Profilaxis con levosimendán para la prevención del síndrome de gasto cardíaco bajo y la mortalidad en pacientes pediátricos sometidos a cirugía por una cardiopatía congénita
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial. | |
| Participants | 50 (25 intervention, 25 control), aged 7 to 38 months, with atrial or ventricular septal defects with high systolic pulmonary artery pressure (PAP) exceeding 50% of systemic systolic pressure, assigned for surgical correction of the defect by cardiopulmonary bypass (CPB). | |
| Interventions | Intervention: levosimendan infusion started immediately after declamping of the aorta; an initial loading dose of 15 µg/kg given over a ten‐minute period, followed by infusion at 0.1 to 0.2 µg/kg/min. Control: dobutamine infusion started immediately after declamping of the aorta, at 4 to 10 µg/kg/min. | |
| Outcomes | Recorded until 20 hours after ICU admission: 1. mean PAP recorded preoperatively by transthoracic echocardiography, intraoperatively by pulmonary artery catheter, postoperatively by transoesophageal echocardiography; assessment until 20 hours after ICU admission (lower score = improvement) 2. mean cardiac index recorded by transoesophageal 4‐MHz Doppler probe (Cardio Q, Deltex Medical), assessment until 20 hours after ICU admission (higher score = improvement) | |
| Notes | Exact rate of infusion for intervention and control drug was titrated according to haemodynamic data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomized using sealed envelopes and allocated to two equal groups". No further details of random sequence generation stated. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was performed using sealed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding. |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes listed in the methods section reported. |
| Selective reporting (reporting bias) | Low risk | No incomplete reporting suspected. |
| Other bias | Low risk | Note: Measurement of cardiac index by transoesophageal Doppler is not considered to be gold standard. Especially in the setting of an unblinded study, there is a certain risk of detection bias. |
| Methods | Randomised controlled trial. | |
| Participants | 40 (20 intervention, 20 control), aged under one year, undergoing corrective open‐heart surgery for their congenital heart defects, children with tetralogy of Fallot excluded | |
| Interventions | Intervention: levosimendan continuous infusion of 0.1 µg/kg/min, started at the time of weaning from CPB, for the first 24 hours postoperative. Control: milrinone continuous infusion of 0.5 µg/kg/min, started at the time of weaning from CPB, for the first 24 hours postoperative. | |
| Outcomes | Recorded until 48 hours after initiation of the study drug: 1. cardiac index calculated from cardiac output and the patient's body surface area, cardiac output measurement using transoesophageal Doppler technique (Cardio Q, Deltex Medical) (higher score = improvement). 2. heart rate, systemic arterial pressure, left atrial pressure, mixed venous saturation, lactate concentrations, inotrope score (dopamine + dobutamine + epinephrine x 100 + norepinephrine x 100 (dosages in µg/kg/min)), cerebral near infrared spectroscopy, occurrence of LCOS (defined as tachycardia, acidosis, oliguria, a widened arterial‐mixed venous oxygen saturation difference, need for mechanical circulatory support). | |
| Notes | 1 patient (intervention group) had to be excluded from the intention‐to‐treat analysis (did not receive the intervention because of immediate reoperation), 2 additional patients (intervention group) had to be excluded from the per‐protocol analysis (because of serious violation of the study protocol) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Computer‐generated random numbers. |
| Blinding of participants and personnel (performance bias) | Low risk | Study drugs prepared and labelled blinded by the local pharmacy, both drugs administered at the same infusion rates, opaque black syringes and catheters used. |
| Blinding of outcome assessment (detection bias) | Low risk | Study drugs labelled blinded, both drugs administered at same infusion rates, opaque black syringes and catheters used. |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis available for N = 39, per‐protocol analysis available for N = 37. |
| Selective reporting (reporting bias) | Low risk | No incomplete reporting suspected. |
| Other bias | Low risk | Note: measurement of CI by transoesophageal Doppler is not considered to be gold standard. Furthermore it was not possible to obtain reliable standard deviations for inotropic score, as the information extracted from a figure (Figure 4 in this source) deviated from information provided by personal communication with the study author. |
| Methods | Randomised controlled trial. | |
| Participants | 41 (20 intervention, 21 control), aged under five years, undergoing corrective surgery for congenital heart defects using CPB and in need of inotropic support. | |
| Interventions | Intervention: 0.05 µg/kg/min of levosimendan, started at onset of CPB, allowed to be doubled, for a maximum of 48 hours. Control: 0.4 µg/kg/min of milrinone, started at onset of CPB, allowed to be doubled, for a maximum of 48 hours. | |
| Outcomes | Recorded until 48 hours after admission to paediatric ICU: 1. Lactate level at four hours postoperatively. 2. Biologic markers and haemodynamic data (heart rate, mean arterial pressure, lactate, difference between arterial and mixed venous oxygen saturations, troponin, creatinine, alanine aminotransferase, aspartate aminotransferase). | |
| Notes | One patient (control group) excluded who did not receive study medication because of modification of the surgical plan, two patients (1 intervention group, 1 control group) excluded because they required extracorporeal membrane oxygenation at the end of CPB, two patients (1 intervention group, 1 control group) excluded because of intraoperative death. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random code. |
| Allocation concealment (selection bias) | Low risk | Allocation code was concealed in an envelope opened by the study nurse in charge of preparing the medication. |
| Blinding of participants and personnel (performance bias) | Low risk | Syringes and tubing system covered with aluminium foil, both drugs administered at same infusion rates. |
| Blinding of outcome assessment (detection bias) | Low risk | Syringes and tubing system covered with aluminium foil, both drugs administered at same infusion rates. |
| Incomplete outcome data (attrition bias) | High risk | Two patients (1 intervention group, 1 control group) excluded because they required extracorporeal membrane oxygenation at the end of CPB, two patients (1 intervention group, 1 control group) excluded because of intraoperative death. |
| Selective reporting (reporting bias) | Low risk | No incomplete reporting suspected. |
| Other bias | Low risk | No other bias suspected. |
| Methods | Randomised controlled trial. | |
| Participants | 20 (11 intervention, 9 control) neonates undergoing cardiovascular surgery with CPB. | |
| Interventions | Intervention: continuous intravenous infusion of levosimendan started intraoperatively and increased stepwise: dose 1 (0.1 µg/kg/min) starting immediately after central line placed and maintained for duration of surgical procedure, dose 2 (0.15 µg/kg/min) upon admission to neonatal ICU, dose 3 (0.2 µg/kg/min) starting after two hours of stability with dose 2; infusion stopped after 48 hours. Control: continuous intravenous infusion of milrinone started intraoperatively and increased stepwise: dose 1 (0.5 µg/kg/min) starting immediately after central line placed and maintained for duration of surgical procedure, dose 2 (0.75 µg/kg/min) upon admission to neonatal ICU, dose 3 (1 µg/kg/min) starting after two hours of stability with dose 2, until 48 hours after infusion started, afterwards infusion rate tapered according to attending physician. | |
| Outcomes | Recorded until day 6 postsurgery: 1. Heart rate, breathing rate, central and peripheral temperature, arterial blood pressure, SaO2, cerebral and peripheral perfusion‐oxygenation (using NIRS instrument NIRO‐3000). 2. Blood gases, acid‐base status, CO‐oximetry, lactate, glucose, haemoglobin‐concentration, creatinine, N‐terminal probrain natriuretic peptide, troponine I, pro‐inflammatory and anti‐inflammatory factors. | |
| Notes | Beyond 48 hours open study. Financial support of Orion Pharma Spanish Division for the pharmacokinetic studies (not part of outcome measures). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list. Randomisation stratified by type of congenital heart defects and risk adjustment (using the congenital heart surgery method) |
| Allocation concealment (selection bias) | Low risk | Study nurse not involved in the clinical care of the infants was custodian of the allocation code. |
| Blinding of participants and personnel (performance bias) | Low risk | Study medications prepared in identical opaque syringes, infusion tubes covered by aluminium foil, same infusion rates. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear risk due to unblinding of study after 48 hours, potential risk of detection bias after unblinding (study time point T3 and T4). |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes listed in the methods section reported. |
| Selective reporting (reporting bias) | Low risk | No incomplete reporting suspected. |
| Other bias | Low risk | Financial support of Orion Pharma Spanish Division for the pharmacokinetic studies (not part of outcome measures). Not deemed an important source of bias. |
| Methods | Randomised controlled trial. | |
| Participants | 63 (32 intervention, 31 control) neonates undergoing risk‐adjusted classification for congenital heart surgery (RACHS) 3 and 4 procedures | |
| Interventions | Intervention: continuous infusion of 0.1 µg/kg/min levosimendan for 72 hours added to standard inotropic support, started while weaning from CPB. Control: standard post‐CPB inotrope infusion (milrinone 0.75 µg/kg/min and dopamine 5 to 10 µg/kg/min, adrenaline 0.05 to 0.3 µg/kg/min if necessary). | |
| Outcomes | Recorded until 72 hours postoperatively: 1. Incidence of LCOS (defined as tachycardia (heart rate > 170 beats/min), oliguria (urine output < 0.5 mL/kg/h), cold extremities (peripheral temperature < 27°C), with or without at least 30% difference in arterial to mixed venous oxygen saturation or metabolic acidosis (an increase in base deficit of greater than 4 or an increase in lactate of more than 2 mg/dL) on two successive blood gas measurements, cardiac arrest, need for extracorporeal membrane oxygenation 2. Lactate, heart rate, mean arterial pressure, inotropic score, diuresis, need for peritoneal dialysis, mixed venous oxygen saturation, brain natriuretic peptide (BNP), number of ventilation days, paediatric cardiac ICU length of stay and survival, side effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random‐generation program. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes containing the allocation group opened by a nurse in charge of preparing the infusions. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding. |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes listed in the methods section reported. |
| Selective reporting (reporting bias) | Low risk | No incomplete reporting suspected. |
| Other bias | Low risk | No other bias suspected. |
ICU = intensive care unit
LCOS = low cardiac output syndrome
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Uncontrolled case series study, not RCT. Patients with LCOS of any origin, not exclusively postoperative LCOS. | |
| Preoperative administration of levosimendan for a cohort of selected neonates with hypoplastic left heart syndrome, not RCT. | |
| Retrospective observational study, not RCT. | |
| Case‐control retrospective study, not RCT. | |
| Retrospective study, not RCT. | |
| Single group phase II study, not RCT. Patients in preoperative setting without LCOS. |
LCOS = low cardiac output syndrome
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Double‐blind randomised clinical trial to evaluate the efficacy and safety of levosimendan as pre‐ischaemic myocardial conditioner in paediatric cardiac surgery |
| Methods | RCT |
| Participants | 36 patients of both genders, aged one month to 14 years, who are to undergo cardiac surgery, with high risk factors of postoperative acute heart failure |
| Interventions | Intervention: levosimendan; control: placebo. |
| Outcomes | HR, MAP, central venous pressure, thermal gradient, capillary refill time, diuresis, inotropic score, inotropic medication, atrial natriuretic peptide, troponine‐I, myoglobine, lactate, central venous oxygen saturation, oxygen transport, changes in the neurohormonal profile, adverse events, all‐cause mortality, analysis of economic impact of levosimendan, days in ICU, days of mechanical ventilation, survival at 30 days |
| Starting date | 05 June 2013 |
| Contact information | Hospital Universitario Virgen de las Nieves, Granada, Spain |
| Notes | Anticipated date of completion: unknown |
HR = heart rate
MAP = mean arterial pressure
ICU = intensive care unit
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 3 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.12, 1.82] |
| Analysis 1.1  Comparison 1 Mortality, Outcome 1 Mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 LCOS Show forest plot | 2 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.39, 1.04] |
| Analysis 2.1 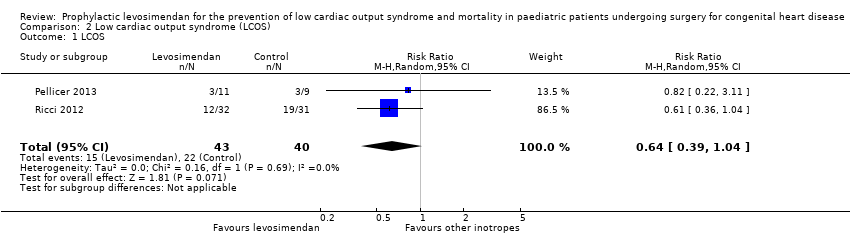 Comparison 2 Low cardiac output syndrome (LCOS), Outcome 1 LCOS. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Length of ICU stay (days) Show forest plot | 4 | 188 | Mean Difference (IV, Random, 95% CI) | 0.33 [‐1.16, 1.82] |
| Analysis 3.1  Comparison 3 Length of ICU stay, Outcome 1 Length of ICU stay (days). | ||||
| 2 Length of ICU stay (days) Show forest plot | 4 | 188 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.54, 0.44] |
| Analysis 3.2  Comparison 3 Length of ICU stay, Outcome 2 Length of ICU stay (days). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Length of hospital stay (days) Show forest plot | 2 | 75 | Mean Difference (IV, Random, 95% CI) | 0.26 [‐3.50, 4.03] |
| Analysis 4.1 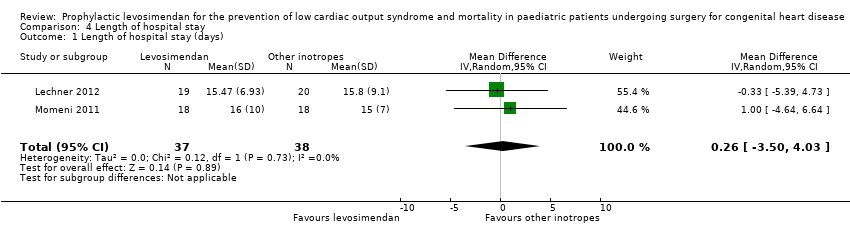 Comparison 4 Length of hospital stay, Outcome 1 Length of hospital stay (days). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of mechanical ventilation (days) Show forest plot | 5 | 208 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.08, 0.00] |
| Analysis 5.1 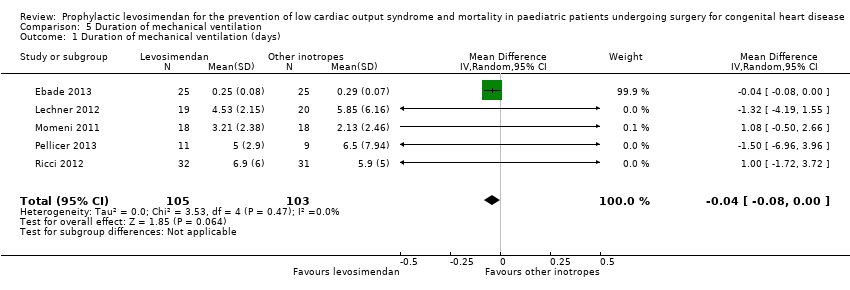 Comparison 5 Duration of mechanical ventilation, Outcome 1 Duration of mechanical ventilation (days). | ||||
| 2 Duration of mechanical ventilation (days) Show forest plot | 5 | 208 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.43, 0.27] |
| Analysis 5.2 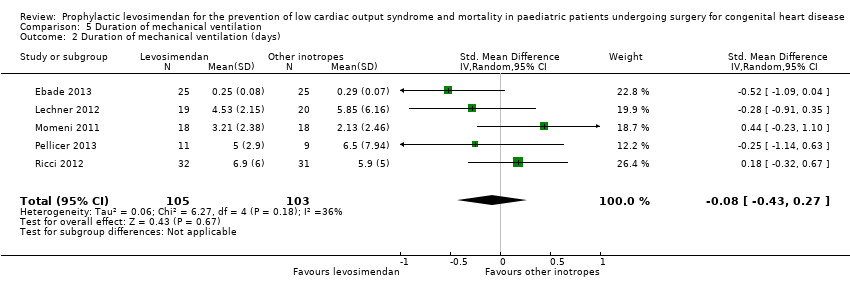 Comparison 5 Duration of mechanical ventilation, Outcome 2 Duration of mechanical ventilation (days). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mechanical circulatory support or cardiac transplantation Show forest plot | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.19, 11.37] |
| Analysis 6.1 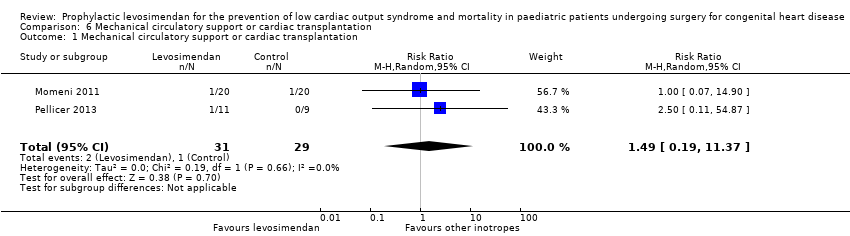 Comparison 6 Mechanical circulatory support or cardiac transplantation, Outcome 1 Mechanical circulatory support or cardiac transplantation. | ||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
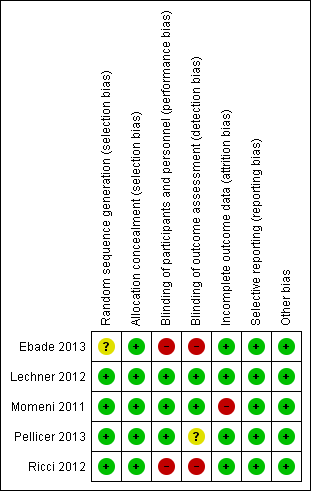
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Mortality, outcome: 1.1 Mortality.

Forest plot of comparison: 2 Low cardiac output syndrome (LCOS), outcome: 2.1 LCOS.

Comparison 1 Mortality, Outcome 1 Mortality.

Comparison 2 Low cardiac output syndrome (LCOS), Outcome 1 LCOS.

Comparison 3 Length of ICU stay, Outcome 1 Length of ICU stay (days).
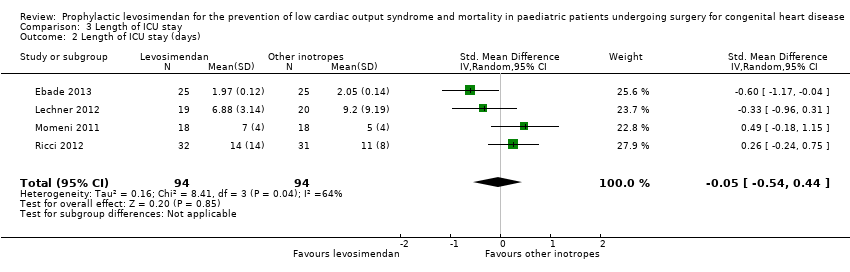
Comparison 3 Length of ICU stay, Outcome 2 Length of ICU stay (days).

Comparison 4 Length of hospital stay, Outcome 1 Length of hospital stay (days).
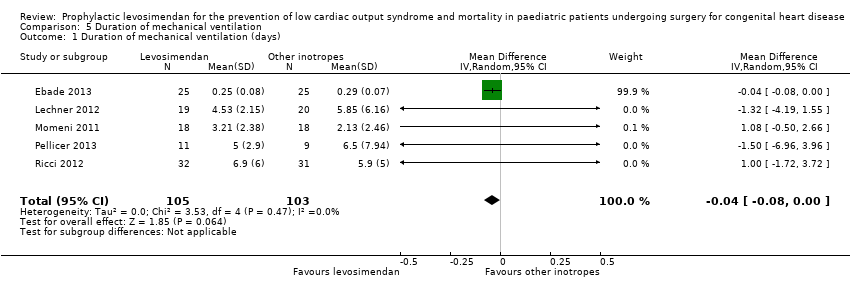
Comparison 5 Duration of mechanical ventilation, Outcome 1 Duration of mechanical ventilation (days).

Comparison 5 Duration of mechanical ventilation, Outcome 2 Duration of mechanical ventilation (days).
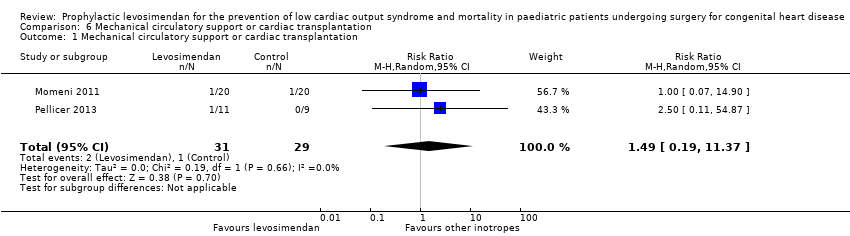
Comparison 6 Mechanical circulatory support or cardiac transplantation, Outcome 1 Mechanical circulatory support or cardiac transplantation.
| Prophylactic levosimendan compared to standard treatment in children following heart surgery | ||||||
| Patient or population: paediatric patients following heart surgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with standard inotropes | Risk with levosimendan | |||||
| Mortality | Study population | RR 0.47 | 123 | ⊕⊕⊝⊝ | ||
| 57 per 1000 | 27 per 1000 | |||||
| Low cardiac output syndrome (LCOS) | Study population | RR 0.64 | 83 | ⊕⊕⊝⊝ | ||
| 367 per 1000 | 235 per 1000 | |||||
| Length of intensive care unit stay (Length of ICU stay) | The mean length of stay in the ICU in the control groups ranged from 2.05 to 11 days | The mean length of stay in ICU in the intervention groups was 0.33 days higher (1.16 lower to 1.82 higher) | ‐ | 188 | ⊕⊕⊝⊝ | |
| Length of hospital stay | The mean length of hospital stay in the control groups ranged from 15 to 15.8 days | The mean length of hospital stay in the intervention groups was 0.26 days higher | ‐ | 75 | ⊕⊕⊝⊝ | |
| Duration of mechanical ventilation | The mean duration of mechanical ventilation in the control groups ranged from 0.29 to 6.5 days | The mean duration of mechanical ventilation in the intervention groups was 0.04 days lower | ‐ | 208 | ⊕⊕⊝⊝ | |
| Mechanical circulatory support or cardiac transplantation | Study population | RR 1.49 | 60 | ⊕⊕⊝⊝ | ||
| 10 per 1000 | 14 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded for imprecision due to small sample size. 2 Downgraded for imprecision due to few number of events (less than 300). 3 Detection of outcome potentially dependent on study personnel: risk of detection bias in two studies due to unblinded setting. 4 Considerable inconsistency between study results. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 3 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.12, 1.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 LCOS Show forest plot | 2 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.39, 1.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Length of ICU stay (days) Show forest plot | 4 | 188 | Mean Difference (IV, Random, 95% CI) | 0.33 [‐1.16, 1.82] |
| 2 Length of ICU stay (days) Show forest plot | 4 | 188 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.54, 0.44] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Length of hospital stay (days) Show forest plot | 2 | 75 | Mean Difference (IV, Random, 95% CI) | 0.26 [‐3.50, 4.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of mechanical ventilation (days) Show forest plot | 5 | 208 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.08, 0.00] |
| 2 Duration of mechanical ventilation (days) Show forest plot | 5 | 208 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.43, 0.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mechanical circulatory support or cardiac transplantation Show forest plot | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.19, 11.37] |

