استفاده از تیمهای تخصصی باز کننده عروق برای قرار دادن دستگاه و پیشگیری از شکست آن
چکیده
پیشینه
اغلب افرادی که در سرتاسر جهان در بیمارستانها پذیرش میشوند، به دستگاه دسترسی به عروق (vascular access device; VAD) نیاز دارند. سالانه صدها میلیون VAD در ایالات متحده آمریکا وارد میشود و گزارش شده سالانه بیش از یک میلیارد کاتتر داخل وریدی محیطی (peripheral intravenous catheters) در جهان استفاده میشود. گزارشهای متعددی نشان میدهد که رویکرد تیمی برای بررسی، وارد کردن، و نگهداری VADها، پیامدهای بالینی، تجربه بیمار و فرآیندهای مراقبت سلامت را بهبود میبخشد.
اهداف
مقایسه استفاده از تیم متخصص در دسترسی به عروق (vascular access specialist teams; VAST) برای قرار دادن VAD با رویکرد مدل عمومی برای شرکتکنندگان در بیمارستان یا جامعه که به VAD نیاز دارند از نظر موفقیت وارد کردن، شکست دستگاه، و هزینه‐اثربخشی آن.
روشهای جستوجو
پایگاه ثبت مرکزی کارآزماییهای کنترل شده کاکرین (CENTRAL؛ 2018، شماره 1)؛ Ovid MEDLINE (از سال 1950 تا 7 فوریه 2018)؛ Ovid Embase (از سال 1980 تا 7 فوریه 2018)؛ EBSCO CINAHL (از سال 1982 تا 7 فوریه 2018)؛ نمایه استنادی چکیده مقالات کنفرانسهای علوم ‐ علوم انسانی و اجتماعی (از سال 1990 تا 7 فوریه 2018)؛ و Google Scholar را جستوجو کردیم. پایگاه ثبت کارآزماییهای زیر را نیز جستوجو کردیم: پایگاه ثبت کارآزماییهای بالینی استرالیا و نیوزیلند (www.anzctr.org.au)؛ ClinicalTrials.gov (www.clinicaltrials.gov)؛ کارآزماییهای کنترل شده در حال انجام (Current Controlled Trials) (www.controlled-trials.com/mrct)؛ پایگاه ثبت کارآزماییهای بالینی HKU (www.hkclinicaltrials.com)؛ پایگاه ثبت کارآزماییهای بالینی هند (ctri.nic.in/Clinicaltrials/login.php)؛ دروازه کارآزماییهای بالینی انگلستان (www.controlled-trials.com/ukctr/)؛ و پلتفرم بینالمللی پایگاه ثبت کارآزماییهای بالینی سازمان جهانی بهداشت (WHO ICTRP) (www.who.int/trialsearch). تمام بانکهای اطلاعاتی را تا 7 فوریه 2018 جستوجو کردیم.
معیارهای انتخاب
ما برنامهریزی کردیم تا کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) را وارد کنیم که اثربخشی تاثیر VAST یا استفاده از تیم تخصصی را برای قرار دادن لوله در ورید بر پیامدهای بالینی ارزیابی کنیم.
گردآوری و تجزیهوتحلیل دادهها
از پروسیجرهای روششناسی استاندارد توصیه شده توسط کاکرین استفاده کرده و از نرمافزار Covalence برای مدیریت فایلها کمک گرفتیم.
نتایج اصلی
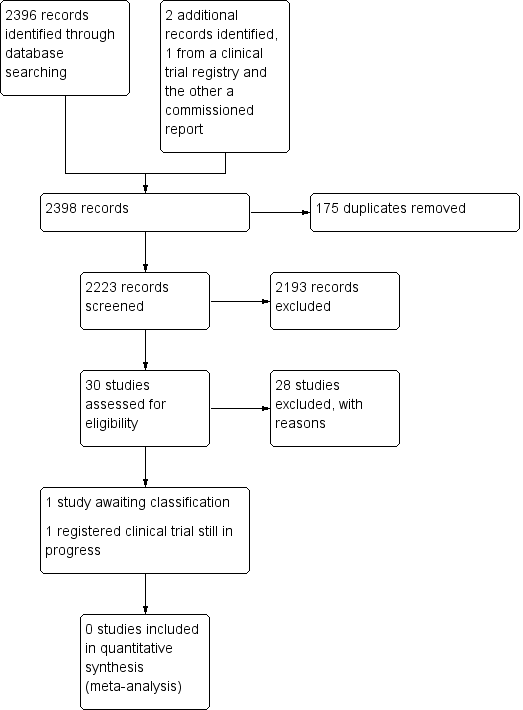
2398 استناد را بازیابی کردیم: 30 مطالعه واجد شرایط برای بررسی متن کامل خود بودند، و یک کارآزمایی بالینی در حال انجام پیدا کردیم. هیچ مطالعهای نتوانست وارد تجزیهوتحلیل یا مرور شود. یک مطالعه در انتظار طبقهبندی بود و برای انتشار پذیرفته نشده بود.
نتیجهگیریهای نویسندگان
این مرور سیستماتیک نتوانست کارآزماییهای منتشر شده مرتبط را برای حمایت یا رد ادعای تیمهای تخصصی باز کننده عروق که برتر از مدل عمومی هستند، پبدا کند. یک تیم تخصصی باز کننده عروق، نسبت به مدل جنرالیست که شامل پرستاران، پزشکان یا دیگر متخصصان مراقبت سلامت در تسهیلات مراقبت سلامت هستند و ممکن است دانش کمتری در روشهای پیشرفته جایگذاری داشته باشند، دانش پیشرفتهای را در مورد روشهای جایگذاری، مراقبت بالینی، و مدیریت دستگاه باز کننده عروق دارند. با این وجود، ممکن است وقتی که نتایج یک مطالعه در انتظار طبقهبندی و یک مطالعه در حال انجام منتشر شوند، این نتیجهگیری تغییر کند. برای ارزیابی اثربخشی روش تیم تخصصی باز کننده عروق برای قرار دادن دستگاه باز کننده عروق و مراقبت به منظور پیشگیری از شکست آن، کارآزماییهای با کیفیت خوبی مورد نیاز است.
PICO
خلاصه به زبان ساده
استفاده از تیمهای تخصصی باز کننده عروق برای قرار دادن دستگاه باز کننده عروق و پیشگیری از شکست آن
سوال مطالعه مروری
ما شواهد را با توجه به اثربخشی تیمهای تخصصی باز کننده عروق (vascular access specialist teams; VAST) در مقایسه با مدلهای عمومی از نظر موفقیت در قرار دادن دستگاه باز کننده عروق، شکست دستگاه و هزینه‐اثربخشی آن مرور کردیم.
هیچ مطالعه واجد شرایطی برای مرور خود پیدا نکردیم.
پیشینه
ما VAST را به عنوان گروهی از کارکنان مراقبت سلامت که دانش و مهارت پیشرفتهای در ارزیابی، قرار دادن، مراقبت و مدیریت دستگاه باز کننده عروق دارند، توصیف کردیم، از جمله تیمهای اینفیوژن/تزریق داخل وریدی، داروهای تزریقی داخل وریدی مثل متخصصان باز کردن عروق افراد (پرستار، دکتر، درمانگر (therapist)، تکنسین، و دستیار پزشک).
هدف ما ارزیابی این بود که رویکرد VAST برتر از رویکرد عمومی است یا خیر.
مدل یا رویکرد عمومی را به عنوان گروه بزرگتری از پرستاران، پرشکان، یا سایر متخصصان مراقبت سلامت مشخص در تسهیلات مراقبت سلامت (healthcare facility) تعریف کردیم که مهارت و دانش پیشرفته کمتری در قرار دادن و مدیریت دستگاه باز کننده عروق داشتند.
دستگاه باز کننده عروق (vascular access device; VAD) را به عنوان یک کاتتر (لوله باریک) تعریف کردیم که در وریدها یا پورتهایی (ports) که میتواند زیر پوست قرار گیرد، گذاشته شده و به مایعات و داروها اجازه میدهد وارد وریدها شوند. کاتترها داخل شریانهایی گذاشته میشوند که بتوانند برای نظارت بر درمان استفاده شوند. شایعترین VAD، کاتتر داخل وریدی محیطی (peripheral intravenous catheter; PIVC) است، که ممکن است در چندین روز پیش از برداشته شدن، در محل باقی بماند. VADها یا کاتترهای کاشته شده در وریدهای مرکزی معمولا میتواند چندین هفته، ماه، و در بعضی موارد، به ویژه با پورت، سالها باقی بماند. دستگاههای باز کننده عروق برای دادن مایعات (درمان اینفیوژن) و داروهای داخل وریدی (تزریق به داخل یک ورید، نمونهبرداری از خون، و پایش ویژه استفاده میشوند، و اغلب برای ارائه مراقبت و درمان مهم هستند. VADها و درمان اینفیوژن تقریبا در تمام تخصصهای مراقبتهای ویژه، جراحی، و پزشکی استفاده میشود که در بیمارستان، مراقبت طولانیمدت و مراقبت در منزل اتفاق میافتند.
خطرات بسیاری در ارتباط با قرار دادن VAD و ادامه درمان با آن وجود دارد که میتواند منجر به شکست دستگاه شود (که دیگر برای ادامه مراقبت مناسب نباشد). یکی از عوارض مهم بعد از گذاشتن VAD، شامل ترومبوز وریدی (venous thrombosis) مربوط به کاتتر (تشکیل لخته خونی) است. افراد مبتلا به سرطان یا کسانی که بیماری وخیم دارند و ممکن است به مداخلات پزشکی اضافی برای درمان ترومبوز نیاز پیدا کنند، به طور ویژه در معرض خطر این عارضه هستند. یکی از خطرات مربوط به اینفیوژن، التهاب ورید است (فلبیت یا ترومبوفلبیت) (phlebitis or thrombophlebitis) که به علت PIVC به وجود میآید، وقتی است که ورید لوله گذاری شده (cannulated) دردناک میشود و علائم بالقوه دیگری مثل قرمز شدن محل تزریق ظاهر میشود. خطرات تزریق مثل عفونتهای خونی مرتبط با کاتتر، به تمام VADها مربوط میشود؛ پیشگیری از وقوع چنین وقایعی، اولویت مراقبت سلامت است. عفونتهای خونی مرتبط با کاتتر با اقامت طولانیمدت در بیمارستان، بیماری جدی، مرگومیر و افزایش هزینههای خدمات سلامت همراه هستند.
ویژگیهای مطالعه
در طیف گستردهای از بانکهای اطلاعاتی پزشکی تا 7 فوریه 2018 جستوجو کردیم. 2398 مطالعه محتمل پیدا کردیم، که جزئیات 30 مطالعه بررسی شد. یک مطالعه مناسب پیدا کردیم، در حالی که هنوز پیشنویس این مطالعه کامل نشده و برای چاپ پذیرفته نشده بود، بنابراین نتوانستیم دادههای آن را تجزیهوتحلیل کنیم. ما این مطالعه را به عنوان یک مطالعه در انتظار طبقهبندی قرار دادیم، تا وقتی که نتایج آن منتشر شد، مجددا آن را بررسی کرده و در مورد واجد شرایط بودن آن برای ورود به این مرور، تصمیم بگیریم. یک کارآزمایی ثبت شده کشف کردیم که در حال بررسی سوال مرور ما است، اما هنوز در حال انجام بوده و کامل یا منتشر نشده است.
نتایج کلیدی
ما موفق نشدیم هیچ کارآزمایی تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) منتشر شدهای را برای حمایت یا رد این ادعا پیدا کنیم که تیمهای تخصصی باز کردن عروق نسبت به مدل عمومی برای قرار دادن دستگاه باز کننده عروق و پیشگیری از شکست آن، برتری دارند. با این وجود، این نتیجهگیری ممکن است بعد از انتشار یک مطالعه در انتظار طبقهبندی و یک مطالعه در حال انجام، تغییر کند. برای ارزیابی اثربخشی رویکرد VAST برای قرار دادن VAD و پیشگیری از شکست آن، نیاز به کارآزماییهای با کیفیت خوب است. یک RCT، مطالعهای (یا کارآزمایی) است که با هدف کاهش سوگیری، یک درمان جدید را تست میکند. افرادی که در کارآزمایی شرکت میکنند، به صورت تصادفی هم در گروهی که درمان تحت بررسی را دریافت میکنند یا در گروهی که به عنوان گروه کنترل، درمان استاندارد (یا درمان دارونما (placebo)) را دریافت میکنند، قرار داده میشوند.
کیفیت شواهد
کیفیت شواهد را تجزیهوتحلیل نکردیم چون هیچ مطالعه مناسبی را برای وارد کردن به مرور خود، پیدا نکردیم.
Authors' conclusions
Background
Most people admitted to hospital will require a vascular access device (VAD). Hundreds of millions of VADs are inserted annually in the USA alone (O'Grady 2011), with billions inserted in patients worldwide (Rickard 2015). Improving the patient journey with an appropriately placed VAD has been argued as a priority for healthcare delivery services (Moureau 2012). A variety of professionals currently provide VAD insertion and ongoing management of the device, but there is some evidence to suggest that this can contribute to fragmentation of the patient's overall care (Castro‐Sánchez 2014). The question underlying this review was whether a specialist rather than a generalist approach is likely to produce better VAD insertion and care and, therefore, a reduction in complications that contribute to VAD failure.
Description of the condition
The term 'vascular access device' represents a variety of catheters commonly used to access the circulatory system for healthcare treatments. Most VADs used for intravenous therapy are classified by two distinct insertion routes: via peripheral or central veins; however, they can also include arterial, intraosseous, and umbilical routes (Green 1998; Kelly 2009; Reades 2011; Scheer 2002).
Function of a vascular access device
Vascular access devices permit infusion therapy via the circulatory system, blood sampling analysis, and invasive monitoring, and are often crucial in providing treatment and care (Gorski 2016). The use of VADs and infusion therapy extends across almost all medical, surgical, and critical care specialties, and occurs in hospital, long‐term care, and home care settings.
Insertion of vascular access devices
Vascular access device insertion is a patient safety issue (Castro‐Sánchez 2014; Moureau 2013). Several attempts may be made to insert a VAD successfully, with each attempt involving a puncture of the skin by a needle in an attempt to cannulate the desired vessel. Identified rates of first‐time peripheral insertion failure are 12% to 26% in adults and 24% to 54% in children (Sabri 2013). A variety of strategies to improve insertion outcomes for peripheral routes exist, such as assessment tools and clinical prediction rules (Carr 2017), as failure can lead to bruising and pain at the insertion sites (Webster 2008), and multiple punctures of the skin predispose micro‐organism entry into the bloodstream (Mermel 2017). Centrally inserted VADs are associated with more critical procedural complications (e.g. pneumothorax or arterial puncture) and contribute to patient morbidity and mortality, although specialized technology such as ultrasound can be used to reduce the risk of such complications (Wu 2013).
Complications associated with vascular access devices
There are several risks related to VAD insertion and ongoing care. These risks can be either operator or patient related. Postinsertion complications including catheter‐related venous thrombosis (clot formation) can necessitate further medical intervention (Ge 2012). Particularly at risk are people with cancer or who are critically ill (Chopra 2013). A risk of infusion‐related phlebitis (or thrombophlebitis) with peripheral intravenous catheters (PIVCs) exists when the cannulated vein becomes painful with other potential signs such as erythema (red appearance) at the insertion site (Tagalakis 2002). Infection risks such as catheter‐related bloodstream infections are a significant hospital burden and are associated with all VADs, but particularly with central venous catheters (CVCs); preventing such occurrences is another healthcare priority (O'Grady 2011). Catheter‐related bloodstream infection increases hospital stay, morbidity, mortality, and health service costs (Ge 2012).
Vascular access specialist team
For the purposes of this review, the term vascular access specialist team (VAST) represents any grouping of personnel specifically associated with VAD insertion and care, and is synonymous with titles such as infusion teams, intravenous teams, or intravenous therapy teams, as well as individual vascular access specialists (nurse, doctor, respiratory therapist, technician, and physician assistant) who have advanced knowledge and skills and who frequently insert or manage VADs, or both. Positive reports of a VAST approach include the use of nurse‐led teams and advanced nurse practitioners who have inserted CVCs in critical care environments (Alexandrou 2014; Gopal 2006; Yacopetti 2010). The use of a team approach for inserting PIVCs has increased first‐time insertion success (Carr 2010), and historically is associated with decreased device‐related complications (Tomford 1984). The alternative to VAST is the generalist model, where larger groups of nurses, doctors, or other designated healthcare professionals in the healthcare facility who have less advanced skills, insert and care for VADs.
How the intervention might work
The argument for VAD insertion by a VAST is that best‐practice care is supported by a consistent, knowledgeable, and skilled approach. Higher levels of inserter knowledge and confidence, built upon experience and procedural competence, suggest the VAST approach has positive insertion outcomes for patients (Alexandrou 2014; Harnage 2012; Jackson 2012). While some VAST models focus on VAD insertion only, others include follow‐up care, which can include clinical tasks such as dressing replacement and daily assessment for potential removal. Even with a limited scope of 'insertion only', VASTs have reported better outcomes for first‐time insertion success (Carr 2010). Reducing the number of failed needle insertions is an important infection prevention strategy (da Silva 2010), and one that can reduce patient stress and length of hospital stay (Barton 1998).
Vascular access specialist team impact on device‐related complications
Peripheral intravenous catheters inserted using a VAST approach have been associated with less phlebitis, erythema, induration, and infiltration (Soifer 1998). This may be related to an increased first‐time insertion success with a VAST, since multiple insertion attempts have been associated with complications and failure (Wallis 2014). Central venous catheters inserted using the VAST approach have low iatrogenic complications with reports of as little as 1% of insertions developing pneumothorax, arterial puncture, and subsequent catheter‐related infection (Alexandrou 2012). Reduced catheter‐related bloodstream infection rates and VAD bacteraemia rates have been attributed to the adoption of a VAST approach (Brunelle 2003; Legemaat 2015)
Why it is important to do this review
Vascular access device insertion success and reduction of subsequent failure are important objectives that can positively impact on patient experience and clinical outcome. It is important to understand if the VAST approach does improve insertion success and reduce VAD failure, iatrogenic procedural complications, and device‐related infection. However, there is no clear evidence to date of the effectiveness of VAST compared with the generalist approach to VAD insertion and no prior systematic review on this topic. Although VASTs themselves do incur costs to pay staff, considering the adverse outcomes that may be avoided, VAST may be the more cost‐effective model. Establishing whether clinical outcomes of VAST are superior to generalist VAD insertion and management is of initial importance for clinicians, consumer groups, policymakers, and healthcare systems.
Objectives
To compare the use of the vascular access specialist team for vascular access device insertion and care to a generalist model approach for hospital or community participants requiring a vascular access device in terms of insertion success, device failure, and cost‐effectiveness.
Methods
Criteria for considering studies for this review
Types of studies
We intended to include all randomized controlled trials (RCTs) that evaluated the effectiveness of VASTs or specialist inserters for their impact on clinical outcomes. We excluded cluster RCTs, where the cluster represented randomization at the ward or hospital level.
We intended to include controlled clinical trials if we did not find any RCTs. Controlled clinical trials refer to quasi‐randomized studies where, although the trial involves testing an intervention with a control, with concurrent enrolment and follow‐up of test and control‐treated groups, the method of allocation is not considered truly random.
Types of participants
We included hospitalized or community participants requiring vascular access. Age was not an excluding factor.
Types of interventions
Intravenous/vascular access teams or specialist inserters (as in VAST) providing insertion or maintenance (or both) of VADs.
Types of outcome measures
Primary outcomes
-
First‐time insertion success (generally insertion success is reported as a percentage): insertion of a PIVC where venous return occurs, a saline flush passes, and the PIVC is secured and ready for use (Riker 2011), or as defined by the study authors; in CVCs, the number of reported insertion attempts (generally insertion success is reported as a percentage) and where the tip of the CVC is confirmed in the lower third of the superior vena cava (Moureau 2013), or as defined by the study authors.
-
Insertion‐related adverse events/complications:
-
pneumothorax: inadvertent injury to the pleura of the lung during cannulation of a large vein (defined by each study) (Ayas 2007);
-
arterial puncture: inadvertent puncture of an artery during cannulation of a large vein (defined by each study) (Guilbert 2008);
-
nerve damage: inadvertent damage to the nerve that is adjacent to the vein/artery (defined by each study) (Guilbert 2008);
-
other: as defined by the study author(s).
-
-
Cost as defined by the study authors (in the currency of the country where the publication originated).
Secondary outcomes
-
Premature device failure rates as a result of the following:
-
phlebitis/thrombophlebitis: pain, induration, and erythema with a palpable thrombosis of the cannulated vein (Tagalakis 2002), or as defined by the study authors;
-
infiltration/extravasation: infiltration is the unintentional leaking of non‐vesicant medication or solution into surrounding tissues, and extravasation is the unintentional leaking of vesicant medication or solution into surrounding tissues (Dougherty 2008);
-
occlusion: inability to infuse fluid into the VAD or to aspirate blood (Camp‐Sorrell 2007);
-
thrombosis: central venous thrombosis characterized by neck and arm swelling with associated pain (Ge 2012);
-
catheter‐related or catheter‐associated bloodstream infection: laboratory‐confirmed bloodstream infection attributed to the catheter (Chopra 2013; Maki 2006);
-
dislodgement or accidental removal: as defined by the study authors.
-
-
Patient satisfaction: as defined by the study authors.
-
Staff satisfaction: as defined by the study authors.
-
Dwell time of VAD: in hours from insertion to removal.
-
Length of hospital stay: in days from hospital admission to discharge.
Search methods for identification of studies
We adapted an Ovid MEDLINE search strategy to search CENTRAL, Ovid Embase, and EBSCO CINAHL and ISI Web of Science. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE and Embase: sensitivity and precision‐maximizing version (Lefebvre 2011). We placed no date, language, or publication restrictions. We performed our search on 7 February 2018.
Electronic searches
We identified RCTs through literature searching with systematic and sensitive search strategies as outlined in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We applied no restrictions to language or publication status.
We searched the following databases for relevant trials.
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 1) (Appendix 1)
-
MEDLINE (Ovid SP, 1966 to 7 February 2018) (Appendix 2)
-
Embase (Ovid SP, 1988 to 7 February 2018) (Appendix 3)
-
CINAHL (Cumulative Index to Nursing and Allied Health Literature) (EBSCO, 1982 to 7 February 2018) (Appendix 4)
-
ISI Web of Science (1990 to 7 February 2018) (Appendix 5)
We developed a subject‐specific search strategy in MEDLINE and used that as the basis for the search strategies in the other databases listed. Where appropriate, we expanded the search strategy with search terms for identifying RCTs.
We scanned the following trials registries for ongoing and unpublished trials (7 February 2018).
-
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/trialsearch)
-
ClinicalTrials.gov (www.clinicaltrials.gov)
-
Australian and New Zealand Clinical Trials Register (www.anzctr.org.au)
-
Current Controlled Trials (www.controlled‐trials.com/mrct)
-
HKU Clinical Trials Registry (www.hkclinicaltrials.com)
-
Clinical Trials Registry ‐ India (ctri.nic.in/Clinicaltrials/login.php)
-
UK Clinical Trials Gateway (www.controlled‐trials.com/ukctr/)
We developed the search strategy in consultation with the Information Specialist.
Searching other resources
We scanned the reference lists and citations of included trials and any relevant systematic reviews identified for further references to additional trials. We contacted trial authors for additional information when necessary.
Data collection and analysis
Selection of studies
We merged all the electronically retrieved studies from individual databases into Covidence. Following this we removed any duplicate references found employing the filter using icons imports; manage imports then check duplicates. Two review authors (PC, NH) independently assessed titles and abstracts of retrieved studies for relevance. We retrieved full versions of all potentially eligible studies, which the same two review authors independently checked for eligibility. Any discrepancies between review authors were resolved either through mutual discussion, or on two occasions by consulting other review authors (CR, MC) to arbitrate.
Data extraction and management
Two review authors (PC, NH) intended to extract data from each study using our data extraction sheet (Appendix 6). The data extraction sheet was developed in conjunction with the Cochrane Anaesthesia, Critical and Emergency Care Group, and we had planned to pilot test the first two identified studies. Both PC and NH intended to independently extract data and then perform cross‐checking for accuracy and agreement. We intended to include only studies reported in one publication. If we located studies that had been published in duplicate, we intended to maximally extract data from all relevant publications but not to duplicate data in analyses. If we believed any data were missing from the papers, we planned to contact study authors to retrieve this missing information. See Appendix 6 for more information regarding the data that we intended to extract. As we did not locate any published studies, we could not perform data extraction.
Assessment of risk of bias in included studies
Two review authors (PC, NH), intended to independently assess the risk of bias for each of the studies using the 'Risk of bias' assessment tool described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The tool addresses six specific domains: sequence generation, allocation and concealment, blinding, incomplete outcome data, selective outcome reporting, and other potential sources of bias (Higgins 2011). We planned to express judgements as 'low risk', 'high risk', or 'unclear risk' of bias. We intended to resolve any disagreements by discussion; if we could not reach consensus, a third review author (CR) would arbitrate. We intended to conduct sensitivity analyses to determine whether excluding studies at high risk of bias affected the results of a planned meta‐analysis. We intended to report the 'Risk of bias' table as part of the 'Characteristics of included studies' table and present a 'Risk of bias' summary figure detailing all of the judgements made for all studies included in the review. As we did not locate any published studies, we were unable to assess the risk of bias.
Measures of treatment effect
We intended to calculate dichotomous outcomes using risk ratio (RR) with 95% confidence intervals (CI). We intended to calculate continuous outcomes using the mean difference (MD) with 95% CI. If the results were expressed as rate data (e.g. incidence rates), we planned to calculate a rate ratio. We intended to extract data from time‐to‐event (i.e. dwell time) studies, if the estimates were presented as log‐rank or Cox proportional models. We would not analyse time‐to‐event data that were incorrectly presented as continuous data. As we did not locate any published studies, we could not measure treatment effect.
Unit of analysis issues
Ideally, a study would be designed with participant‐level randomization and analysis, and only one device per participant (adjustment for clustering not necessary in this case). However, we expected to find studies that reported on multiple devices per participant, randomized or analysed at device level, or both, and unadjusted for clustering. We expected to find the following differences.
-
Number of devices and observed per participant: one or more than one (e.g. after the removal of the initial device the consecutive device(s) was (were) also observed).
-
Randomization methods: device level, participant level, or ward (or similar) level.
-
Unit of analysis: device level, participant level, or ward (or similar) level.
-
Analysis methods: adjusted for clustering or not adjusted for clustering.
In such cases, we intended to attempt to obtain the following from the study authors: participant‐level data or results; data or results for one device per participant; or device‐level data and perform multilevel regression to calculate the adjusted effect. We intended to combine the adjusted results in the meta‐analysis with those of participant‐level trials (using the generic inverse method) and perform sensitivity analyses (Higgins 2011). If we were unsuccessful in obtaining the additional necessary data, then we planned to exclude the study from the meta‐analysis. We excluded cluster RCTs where the cluster represents randomization at the ward or hospital level. We did not locate any published studies to assess unit of analysis.
Dealing with missing data
Whenever possible, we intended to contact the original investigators to request missing data and methodological details. If we considered that data were missing at random, we intended to analyse the available information. If we considered that data were not missing at random, we intended to analyse the available information and assess the potential impact of the missing data on the findings of the review in the Discussion section. However, we did not locate any published studies.
Assessment of heterogeneity
We intended to consider clinical, methodological, and statistical heterogeneity. We planned to undertake an assessment of comparability of the studies prior to meta‐analysis. We planned to assess heterogeneity of selected studies visually and by using the Chi2 test with significance level set at P value less than 0.10. This assesses whether observed differences in results are compatible with chance alone. In addition, we planned to investigate the degree of heterogeneity by calculating the I2 statistic (an equation combining the Chi2 statistic relative to its degree of freedom). This describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance). If studies were sufficiently similar to consider pooling, we planned to use a fixed‐effect model for low‐to‐moderate levels of heterogeneity (I2 = 0% to 50%). Where appropriate, in the absence of clinical heterogeneity and in the presence of statistical heterogeneity (I2 greater than 50%), we planned to use a random‐effects model. However, studies where heterogeneity exceeded 75% were not going to be pooled (Higgins 2011). As we did not locate any published studies, we did not need to assess heterogeneity.
Assessment of reporting biases
We intended to use visual asymmetry on funnel plots to assess reporting biases if at least 10 studies were available for a meta‐analysis (Sterne 2011). We intended to report each outcome separately. We planned to undertake an observation of small‐study effects if required.
We did not locate any published studies to assess reporting biases.
Data synthesis
We intended to enter into Review Manager 5 all trials included in the systematic review and insert and analyse quantitative data (RevMan 2014). The decision to pool data in a meta‐analysis depended upon the availability of outcome data and assessment of between‐trial heterogeneity. If we identified evidence of substantial heterogeneity (i.e. greater than 50%), we planned to explore potential causes and use a random‐effects model, and a fixed‐effect model to explore any differences between these two estimates. As no studies were included in the review, and synthesis was inappropriate, we have presented a structured narrative review in the Results of the search and Discussion sections.
Subgroup analysis and investigation of heterogeneity
If sufficient data were available, we planned to perform the following subgroup analyses.
-
Adult VAST versus paediatric VAST.
-
Device type: peripheral versus central.
-
Central device type: peripherally inserted central catheter versus CVC versus tunnelled VAD versus totally implanted.
-
VAST model: insertion only versus insertion and follow‐up care services.
-
VAST team versus individual specialists.
As we did not locate any studies, we did not undertake any subgroup analysis and investigation of heterogeneity.
Sensitivity analysis
We planned to initially perform a sensitivity analysis by excluding studies at high risk of bias. We intended to only include studies that were assessed as having a low risk of bias for the estimates of treatment effect in all key domains, namely adequate generation of the randomization sequence, adequate allocation concealment, and blinding of outcome assessor. We also planned to perform sensitivity analysis on:
-
size of study (fewer than 100 participants);
-
missing data (worst‐case/best‐case scenario).
'Summary of findings' table and GRADE
We intended to present the main results of the review using the principles of the GRADE system to assess the quality of the body of evidence associated with specific outcomes in our review (Guyatt 2008), and construct a 'Summary of findings' table using GRADEpro GDT software (Appendix 7) (GRADEpro GDT).
We planned to present the following specific primary outcomes of interest in the 'Summary of findings' tables.
-
First‐time insertion success.
-
Insertion‐related adverse events/complications.
-
Cost.
-
Device failure with dwell time.
-
Patient satisfaction.
-
Staff satisfaction.
-
Length of hospital stay.
We intended to present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). We intended that the 'Summary of findings' tables would include an overall grading of the body of evidence related to each of the main outcomes using the GRADE approach (Schünemann 2011b). The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates, and risk of publication bias (Schünemann 2011b).
Results
Description of studies
See Figure 1.
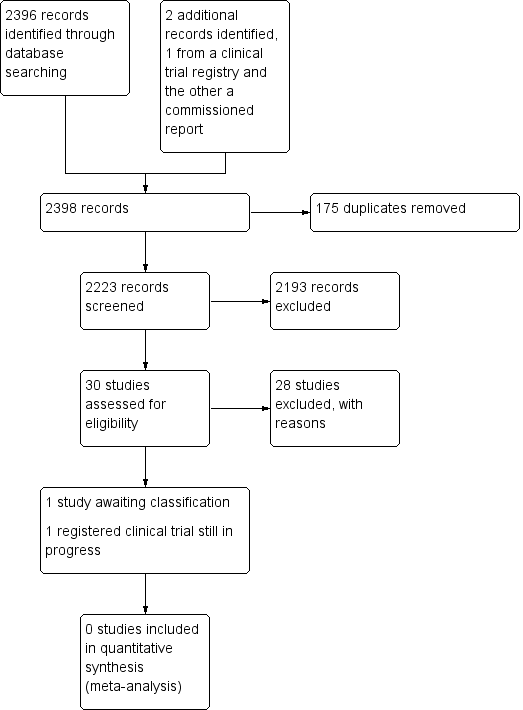
Study search flowchart.
Results of the search
We aimed to identify RCTs pertaining to VAST where the unit of randomization was the participant. We updated our search on the 7 February 2018 as per our protocol search strategy (Carr 2014).
We identified 30 studies that were potentially relevant to the interventional review question posed. We were unable to locate two papers (Tomford 1982; Ward 2000). We retrieved full‐text versions of 26 studies (Boland 2003; Casey 2003; Fong 2001; Gilbert 2016; Hammes 2015; Hockley 2007; Huraib 1994; Keohane 1983; King 2010; Larson 1984; Meier 1998; Mokrzycki 2006; Moretti 2005; Møller 2005; Nehme 1980; Neuman 1998; Puri 1982; Robinson 2005; Secola 2011; Secola 2012; Sherertz 1997; Soifer 1998; Tadokoro 2015; Taylor 2011; Tomford 1984; Treacy 2002).
We obtained one unpublished manuscript (Garate‐Echenique 2014), and we found one ongoing clinical trial (ACTRN12616001675415). Two review authors (PC, NH) independently checked the 26 full‐text papers for eligibility using the Covidence tool (Covidence), and excluded all of them. For transparency, we have provided a summary of studies assessed for full‐text review in our PRISMA flowchart as recommended (see Figure 1) (Liberati 2009).
Included studies
We included no studies in this review.
Excluded studies
We excluded 28 studies (Boland 2003; Casey 2003; Fong 2001; Gilbert 2016; Hammes 2015; Hockley 2007; Huraib 1994; Keohane 1983; King 2010; Larson 1984; Meier 1998; Mokrzycki 2006; Moretti 2005; Møller 2005; Nehme 1980; Neuman 1998; Puri 1982; Robinson 2005; Secola 2011; Secola 2012; Sherertz 1997; Soifer 1998; Tadokoro 2015; Taylor 2011; Tomford 1982; Tomford 1984; Treacy 2002; Ward 2000).
We have documented our reasons for excluding studies in the Characteristics of excluded studies table.
Studies awaiting classification
One unpublished study is awaiting classification, as the manuscript has not yet been accepted for publication, and further data analysis is pending (Garate‐Echenique 2014). See Characteristics of studies awaiting classification.
Ongoing studies
We identified one ongoing clinical trial that has not finished recruitment (ACTRN12616001675415). See Characteristics of ongoing studies.
Risk of bias in included studies
As no studies fulfilled the inclusion criteria for the review, we were unable to assess risk of bias.
Effects of interventions
As we identified no studies to include in the meta‐analysis, we were unable to generate an analysis.
Discussion
Summary of main results
The effectiveness of the VAST approach for VAD insertion and prevention of failure has not yet been evaluated in randomized controlled trials where the unit of randomization represents the participant.
Overall completeness and applicability of evidence
At present, there are no published RCTs evaluating the efficacy of VAST versus the generalist approach. We assumed given the proliferation and ubiquity of both VADs in health care and the various VAST models in use that interventional studies would exist. It may be the case that controlled clinical trials, prospective studies, and quality initiatives are sufficient to convince some healthcare institutions to invest in the VAST concept. Given that VADs are used ubiquitously in health care, that peripheral insertion failure ranges from 12% to 54% (Sabri 2013), and that postinsertion failure rates are reported as up to 50% (Helm 2015), it is unclear why health service researchers have not investigated this topic more stringently. Two early studies (Soifer 1998; Tomford 1984), a cluster RCT and a controlled clinical trial, respectively focused on PIVCs with the primary outcome of device infections, yet many advances since that time limit their current applicability. Both studies took place when routine time‐based removal and insertion of replacement PIVCs was commonly practiced, yet a Cochrane Review now supports clinical indication for removal (Webster 2015). Additionally, vessel‐locating technology to support successful insertions is increasingly reported in the literature, and include ultrasound, transilluminators, and near infrared technology. However, systematic reviews and meta‐analysis have not overwhelmingly proved a clinical benefit with respect to the number of attempts required for success (Egan 2013; Heinrichs 2013; Parker 2016; Stolz 2015). High first‐time insertion has been associated with a VAST approach (Carr 2010; Sabri 2013). Other technological advances such as impregnated catheters and securement technologies are in existence, and may assist with reducing postinsertion VAD complications such as infections (Gilbert 2016). However, a Cochrane Review found no strong evidence to support a particular dressing or securement device technology for the prevention of PIVC dislodgement (Marsh 2015). Other VADs, such as the peripherally inserted central catheter and acute central venous catheter, lack interventional evidence evaluating the VAST approach, despite the increasing prevalence of a specialist team approach with their use (Chopra 2017).
There is an absence of published RCTs evaluating the impact of VAST using current practices and technologies. This systematic review identified one RCT presented at a World Congress in Vascular Access in 2014, which has yet to be published (Garate‐Echenique 2014). Waste in clinical research is of concern (Glasziou 2014), but more importantly if evidence supportive of a VAST approach is left unpublished, it limits application to benefit health services wanting to consider implementing a VAST. An updated review will likely be improved if the work of Garate‐Echenique 2014 (which evaluates a VAST approach with peripherally inserted central catheters) is published, and by expected publications from the pilot RCT registered by Marsh and colleagues (ACTRN12616001675415).
Quality of the evidence
We could not analyse the quality of the evidence as one study is awaiting classification (Garate‐Echenique 2014), and an ongoing trial is not yet completed (ACTRN12616001675415).
Potential biases in the review process
Publication bias may have occurred where only negative studies on this topic have not been published. This seems unlikely given that we found only one registered clinical trial, and it is not yet published. Additionally, and with respect to our correspondence with Garate‐Echenique 2014, non‐publication of their revised manuscript may be owing to resource limitations to complete reviewers' suggestions. Finally, we may have missed a VAST trial represented by a different synonym. However, this is unlikely given that two research librarians assisted with the search strategy. One potential bias is that our inclusion criteria limited the review to RCTs where the unit of randomization was the participant. However, given the lack of eligible studies found with this approach, an opportunity exists for a greater number of similar RCTs on this topic. We attempted to conduct a comprehensive search for studies, but the fact that no studies have as yet been incorporated may be a source of potential bias.
Agreements and disagreements with other studies or reviews
We found reports of the efficacy of a VAST approach using a cluster randomized trial design (Tomford 1984), or quasi‐experimental studies (Soifer 1998). It is unclear if this and other evidence are enough to support change, but it is worthwhile noting that not all VAST approaches have the desired impact in reducing catheter‐related bloodstream infections, even if clinical guidelines are rigorously followed (Secola 2012). Despite a health technology assessment programme in the UK commissioning an RCT investigation examining the clinical and cost‐effectiveness of tunnelled central venous access device insertion with or without vessel‐locating technology in adult cancer patients (Boland 2003), little progress has been made in assessing the effectiveness of VAST. Furthermore, the most recent survey of 'vascular access specialist' reported that clinical practice is not always consistent with contemporary evidence‐based recommendations (Chopra 2017). Additionally, the recent recommendation for defining VASTs by the largest vascular access society (Davis 2016), is perhaps more proof that there exists disconnectedness with this clinical aspect of health care (Castro‐Sánchez 2014). It is conceivable that RCTs would go some way to substantiate or refute the evidence for VAST.
Study search flowchart.

