Intervenciones para los trastornos de los movimientos oculares debidos a una lesión cerebral adquirida
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011290.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 05 marzo 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Salud ocular y de la visión
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
FR proposed the review questions, co‐ordinated the review, organised retrieval of papers and wrote to trial authors for additional information.
FR and KH screened search results, screened retrieved papers against inclusion criteria, appraised quality of papers, extracted data from papers, provided additional data about papers, obtained and screened data on unpublished studies and entered data in Review Manager.
JE provided methodological expertise.
All authors provided additional content expertise, provided clinical, policy and consumer perspectives, read and commented on final drafts of the review.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
-
NIHR have funded the research fellowship programme that encompasses this Review.
-
Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the NIHR to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
-
This review was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK editorial base.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
-
Declarations of interest
Fiona Rowe is an National Institute of Health Research (NIHR) Research Fellow and has received funding from the NIHR to support research relating to visual problems after stroke (including this Cochrane Review).
The work presented here represents the view of the authors and not necessarily those of the funding bodies.
Acknowledgements
Cochrane Eyes and Vision created and executed the electronic search strategies. We thank Catey Bunce and Tracey Shipman for their comments. We thank Anupa Shah and Iris Gordon for their assistance throughout the review process.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Mar 05 | Interventions for eye movement disorders due to acquired brain injury | Review | Fiona J Rowe, Kerry Hanna, Jennifer R Evans, Carmel P Noonan, Marta Garcia‐Finana, Caroline S Dodridge, Claire Howard, Kathryn A Jarvis, Sonia L MacDiarmid, Tallat Maan, Lorraine North, Helen Rodgers | |
| 2014 Sep 10 | Interventions for eye movement disorders due to acquired brain injury | Protocol | Fiona J Rowe, Carmel P Noonan, Marta Garcia‐Finana, Caroline S Dodridge, Claire Howard, Kathryn A Jarvis, Sonia L MacDiarmid, Tallat Maan, Lorraine North, Helen Rodgers | |
Differences between protocol and review
Objectives
During the course of doing the review it became clear that it would not make sense to pool data on the pre‐specified different types of interventions (restitutive, substitutive, compensatory and pharmacological) as they are clinically so different. Our original protocol objectives specified a primary objective which implied that these interventions would be pooled, if data were available. We amended our objectives to make it clear that we considered these different groups of interventions separately.
Inclusion criteria
We clarified the following inclusion criteria.
-
Type of studies: we clarified the inclusion of cross‐over trials.
-
Type of participants: we clarified the inclusion of studies of mixed aetiologies.
-
Type of participants: we clarified the exclusion of participants with multiple sclerosis and degenerative conditions.
Search strategy
We amended the search strategy to include additional terms for nystagmus, nerve palsy and gaze disorders.
Amendment to methods
-
Measures of treatment effect: we used the risk ratio rather than the odds ratio as planned, since this provides a better assessment of the treatment effect. Odds ratios are always more exaggerated (larger or smaller) and in this review there were no analytical issues that would warrant using the odds ratio.
-
Assessment of risk of bias: As four out of the five included studies were cross‐over studies, we amended the protocol to include additional 'Risk of bias' assessment criteria for cross‐over studies (Higgins 2011c).
-
GRADE and 'Summary of findings' table: these were not specified in the protocol but we added them because they have since become mandatory Cochrane methods (methods.cochrane.org/mecir).
Planned methods that were not used
-
We did not undertake any meta‐analyses so the following planned methods were not implemented.
-
Measures of treatment effect: we specified the use of the standardised mean difference and also how we would manage change data and final value data but this was not needed.
-
Dealing with missing data: we planned to assess trials that included intention‐to‐treat analyses to ensure this had been done correctly, ensuring participants had been included even if they did not fully adhere to the protocol and that it was possible to extract the appropriate data for these participants from the results.
-
Assessment of heterogeneity: we planned to examine the forest plots and use the Chi2 test and I2 test to assess heterogeneity.
-
Assessment of reporting bias: we planned to create a funnel plot if there were 10 or more trials in any analysis.
-
Data synthesis: we planned to combine data using a random‐effects model (unless there was a small number of trials in which case we would have used a fixed‐effect model).
-
Subgroup analysis: initially we planned to consider the different types of intervention — restitutive, compensatory, substitutive and pharmacological — as subgroup analyses, however in reality but the interventions in these categories were so different we considered that an overall meta‐analysis would be unlikely to be informative (even if the data were available), so we considered these comparisons separately. Other planned subgroup analyses of gender, type of acquired brain injury, side of brain injury, type of eye movement disorder, deviation of eye movement, and severity of eye movement were also not possible.
-
Sensitivity analyses: we planned sensitivity analyses to test the effect of any assumptions regarding missing data, and effects of publication type and risk of bias.
Additional authors
This review includes the authors Kerry Hanna and Jennifer Evans who were not involved in the protocol authorship.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- 4-Aminopyridine [*analogs & derivatives, therapeutic use];
- Abducens Nerve Diseases [etiology];
- Amifampridine;
- Amines [*therapeutic use];
- Baclofen [*therapeutic use];
- Botulinum Toxins [adverse effects, *therapeutic use];
- Brain Injuries [*complications];
- Cyclohexanecarboxylic Acids [*therapeutic use];
- Gabapentin;
- gamma-Aminobutyric Acid [*therapeutic use];
- Neuromuscular Agents [adverse effects, *therapeutic use];
- Nystagmus, Pathologic [etiology, therapy];
- Ocular Motility Disorders [*drug therapy, etiology];
- Randomized Controlled Trials as Topic;
- Vision, Binocular;
- Watchful Waiting;
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram
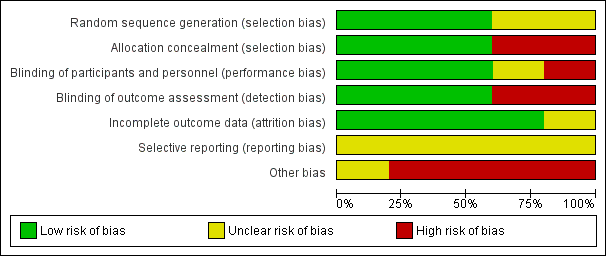
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
| Botulinum toxin versus observation in people with sixth nerve palsy | ||||||
| Participant or population: people with sixth nerve palsy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with observation | Risk with botulinum toxin | |||||
| Improvement in ocular motility (ocular alignment ≤ 10 prism dioptres). Follow‐up to 4 months | 800 per 1,000 | 952 per 1,000 | RR 1.19 | 47 | ⊕⊕⊝⊝ | |
| Achievement of binocular single vision (fusion and stereopsis present). Follow‐up to 4 months | 800 per 1,000 | 952 per 1,000 | RR 1.19 | 47 | ⊕⊕⊝⊝ | |
| Improvement in functional ability | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Adverse events. Follow‐up to 4 months | In the injection group only, there were 2/22 (9%) cases of transient ptosis and 4/22 (18%) with transient vertical deviation, with a total complication rate of 24% per injection and 27% per participant. All adverse events recovered within the follow‐up time period of 6 months with no lasting adverse effects. | 47 (1 RCT) | ⊕⊕⊝⊝ | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for risk of bias (investigators were aware of the randomisation and it was not possible to mask investigators or participants to the allocation and there was variable follow‐up between groups) and downgraded one level for imprecision (confidence intervals include 1, no effect). | ||||||
| Pharmacological treatments (Gabapentin / Baclofen / 3,4‐DAP / 4‐AP) for people with acquired nystagmus | |||
| Participant or population: people with acquired nystagmus | |||
| Comparison | Main findings | № of participants | Certainty of the evidence |
| Gabapentin up to 900 mg/day) versus baclofen (up to 30 mg/day). Follow‐up 2 weeks | Gabapentin may work better than baclofen in improving ocular motility and reducing participant‐reported symptoms (oscillopsia). These effects may be different in pendular and jerk nystagmus but there was no formal subgroup analysis so it is unclear if the difference between the two types of nystagmus was a chance finding. Quality of life was not reported but ten participants with pendular nystagmus chose to continue treatment with gabapentin and one with baclofen. Two participants with jerk nystagmus chose to continue treatment with gabapentin and one with baclofen. Drug intolerance was reported in one person for gabapentin and four participants for baclofen. Increased ataxia was reported in three participants for gabapentin and two participants for baclofen. | 21 | ⊕⊝⊝⊝ |
| 3,4‐DAP (20 mg, single dose) versus placebo. Assessments made 30 minutes after taking the drug or placebo | 3,4‐DAP may reduce the mean peak slow‐phase velocity in people with downbeat nystagmus. In 10 of the 17 participants, mean peak slow‐phase velocity decreased by more than 50% and these 10 people reported having less oscillopsia. No significant adverse events were reported. Nine participants continued treatment. Three participants reported transient side effects of minor perioral/distal paraesthesia. | 17 | ⊕⊝⊝⊝ |
| 4‐AP (10 mg, single dose) versus 3,4‐DAP (10 mg, single dose) Assessments made at 45 and 90 minutes after taking the drug | 3,4 DAP and 4‐AP may reduce mean slow‐phase velocity in people with downbeat nystagmus. This effect may be stronger with 4‐AP. All participants reported mild paraesthesias with both medications. | 8 | ⊕⊝⊝⊝ |
| GRADE Working Group grades of evidence | |||
| 1 Downgraded two levels for imprecision (due to small number of participants) and one level for serious risk of bias (cross‐over study with analysis that did not permit estimation of effect size). | |||
| Study ID | Total participants | Primary: improved ocular motility | Secondary: improved binocular single vision | Secondary: improved symptoms | Secondary: adverse events |
| Lee 1994 | 47, parallel arm RCT 22 ‐ botulinum toxin 25 ‐ observation 6 month follow‐up | 21 (95.5%) ‐ botulinum toxin 20 (80%) ‐ observation | Success: 21 (95.5%) ‐ botulinum toxin 20 (80%) ‐ observation Partial: 3 (12%) ‐ observation Fail: 1 (4.5%) ‐ botulinum toxin 2 (8%) ‐ observation | 21 (95.5%) ‐ botulinum toxin 20 (80%) ‐ observation | 9% ptosis 18% vertical deviation |
| RCT: randomised controlled trial | |||||
| Study ID | Total participants | Primary: improved ocular motility | Secondary: improved functional vision | Secondary: improved symptoms | Secondary: adverse events |
| Thiagarajan 2014 | 12, cross‐over RCT 13‐week follow‐up | Baseline 2.1 saccadic ratio reducing to 1.7, P < 0.05 — OM rehabilitation Control group change not reported | Reading rate: Baseline 142 (10) wpm improving to 177 (14). Reading level: Baseline 4.1 (0.7) grade level improving to 6.3 (1.2), P < 0.01 Fixations per 100 words: Baseline 164 (10) improving to 135 (11), P = 0.02 Regressions per 100 words: Baseline 30 (3) improving to 23 (4) Control group changes not reported [means (SEM)] | Improved for OM rehabilitation. Control group changes not reported | Nil reported |
| SEM: standard error mean | |||||
| Study ID | Total participants | Primary: improved ocular motility | Secondary: improved visual acuity | Secondary: improved symptoms | Secondary: adverse events |
| Averbuch‐Heller 1997 | 21, crossover RCT 15 ‐ pendular 6 ‐ jerk 6‐week trial duration | 15 pendular ‐ gabapentin | 15 pendular ‐ gabapentin 1 jerk ‐ gabapentin 1 jerk ‐ baclofen | 6 pendular ‐ gabapentin 1 jerk ‐ gabapentin 1 jerk ‐ baclofen | 1 drug intolerance ‐ gabapentin 4 drug intolerance ‐ baclofen 3 ataxia ‐ gabapentin 2 ataxia ‐ baclofen |
| Kalla 2011 | 8, crossover RCT 8 ‐ downbeat 8‐day trial duration | Baseline ‐6.04; 45 mins ‐1.58; 90 mins ‐1.21 (4‐aminopyridine) Baseline ‐5.68; 45 mins ‐3.29; 90 mins ‐2.96 (3,4‐diaminopyridine) | ‐ | ‐ | All with mild paraesthesia |
| Strupp 2003 | 17, crossover RCT 17 ‐ downbeat 16‐day trial duration | Baseline 7.2 ± 4.2 °/sec reducing to 3.1 ± 2.5 (3,4‐diaminopyridine) Baseline 7.4 ± 4.1 °/sec reducing to 7.3 ± 3.7 (placebo) | ‐ | 10 ‐ reduced symptoms (3,4‐diaminopyridine) 0 ‐ reduced symptoms (placebo) | 3 ‐ mild paraesthesia (3,4‐diaminopyridine) 1 ‐ nausea/headache (3,4‐diaminopyridine) |
| RCT: randomised controlled trial | |||||

