Profilaxis con levosimendán para la prevención del síndrome de gasto cardíaco bajo y la mortalidad en pacientes pediátricos sometidos a cirugía por una cardiopatía congénita
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011312.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 02 agosto 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Corazón
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
JH, BS and GR: conceived and designed the review.
JH and BS: collected, screened, extracted, and interpreted data.
JH: co‐ordinated the review, wrote authors for additional data, wrote the protocol and the review.
BS: revised the review critically for important intellectual content.
GR: provided methodological input, analysed data, revised the review critically for important intellectual content.
All authors read and approved the final version of the review.
Sources of support
Internal sources
-
Department of Congenital Heart Defects and Pediatric Cardiology, Heart Center, University of Freiburg, Freiburg, Germany.
Consists of providing free access to computers, data bases, full‐text literature, collaboration with experts of different faculties and partial release from clinical work to the authors. No additional financial grants.
External sources
-
No sources of support supplied
Declarations of interest
JH: None known.
GR: received payment for a one‐day course on statistical methods in meta‐analysis by Grünenthal Group, Aachen, Germany.
BS: None known.
Acknowledgements
We thank Nicole Martin for her valuable help with the design of the search strategy and the electronic literature searches across databases, as well as her helpful editorial comments. We would also like to thank the authors of the included studies who provided additional data about their trials.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Aug 02 | Prophylactic levosimendan for the prevention of low cardiac output syndrome and mortality in paediatric patients undergoing surgery for congenital heart disease | Review | Johanna Hummel, Gerta Rücker, Brigitte Stiller | |
| 2017 Mar 06 | Prophylactic levosimendan for the prevention of low cardiac output syndrome and mortality in paediatric patients undergoing surgery for congenital heart disease | Review | Johanna Hummel, Gerta Rücker, Brigitte Stiller | |
| 2014 Sep 25 | Prophylactic levosimendan for the prevention of low cardiac output syndrome and mortality in children undergoing surgery for congenital heart disease | Protocol | Johanna Hummel, Gerta Rücker, Brigitte Stiller | |
Differences between protocol and review
'Children' was replaced by 'paediatric patients' in the review title, since neonates were a relevant part of the included study population.
Definitions of LCOS in the included studies varied and did not necessarily meet the definition stated in the protocol.
The data extraction form that was used was not piloted as mentioned in the protocol, as the number of included studies was very small. We returned to the reports that had already undergone data extraction whenever data were missing from the data extraction form.
Subgroup analyses, as mentioned in the protocol (age‐based and cardiovascular‐physiology‐based), were not feasible due to the small number of events.
In addition to the planned methods stated in the protocol, we provided a ‘Summary of findings’ table in the review to present the main findings in a transparent and simple tabular format. In addition, we conducted a GRADE assessment of the quality of evidence which we also presented in the ‘Summary of findings’ table.
Notes
26 July 2017: The abstract was edited and this amendment is being published as a new citation.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Assisted Circulation [statistics & numerical data];
- Cardiac Output, Low [etiology, mortality, *prevention & control];
- Cardiotonic Agents [*therapeutic use];
- Heart Defects, Congenital [mortality, *surgery];
- Hydrazones [*therapeutic use];
- Intensive Care Units, Pediatric [statistics & numerical data];
- Length of Stay [statistics & numerical data];
- Postoperative Complications [mortality, *prevention & control];
- Pyridazines [*therapeutic use];
- Randomized Controlled Trials as Topic;
- Respiration, Artificial [statistics & numerical data];
- Simendan;
- Syndrome;
Medical Subject Headings Check Words
Child, Preschool; Humans; Infant;
PICO

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
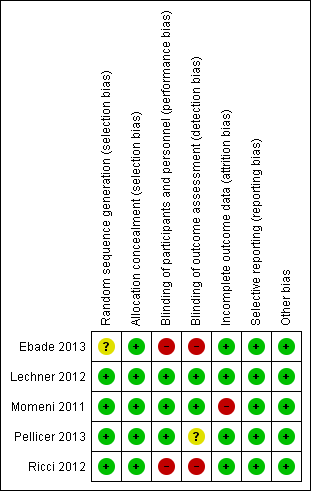
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Mortality, outcome: 1.1 Mortality.

Forest plot of comparison: 2 Low cardiac output syndrome (LCOS), outcome: 2.1 LCOS.

Comparison 1 Mortality, Outcome 1 Mortality.

Comparison 2 Low cardiac output syndrome (LCOS), Outcome 1 LCOS.

Comparison 3 Length of ICU stay, Outcome 1 Length of ICU stay (days).
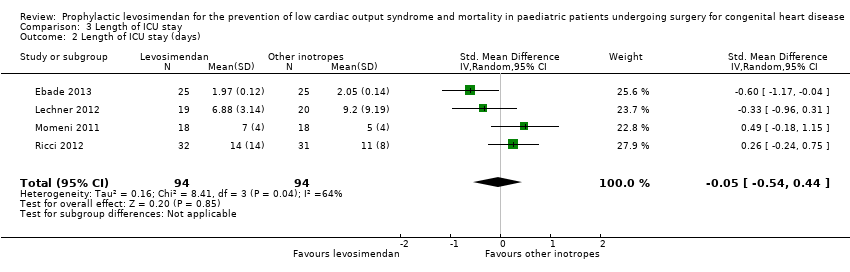
Comparison 3 Length of ICU stay, Outcome 2 Length of ICU stay (days).

Comparison 4 Length of hospital stay, Outcome 1 Length of hospital stay (days).
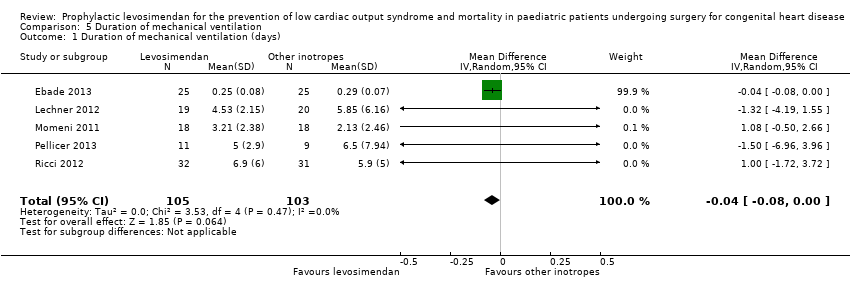
Comparison 5 Duration of mechanical ventilation, Outcome 1 Duration of mechanical ventilation (days).

Comparison 5 Duration of mechanical ventilation, Outcome 2 Duration of mechanical ventilation (days).
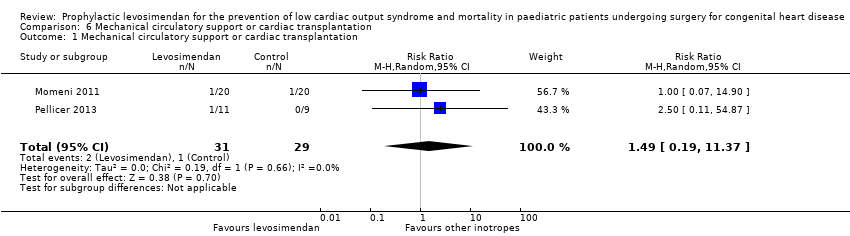
Comparison 6 Mechanical circulatory support or cardiac transplantation, Outcome 1 Mechanical circulatory support or cardiac transplantation.
| Prophylactic levosimendan compared to standard treatment in children following heart surgery | ||||||
| Patient or population: paediatric patients following heart surgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with standard inotropes | Risk with levosimendan | |||||
| Mortality | Study population | RR 0.47 | 123 | ⊕⊕⊝⊝ | ||
| 57 per 1000 | 27 per 1000 | |||||
| Low cardiac output syndrome (LCOS) | Study population | RR 0.64 | 83 | ⊕⊕⊝⊝ | ||
| 367 per 1000 | 235 per 1000 | |||||
| Length of intensive care unit stay (Length of ICU stay) | The mean length of stay in the ICU in the control groups ranged from 2.05 to 11 days | The mean length of stay in ICU in the intervention groups was 0.33 days higher (1.16 lower to 1.82 higher) | ‐ | 188 | ⊕⊕⊝⊝ | |
| Length of hospital stay | The mean length of hospital stay in the control groups ranged from 15 to 15.8 days | The mean length of hospital stay in the intervention groups was 0.26 days higher | ‐ | 75 | ⊕⊕⊝⊝ | |
| Duration of mechanical ventilation | The mean duration of mechanical ventilation in the control groups ranged from 0.29 to 6.5 days | The mean duration of mechanical ventilation in the intervention groups was 0.04 days lower | ‐ | 208 | ⊕⊕⊝⊝ | |
| Mechanical circulatory support or cardiac transplantation | Study population | RR 1.49 | 60 | ⊕⊕⊝⊝ | ||
| 10 per 1000 | 14 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded for imprecision due to small sample size. 2 Downgraded for imprecision due to few number of events (less than 300). 3 Detection of outcome potentially dependent on study personnel: risk of detection bias in two studies due to unblinded setting. 4 Considerable inconsistency between study results. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 3 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.12, 1.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 LCOS Show forest plot | 2 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.39, 1.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Length of ICU stay (days) Show forest plot | 4 | 188 | Mean Difference (IV, Random, 95% CI) | 0.33 [‐1.16, 1.82] |
| 2 Length of ICU stay (days) Show forest plot | 4 | 188 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.54, 0.44] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Length of hospital stay (days) Show forest plot | 2 | 75 | Mean Difference (IV, Random, 95% CI) | 0.26 [‐3.50, 4.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of mechanical ventilation (days) Show forest plot | 5 | 208 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.08, 0.00] |
| 2 Duration of mechanical ventilation (days) Show forest plot | 5 | 208 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.43, 0.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mechanical circulatory support or cardiac transplantation Show forest plot | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.19, 11.37] |

