cáncer esofágico aptos para cirugía a largo plazo. No hubo diferencias en la recidiva del cáncer a largo plazo entre el tratamiento no quirúrgico y la cirugía. La diferencia entre los tratamientos no quirúrgicos y quirúrgicos no fue precisa para las muertes a corto plazo, el porcentaje de participantes con eventos adversos graves en el transcurso de tres meses y el porcentaje de participantes con recidiva del cáncer en o los alrededores del conducto de alimentación. La calidad de vida relacionada con la salud (que abarca aspectos como la actividad, la vida cotidiana, la salud, el apoyo de la familia y los amigos y las perspectivas) fue mayor en el tratamiento no quirúrgico entre las cuatro semanas y los tres meses después del tratamiento, aunque no está claro lo que significa esta diferencia para el paciente. La diferencia entre los tratamientos no quirúrgicos y quirúrgicos no fue precisa para la calidad de vida relacionada con la salud a medio plazo (tres meses a dos años después del tratamiento). La quimiorradioterapia solamente parece ser al menos equivalente a la cirugía en cuanto a la supervivencia a corto y a largo plazo en los pacientes con un tipo de cáncer esofágico llamado cáncer escamocelular y que son aptos para cirugía. Hay más incertidumbre en la comparación de quimiorradioterapia solamente versus cirugía para otro tipo de cáncer esofágico llamado adenocarcinoma y no se pueden descartar los efectos beneficiosos o perjudiciales significativos de la quimiorradioterapia definitiva versus la cirugía en este tipo de cáncer esofágico. Más pacientes tuvieron dificultad para tragar antes de la muerte después del tratamiento con quimiorradioterapia en comparación con el tratamiento quirúrgico.
La radioterapia solamente da lugar a una menor supervivencia a largo plazo que la cirugía (cerca de un aumento del 40% en el riesgo de muerte). Se necesitan estudios adicionales bien diseñados que midan resultados que son importantes para los pacientes.
Calidad de la evidencia
La calidad de la evidencia fue baja o muy baja debido a que los estudios incluidos eran pequeños y presentaban errores en el diseño. Como resultado, hay mucha incertidumbre con respecto a los resultados.
Authors' conclusions
Summary of findings
| Non‐surgical versus surgical treatment of oesophageal cancer | |||||
| Patient or population: people with oesophageal cancer | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Surgical treatment | Non‐surgical treatment | ||||
| Short‐term mortality | 78 per 10001 | 30 per 1000 | RR 0.39 | 689 | ⊕⊝⊝⊝ |
| Long‐term mortality (binary outcome) | 691 per 10001 | 712 per 1000 | RR 1.03 | 511 | ⊕⊕⊝⊝ |
| Long‐term mortality (time‐to‐event outcome): chemoradiotherapy versus surgery | 349 per 10001 | 314 per 1000 | HR 0.88 | 602 | ⊕⊕⊝⊝ |
| Long‐term mortality (time‐to‐event outcome): radiotherapy versus surgery | 350 per 10001 | 451 per 1000 | HR 1.39 | 512 | ⊕⊝⊝⊝ |
| Long‐term mortality (binary): definitive chemoradiotherapy versus surgery with neoadjuvant chemotherapy All‐cause mortality for the duration of follow‐up | 740 per 1000 | 769 per 1000 | RR 1.04 | 431 | ⊕⊝⊝⊝ |
| Long‐term mortality (time‐to‐event): definitive chemoradiotherapy versus surgery with neoadjuvant chemotherapy or chemoradiotherapy All‐cause mortality for the duration of follow‐up | 349 per 1000 | 346 per 1000 | HR 0.99 | 431 | ⊕⊝⊝⊝ |
| Proportion with a serious adverse event within 3 months | 273 per 10001 | 166 per 1000 | RR 0.61 | 80 | ⊕⊝⊝⊝ |
| Short‐term health‐related quality of life | The mean short‐term health‐related quality of life in the control groups was | The mean short‐term health‐related quality of life in the intervention groups was | ‐ | 165 | ⊕⊝⊝⊝ |
| Medium‐term health‐related quality of life | The mean medium‐term health‐related quality of life in the control groups was | The mean medium‐term health‐related quality of life in the intervention groups was | ‐ | 62 | ⊕⊝⊝⊝ |
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1The basis for control risk is the event rate across all studies (i.e. the sum of all events in the surgical group across all studies reporting the outcome divided by the sum of all people in the surgical group in the trials reporting the outcome) for all outcomes except long‐term mortality (time‐to‐event) where a control group risk of 0.35 was used (based on similar control group risks at 2 years in a number of trials included in this analysis) and long‐term recurrence (time‐to‐event) where a control group risk of 0.4 was used (based on similar control group risk at 2 year in a trial included for this analysis). | |||||
| Non‐surgical versus surgical treatment of oesophageal cancer | ||||||
| Patient or population: people with oesophageal cancer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Surgical treatment | Non‐surgical treatment | |||||
| Long‐term recurrence (binary outcome) | 526 per 10001 | 552 per 1000 | RR 1.05 | 339 | ⊕⊝⊝⊝ | ‐ |
| Long‐term recurrence (time‐to‐event outcome) | 508 per 10001 | 494 per 1000 | HR 0.96 | 349 | ⊕⊕⊝⊝ | ‐ |
| Local recurrence (binary) | 381 per 1000 | 339 per 1000 | RR 0.89 | 449 | ⊕⊝⊝⊝ | ‐ |
| Proportion with any adverse event within 3 months | 386 per 10001 | 668 per 1000 | RR 1.73 | 80 | ⊕⊝⊝⊝ | ‐ |
| Length of hospital stay (days) | See comment | See comment | Not estimable | 342 | ⊕⊝⊝⊝ | Significant heterogeneity present (I² statistic = 93%, P = 0.0001) making meta‐analysis inappropriate. The mean hospital stay was 16 days shorter (3 days shorter to 29 days shorter) in non‐surgical treatment than surgical treatment in 1 trial (Bedenne 2007) and 14 days longer (5 days longer to 23 days longer) in non‐surgical treatment than surgical treatment in another trial (Chiu 2005). |
| Dysphagia at maximal follow‐up | 367 per 1000 | 543 per 1000 | RR 1.48 | 139 | ⊕⊝⊝⊝ | ‐ |
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The basis for control risk is the event rate across all studies (i.e. the sum of all events in the surgical group across all studies reporting the outcome divided by the sum of all people in the surgical group in the trials reporting the outcome). | ||||||
Background
Description of the condition
See Appendix 1 for a glossary of terms we have used in the Background.
Oesophageal cancer is the sixth most common cause of cancer‐related mortality in the world with an incidence varying from an age‐standardised annual incidence rate of less than one per 100,000 population in parts of Western Africa (Nigeria, Guinea, and Guinea‐Bissau) to an age‐standardised annual incidence rate of 17 to 24 per 100,000 population in parts of Eastern Africa, such as Malawi and Kenya, and parts of Central Asia (Turkmenistan) and East Asia (Mongolia) (IARC 2014); and worldwide incidence is thought to be increasing (Pennathur 2013). In 2012, there were about 455,000 new people diagnosed with oesophageal cancer and 400,000 deaths due to oesophageal cancer globally (IARC 2014). Treatment depends on the cancer stage. The American Joint Committee on Cancer (AJCC) oesophageal cancer staging system is used for this purpose (AJCC Cancer Staging Manual 2010; Rice 2010). The AJCC staging system takes into account several factors: involvement of tissue layers by the tumour (T), involvement of nodes (N), presence of metastases (M) (TNM classification), grade of the tumour (G), and histological type (AJCC Cancer Staging Manual 2010; Rice 2010; Stahl 2013). Whilst there are two histological types, namely squamous cell carcinoma and adenocarcinoma, there is only a marginal difference in their management (Berry 2014). Presence of metastases, on the other hand, has a large impact on treatment. If present, the cancer is stage IV and only palliative treatment is possible (Stahl 2013). In the absence of metastases and when the person is fit for surgery, endoscopic treatment or surgical treatment is recommended for carcinoma in situ and stage Ia oesophageal cancer (Stahl 2013). For other stages without metastases, surgical treatment with or without perioperative chemoradiotherapy depending upon the tumour stage and resection status is recommended when the person is fit for surgery (Stahl 2013). Five‐year survival depends on the stage, and ranges from 70% in Stage Ia squamous cell carcinoma and 80% in Stage Ia adenocarcinoma to 15% in Stage IV squamous cell carcinoma or adenocarcinoma (AJCC Cancer Staging Manual 2010; Rice 2010).
Description of the intervention
Surgical intervention involves mobilisation of the upper and lower oesophageal tract, oesophagectomy, and reconstruction of the oesophageal tube using a section of colon or bowel. There are two main methods to achieve this: transthoracic oesophagectomy and transhiatal oesophagectomy. Transthoracic oesophagectomy involves an abdominal incision followed by a thoracic incision to complete the oesophagectomy. A third incision may be used in the neck (McKeown 1976). Transhiatal oesophagectomy only requires an abdominal and cervical incision (Orringer 1978; Yamamoto 2013). Minimally‐invasive alternatives are laparoscopic or thoracoscopic adaptations of open oesophagectomy (Cuschieri 1992). Abdominal and thoracic incisions can be replaced by a series of 5 mm to 10 mm portholes for laparoscopic or thoracoscopic instruments (Luketich 2000; Yamamoto 2013). Chemoradiotherapy in conjunction with surgical intervention has been established as an effective treatment option for people with oesophageal cancer (van Hagen 2012), and is recommended for all people with node‐positive adenocarcinoma or adenocarcinoma that extends beyond muscularis propria undergoing surgical resection; and for people with squamous cell carcinoma who have undergone incomplete surgical resection (Berry 2014; Stahl 2013). Definitive chemoradiotherapy (chemoradiotherapy as the sole treatment with curative intent) is currently only advocated in people with localised cancer of the oesophagus who are unfit for surgery (Stahl 2013). Definitive chemoradiotherapy normally involves a 5‐fluorouracil and cisplatin chemotherapy treatment alongside a radiation dose of 46 to 65 grays (Bedenne 2007; Chiu 2005; Stahl 2005). There is a second alternative to surgical treatment for early stage oesophageal cancer (carcinoma in situ and stage Ia oesophageal cancer): endoscopic mucosal resection involves removing the cancerous tissue from within the oesophagus using endoscopic access (Stahl 2013). Only the affected tissue is removed, avoiding complete resection of the oesophagus which requires an open surgical procedure. A variation of the endoscopic mucosal resection is the endoscopic submucosal dissection, which involves injecting saline and dissecting the submucosal connective tissue just beneath the lesion from the muscularis propria (Ishihara 2008). The complication rates are considered to be low with endoscopic submucosal dissection (Sun 2014).
How the intervention might work
Surgery, endoscopic mucosal resection, and endoscopic submucosal dissection work by removal of the cancer tissue. Definitive chemoradiotherapy destroys the cancer cells using radiation and substances which are toxic to cells.
Why it is important to do this review
Definitive chemoradiotherapy may be comparable to surgery in locally‐advanced, non‐metastatic oesophageal squamous cell carcinoma (Bedenne 2007; Chiu 2005; Stahl 2005). Whilst associated with some side effects (renal toxicity, infection, and vomiting), definitive chemoradiotherapy is less invasive than oesophagectomy. Whether surgery is actually superior to definitive chemoradiotherapy is unclear (Yamashita 2008). Oesophagectomy carries a significant morbidity risk (such as surgical site infections, pneumonia, anastomotic stenosis, anastomotic dehiscence, intra‐abdominal abscess, and dysphagia) and mortality risk, particularly in smaller centres (Bedenne 2007; Goh 2015; Migliore 2007). Definitive chemoradiation may reduce mortality and may result in a shorter hospital stay compared to surgery. The same may be true for endoscopic mucosal resection. Since it does not rely on complete removal of the oesophagus, it is much less invasive. Therefore, these less invasive procedures may be preferred by people with oesophageal cancer, their families and carers, and healthcare providers if these procedures are as effective as surgery in terms of long‐term survival. The most recent National Comprehensive Cancer Network guidelines on treatment of oesophageal cancer reflect this and therefore allow a spectrum of treatment options that involve surgery, surgery with chemoradiotherapy, chemoradiotherapy alone, and endoscopic mucosal resection (Ajani 2011). This Cochrane review aims to assess the comparative roles of surgical and non‐surgical management in people with different stages of oesophageal cancer in order to develop treatment pathways to streamline clinical decisions.
Objectives
To assess the benefits and harms of non‐surgical treatment versus oesophagectomy for people with oesophageal cancer. In particular we planned to investigate the effects by participant groups (such as cancer stage and cancer type) and by intervention types (such as definitive chemoradiotherapy, definitive radiotherapy, and endoscopic treatment).
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs). We included studies reported as full‐text articles, those published as abstracts only and unpublished data, irrespective of the language in which they were published.
Types of participants
We included adults undergoing treatment for stages I to III oesophageal cancer (squamous cell carcinoma or adenocarcinoma) in the hospital setting (including palliative treatment centres such as hospices).
Types of interventions
We included trials that compared oesophagectomy (irrespective of whether it was performed by open method, laparoscopic method, or minimally invasive method and whether adjuvant chemotherapy, adjuvant radiotherapy, or adjuvant chemoradiotherapy was used) of any type with solely non‐surgical treatment for oesophageal cancer. Non‐surgical treatment included definitive chemoradiotherapy, endoscopic mucosal resection, and endoscopic submucosal dissection.
We aimed to perform the following comparisons and two meta‐analyses.
-
Definitive chemoradiotherapy, definitive chemotherapy, or definitive radiotherapy versus oesophagectomy.
-
Endoscopic treatment versus oesophagectomy.
However we only found trials that compared definitive chemoradiotherapy or definitive radiotherapy versus oesophagectomy. Therefore we only completed the first comparison.
Types of outcome measures
Primary outcomes
-
All‐cause mortality.
-
Short‐term mortality (in‐hospital mortality or mortality within three months).
-
Long‐term mortality.
-
-
Serious adverse events (within three months). We planned to accept the following definitions of serious adverse events.
-
ICH‐GCP International Conference on Harmonisation ‐ Good Clinical Practice guideline (ICH‐GCP 1996): serious adverse events defined as any untoward medical occurrence that results in death, is life‐threatening, requires inpatient hospitalisation or prolongation of existing hospitalisation, or results in persistent or significant disability/incapacity.
-
Other variations of ICH‐GCP classifications such as the U.S. Food and Drug Administration (FDA) classification (FDA 2006), or Medicines and Healthcare products Regulatory Agency (MHRA) classification (MHRA 2013).
-
-
Health‐related quality of life (using any validated scale).
-
Short‐term (four weeks to three months).
-
Medium‐term (more than three months to two years).
-
Long‐term (more than two years).
-
Secondary outcomes
-
Recurrence (local recurrence, surgical wound recurrence (also known as port site metastases in the endoscopic group) or distal metastases).
-
Short‐term recurrence (within six months).
-
Long‐term recurrence.
-
-
Adverse events (within three months). We accepted all adverse events reported by the study author(s) irrespective of the severity of the adverse event.
-
Measures of recovery.
-
Length of hospital stay (including the index admission for oesophagectomy (the hospital admission during which the oesophagectomy is performed) and any surgical complication‐related readmissions).
-
Time to return to normal activity (return to pretreatment mobility without any additional carer support; however defined by the trial authors).
-
Time to return to work (in those participants who were employed previously).
-
-
Dysphagia at maximal follow‐up (however defined by the trial authors).
We based the choice of the above clinical outcomes on the necessity to assess whether non‐surgical treatment is safe and effective in terms of short‐term results and long‐term cancer control.
Reporting of the outcomes listed here was not an inclusion criterion for this Cochrane review.
Search methods for identification of studies
Electronic searches
We conducted a literature search to identify all published and unpublished RCTs. The literature search identified potential studies in all languages. We translated the non‐English language papers and assessed them for potential inclusion in the review as necessary.
We searched the following electronic databases on 4th March 2016 for potential studies for inclusion.
-
The Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 3, 2016) (Appendix 2).
-
MEDLINE (1966 to March 2016) (Appendix 3).
-
EMBASE (1988 to March 2016) (Appendix 4).
-
Science Citation Index (1982 to March 2016) (Appendix 5).
We conducted a search of ClinicalTrials.gov (Appendix 6) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (Appendix 7) up to 4th March 2016.
Searching other resources
We checked reference lists of all included studies and review articles for additional references. We contacted authors of included trials and asked them to identify other published and unpublished studies.
We searched for errata or retractions from eligible trials on PubMed but did not find any.
Data collection and analysis
Selection of studies
Two review authors (LB and KG) independently screened for inclusion all the potential studies identified from the literature searches and coded them as either 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports and two review authors (LB and KG) independently screened the full‐text articles, identified studies for inclusion, and identified and recorded reasons for exclusion of ineligible studies. We resolved any disagreement through discussion. We identified and excluded duplicate references and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and a 'Characteristics of excluded studies' table.
Data extraction and management
We used a standardised data collection form for extraction of study characteristics and outcome data, which we had piloted on at least one study included in the review. Two review authors (LB and KG) extracted study characteristics from the included studies. We extracted the following study characteristics.
-
Methods: study design, total duration of study, number of study centres and location, study setting, withdrawals, and date of study.
-
Participants: number (N), mean age, age range, gender, tumour stage, tumour location, histological subtype, performance status, American Society of Anesthesiologists (ASA) status (ASA 2014), inclusion criteria, and exclusion criteria.
-
Interventions: intervention, comparison, and concomitant interventions.
-
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
-
Notes: funding for trial, and notable conflicts of interest of trial authors.
Two review authors (LB and KG) independently extracted outcome data from included studies. If the included studies reported outcomes multiple times for the same time frame (e.g. short‐term health‐related quality of life was reported at six weeks and three months), we chose the later time point (i.e. three months) for data extraction. For time‐to‐event outcomes, we extracted data to calculate the natural logarithm of the hazard ratio (HR) and its standard error (SE) using the methods suggested by Parmar 1998 if possible.
We included all randomised participants for medium‐ and long‐term outcomes (e.g. mortality or quality of life) and this was not conditional upon the short‐term outcomes (e.g. being alive at three months or having a low or high quality of life index at three months).
We have noted in the 'Characteristics of included studies' table if the included trials reported outcome data in an unusable way. We resolved disagreements by consensus. One review author (LB) copied the data from the data collection form into the Review Manager (RevMan) file (Review Manager 2014). We double‐checked that LB had entered the data correctly by comparing the study reports with how we presented the data in the systematic review.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each included study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion. We assessed the risk of bias according to the following domains.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Other bias.
We graded each potential source of bias as either 'high', 'low', or 'unclear' and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' tables. We summarised the 'Risk of bias' judgements across different studies for each listed domain. We acknowledge that blinding of participants and personnel is impossible but blinding of outcome assessors is possible. We considered blinding separately for different important outcomes where necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a patient‐reported quality of life scale since lack of blinding is unlikely to result in bias in all‐cause mortality while lack of blinding is likely to introduce a significant bias in quality of life). Where information on risk of bias relates to unpublished data or correspondence with a trial author, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol, Best 2015, and reported any deviations from it in the 'Differences between protocol and review' section.
Measures of treatment effect
We analysed dichotomous data (short‐term mortality, proportion of people with adverse and serious adverse events, short‐term recurrence) as risk ratios (RRs), and continuous data as mean differences (MDs) when all the trials reported the outcome or converted it to the same units (e.g. hospital stay), or standardised mean differences (SMDs) when trials used different scales to measure the outcome (e.g. quality of life). We have ensured that higher scores for continuous outcomes have the same meaning for the particular outcome, have explained the direction to the reader, and have reported where the directions were reversed if this was necessary. We calculated the rate ratio for outcomes such as adverse events and serious adverse events, where it was possible for the same person to develop more than one adverse event (or serious adverse event). If the trial authors calculated the rate ratio of adverse events (or serious adverse events) in the intervention versus control based on Poisson regression, we obtained the rate ratio by the Poisson regression method in preference to rate ratio calculated based on the number of adverse events (or serious adverse events) during a certain period. We calculated the HR for time‐to‐event outcomes such as long‐term mortality, long‐term recurrence, and time‐to‐first adverse event (or serious adverse event).
We undertook meta‐analyses only where meaningful i.e. if the treatments, participants, and the underlying clinical question were similar enough for pooling to make sense.
A common way that trial authors indicate they have skewed data is by reporting medians and interquartile ranges. When we encountered this we noted that the data were skewed and considered the implication of this. If the data were skewed, then we did not perform a meta‐analysis but performed a narrative summary instead.
Where a single trial reported multiple trial arms, we included only the relevant arms. If we had to enter two comparisons (e.g. laparoscopic oesophagectomy versus definitive chemoradiotherapy and open oesophagectomy versus definitive chemoradiotherapy) into the same meta‐analysis, we planned to half the control group to avoid double counting. The alternative way of including such trials with multiple arms is to pool the results of the oesophagectomy irrespective of the method and compare it with definitive chemoradiotherapy. We performed a sensitivity analysis to determine if the results of the two methods of dealing with multi‐arm trials led to different conclusions.
Unit of analysis issues
The unit of analysis was individual participants undergoing treatment for oesophageal cancer. We did not encounter any cluster‐RCTs for this comparison and therefore did not require any specific methodology for this trial type.
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study is identified as abstract only). If we were unable to obtain the information from the trial authors or study sponsors, we imputed the mean from the median (i.e. consider median as the mean) and standard deviation (SD) from the SE, interquartile range, or P values according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) when the data did not appear to be skewed but we assessed the impact of including such studies as indicated in a sensitivity analysis. If we were unable to calculate the SD from SE, interquartile range, or P values, we imputed SD as the highest SD in the remaining trials included in the outcome, fully aware that this method of imputation decreases the weight of the studies in the meta‐analysis of MD and shifts the effect towards no effect for SMD.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis. If we identified substantial heterogeneity according to the Cochrane Handbook for Systematic Reviews of Interventions (greater than 50% to 60%), we explored it by prespecified subgroup analysis. We also assessed heterogeneity by evaluating whether there was good overlap of confidence intervals (CIs).
Assessment of reporting biases
We attempted to contact study authors by asking them to provide missing outcome data. Where this was not possible, and we thought the missing data may have introduced serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis. We also sought published protocols of trials to determine if outcomes mentioned in the protocol were not reported in order to determine selective outcome reporting bias. However, we were unable to locate the published protocol of any included trial.
If we were able to pool more than 10 trials, we planned to create and examine a funnel plot to explore possible publication biases. We planned to use Egger's test to determine the statistical significance of the reporting bias (Egger 1997). We considered a P value of less than 0.05 statistically significant reporting bias. However, we did not explore reporting biases since only eight trials met the inclusion criteria of this Cochrane review.
Data synthesis
We performed the analysis using RevMan (Review Manager 2014). We used the Mantel‐Haenszel method for dichotomous data, inverse variance method for continuous data, and generic inverse variance for count and time‐to‐event data. We used both a random‐effects model (DerSimonian 1986) and a fixed‐effect model (Demets 1987) for the analysis. In case of discrepancy between the two models, we reported both results; otherwise we only reported the results from the fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses.
-
Different cancer stages (stage I, stage II, stage III).
-
Different histological types (squamous cell carcinoma and adenocarcinoma).
-
Different locations (upper third, middle third, lower third).
-
Different non‐surgical treatments: endoscopic mucosal resection and endoscopic submucosal dissection.
-
Different anaesthetic risk patients (ASA I or II (a healthy participant or one with mild systemic disease) versus ASA III or more (a participant with severe systemic disease or worse) (ASA 2014).
We planned to use all the primary outcomes in the subgroup analysis.
We used the formal Chi² test for subgroup differences to test for subgroup interactions.
Sensitivity analysis
We planned to perform the following sensitivity analyses, which we defined a priori, to assess the robustness of our conclusions.
-
Exclusion of trials at unclear or high risk of bias (one of more of the risk of bias domains (other than blinding of surgeon) classified as unclear or high).
-
Exclusion of trials in which we imputed either mean or SD or both.
-
Exclusion of cluster RCTs in which the trial authors did not report the adjusted effect estimates.
-
Different methods of dealing with multi‐arm trials (please see the 'Measures of treatment effect' section).
'Summary of findings' table
We created a 'Summary of findings' table using all the outcomes. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies that contribute data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and used GRADEpro Guidelines Development Tool (GDT) software (www.gradepro.org). We justified all decisions to downgrade or upgrade the quality of the evidence from included studies using footnotes, and we made comments to aid the reader's understanding of the review where necessary. We considered whether there was any additional outcome information that we could not incorporate into meta‐analyses, and planned to note this in the comments and state if it supported or contradicted the information from the meta‐analyses.
Reaching conclusions
We based our conclusions only on findings from the quantitative or narrative synthesis of included studies for this Cochrane review. We avoided making recommendations for practice and our implications for research will give the reader a clear sense of where the focus of any future research in the area should be and what the remaining uncertainties are.
Results
Description of studies
Results of the search
We identified 10,952 references through electronic searches of the Cochrane Central Register of Controled trials (CENTRAL), MEDLINE, EMBASE, Science Citation Index, ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP). After we removed duplicate references there were 7849 references. We excluded 7823 irrelevant references after we screened abstracts. We sought the full text of 26 references for further assessment. We did not identify any additional references to trials by searching the trial registry. We excluded six references because of the reasons mentioned in the 'Characteristics of excluded studies' table and 'Excluded studies' section. Eight trials (20 references) met the inclusion criteria (Badwe 1999; Bedenne 2007; Blazeby 2014; Carstens 2007; Chiu 2005; Fok 1994; Stahl 2005; Sun 2006). We have presented the study flow diagram in Figure 1.

Study flow diagram.
Included studies
The eight included trials compared definitive radiotherapy or definitive chemoradiotherapy with surgery (Badwe 1999; Bedenne 2007; Blazeby 2014; Carstens 2007; Chiu 2005; Fok 1994; Stahl 2005; Sun 2006). We did not identify any trials that compared endoscopic mucosal resection or endoscopic submucosal dissection with surgery. Seven trials were two armed randomised controlled trials (RCTs) (Badwe 1999; Bedenne 2007; Blazeby 2014; Carstens 2007; Chiu 2005; Stahl 2005; Sun 2006). The eighth trial was a four‐armed trial (Fok 1994). Three of the arms involved surgery (surgery alone, surgery with preoperative radiotherapy, and surgery with postoperative radiotherapy) and the fourth arm was non‐surgical (definitive radiotherapy). In total, the eight included trials randomised 1132 participants. One trial, which included five participants, did not contribute any data to this Cochrane review, because the trial authors did not report any of the outcomes included in this review (Blazeby 2014). Therefore, the seven trials that contributed data to this Cochrane review randomised 1127 participants, of which 13 participants were withdrawn postrandomisation, which left a total of 1114 participants for whom data were available. Of these 1114 participants, 510 were randomised to non‐surgical treatment and 604 to surgical treatment (Badwe 1999; Bedenne 2007; Carstens 2007; Chiu 2005; Fok 1994; Stahl 2005; Sun 2006).
Of the eight included trials, three used radiotherapy alone (Badwe 1999; Fok 1994; Sun 2006), five used chemoradiotherapy (Bedenne 2007; Blazeby 2014; Carstens 2007; Chiu 2005; Stahl 2005), and none used chemotherapy alone as the non‐surgical treatment. None of the trials compared endoscopic treatment with surgical treatment. Regarding the surgical arm, three trials included surgical treatment without any adjuvant therapy (Badwe 1999; Carstens 2007; Sun 2006), three trials used preoperative chemotherapy or chemoradiotherapy in addition to surgery (Bedenne 2007; Blazeby 2014; Stahl 2005), and one trial used postoperative chemoradiotherapy or radiotherapy (Chiu 2005). In one trial, surgical treatment consisted of three groups of which one group received preoperative radiotherapy, another received postoperative radiotherapy, and the last group received surgery alone (Fok 1994).
Three trials reported the cancer stage (Bedenne 2007; Blazeby 2014; Stahl 2005) and these all included T2‐4N0‐1M0 tumours (or a subset of this range). The remaining trials did not state the tumour stage of participants, but it is likely that these trials included only resectable cancers as surgical resection was one of the arms in the trials. Seven trials reported the histological classification of tumours, with five of these being squamous cell carcinoma only (Badwe 1999; Blazeby 2014; Chiu 2005; Fok 1994; Stahl 2005) and two either adenocarcinoma or squamous cell carcinoma (Bedenne 2007; Carstens 2007). The remaining trial, Sun 2006, did not report the histological cancer type. There was little concordance between studies in the terminology they used to describe the tumour location. We have stated the term the study authors used in the 'Characteristics of included studies' table. None of the studies specifically reported the American Society of Anesthesiologists (ASA) grade of participants, although it is likely that trials only included participants who were fit to undergo major surgery. In terms of the surgical treatment used, six trials used open oesophagectomy (Badwe 1999; Carstens 2007; Chiu 2005; Fok 1994; Stahl 2005; Sun 2006), and the remaining two trial did not report whether the surgery was performed by open method or laparoscopic method (Bedenne 2007; Blazeby 2014).
The follow‐up period in the trials were as follows.
-
Badwe 1999 had a maximum follow‐up of three years.
-
Bedenne 2007 had a median follow‐up of four years.
-
Blazeby 2014 did not state the follow‐up period.
-
Carstens 2007 did not specify the follow‐up period but reported four year follow‐up results.
-
Chiu 2005 had a median follow‐up 1.5 years.
-
Fok 1994 stated that long‐term follow‐up with partial data were available at 10 years, but did not specify details.
-
Stahl 2005 had a median follow‐up of six years.
-
Sun 2006 had a median follow‐up of 4.8 years.
Excluded studies
We excluded six full‐text articles (five studies) in total. We excluded two articles because they were not RCTs (Desai 1987; Ilson 2007). Two trials changed their protocol to a non‐randomised study following poor recruitment (Earlam 1991; Hainsworth 2007). We excluded one article because this trial is expected to completed in 2018 and the current report (a conference abstract) included details of the safety of surgery in participants randomised to surgical arm and the report included non‐randomised patients undergoing surgery as well (Nozaki 2014).
Risk of bias in included studies
All included trials were at high risk of bias, as we have shown in Figure 2 and Figure 3.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
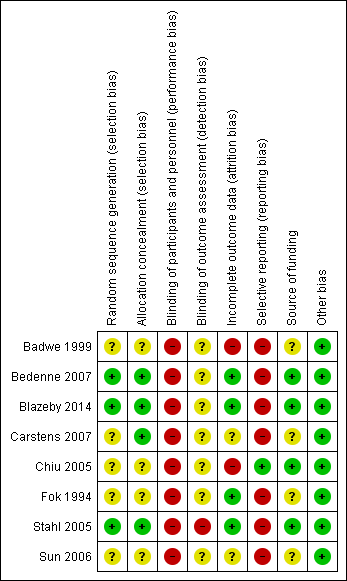
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
Three trials were free from selection bias (Bedenne 2007; Blazeby 2014; Stahl 2005). These trials had low risk of bias regarding random sequence generation and allocation concealment. The remaining trials were at unclear risk of bias in at least one aspect of random sequence generation or allocation concealment.
Blinding
All eight trials were at high risk of performance bias because it is almost impossible to blind the participants and healthcare providers in a surgical versus non‐surgical trial. One trial reported that it was unblinded and therefore was at high risk of detection bias (Stahl 2005). The other seven trials did not address this aspect and we considered them to be at an unclear risk of detection bias.
Incomplete outcome data
We classified four trials as at low risk of attrition bias as they described no postrandomisation exclusions (Bedenne 2007; Blazeby 2014; Fok 1994; Stahl 2005). Two trials were at unclear risk of attrition bias as the reports do not clearly describe whether there were any postrandomisation exclusions (Carstens 2007; Sun 2006). Two trials were at high risk of attrition bias as they had postrandomisation exclusions, which was likely to affect the results (Badwe 1999; Chiu 2005).
Selective reporting
Protocols were not available for any of the included trials. Only one trial reported all important outcomes and we therefore considered it at low risk of reporting bias (Chiu 2005). The remaining seven trials were at high risk of reporting bias.
Other potential sources of bias
Four trials described an impartial source of funding, and were therefore at low risk of bias (Bedenne 2007; Blazeby 2014; Chiu 2005; Stahl 2005). The other four trials did not describe how they were funded and we therefore considered them at unclear risk of bias regarding the funding source (Badwe 1999; Carstens 2007; Fok 1994; Sun 2006). We considered all the included trials as free from any other sources of bias.
Effects of interventions
See: Summary of findings for the main comparison Non‐surgical versus surgical treatment of oesophageal cancer (primary outcomes); Summary of findings 2 Non‐surgical versus surgical treatment of oesophageal cancer (secondary outcomes)
The included trials reported the following outcomes: short‐term mortality, long‐term mortality, serious adverse events within three months, short‐term quality of life, medium‐term quality of life, long‐term recurrence, adverse events within three months, and length of hospital stay. None of the trials reported the remaining outcomes of interest in this Cochrane review, i.e. long‐term quality of life, recurrence within six months, time to return to normal activity, and time to return to work. We have summarised the results in 'Summary of findings' table 1 (summary of findings Table for the main comparison) and 'Summary of findings' table 2 (summary of findings Table 2).
Short‐term mortality
Five trials reported short‐term mortality, which is defined as mortality in hospital or within 30 days of treatment (Badwe 1999; Bedenne 2007; Carstens 2007; Chiu 2005; Stahl 2005). We pooled the trials and used a fixed‐effect model. There was a statistically significant lower proportion of participants who died within 30 days of treatment between the non‐surgical group (30 per 1000) compared to the surgical group (78 per 1000) (risk ratio (RR) 0.33, 95% confidence interval (CI) 0.16 to 0.69; 689 participants; five studies; I² statistic = 46%) . However we applied when a random‐effects model this became statistically non‐significant (RR 0.39, 95% CI 0.11 to 1.35; 689 participants; five studies; Analysis 1.1).
Long‐term mortality
Three trials reported long‐term mortality, which included deaths at maximal follow‐up as binary outcomes (Bedenne 2007; Chiu 2005; Stahl 2005). We pooled the trials using a fixed‐effect model. There was no significant difference in the proportion of participants who died beyond three months after treatment between the non‐surgical group (712 per 1000) compared to the surgical group (691 per 1000) (RR 1.03, 95% CI 0.92 to 1.14; 511 participants; three studies; I² statistic = 0%; Analysis 1.2). There was no change to the conclusions when we used a random‐effects model.
Seven trials reported long‐term mortality, which we could analyse as a time‐to‐event analysis (Badwe 1999; Bedenne 2007; Carstens 2007; Chiu 2005; Fok 1994; Stahl 2005; Sun 2006). We pooled the trials using a fixed‐effect model. There was no significant difference in long‐term mortality between the groups (hazard ratio (HR) 1.09, 95% CI 0.97 to 1.22; 1114 participants; seven studies; I² statistic = 79%; Analysis 1.3). There was no change to the conclusions when we used a random‐effects model.
Serious adverse events within three months
One trial reported serious adverse events within three months of treatment (Chiu 2005). There was no significant difference in the proportion of participants who suffered a serious adverse event within three months of treatment between the non‐surgical group (166 per 1000) compared to the surgical group (273 per 1000) (RR 0.61, 95% CI 0.25 to 1.47; 80 participants; one study; Analysis 1.4). Since only one trial reported this outcome, the issue of fixed‐effect model versus random‐effects model did not arise and studies of heterogeneity are irrelevant. None of the trials reported the number of serious adverse events within three months.
Short‐term health‐related quality of life
One trial reported short‐term health‐related quality of life, which is defined as any validated quality of life measurement (in this case Spitzer score) recorded four weeks to three months after treatment (Bedenne 2007). Lower scores indicate poorer quality of life (Spitzer 1981). There was a statistically significantly higher quality of life score in non‐surgical treatment between four weeks and three months after treatment (MD 0.93, 95% CI 0.24 to 1.62; 165 participants; one study; Analysis 1.5). Since only one trial reported this outcome, the issue of fixed‐effect model versus random‐effects model did not arise.
Medium‐term health‐related quality of life
One trial reported medium‐term health‐related quality of life, which is defined as any validated quality of life measurement (in this case Spitzer score) recorded three months to two years after treatment (Bedenne 2007). There was no significant difference in the quality of life score between three months and two years after treatment (MD −0.95, 95% CI −2.10 to 0.20; 62 participants; one study; Analysis 1.6). Since only one trial reported this outcome, the issue of fixed‐effect model versus random‐effects model did not arise.
Medium‐term health‐related quality of life
None of the trials reported this outcome.
Recurrence within six months
None of the trials reported this outcome.
Long‐term recurrence
Two trials reported recurrence as a binary outcome (Bedenne 2007; Chiu 2005). We pooled the trials using a fixed‐effect model. There was no significant difference in the proportion of participants who suffered recurrence more than six months after treatment between the non‐surgical group (552 per 1000) compared to the surgical group (526 per 1000) (RR 1.05, 95% CI 0.87 to 1.28; 339 participants; two studies; I² statistic = 0%; Analysis 1.7). There was no change to the conclusions when we used a random‐effects model.
Two trials reported long‐term recurrence as a time‐to‐event outcome (Chiu 2005; Sun 2006). We pooled the trials using a fixed‐effect model. There was no significant difference in the HR for recurrence after more than six months after treatment between the two groups (HR 0.96, 95% CI 0.80 to 1.16; 349 participants; two studies; I² statistic = 41%; Analysis 1.8). There was no change to the conclusions when we used a random‐effects model.
Three trials reported local recurrence as a binary outcome (Bedenne 2007; Chiu 2005; Fok 1994). There was no statistically significant difference in the local recurrence between the non‐surgical and surgical groups (RR 0.89, 95% CI 0.70 to 1.12; 449 participants; three studies; I² statistic = 90%; Analysis 1.9).
Adverse events within three months
One trial reported adverse events within three months of treatment (Chiu 2005). There were statistically significantly more adverse events within three months of treatment (RR 1.73, 95% CI 1.11 to 2.67; 80 participants; one study) in the non‐surgical group (668 per 1000) compared to the surgical group (386 per 1000) (Analysis 1.10). Since only one trial reported this outcome, the issue of fixed‐effect model versus random‐effects model did not arise and studies of heterogeneity are irrelevant. None of the included trials reported the number of adverse events within three months.
Length of hospital stay
Two trials reported length of hospital stay (Bedenne 2007; Chiu 2005). However these two trials showed completely opposite results and meta‐analysis had an I² statistic value of 93%. An I² statistic value of greater than 80% indicates significant underlying heterogeneity (Higgins 2011). The heterogeneity in the results between the trials was unexplained, although this could be because of the way the trials measured the length of hospital stay (please see the 'Discussion' section). Therefore meta‐analysis was invalid and we have only shown point estimates for individual studies (Analysis 1.11).
Time to return to normal activity
None of the trials reported this outcome.
Time to return to work
None of the trials reported this outcome.
Dysphagia at maximal follow‐up
Only one trial reported dysphagia at maximal follow‐up (Bedenne 2007). People without dysphagia were asymptomatic or were able to eat solids with some dysphagia at the last clinic visit prior to death. The dysphagia at maximal follow‐up prior to death was statistically significantly higher in the non‐surgical group than surgical group (RR 1.48, 95% CI 1.01 to 2.19; 139 participants; one study; Analysis 1.12). Since only one trial reported this outcome, the issue of fixed‐effect model versus random‐effects model did not arise and studies of heterogeneity are irrelevant.
Subgroup analysis
We had planned to perform five subgroup analyses but only one was possible. The first planned subgroup analysis was not possible because only three trials reported cancer stage and they were all within a narrow range (T2‐4, N0‐1, M0). Analysis by histological type was not possible because five trials included squamous cell carcinoma only (Badwe 1999; Blazeby 2014; Chiu 2005; Fok 1994; Stahl 2005), two trials included either adenocarcinoma or squamous cell carcinoma (Bedenne 2007; Carstens 2007), and one trial did not report the histology (Sun 2006). The third analysis we had planned was by tumour location. However, only a few of the included trials reported this but did not report it in a standardised way, which made comparisons impossible.
Comparison of different types of non‐surgical treatment was the only subgroup analysis which was possible. The two different treatments were definitive chemoradiotherapy and definitive radiotherapy. The only outcome reported, which contained at least two trials for both definitive chemoradiotherapy and definitive radiotherapy, was long‐term mortality with time‐to‐event analysis (the presence of at least two trials allows meta‐analysis and assessment of heterogeneity within the subgroup to explore whether the heterogeneity in overall results could be explained because of the clinical differences). The results of the subgroup analysis show that there are statistically significant subgroup differences (test for subgroup differences: Chi² test = 16.15, df = 1 (P < 0.0001), I² statistic = 93.8%). There was a difference in both the magnitude and direction in the subgroups with definitive chemoradiotherapy having a HR of 0.88 (95% CI 0.76 to 1.03; 602 participants; four trials; Analysis 1.13) and definitive radiotherapy having a HR of 1.39 (95% CI 1.18 to 1.64; 512 participants; three trials; Analysis 1.13) compared to surgical treatment with respect to long‐term mortality. We performed a post‐hoc subgroup analysis in which we compared definitive chemoradiotherapy versus oesophagectomy with neoadjuvant chemotherapy or chemoradiotherapy. As shown in Analysis 1.14 and Analysis 1.15, there was no statistically significant difference in the long‐term survival between definitive chemoradiotherapy versus oesophagectomy with neoadjuvant chemotherapy or chemoradiotherapy whether analysed as binary outcome (RR 1.04, 95% CI 0.93 to 1.16; 431 participants; two trials; Analysis 1.14) or as time‐to‐event outcome (HR 0.99, 95% CI 0.78 to 1.26; 431 participants; two trials; Analysis 1.15).
Finally none of the included trials explicitly reported the ASA grade of included participants, which made subgroup analysis impossible.
Sensitivity analysis
We could not perform any of the planned sensitivity analyses. Regarding the first sensitivity analysis, all the trials were at unclear or high risk of bias and thus we could not perform the analysis. We could not perform the second sensitivity analysis since we did not impute the mean or SD for short‐term or medium‐term health‐related quality and imputed the mean or SD or both for all the trials included for the length of hospital stay. We did not perform the third sensitivity analysis since there were no cluster RCTs. Regarding the different ways of dealing with multi‐arm trials, one trial had four arms (three surgical treatments: surgery alone, surgery combined with preoperative radiotherapy, and surgery combined with postoperative radiotherapy; and definitive radiotherapy) (Fok 1994). Fok 1994 only reported the outcome of long‐term mortality, and presented the long‐term mortality for the three surgical groups together rather than for each subgroup individually. Thus, we could not perform this sensitivity analysis.
Discussion
Summary of main results
In this Cochrane review, we compared non‐surgical versus surgical treatment for people with non‐metastatic oesophageal cancer. The included trials compared the treatments of definitive chemoradiotherapy versus surgery and definitive radiotherapy versus surgery. There were no statistically significant differences in short‐term mortality, long‐term mortality, proportion of people with serious adverse events, medium‐term health‐related quality of life, proportion of people with recurrence after six months, long‐term recurrence (time‐to‐event), or proportion of people with local recurrence. However, the subgroup analysis of long‐term mortality showed that the results differed for chemoradiotherapy versus surgery and radiotherapy versus surgery. While radiotherapy treatment resulted in more long‐term mortality than surgery group (hazard ratio (HR) 1.39, 95% confidence interval (CI) 1.18 to 1.64), there was no statistically significant difference in long‐term mortality in the chemoradiotherapy group compared with the surgery group (HR 0.88, 95% CI 0.76 to 1.03). If anything, there was a trend that favoured chemoradiotherapy and there was good overlap of CIs in this subgroup (chemoradiotherapy versus surgery). We performed the post‐hoc subgroup analysis of definitive chemoradiotherapy versus neoadjuvant chemotherapy or chemoradiotherapy along with surgery since neoadjuvant chemotherapy or chemoradiotherapy along with surgery provides better survival than surgery alone (Sjoquist 2011) and the European Society for Medical Oncology (ESMO) guidelines recommend this treatment (Stahl 2013).
There was higher proportion of people with any adverse events in the non‐surgical treatment group than the surgical treatment group, while the short‐term health‐related quality of life was better in the non‐surgical treatment group than surgical treatment group. One might expect lower health‐related quality of life if there are more adverse events. However, we did not observe this. The conflicting results in this Cochrane review could be because of the way that trial authors reported the adverse events in the two groups or because of the inconsistent results in the studies that reported these outcomes. In either case, one cannot attach much clinical significance to the difference in the adverse events favouring surgical treatment since this was based on a single trial (Chiu 2005). Also there were no statistically significant differences between the groups for other outcomes such as mortality or recurrence. One cannot attach much clinical significance to the difference in the short‐term quality of life favouring non‐surgical treatment either since this was based on a single trial (Bedenne 2007) and it is unclear whether or not the difference was clinically significant. The length of hospital stay was lower in the non‐surgical treatment group than the surgical treatment group in one trial (Bedenne 2007), and higher in the non‐surgical treatment group than non‐surgical treatment group in another trial (Chiu 2005). The length of hospital stay was the mean hospital stay during the entire follow‐up period in Bedenne 2007, while this was the median hospital stay during the treatment in Chiu 2005. The inconsistent results in length of hospital stay between the studies are likely to be due to the different ways the two trials measured the length of hospital stay (Bedenne 2007; Chiu 2005).
Overall, chemoradiotherapy appears to be equivalent to surgery regarding long‐term survival (as the long‐term mortality of the non‐surgical treatment that was equivalent to surgery was chemoradiotherapy; radiotherapy resulted in higher long‐term mortality than non‐surgical treatment. In addition, most included trials, for the outcomes other than long‐term mortality, used chemoradiotherapy as the non‐surgical treatment). The major question is whether the lack of statistically significant difference in the outcomes between chemoradiotherapy and surgery was because of a lack of difference in the outcomes or the lack of evidence of difference. There was a trend that suggested that chemoradiotherapy resulted in less long‐term mortality but there was no statistical significance. We considered 25% relative change as clinically important since there was no evidence from literature regarding the clinically important difference in long‐term mortality. The CIs did not overlap a 25% relative increase in long‐term mortality (i.e. the confidence intervals did not overlap RR of 1.25) whether this was analysed as a binary outcome or a time‐to‐event outcome i.e. one can rule out a 25% relative increase in long‐term mortality with chemoradiotherapy based on the results reported in the study. The short‐term mortality was lower in the non‐surgical treatment group compared to surgical treatment group when we used the fixed‐effect model, but there was no statistically significant difference between the groups when we used the random‐effects model. Thus, there is nothing to suggest that the lack of statistical significance is because of lack of evidence of beneficial effect of surgery. The most likely interpretation of the data is that chemoradiotherapy is at least equivalent to surgery in terms of survival. In the absence of beneficial effect of surgery in other outcomes, there is no reason to prefer surgery over chemoradiotherapy based on the current data. Importantly, of the five trials that used chemoradiotherapy as the intervention arm (Bedenne 2007; Blazeby 2014; Carstens 2007; Chiu 2005; Stahl 2005), the surgical arm received adjuvant chemotherapy or chemoradiotherapy in three trials (Bedenne 2007; Blazeby 2014; Stahl 2005). Thus the control group is a contemporary control group. We performed another subgroup analysis to compare definitive chemoradiotherapy versus oesophagectomy with neoadjuvant therapy. There was no statistically significant difference in the long‐term mortality between the two groups irrespective of whether we analysed this as a binary outcome (RR 1.04, 95% CI 0.93 to 1.16) or as a time‐to‐event outcome (HR 0.99, 95% CI 0.78 to 1.26). When we analysed it as a binary outcome, the CIs did not overlap a 25% relative increase or decrease. When we analysed it as a time‐to‐event outcome, the CIs overlapped 25% relative increase. Overall, there does not appear to be any difference in the long‐term mortality between the two groups.
The proportion of people with dysphagia at the last follow‐up visit prior to death was higher with chemoradiotherapy than surgery in the only trial that reported dysphagia (Bedenne 2007). While dysphagia is an important patient‐oriented outcome and is likely to have a significant impact on the quality of people, we were unable to assess the impact of dysphagia on the quality of life since the trial (or any other trial) did not report long‐term quality of life. However, this must be confirmed in further trials before we can be definite that surgery is better than definitive chemoradiotherapy regarding dysphagia.
Overall completeness and applicability of evidence
This Cochrane review included participants either undergoing surgery of oesophageal cancer or non‐surgical treatment of different histological types and stages of oesophageal cancer. However, most participants had squamous cell carcinoma and thus, the results are applicable mainly to squamous cell carcinoma. Only two trials included adenocarcinoma (Bedenne 2007; Carstens 2007). In Bedenne 2007, only 10% of 259 participants had adenocarcinoma, while in Carstens 2007 50% of 91 participants had adenocarcinoma. The effect estimates observed in these trials did not differ from the other trials. So, there is no evidence to suggest that the effect estimates of definitive chemoradiotherapy versus surgery will be different for squamous cell carcinoma and adenocarcinoma. However, there is more uncertainty about equivalence of definitive chemoradiotherapy and surgery in terms of long‐term survival because of the low number of participants with adenocarcinoma. One of the trials that contributed significantly to the different meta‐analysis, only participants who responded to initial induction chemoradiotherapy (defined as least 30% decrease in tumour length following induction chemotherapy) were randomised to chemoradiotherapy and surgery. Those who did not respond were offered surgery. So, the findings of this review are applicable mainly to people who respond to chemoradiotherapy. However, failure of response to induction chemoradiotherapy does not prevent people with oesophageal cancer from having surgery; so, surgery can be offered to such people as definitive treatment.
Although the included trials did not report the American Society of Anesthesiologists (ASA) status, all participants must have been fit for major surgery if the randomisation procedures were performed adequately. Thus, the results of this review are applicable only to people with non‐metastatic oesophageal cancer (of different tumour histological types and stages) and are not applicable to people who are unsuitable for surgery either because of their anaesthetic risk or because of the location and extent of the cancer.
Quality of the evidence
All included trials were at high risk of bias. None of the included trials reported blinding of participants and personnel and it is unrealistic in trials that compare non‐surgical and surgical treatments. However, blinding of the outcome assessors can be performed and is necessary to decrease the detection bias. Only one trial, Stahl 2005, appeared to have blinded the outcome assessors. However, there is no evidence that lack of blinding will lead to bias in an outcome such as all‐cause mortality while it is likely to affect most of the other outcomes, including adverse events and quality of life. Every effort should be taken to ensure blinding of outcome assessors. All included trials except Chiu 2005 were at high risk of bias for selective reporting because they did not report treatment related complications, an important consideration when comparing treatment regimes. In addition two trials suffered high risk of attrition bias because of postrandomisation exclusions (Badwe 1999; Chiu 2005). The selection bias and attrition bias can be easily reduced by reporting the most important clinical outcomes in all randomised participants according to the group to which they were randomised (intention‐to‐treat analysis).
Another reason for low or very low quality of evidence was the small sample size for many outcomes. There was also inconsistency in the results for some outcomes. The heterogeneity in long‐term mortality for radiotherapy was only in the magnitude of effect and there was reasonable overlap of CIs for long‐term recurrence. However, the heterogeneity in the length of hospital stay was both in magnitude and in direction, and resulted in decreased confidence in the results of the length of hospital stay.
Potential biases in the review process
We followed the guidance of the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011). There were no language, publication status, or sample size restrictions. Thus, we minimised the bias due to selection of trials. However, we used median values for the meta‐analyses when the mean value was unavailable. We also imputed the standard deviation from P values, according to the formulae stated in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011). This was only for the length of hospital stay which did not contribute to our conclusion because of the heterogeneity (and hence uncertainty) in the results. Thus, this did not impact on our conclusions. We were unable to assess the reporting bias because fewer than 10 trials met the inclusion criteria of this review. Since there was no restriction on the publication date, we included trials from the pre‐mandatory trial registration era. There is a possibility that some trials were not reported because of the direction of results. However, we have to be pragmatic and accept that it will be difficult to obtain useful data from these trials after such a long period of time. Therefore, we must base our conclusions on the trials that have been published or reported in conferences.
Agreements and disagreements with other studies or reviews
This is the first Cochrane review to assess non‐surgical versus surgical treatment for oesophageal cancer. We identified one previous systematic review on this topic (Pöttgen 2012). The authors of that systematic review concluded that surgery along with chemotherapy or chemoradiotherapy offers better results in terms of locoregional control than surgery alone or definitive chemoradiotherapy, and that definitive chemoradiotherapy is a reasonable choice especially in people with oesophageal squamous cell carcinoma and co‐morbidities. However, our conclusions are that chemoradiotherapy appears at least equivalent to surgery in terms of short‐term and medium‐term survival in people with oesophageal cancer (squamous type) who are fit for surgery. One possible reason for the difference in conclusions between the two systematic reviews is that Pöttgen 2012 did not perform a subgroup analysis of chemoradiotherapy versus surgery, which we had planned a priori in our review. However, it is unclear why the authors concluded that chemoradiotherapy is a reasonable choice in people with co‐morbidities, since the participants included in most included studies in Pöttgen 2012 were fit to undergo surgery.
Another question that has to be answered before arriving at any conclusions is whether the evidence from these trials is better than many observational studies that demonstrate that surgery (in combination with adjuvant therapy) offers the best outcome for most stages of operable oesophageal cancer. The major problem with such observational studies is the selection bias since the participants who receive chemoradiotherapy in such studies are unsuitable for surgery either in terms of their anaesthetic risk or in terms of the location or extent of cancer. This is because of the strong prejudice of surgeons in favouring surgery over other options. This prejudice was evident in a randomised controlled trial (RCT) where surgeons expressed preference to the surgery arm while recruiting participants in a RCT that compared definitive chemoradiotherapy with surgery (Blazeby 2014). This prejudice is also reflected in the current EMSO guidelines, which recommend definitive chemoradiotherapy only in those who are unfit for surgery (Stahl 2013). No statistical adjustment can account for the differences in the types of people who receive definitive chemoradiotherapy and surgery in such a prejudiced scenario because of the risk of residual confounding. The only study design that can overcome this prejudiced selection of participants for definitive chemoradiotherapy versus surgery is the RCT design. We have identified all RCTs on this topic. Despite the shortcomings in the studies included in this review, these studies constitute the best level of evidence that is currently available. Overall, the evidence from this systematic review is more trustworthy than observational studies and expert opinions.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.

Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 1 Short‐term mortality.

Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 2 Long‐term mortality (binary).

Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 3 Long‐term mortality (time‐to‐event).

Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 4 Proportion with a serious adverse event within 3 months.

Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 5 Short‐term health‐related quality of life.

Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 6 Medium‐term health‐related quality of life.

Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 7 Long‐term recurrence (binary).

Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 8 Long‐term recurrence (time‐to‐event).

Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 9 Local recurrence (binary).

Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 10 Proportion with any adverse event within 3 months.

Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 11 Length of hospital stay (days).

Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 12 Dysphagia at maximal follow‐up.

Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 13 Long‐term mortality (time‐to‐event): stratified by treatment.

Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 14 Long‐term mortality (binary): definitive chemoradiotherapy versus surgery with neoadjuvant chemotherapy.

Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 15 Long‐term mortality (time‐to‐event): definitive chemoradiotherapy versus surgery with neoadjuvant chemotherapy or chemoradiotherapy.
| Non‐surgical versus surgical treatment of oesophageal cancer | |||||
| Patient or population: people with oesophageal cancer | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Surgical treatment | Non‐surgical treatment | ||||
| Short‐term mortality | 78 per 10001 | 30 per 1000 | RR 0.39 | 689 | ⊕⊝⊝⊝ |
| Long‐term mortality (binary outcome) | 691 per 10001 | 712 per 1000 | RR 1.03 | 511 | ⊕⊕⊝⊝ |
| Long‐term mortality (time‐to‐event outcome): chemoradiotherapy versus surgery | 349 per 10001 | 314 per 1000 | HR 0.88 | 602 | ⊕⊕⊝⊝ |
| Long‐term mortality (time‐to‐event outcome): radiotherapy versus surgery | 350 per 10001 | 451 per 1000 | HR 1.39 | 512 | ⊕⊝⊝⊝ |
| Long‐term mortality (binary): definitive chemoradiotherapy versus surgery with neoadjuvant chemotherapy All‐cause mortality for the duration of follow‐up | 740 per 1000 | 769 per 1000 | RR 1.04 | 431 | ⊕⊝⊝⊝ |
| Long‐term mortality (time‐to‐event): definitive chemoradiotherapy versus surgery with neoadjuvant chemotherapy or chemoradiotherapy All‐cause mortality for the duration of follow‐up | 349 per 1000 | 346 per 1000 | HR 0.99 | 431 | ⊕⊝⊝⊝ |
| Proportion with a serious adverse event within 3 months | 273 per 10001 | 166 per 1000 | RR 0.61 | 80 | ⊕⊝⊝⊝ |
| Short‐term health‐related quality of life | The mean short‐term health‐related quality of life in the control groups was | The mean short‐term health‐related quality of life in the intervention groups was | ‐ | 165 | ⊕⊝⊝⊝ |
| Medium‐term health‐related quality of life | The mean medium‐term health‐related quality of life in the control groups was | The mean medium‐term health‐related quality of life in the intervention groups was | ‐ | 62 | ⊕⊝⊝⊝ |
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1The basis for control risk is the event rate across all studies (i.e. the sum of all events in the surgical group across all studies reporting the outcome divided by the sum of all people in the surgical group in the trials reporting the outcome) for all outcomes except long‐term mortality (time‐to‐event) where a control group risk of 0.35 was used (based on similar control group risks at 2 years in a number of trials included in this analysis) and long‐term recurrence (time‐to‐event) where a control group risk of 0.4 was used (based on similar control group risk at 2 year in a trial included for this analysis). | |||||
| Non‐surgical versus surgical treatment of oesophageal cancer | ||||||
| Patient or population: people with oesophageal cancer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Surgical treatment | Non‐surgical treatment | |||||
| Long‐term recurrence (binary outcome) | 526 per 10001 | 552 per 1000 | RR 1.05 | 339 | ⊕⊝⊝⊝ | ‐ |
| Long‐term recurrence (time‐to‐event outcome) | 508 per 10001 | 494 per 1000 | HR 0.96 | 349 | ⊕⊕⊝⊝ | ‐ |
| Local recurrence (binary) | 381 per 1000 | 339 per 1000 | RR 0.89 | 449 | ⊕⊝⊝⊝ | ‐ |
| Proportion with any adverse event within 3 months | 386 per 10001 | 668 per 1000 | RR 1.73 | 80 | ⊕⊝⊝⊝ | ‐ |
| Length of hospital stay (days) | See comment | See comment | Not estimable | 342 | ⊕⊝⊝⊝ | Significant heterogeneity present (I² statistic = 93%, P = 0.0001) making meta‐analysis inappropriate. The mean hospital stay was 16 days shorter (3 days shorter to 29 days shorter) in non‐surgical treatment than surgical treatment in 1 trial (Bedenne 2007) and 14 days longer (5 days longer to 23 days longer) in non‐surgical treatment than surgical treatment in another trial (Chiu 2005). |
| Dysphagia at maximal follow‐up | 367 per 1000 | 543 per 1000 | RR 1.48 | 139 | ⊕⊝⊝⊝ | ‐ |
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The basis for control risk is the event rate across all studies (i.e. the sum of all events in the surgical group across all studies reporting the outcome divided by the sum of all people in the surgical group in the trials reporting the outcome). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term mortality Show forest plot | 5 | 689 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.11, 1.35] |
| 2 Long‐term mortality (binary) Show forest plot | 3 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.92, 1.14] |
| 3 Long‐term mortality (time‐to‐event) Show forest plot | 7 | 1114 | Hazard Ratio (Fixed, 95% CI) | 1.09 [0.97, 1.22] |
| 4 Proportion with a serious adverse event within 3 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Short‐term health‐related quality of life Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Medium‐term health‐related quality of life Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Long‐term recurrence (binary) Show forest plot | 2 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.87, 1.28] |
| 8 Long‐term recurrence (time‐to‐event) Show forest plot | 2 | 349 | Hazard Ratio (Fixed, 95% CI) | 0.96 [0.80, 1.16] |
| 9 Local recurrence (binary) Show forest plot | 3 | 449 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.70, 1.12] |
| 10 Proportion with any adverse event within 3 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Length of hospital stay (days) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12 Dysphagia at maximal follow‐up Show forest plot | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.01, 2.19] |
| 13 Long‐term mortality (time‐to‐event): stratified by treatment Show forest plot | 7 | 1114 | Hazard Ratio (Fixed, 95% CI) | 1.09 [0.97, 1.22] |
| 13.1 Chemoradiotherapy | 4 | 602 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.76, 1.03] |
| 13.2 Radiotherapy | 3 | 512 | Hazard Ratio (Fixed, 95% CI) | 1.39 [1.18, 1.64] |
| 14 Long‐term mortality (binary): definitive chemoradiotherapy versus surgery with neoadjuvant chemotherapy Show forest plot | 2 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.93, 1.16] |
| 15 Long‐term mortality (time‐to‐event): definitive chemoradiotherapy versus surgery with neoadjuvant chemotherapy or chemoradiotherapy Show forest plot | 2 | 431 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.78, 1.26] |

