Intervenciones para los trastornos de los movimientos oculares debidos a una lesión cerebral adquirida
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Pharmacological interventions for acquired pendular and jerk nystagmus Allocation: double‐masked Masking: double‐masked Exclusions: 0 Losses: 1 Design: cross‐over RCT | |
| Participants | Country: 4 sites in USA and Germany Number of participants randomised: 21 (15 with pendular nystagmus and 6 with jerk nystagmus) Age: 25‐73 years Gender: 10 female, 11 male Aetiologies: multiple sclerosis (9), degeneration (1), cerebellar atrophy (1), stroke (5), idiopathic (2), encephalitis (1), tonsillar herniation (1), AIDS (1) Ocular motility condition: acquired pendular nystagmus and horizontal jerk nystagmus Inclusion criteria: adult nystagmus Exclusion criteria: not specified | |
| Interventions | Intervention 1: gabapentin Dose: 300 mg up to 900 mg/day Intervention 2: baclofen Dose: 10 mg up to 30 mg/day Duration: 2 weeks of intervention, 1‐2 weeks for wash‐out period, 2 weeks of intervention | |
| Outcomes | Measurements: Landolt C, eye movement recordings, perceived motion of target, drug effects by participant recall Timepoints: Baseline, 2 weeks, 4 weeks and 6 weeks Adverse events: Drug intolerance, Increased ataxia | |
| Notes | Health economic costs: not reported Quality of life measures: not reported Funding: USPHS grant E706717, Office of Research and development, Medical research Service, Department of Veteran Affairs and Evenon Arlington Fund and Deutsche Forschungsgemeinschaft Declaration of interests: Parke‐Davis Co provided transport and participant insurance fees in Germany Dates of study: not specified Trial registration ID: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomly assigned to [gabapentin or baclofen]; these drugs were administered in opaque capsules that were identical in appearance, and both the primary investigators and the patients were blinded as to their identify". Judgment comment: primary investigators were masked which suggests that the allocation was concealed. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Patients were randomly assigned to [gabapentin or baclofen]; these drugs were administered in opaque capsules that were identical in appearance, and both the primary investigators and the patients were blinded as to their identify". |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Patients were randomly assigned to [gabapentin or baclofen]; these drugs were administered in opaque capsules that were identical in appearance, and both the primary investigators and the patients were blinded as to their identify". |
| Incomplete outcome data (attrition bias) | Low risk | 21 people were recruited and 20 completed both 2‐week test periods. The one person who dropped out did so because of an unrelated condition. |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol or trials registry entry. |
| Other bias | High risk | Additional 'Risk of bias' assessment for cross‐over study Was the cross‐over design suitable: probably Was there a carry‐over effect: uncertain, no analysis done. Was only first period data available: no, first period data were not available Was the analysis correct: unclear, no estimates of effect reported Comparability of results with those from parallel‐group trials: no parallel group trials. |
| Methods | Pharmacological interventions for acquired downbeat nystagmus Allocation: double‐masked Masking: double‐masked Exclusions: 0 Losses: 0 Design: cross‐over RCT | |
| Participants | Country: Germany Number of participants randomised: 8 Age: mean 68 years ± 5.93, 58‐76 years Gender: 6 females, 2 males Aetiologies: degeneration (2), Arnold‐Chiari malformation (1), cryptogenic cerebellar ataxia (4), inflammation (1) Ocular motility condition: downbeat nystagmus Inclusion criteria: adult nystagmus Exclusion criteria: not specified | |
| Interventions | Intervention 1: 4‐aminopyridine Dose: 10 mg Intervention 2: 3,4‐diaminopyridine Dose: 10 mg Duration: 1 day for intervention with 6‐day wash‐out period between interventions | |
| Outcomes | Measurements: 3D video‐oculography, drug effects by participant recall Adverse events: Mild paraesthesia | |
| Notes | Health economic costs: not reported Quality of life measures: not reported Funding: German Ministry of Education and Research Declaration of interests: authors declare no conflicts of interest Dates of study: not specified Trial registration ID: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned capsules of 10 mg of 3,4‐DAP or 4‐AP; they received 1 single capsule of either substance. There was a washout period of 6 days when no medication was given. One week later, the treatment was switched (i.e., they received a single capsule of the other substance)." Judgement comment: it was not reported how the allocation sequence was generated. |
| Allocation concealment (selection bias) | Low risk | Judgement comment: study was described as "double‐blind" and identical single 10 mg doses used so we judge it was likely that the allocation was concealed. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "...identical single 10‐mg doses of both aminopyridines were compared in our double‐blind study with crossover design" Judgement comment: although this information was only provided in the discussion section of the article we judge that masking of participants was likely to have been done with identical tablets. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "...identical single 10‐mg doses of both aminopyridines were compared in our double‐blind study with crossover design" Judgement comment: although this information was only provided in the discussion section of the article we judge that masking of outcome assessors was likely to have been done with identical tablets and description of the study as double‐masked. |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: the article describes a study of 8 patients. Loss to follow‐up was not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol or trials registry entry. |
| Other bias | High risk | Additional 'Risk of bias' assessment for cross‐over study Was the cross‐over design suitable: probably Was there a carry‐over effect: uncertain, no analysis done. Was only first period data are available: no, first period data not available Was the analysis correct: unclear, no estimates of effect reported Comparability of results with those from parallel‐group trials: no parallel group trials |
| Methods | Botulinum toxin versus observation of acute onset sixth nerve palsy Allocation: random number table | |
| Participants | Country: UK Aetiologies: Controls: multiple sclerosis (2) microvascular (16), sarcoidosis (1), ectatic basilar artery (1), unknown (5) BT: multiple sclerosis (1), microvascular (18), unknown (3) Exclusion criteria: change in diagnosis | |
| Interventions | Treatment: | |
| Outcomes | Measurements: Ocular motility range — abduction deficit. Binary response of yes/no. Angle of deviation by prism cover test. Field of binocular single vision. Participant‐reported symptoms. Adverse reactions. | |
| Notes | Health economic costs: not reported Quality of life measures: not reported Funding: None specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "...patients were randomly assigned to "treatment" or "control" groups by reference to a random number table." |
| Allocation concealment (selection bias) | High risk | Judgement comment: as the treatments were quite different ‐ botulinum toxin versus observation ‐ it is likely that the Investigators were aware of participant allocation. |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: as the treatments were quite different ‐ botulinum toxin versus observation ‐ it is likely that the participants and their carers were aware of participant allocation. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: as the treatments were quite different ‐ botulinum toxin versus observation ‐ it is likely that the outcome assessors were aware of participant allocation. |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote "Of the initial 54 patients, five (four controls, one injection) were lost to follow‐up. A further two patients (both controls) were later excluded because of a change in diagnosis." Judgement comment: follow‐up was clearly described and most participants were followed‐up (47/54, 87%). However, loss to follow‐up appeared to occur predominantly in the control group. It is unclear what impact that would have had. |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol or trials registry entry. |
| Other bias | Unclear risk | Not applicable |
| Methods | Pharmacological interventions for acquired downbeat nystagmus Allocation: double‐masked Masking: double‐masked Exclusions: 1 (chronic alcohol use) Losses: 0 Design: cross‐over RCT | |
| Participants | Country: Germany Dates of recruitment: March 2002 to September 2002 Number of participants randomised: 18 Age: 50‐85 years Gender: 9 female, 9 male Aetiologies: Arnold‐Chiari malformation (1), degeneration (4), cerebellar ataxia (1), stroke (3), unknown (8) Ocular motility condition: acquired downbeat nystagmus Inclusion criteria: pure downbeat nystagmus, downbeat nystagmus with associated central vestibular or ocular motility disorders Exclusion criteria: epileptic seizures, cardiac arrhythmia, taking drugs affecting the central nervous system or vestibular system | |
| Interventions | Intervention: 3,4‐diaminopyridine Dose: 20 mg Control: lactose placebo Duration: 1 day of intervention, 1‐2 weeks for wash‐out period, 1 day of control | |
| Outcomes | Measurements: 2‐dimensional video‐oculography, perceived motion of target, drug effects by participant recall Timepoints: Adverse events: Transient minor perioral or digital paraesthesia, nausea | |
| Notes | Health economic costs: not reported Quality of life measures: not reported Funding: not specified Declaration of interests: not specified Trial registration ID: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "With use of a computer‐generated randomization list" |
| Allocation concealment (selection bias) | Low risk | Quote: "Code envelopes were kept by the investigator during the trial and returned unopened to the monitor after termination of the study. The blind was maintained until data analysis had been completed." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Capsules with 20 mg of 3,4‐DAP and lactose or placebo (a capsule with lactose alone) were manufactured and delivered by the pharmacy of the University of Munich (Klinikum Grosshadern). The shape and color of the capsules with 3,4‐DAP or placebo were identical." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Capsules with 20 mg of 3,4‐DAP and lactose or placebo (a capsule with lactose alone) were manufactured and delivered by the pharmacy of the University of Munich (Klinikum Grosshadern). The shape and color of the capsules with 3,4‐DAP or placebo were identical." Quote: "Code envelopes were kept by the investigator during the trial and returned unopened to the monitor after termination of the study. The blind was maintained until data analysis had been completed." |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Seventeen patients (nine men; aged 50 to 85 years) with DBN were included in the study; one patient had to be excluded because of chronic alcohol consumption even on the day of the planned examination" Judgement comment: this excluded participant appeared to be excluded before randomisation. All 17 participants completed the study. |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol or trials registry entry. |
| Other bias | High risk | Additional 'Risk of bias' assessment for cross‐over study Was the cross‐over design suitable: probably Was there a carry‐over effect: uncertain, no analysis done. Was only first period data are available: no, first period data not available Was the analysis correct: unclear, no estimates of effect reported Comparability of results with those from parallel‐group trials: no parallel group trials |
| Methods | Oculomotor rehabilitation versus sham training for traumatic brain injury Allocation: single masked Masking: single masked Exclusions: 0 Losses: 0 Design: cross‐over RCT | |
| Participants | Country: USA Number of participants randomised: 12 Age: 29 ± 3 years Gender: not specified Aetiologies: type of acquired brain injury not specified Ocular motility condition: any acquired disorder Inclusion criteria: TBI onset at least one year post‐incident to ensure that any subsequent changes during training are not secondary to their natural neurological recovery function period (6‐9 months). Participants exhibit at least one symptom (e.g. skipping lines while reading, blur, diplopia, etc.) and one clinical sign (e.g. receded near point of convergence) of a non‐strabismic oculomotor dysfunction related to impaired sustained reading. Intact cognitive ability to perform the required tasks for the study. Stable systemic health. Exclusion criteria: persons over the age of 40 years, as they typically will not have sufficient accommodation to measure reliably. Best corrected visual acuity poorer than 20/30 in either eye. Constant strabismus, amblyopia, or ocular disease in either eye. Medications that alter oculomotor function or attentional state (or both) | |
| Interventions | Intervention: ocular motor rehabilitation — training of versions, vergence and accommodation for 15 minutes each interspaced with 5 minute rest intervals. Dose: 2 sessions of 60 minutes training per week, block of 6 weeks Control: sham treatment of basic reading tasks Dose: 2 sessions of 60 minutes training per week, block of 6 weeks Duration: 2 blocks of 6 weeks with one‐week interim wash‐out | |
| Outcomes | Measurements: Reading rate, infra‐red eye recording of reading eye movements, saccade ratio — progression and regression saccades by eye movement recording, binocular accommodative amplitude, near point of convergence, convergence insufficiency symptom survey questionnaire Timepoints: Baseline, 6 weeks and post final block | |
| Notes | Health economic costs: not reported Funding: US Army, DoD award, College of Optometrists in Vision Development and SUNY Graduate programme Dates of study: not reported Declaration of interests: no interests to declare Trial registration ID: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "During phase 1, every odd‐numbered subject first received OMT, and every even‐numbered subject first received ST, and vice‐versa during phase 2." |
| Allocation concealment (selection bias) | High risk | Single‐masked |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "A cross‐over, interventional experimental design of a single‐blinded nature (for the subject) was used." Judgement comment: this implies the participants were masked to the intervention, but the intervention and control are so different it is likely that the participants may be influenced by knowledge of the intervention. It is unclear what the impact of this would have been and may be considered to be part of the intervention so we have graded this as unclear risk of bias. |
| Blinding of outcome assessment (detection bias) | High risk | Not masked |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol or trials registry entry. |
| Other bias | High risk | Additional risk of bias assessment for cross‐over study Was the cross‐over design suitable: probably not Was there a carry‐over effect: uncertain, no analysis done. Was only first period data are available: no, first period data not available Was the analysis correct: unclear, no estimates of effect reported, data for intervention group only reported Comparability of results with those from parallel‐group trials: no parallel group trials |
A & E: Accident and Emergency
AIDS: acquired immune deficiency syndrome
BT: botulinum toxin
PD: prism dioptre
RCT: randomised controlled trial
SEM: standard error mean
TBI: traumatic brain injury
USPHS: United States Public Health Service
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Six of seven cases were either multiple sclerosis or degenerative; only one case of potential acquired brain injury | |
| Not a randomised controlled trial | |
| 25 of 27 cases were either idiopathic or degenerative; only two cases of potential acquired brain injury | |
| Cases were normal participants with induced vestibular imbalance; no acquired brain injury | |
| Cases were normal participants with induced imbalance; no acquired brain injury | |
| No cases of acquired brain injury; all participants were idiopathic or degenerative | |
| Not a randomised controlled trial | |
| Seven of ten cases were either multiple sclerosis or degenerative; only three cases of acquired brain injury | |
| Not a randomised controlled trial | |
| No cases of acquired brain injury; all with essential tremor | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| No cases of acquired brain injury; all with progressive supranuclear palsy |

Study flow diagram
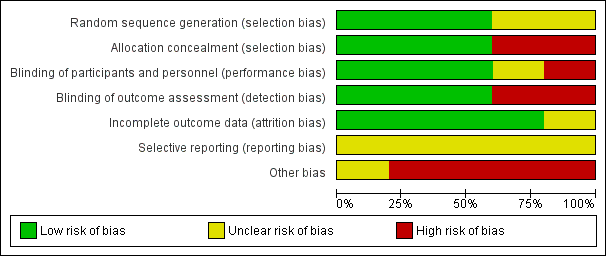
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
| Botulinum toxin versus observation in people with sixth nerve palsy | ||||||
| Participant or population: people with sixth nerve palsy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with observation | Risk with botulinum toxin | |||||
| Improvement in ocular motility (ocular alignment ≤ 10 prism dioptres). Follow‐up to 4 months | 800 per 1,000 | 952 per 1,000 | RR 1.19 | 47 | ⊕⊕⊝⊝ | |
| Achievement of binocular single vision (fusion and stereopsis present). Follow‐up to 4 months | 800 per 1,000 | 952 per 1,000 | RR 1.19 | 47 | ⊕⊕⊝⊝ | |
| Improvement in functional ability | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Adverse events. Follow‐up to 4 months | In the injection group only, there were 2/22 (9%) cases of transient ptosis and 4/22 (18%) with transient vertical deviation, with a total complication rate of 24% per injection and 27% per participant. All adverse events recovered within the follow‐up time period of 6 months with no lasting adverse effects. | 47 (1 RCT) | ⊕⊕⊝⊝ | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for risk of bias (investigators were aware of the randomisation and it was not possible to mask investigators or participants to the allocation and there was variable follow‐up between groups) and downgraded one level for imprecision (confidence intervals include 1, no effect). | ||||||
| Pharmacological treatments (Gabapentin / Baclofen / 3,4‐DAP / 4‐AP) for people with acquired nystagmus | |||
| Participant or population: people with acquired nystagmus | |||
| Comparison | Main findings | № of participants | Certainty of the evidence |
| Gabapentin up to 900 mg/day) versus baclofen (up to 30 mg/day). Follow‐up 2 weeks | Gabapentin may work better than baclofen in improving ocular motility and reducing participant‐reported symptoms (oscillopsia). These effects may be different in pendular and jerk nystagmus but there was no formal subgroup analysis so it is unclear if the difference between the two types of nystagmus was a chance finding. Quality of life was not reported but ten participants with pendular nystagmus chose to continue treatment with gabapentin and one with baclofen. Two participants with jerk nystagmus chose to continue treatment with gabapentin and one with baclofen. Drug intolerance was reported in one person for gabapentin and four participants for baclofen. Increased ataxia was reported in three participants for gabapentin and two participants for baclofen. | 21 | ⊕⊝⊝⊝ |
| 3,4‐DAP (20 mg, single dose) versus placebo. Assessments made 30 minutes after taking the drug or placebo | 3,4‐DAP may reduce the mean peak slow‐phase velocity in people with downbeat nystagmus. In 10 of the 17 participants, mean peak slow‐phase velocity decreased by more than 50% and these 10 people reported having less oscillopsia. No significant adverse events were reported. Nine participants continued treatment. Three participants reported transient side effects of minor perioral/distal paraesthesia. | 17 | ⊕⊝⊝⊝ |
| 4‐AP (10 mg, single dose) versus 3,4‐DAP (10 mg, single dose) Assessments made at 45 and 90 minutes after taking the drug | 3,4 DAP and 4‐AP may reduce mean slow‐phase velocity in people with downbeat nystagmus. This effect may be stronger with 4‐AP. All participants reported mild paraesthesias with both medications. | 8 | ⊕⊝⊝⊝ |
| GRADE Working Group grades of evidence | |||
| 1 Downgraded two levels for imprecision (due to small number of participants) and one level for serious risk of bias (cross‐over study with analysis that did not permit estimation of effect size). | |||
| Study ID | Total participants | Primary: improved ocular motility | Secondary: improved binocular single vision | Secondary: improved symptoms | Secondary: adverse events |
| Lee 1994 | 47, parallel arm RCT 22 ‐ botulinum toxin 25 ‐ observation 6 month follow‐up | 21 (95.5%) ‐ botulinum toxin 20 (80%) ‐ observation | Success: 21 (95.5%) ‐ botulinum toxin 20 (80%) ‐ observation Partial: 3 (12%) ‐ observation Fail: 1 (4.5%) ‐ botulinum toxin 2 (8%) ‐ observation | 21 (95.5%) ‐ botulinum toxin 20 (80%) ‐ observation | 9% ptosis 18% vertical deviation |
| RCT: randomised controlled trial | |||||
| Study ID | Total participants | Primary: improved ocular motility | Secondary: improved functional vision | Secondary: improved symptoms | Secondary: adverse events |
| Thiagarajan 2014 | 12, cross‐over RCT 13‐week follow‐up | Baseline 2.1 saccadic ratio reducing to 1.7, P < 0.05 — OM rehabilitation Control group change not reported | Reading rate: Baseline 142 (10) wpm improving to 177 (14). Reading level: Baseline 4.1 (0.7) grade level improving to 6.3 (1.2), P < 0.01 Fixations per 100 words: Baseline 164 (10) improving to 135 (11), P = 0.02 Regressions per 100 words: Baseline 30 (3) improving to 23 (4) Control group changes not reported [means (SEM)] | Improved for OM rehabilitation. Control group changes not reported | Nil reported |
| SEM: standard error mean | |||||
| Study ID | Total participants | Primary: improved ocular motility | Secondary: improved visual acuity | Secondary: improved symptoms | Secondary: adverse events |
| Averbuch‐Heller 1997 | 21, crossover RCT 15 ‐ pendular 6 ‐ jerk 6‐week trial duration | 15 pendular ‐ gabapentin | 15 pendular ‐ gabapentin 1 jerk ‐ gabapentin 1 jerk ‐ baclofen | 6 pendular ‐ gabapentin 1 jerk ‐ gabapentin 1 jerk ‐ baclofen | 1 drug intolerance ‐ gabapentin 4 drug intolerance ‐ baclofen 3 ataxia ‐ gabapentin 2 ataxia ‐ baclofen |
| Kalla 2011 | 8, crossover RCT 8 ‐ downbeat 8‐day trial duration | Baseline ‐6.04; 45 mins ‐1.58; 90 mins ‐1.21 (4‐aminopyridine) Baseline ‐5.68; 45 mins ‐3.29; 90 mins ‐2.96 (3,4‐diaminopyridine) | ‐ | ‐ | All with mild paraesthesia |
| Strupp 2003 | 17, crossover RCT 17 ‐ downbeat 16‐day trial duration | Baseline 7.2 ± 4.2 °/sec reducing to 3.1 ± 2.5 (3,4‐diaminopyridine) Baseline 7.4 ± 4.1 °/sec reducing to 7.3 ± 3.7 (placebo) | ‐ | 10 ‐ reduced symptoms (3,4‐diaminopyridine) 0 ‐ reduced symptoms (placebo) | 3 ‐ mild paraesthesia (3,4‐diaminopyridine) 1 ‐ nausea/headache (3,4‐diaminopyridine) |
| RCT: randomised controlled trial | |||||

