Intervenciones para los trastornos de los movimientos oculares debidos a una lesión cerebral adquirida
Resumen
Antecedentes
La lesión cerebral adquirida puede provocar trastornos de los movimientos oculares que pueden incluir: estrabismo, dificultad para mirar fijamente y nistagmo, lo que causa síntomas de visión doble, empañada o "saltarina" y dificultades para la lectura. Existe una variedad amplia de intervenciones que tienen la posibilidad de aliviar o mejorar estos síntomas. Es necesario evaluar la efectividad de estas intervenciones y el momento adecuado para su realización.
Objetivos
Se intentó evaluar la efectividad de cualquier intervención y determinar el efecto del momento de la intervención en el tratamiento del estrabismo, la dificultad para mirar fijamente y el nistagmo debido a una lesión cerebral adquirida. Se consideraron las intervenciones restauradoras, sustitutivas, compensatorias o farmacológicas por separado y se compararon con control, placebo, tratamiento alternativo o ningún tratamiento para mejorar la alineación o la motilidad ocular (o ambas).
Métodos de búsqueda
Se hicieron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials, CENTRAL) (que contiene el registro de ensayos del Grupo Cochrane de Trastornos de los Ojos y la Visión [Cochrane Eyes and Vision Group]) (2017, número 5), MEDLINE Ovid, Embase Ovid, CINAHL EBSCO, AMED Ovid, PsycINFO Ovid, Dissertations & Theses (PQDT) database, PsycBITE (Psychological Database for Brain Impairment Treatment Efficacy), ISRCTN registry, ClinicalTrials.gov, Health Services Research Projects in Progress (HSRProj), National Eye Institute Clinical Studies Database and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP). Se buscó por última vez en las bases de datos el 26 de junio de 2017. No se utilizaron restricciones de fecha ni idioma en las búsquedas electrónicas de ensayos. Se realizó una búsqueda manual en el Australian Orthoptic Journal, British and Irish Orthoptic Journal y en ESA, ISA y actas de congresos del IOA. Se estableció contacto con investigadores activos en este campo para obtener información sobre estudios adicionales publicados o no publicados.
Criterios de selección
Se incluyeron los ensayos controlados aleatorios (ECA) de cualquier intervención para la alineación ocular o los déficits de motilidad (o ambos) debidos a una lesión cerebral adquirida.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, seleccionaron los estudios y extrajeron los datos. Se utilizaron los métodos estándar previstos por Cochrane. Se empleó el enfoque GRADE para interpretar los hallazgos y evaluar la calidad de la evidencia.
Resultados principales
Se encontraron cinco ECA (116 participantes) elegibles para la inclusión. Estos ensayos incluyeron afecciones de nistagmo adquirido, parálisis del sexto nervio craneal y defectos de la motilidad ocular inducidos por lesión cerebral traumática. No se identificaron estudios relevantes de intervenciones restauradoras.
Se identificó un ensayo realizado en el Reino Unido de una intervención sustitutiva, que comparó toxina botulínica con observación en 47 pacientes con parálisis aguda del sexto nervio craneal. Cuatro meses después de la entrada al ensayo, los pacientes que recibieron toxina botulínica tuvieron mayores probabilidades de tener una recuperación total (reducción del ángulo de desviación dentro de 10 dioptrías del prisma), en comparación con los que recibieron observación (cociente de riesgos 1,19; IC del 95%: 0,96 a 1,48; evidencia de certeza baja). Estos participantes también lograron una visión binocular única. En el grupo de inyección solamente, dos de 22 participantes (9%) presentaron ptosis transitoria, y cuatro de 22 participantes (18%) presentaron desviación vertical transitoria, una tasa total de complicación del 24% por inyección y del 27% por participante. Se recuperaron todos los eventos adversos. La certeza de la evidencia se consideró baja y se disminuyó por el riesgo de sesgo y la imprecisión. No fue posible ocultar la asignación a los investigadores ni a los participantes, y el seguimiento entre los grupos varió.
Se identificó un ensayo cruzado realizado en los EE.UU. de una intervención compensatoria. La rehabilitación oculomotora se comparó con el entrenamiento simulado en 12 pacientes con lesión cerebral traumática leve al menos un año después de la lesión. La evidencia de este estudio se consideró de certeza muy baja. El estudio fue pequeño, los datos del grupo de entrenamiento simulado no se informaron completamente y no estuvo claro si el diseño cruzado del estudio fue apropiado, ya que es posible que esta intervención tenga un efecto permanente.
Se identificaron tres estudios cruzados de intervenciones farmacológicas para el nistagmo adquirido, que se realizaron en Alemania y los EE.UU. Estos estudios investigaron dos clases de intervenciones farmacológicas: fármacos gabaérgicos (gabapentina, baclofeno) y aminopiridinas (4‐aminopiridinas [AP], 3,4‐ diaminopiridina). La evidencia de los tres estudios se consideró de certeza muy baja debido al escaso número de participantes (lo que dio lugar a imprecisión) y al riesgo de sesgo (fueron estudios cruzados que no informaron los datos de una manera que permitiera la estimación del tamaño del efecto).
Un estudio comparó gabapentina (hasta 900 mg/día) con baclofeno (hasta 30 mg/día) en 21 pacientes con nistagmo pendular y en resorte. El período de seguimiento fue de dos semanas. Este estudio proporciona evidencia de certeza muy baja de que la gabapentina puede funcionar mejor que el baclofeno para mejorar la motilidad ocular y reducir los síntomas informados por los participantes (oscilopsia). Estos efectos pueden ser diferentes en el nistagmo pendular y en resorte, pero sin un análisis de subgrupos formal no está claro si la diferencia entre los dos tipos de nistagmo se debe al azar. No se informó la calidad de vida. Diez participantes con nistagmo pendular decidieron continuar el tratamiento con gabapentina, y uno con baclofeno. Dos participantes con nistagmo en resorte decidieron continuar el tratamiento con gabapentina, y uno con baclofeno. La intolerancia al fármaco se informó en un paciente que recibió gabapentina y en cuatro pacientes que recibieron baclofeno. Se informó un aumento de la ataxia en tres pacientes que recibieron gabapentina y dos pacientes que recibieron baclofeno.
Un estudio comparó una dosis única de 3,4‐DAP (20 mg) con placebo en 17 pacientes con nistagmo vertical hacia abajo. Las evaluaciones se realizaron a los 30 minutos después de tomar el fármaco. Este estudio aporta evidencia de certeza baja de que 3,4‐DAP puede reducir la media de la velocidad máxima de la fase lenta, con menos oscilopsia, en los pacientes con nistagmo vertical hacia abajo. Tres pacientes informaron efectos secundarios transitorios de parestesia perioral/distal leve.
Un estudio comparó una dosis única de 4‐AP con una dosis única de 3,4‐DAP (ambas dosis de 10 mg) en ocho pacientes con nistagmo vertical hacia abajo. Las evaluaciones se realizaron a los 45 y 90 minutos después de la administración del fármaco. Este estudio aporta evidencia de certeza muy baja de que 3,4‐DAP y 4‐AP pueden reducir la media de la velocidad máxima de la fase lenta en los pacientes con nistagmo vertical hacia abajo. Este efecto puede ser más fuerte con 4‐AP.
Conclusiones de los autores
Los estudios incluidos no proporcionan evidencia suficiente para informar las decisiones acerca de tratamientos específicos para los trastornos de los movimientos oculares que ocurren después de una lesión cerebral adquirida. No se obtuvo información sobre el costo del tratamiento ni las medidas de satisfacción de los participantes con respecto a las opciones de tratamiento ni la efectividad. Fue posible describir el resultado del tratamiento en cada ensayo y evaluar la incidencia de eventos adversos.
PICO
Resumen en términos sencillos
Intervenciones para los trastornos de los movimientos oculares debidos a una lesión cerebral adquirida
¿Cuál es el objetivo de esta revisión?
El objetivo de esta revisión Cochrane fue determinar los tratamientos que funcionan mejor para corregir la posición de los ojos y los trastornos de los movimientos oculares debidos a una lesión cerebral adquirida, y cuándo es el mejor momento para aplicarlos.
Mensajes clave
Actualmente la evidencia sobre los efectos beneficiosos y perjudiciales de los tratamientos para los trastornos de los movimientos oculares debidos a una lesión cerebral adquirida es de certeza muy baja.
¿Qué se estudió en esta revisión?
Se denomina lesión cerebral adquirida a cualquier lesión que se presenta después del nacimiento y daña la función del cerebro. El estrabismo es una afección en que los ojos están fuera de alineación, y uno o ambos ojos están girados hacia afuera, adentro, arriba o abajo. Los trastornos de la motilidad ocular (movimiento ocular) son defectos que impiden el movimiento normal de los ojos. El nistagmo es una afección donde los movimientos oculares no son constantes y, en su lugar, los ojos tiemblan. Las opciones de tratamiento incluyen terapia ocular, lentes, prismas, oclusión, toxina botulínica o cirugía, para reducir la desviación o el movimiento de los ojos. Actualmente no hay recomendaciones claras sobre el mejor momento para proporcionar estos tratamientos, cuál es el costo de estos tratamientos ni si los tratamientos son beneficiosos para los pacientes con alineación ocular y trastornos de los movimientos oculares que ocurren después de una lesión cerebral adquirida.
¿Cuáles son los principales resultados de esta revisión?
Los investigadores de Cochrane encontraron cinco estudios relevantes con un total de 116 participantes. Un estudio se realizó en el Reino Unido y analizó la toxina botulínica en comparación con observación en pacientes con parálisis del sexto nervio craneal de aparición reciente. Un estudio de los EE.UU. comparó el entrenamiento de los movimientos oculares con el entrenamiento simulado (falso) en pacientes con lesión cerebral traumática leve. Tres estudios se realizaron en Alemania o los EE.UU. y compararon tratamientos médicos farmacológicos en pacientes con nistagmo adquirido. La revisión proporciona;
‐ evidencia de certeza baja de que los pacientes con parálisis del sexto nervio craneal pueden tener ligeramente mejores probabilidades de una reducción de los síntomas visuales cuándo la administración de toxina botulínica se comparó con ningún tratamiento.
‐ evidencia de certeza muy baja sobre el tratamiento de los movimientos oculares en los pacientes con lesión cerebral debido a traumatismo y el uso de fármacos para el nistagmo, donde los tratamientos muestran una ligera mejoría en los síntomas.
¿Cuál es el grado de actualización de esta revisión?
Los investigadores Cochrane buscaron estudios que se habían publicado hasta el 26 junio de 2017.
Authors' conclusions
Summary of findings
| Botulinum toxin versus observation in people with sixth nerve palsy | ||||||
| Participant or population: people with sixth nerve palsy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with observation | Risk with botulinum toxin | |||||
| Improvement in ocular motility (ocular alignment ≤ 10 prism dioptres). Follow‐up to 4 months | 800 per 1,000 | 952 per 1,000 | RR 1.19 | 47 | ⊕⊕⊝⊝ | |
| Achievement of binocular single vision (fusion and stereopsis present). Follow‐up to 4 months | 800 per 1,000 | 952 per 1,000 | RR 1.19 | 47 | ⊕⊕⊝⊝ | |
| Improvement in functional ability | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Adverse events. Follow‐up to 4 months | In the injection group only, there were 2/22 (9%) cases of transient ptosis and 4/22 (18%) with transient vertical deviation, with a total complication rate of 24% per injection and 27% per participant. All adverse events recovered within the follow‐up time period of 6 months with no lasting adverse effects. | 47 (1 RCT) | ⊕⊕⊝⊝ | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for risk of bias (investigators were aware of the randomisation and it was not possible to mask investigators or participants to the allocation and there was variable follow‐up between groups) and downgraded one level for imprecision (confidence intervals include 1, no effect). | ||||||
| Pharmacological treatments (Gabapentin / Baclofen / 3,4‐DAP / 4‐AP) for people with acquired nystagmus | |||
| Participant or population: people with acquired nystagmus | |||
| Comparison | Main findings | № of participants | Certainty of the evidence |
| Gabapentin up to 900 mg/day) versus baclofen (up to 30 mg/day). Follow‐up 2 weeks | Gabapentin may work better than baclofen in improving ocular motility and reducing participant‐reported symptoms (oscillopsia). These effects may be different in pendular and jerk nystagmus but there was no formal subgroup analysis so it is unclear if the difference between the two types of nystagmus was a chance finding. Quality of life was not reported but ten participants with pendular nystagmus chose to continue treatment with gabapentin and one with baclofen. Two participants with jerk nystagmus chose to continue treatment with gabapentin and one with baclofen. Drug intolerance was reported in one person for gabapentin and four participants for baclofen. Increased ataxia was reported in three participants for gabapentin and two participants for baclofen. | 21 | ⊕⊝⊝⊝ |
| 3,4‐DAP (20 mg, single dose) versus placebo. Assessments made 30 minutes after taking the drug or placebo | 3,4‐DAP may reduce the mean peak slow‐phase velocity in people with downbeat nystagmus. In 10 of the 17 participants, mean peak slow‐phase velocity decreased by more than 50% and these 10 people reported having less oscillopsia. No significant adverse events were reported. Nine participants continued treatment. Three participants reported transient side effects of minor perioral/distal paraesthesia. | 17 | ⊕⊝⊝⊝ |
| 4‐AP (10 mg, single dose) versus 3,4‐DAP (10 mg, single dose) Assessments made at 45 and 90 minutes after taking the drug | 3,4 DAP and 4‐AP may reduce mean slow‐phase velocity in people with downbeat nystagmus. This effect may be stronger with 4‐AP. All participants reported mild paraesthesias with both medications. | 8 | ⊕⊝⊝⊝ |
| GRADE Working Group grades of evidence | |||
| 1 Downgraded two levels for imprecision (due to small number of participants) and one level for serious risk of bias (cross‐over study with analysis that did not permit estimation of effect size). | |||
Background
Description of the condition
Acquired brain injury is brain damage caused by events occurring after birth that result in permanent or temporary changes in cognition, physical, emotional and behavioural function. This may be due to trauma, surgery, stroke, brain tumour, infection, inflammation and ischaemia. Eye movement disorders following acquired brain injury may include strabismus, gaze deficits and nystagmus (Rowe 2003). In acquired brain injury, these eye movement disorders are caused by damage to the cranial nerves that supply the extra ocular muscles or to damage of the neurological areas that contribute to the control of eye movements (Pierrot‐Deseilligny 2011). The incidence of visual problems in acquired brain injury is unknown but estimates are available for certain types of acquired brain injury. For example, over half of stroke survivors have visual impairments (Freeman 1988), and up to 68% of stroke survivors with visual symptoms have eye movement disorders (Rowe 2009). These impact on daily life by causing a range of difficulties including inability to maintain normal ocular alignment or move the eyes appropriately (Hepworth 2016; Jones 2006; Pedersen 1981; Rowe 2011a). Functional disabilities occur including loss of depth perception, reduced hand‐eye co‐ordination and reading impairment (Hepworth 2016; MacIntosh 2003; Rowe 2011b), and these may impede the effectiveness of rehabilitation therapy in regaining mobility, activities of daily living and quality of life (Ciuffreda 2007; MacIntosh 2003).
The true incidence of eye movement disorders in acquired brain injury is unknown and the prevalence varies depending on the cause of brain injury and area of brain involved. Strabismus is a deviation of the ocular alignment where one eye turns and which may be intermittent or constant (Fowler 1996; Rowe 2010). Strabismus occurs in a number of forms including: esotropia (in‐turning deviation), exotropia (out‐turning deviation) or less commonly, hypertropia (up‐turning deviation), hypotropia (down‐turning deviation) and cyclotropia (rotatory deviation). Gaze deficits include disorders of the eye movement systems which involve saccades (fast movements of the eyes), smooth pursuits (slow, tracking movements of the eyes), vergence (opposite movements of the eyes such as convergence where both eyes turn inwards symmetrically or divergence where both eyes turn outwards), cranial nerve palsy (impairment of III, IV or VI nerve function causing abnormal eye movement and strabismus in the affected eye), bilateral gaze palsy (impaired horizontal, vertical or combined movements in both eyes), unilateral gaze palsy (internuclear ophthalmoplegia, one and a half syndrome) and vestibulo‐ocular and opto‐kinetic reflexes (involuntary movements of the eyes in response to moving objects and movement of the head and body) (Pedersen 1981; Rowe 2011a; Rowe 2013a). Nystagmus is a condition in which there are frequent involuntary oscillations of the eyes that result in reduced visual function (Rowe 2008).
Symptoms of eye movement disorders can include blurring of vision, diplopia (double vision), impaired depth (3‐dimensional) perception, wobbling and jumbling of images, and reading difficulty (Rowe 2013b).
Description of the intervention
There are various treatments associated with strabismus, eye movement disorders and nystagmus. Primarily, treatment is directed at aligning the visual axes and improving the movement of the eyes.
Treatments for eye movement disorders can be described as restitution, compensation, substitution or pharmacology (Kerkhoff 2000; Pollock 2011). Restitutive treatment aims to restore visual function to normal; compensatory treatments aims to aid adaptation to the persistent visual impairment and substitutive treatments aim to use optical or medical aids to enhance visual function. Pharmacological treatments aim to reduce the effects of visual impairment. Restitutive interventions may include convergence training, pursuit training and saccade training. Compensative interventions may include training eye movements for reading, compensatory head posture or movements, use of eye blinks or colour cues and training in activities of daily living (Rowe 2011a). Substitutive interventions may include prisms, eye patches, lens alteration, extra ocular muscle surgery, botulinum toxin, magnification and environmental modification (Pigassou 1972). Pharmacological interventions may include prescription drugs such as baclofen, memantine and carbamazepine, and local anaesthetic injections (Choudhuri 2007; Thurtell 2010).
Commencement of interventions can be at multiple time points. People are offered interventions at different time points dependent on the time lapse since acquired brain injury onset, severity and type of symptoms and extent of recovery, if any. Some interventions can be used at any time point such as prisms, occlusion and lenses, whereas others — such as surgery — tend to be offered later once a visual condition is stable.
How the intervention might work
Restitution
Restitution includes the biochemical events that help restore functional neural tissue through the reduction of oedema, absorption of blood, restoration of normal neuronal physiology, and restoration of axon transport (Pollock 2011). Treatments of positive fusional amplitudes and stereopsis through repetition training of specific deficient functions, such as convergence insufficiency, have been reported as effective (Kerkhoff 2000). Restitutive interventions will include those where there is direct training of the impaired function or repetitive stimulation of eye movement.
Compensation
Compensation aims to improve the mismatch between the participants' skills and the demands placed on them by their environment, by teaching participants to compensate or adapt using a spared or intact function (Kerkhoff 1999; Kerkhoff 2000).
Substitution
Substitution involves adaptation of visual components that have been lost or disrupted through the use of optic devices, extra‐ocular muscle surgery, botulinum toxin or environmental modifications (Kerkhoff 1999; Kerkhoff 2000).
Pharmacology
Pharmacology aims to improve visual functioning through alteration of biochemical and/or physiological effects in the body. Gabapentin is proposed to interact with a high affinity binding site in brain membranes linked to calcium channels. It increases GABA synthesis. Baclofen is a derivative of GABA. It is proposed to activate GABA receptors and acts as an inhibitory neurotransmitter by blocking monosynaptic and polysynaptic reflexes. Aminopyridines are proposed to bind to open and non‐conducting aspects of potassium ion channels resulting in prolonged action potentials and allowing increased neurotransmitter release at the neuromuscular junction.
Why it is important to do this review
There are many forms of interventions for eye movement disorders that occur following acquired brain injury. Although the natural history of acquired brain injury differs dependent on the cause, the treatment of the resultant eye movement disorder is principally the same regardless of cause. Although the cause may recover or progress, the resultant eye movement disorder may persist and requires treatment to alleviate or reduce visual symptoms. We have considered all interventions for all types of eye movement disorders regardless of cause and evolution of eye movement disorder. We extracted data for improving, stable or deteriorating eye movement disorders.
There are multiple time points at which interventions for eye movement disorders that occur following acquired brain injury may be provided. The timing of provision of treatment for eye movement disorders differs for the various treatment options. In the early stages, treatment may include conservative options such as eye patching or prisms which are used whilst the eye movement disorder is monitored until it improves or stabilises. Where eye movement disorders persist, further treatment options such as botulinum toxin or extra ocular muscle surgery may then be considered. There can be delays in referral of participants with acquired brain injury to eye care services and thus participants may not receive treatment early even though treatment options are available. This delay may impede general rehabilitation.
A recent systematic review of interventions for eye movement disorders in stroke found insufficient evidence to reach conclusions about the effectiveness of those interventions for this group of participants (Pollock 2011). There was an absence of relevant evidence and the authors gave an urgent recommendation for high‐quality research. Furthermore, there is no consensus for what constitutes the optimum timing for commencing interventions for eye movement disorders due to stroke. Timing may vary dependent on the type of treatment being considered (e.g. eye patch versus extra‐ocular muscle surgery) and/or the extent of visual symptoms (minimal versus severe impact on daily life). Because this review only concentrated on stroke populations, one recommendation was for a systematic review of interventions for eye movement disorders in participants with acquired brain injury in order to synthesise the current evidence base, to guide current practice and aid in the development of well‐designed randomised controlled trials (RCTs). Other publications reporting efficacy and timing of interventions for eye movement disorders include populations with varied causes of brain damage such as tumours, inflammation, infection, stroke and metabolic causes.
Purpose
We have undertaken a high‐quality systematic review of the existing evidence base in order to determine the evidence for effectiveness and timing of any treatment or management approaches for all adult participants with acquired brain injury with eye movement disorders. This review directly addressed the recommendations of the Cochrane systematic review on interventions for eye movement disorders in stroke (Pollock 2011), i.e. conduct a systematic review of interventions for eye movement disorders in participants with acquired brain injury in order to synthesise the current evidence base. This review examined the timing of interventions for eye movement disorders. The impact on objective and subjective measures of ocular alignment and motility are reported. This review can be used to guide current practice and aid in the development of well‐designed RCTs which follow the UK Medical Research Council guidance on developing and evaluating complex interventions (Craig 2008).
Objectives
We aimed to assess the effectiveness of any intervention and determine the effect of timing of intervention in the treatment of strabismus, gaze deficits and nystagmus due to acquired brain injury. We considered restitutive, substitutive, compensatory or pharmacological interventions separately and compared them to control, placebo, alternative treatment or no treatment for improving ocular alignment or motility (or both).
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs. We included cross‐over trials if sufficient time was justified for the wash‐out period between interventions to avoid carry‐over effects, or where the condition was stable prior to recruitment, and where long‐term follow‐up was not required.
Types of participants
We included individuals with eye movement disorders due to acquired brain injury. We included trials of mixed aetiologies where more than 50% of participants had acquired brain injury. We excluded participants with primary diagnoses of multiple sclerosis (an immune‐modulated process affecting the central nervous system, causing damage to the myelin sheath of nerve fibres) and degenerative conditions of the brain (such as Parkinson's disease, Huntingdon's disease and Alzheimer's). The type of eye movement disorder could include III, IV, and VI cranial nerve palsy, reduced fixation, gaze holding, horizontal or vertical gaze palsy (or both), internuclear ophthalmoplegia, one and a half syndrome, saccadic problems, smooth pursuit problems, strabismus, nystagmus, reduced convergence or divergence, conjugate deviation and skew deviation. The deviation of eye movement could be horizontal, vertical or torsional and the severity of eye movement disorder could be slight, small, moderate or marked, and could include paralysis or paresis. We included monocular and binocular eye movement disorders.
We accepted studies that included participants based on symptoms which can be assumed to be present as a direct result of an eye movement disorder. Symptoms could include double vision, blurred vision, reading difficulty, wobbling or jumbled vision and excessive head movements. We considered participants of all ages from studies of adults and children.
Types of interventions
We included any intervention that aimed to improve the defects of eye movement, or alleviate or reduce the visual symptoms associated with the disorder. We classified interventions as restitution, substitution, compensation or pharmacological. We included trials that documented the timing (recorded as time period from the onset of eye movement disorder) of any intervention that aimed to improve the defects of eye movement, or alleviate or reduce the visual symptoms associated with the disorder. We classified timing as early or late intervention, where early constituted intervention within one month of eye movement disorder onset.
Types of outcome measures
Where possible, we assessed each outcome as a dichotomous variable (yes or no) at the end of the intervention period and at a follow‐up point (ideally a minimum of three months after the completion of the intervention and a maximum of 12 months after).
Primary outcomes
-
Improvement in ocular motility measured by orthoptic assessments of reduction in the angle of deviation (within 10 prism dioptres of ortho/straight position) and/or extent of eye movement range (improvement of one or more grades of limitation ranging from 1 to 4), such that visual axes are aligned in primary or secondary gaze positions (or both)
Secondary outcomes
-
Achievement of binocular single vision as assessed by cover test, motor fusional vergences and stereoacuity
-
Reduction of, or alleviation of, participant‐reported symptoms assessed by participant record notes or questionnaire
-
Improvement in functional ability measured by validated measures such as activity of daily living questionnaires
-
Quality of life data — any measure of participant or parent satisfaction relating to improvement in appearance or improvement to lifestyle
Adverse events
-
Intractable permanent diplopia, perforating injury, symptomatic over‐ or under‐correction, death
-
Assessed by descriptive documentation
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist conducted systematic searches in the following databases for randomised controlled trials and controlled clinical trials. There were no language or publication year restrictions. The date of the search was 26 June 2017.
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 5) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 26 June 2017) (Appendix 1);
-
MEDLINE Ovid (1946 to 26 June 2017) (Appendix 2);
-
Embase Ovid (1980 to 26 June 2017) (Appendix 3);
-
CINAHL (Cumulative Index to Nursing and Allied Health Literature) (1982 to 26 June 2017) (Appendix 4);
-
AMED (Allied and Complementary Medicine Database) (1985 to 26 June 2017) (Appendix 5);
-
PsycINFO (1967 to 26 June 2017) (Appendix 6);
-
Dissertations & Theses (PQDT) database (http://pqdtopen.proquest.com/search.html; searched 26 June 2017) (Appendix 7);
-
PsycBITE (Psychological Database for Brain Impairment Treatment Efficacy) (http://www.psycbite.com/search.php: searched 26 June 2017) (Appendix 8);
-
ISRCTN registry (www.isrctn.com/editAdvancedSearch; searched 26 June 2017) (Appendix 9);
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 26 June 2017) (Appendix 10);
-
Health Services Research Projects in Progress (HSRProj) (wwwcf.nlm.nih.gov/hsr_project/home_proj.cfm; searched 26 June 2017) (Appendix 11);
-
National Eye Institute Clinical Studies Database (clinicalstudies.info.nih.gov/cgi/protinstitute.cgi?NEI.0.html; searched 26 June 2017) (Appendix 12);
-
World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp; searched 26 June 2017) (Appendix 13).
Searching other resources
We searched the reference lists of included trials to identify any further studies and checked the references of review articles about vision after acquired brain injury. We performed citation tracking using Web of Science Cited Reference Search for all included studies and contacted experts in the field, including authors of included trials and excluded studies identified as possible preliminary or pilot work. We searched reference material supplied by commercial companies who provide interventions aimed at restoration of eye movements.
We handsearched the following resources from their inception to the current date at http://pcwww.liv.ac.uk/˜rowef/index_files/Page646.htm.
-
British and Irish Orthoptic Journal
-
Australian Orthoptic Journal
-
Proceedings of the European Strabismological Association (ESA)
-
International Strabismological Association (ISA)
-
International Orthoptic Association (IOA)
Data collection and analysis
We followed guidance in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions for data collection and Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions for data analysis (Deeks 2011; Higgins 2011a). We also conformed to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) checklist (Moher 2009). The Cochrane Eyes and Vision group Information Specialist ran all the electronic searches, downloaded references into bibliographic software, and removed duplicates. Two review authors excluded any titles or abstracts which were obviously not related to acquired brain injury and vision. They independently considered each of these titles and abstracts and excluded any studies where the intervention was not specifically aimed at improving the eye movement disorder or the participant's ability to cope with the eye movement disorder. We resolved any disagreements through discussion. We obtained the full papers for any studies included at this stage.
Selection of studies
Two review authors independently applied the selection criteria, considering and documenting the type of studies, type of participants, intervention, comparison intervention, and the outcome measures. Each review author classified each study as 'include' or 'exclude'. For disagreement between these two review authors, consensus was planned through discussion involving a third review author. In practice, this was not required.
We listed all excluded studies (that we had obtained a full‐text copy for) that included participants with eye movement disorders in the Characteristics of excluded studies table with the reasons for exclusion. We did not list studies in the Characteristics of excluded studies table that were excluded because they included participants that did not have eye movement disorders (i.e. visual neglect, age‐related visual problems, or visual field loss), unless the two review authors agreed that there was a clear reason to do so.
Data extraction and management
We used a pre‐designed data extraction form to record data from the included studies. Two review authors independently documented information found in the table in Appendix 14. If there were any discrepancies between data extracted by the two review authors, they were resolved through discussion. Data were entered into Review Manager 5 (Review Manager 2014).
Assessment of risk of bias in included studies
We used Cochrane's tool for assessing risk of bias for randomised trials as outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We assessed sequence generation, allocation concealment, masking (blinding) of participants, personnel and outcome assessors, incomplete outcome data and selective outcome reporting. We judged each domain as "low risk of bias", "high risk of bias" or "unclear". We also considered additional potential sources of bias for cross‐over studies (Higgins 2011c), namely: (1) whether the cross‐over design is suitable; (2) whether there is a carry‐over effect; (3) whether only first period data are available; (4) incorrect analysis; and (5) comparability of results with those from parallel‐group trials.
Measures of treatment effect
There was only one study that reported outcomes in a way that could be analysed in this review. This parallel group study reported dichotomous outcomes (reduction in angle of deviation within 10 prism dioptres, achievement of binocular single vision). We analysed these as risk ratios with 95% confidence intervals.
Most (four out of five) studies were cross‐over trials. Ideally we would have extracted the results of paired analyses, but these were not available.
See Differences between protocol and review for further discussion of our planned methods.
Unit of analysis issues
The interventions are normally applied at individual level. Some interventions (e.g. prisms for diplopia or field defect, monocular occlusion) are used to treat only one eye. In these instances, there is no unit of analysis issue as the unit of randomisation is the same as the unit of analysis. However, some interventions (e.g. scanning eye exercises, extra‐ocular muscle surgery, pharmacological interventions) treat both eyes. We report outcome measures for the person.
We followed guidance in the Cochrane Handbook for Systematic Reviews of Interventions for the incorporation of data from cross‐over trials, i.e. estimating mean and standard errors from paired analysis (Higgins 2011c).
Dealing with missing data
If an included study did not report a particular outcome, we did not include that study in the analyses of that outcome. If an included study had missing data (e.g. participants lost to follow‐up) we reported results for included participants based on the raw data provided. We used all trial data even if intention‐to‐treat analysis was not conducted. However, we looked at which participants dropped out or changed treatment to determine if these related to one specific group or were dispersed across all groups. We also contacted trial authors for missing data.
Assessment of heterogeneity
We assessed for heterogeneity primarily by examining the characteristics of the study. We did not conduct a meta‐analysis due to clinical heterogeneity, so we did not implement our other planned methods for assessing heterogeneity. See Differences between protocol and review.
Assessment of reporting biases
We assessed the possibility of selective outcome reporting as detailed in the Assessment of risk of bias in included studies section. We did not conduct any meta‐analyses so we did not use our planned methods for assessing publication bias. See Differences between protocol and review.
Data synthesis
We provide a narrative account of the data available. See Differences between protocol and review for details of planned methods for data synthesis that were not used.
Subgroup and sensitivity analyses
Due to the small number of studies identified, we were unable to conduct our planned subgroup and sensitivity analyses. See Differences between protocol and review.
'Summary of findings' table
We aimed to prepare a 'Summary of findings' table for each relevant comparison including all pre‐specified outcomes:
-
improvement in ocular motility;
-
achievement of binocular single vision;
-
improvement in functional ability;
-
quality of life;
-
adverse effects.
The cross‐over studies were not reported in a way that permitted calculation of effect sizes so we have reported the direction, rather than the size of the effect in these cases. We graded the certainty of the evidence using the GRADE approach (Guyatt 2008; GRADEpro 2014). In the absence of pooled data we were guided by the implementation of GRADE for a narrative summary (Murad 2017). We considered methodological limitations of the included studies, indirectness, imprecision, inconsistency and likelihood of publication bias. We did not pre‐specify the 'Summary of findings' table and GRADE assessment in the protocol. See Differences between protocol and review.
Results
Description of studies
Results of the search
The electronic searches yielded a total of 5049 references (Figure 1). The Cochrane Information Specialist removed 330 duplicate records and we screened the remaining 4719 reports. We rejected 4700 records after reading the abstracts and obtained the full‐text reports of 19 references for further assessment. We included five reports of five studies (Averbuch‐Heller 1997; Kalla 2011; Lee 1994; Strupp 2003; Thiagarajan 2014) and we excluded 14 studies (see Characteristics of excluded studies for details). We did not identify any ongoing studies from our searches of the clinical trials registries.

Study flow diagram
Included studies
We included five trials which are summarised below. Additional details can be found in the Characteristics of included studies tables.
Averbuch‐Heller 1997 randomised 21 participants (10 females and 11 males) aged 25 to 73 years. There were 15 cases of acquired pendular nystagmus and six of jerk nystagmus. The trial was conducted across four sites in the USA and Germany. It was a double‐masked randomised controlled trial with a cross‐over design, which compared two pharmacological interventions: 10 mg baclofen and 30 mg gabapentin. Drug 1 was taken for two weeks, followed by a wash‐out period of two weeks; and drug 2 was then taken for two weeks (six‐week study duration in total). The participant group was considered homogenous in that they had both interventions. There were no exclusions, but one participant was lost to follow‐up. Outcomes were measured at baseline and at two‐, four‐ and six‐week follow‐up periods. They included measurement of Landolt C visual acuity, eye movement recordings, subjective image motion, adverse drug effect reporting and choice of continued treatment. Inclusion criteria were specified, but not exclusion criteria.
Kalla 2011 randomised eight participants (six females and two males) with acquired downbeat nystagmus, aged 58 to 76 years. The trial was conducted at one site in Germany. It was a double‐masked randomised controlled trial with a cross‐over design, which compared two pharmacological interventions: 4‐aminopyridine at a 10 mg dose and 3,4‐diaminopyridine at a 10 mg dose. Drug 1 was taken for one day, followed by a wash‐out period of six days; and Drug 2 was taken for one day (eight‐day study duration in total). The participant group was considered homogenous in that they had both interventions. There were no exclusions or losses to follow‐up. Outcomes included measurement of 3D video‐oculography and participant recall of medical side effects. Inclusion criteria were specified, but not exclusion criteria.
Lee 1994 recruited and randomised 54 people presenting with acute unilateral sixth nerve palsy. Forty‐seven participants were followed up. There two groups: those receiving botulinum toxin (Dysport™) to the isolateral medial rectus muscle (22 participants), and those observed for recovery with no invasive treatment (25 participants). This trial was conducted at one site in the UK. The intervention arm comprised 13 males and 9 females, with a mean age of 63 years (range: 24 to 83). The control arm comprised 12 males and 13 females with a mean age of 61 years (range 24 to 86). This was a parallel design randomised controlled trial. Outcome measurements included range of ocular movements for abduction defect, angle of deviation measured by prism cover test, and field of binocular single vision. These groups were compared to each other for clinical diagnosis of recovery. A full recovery was defined as completely normal ocular rotations with full field of binocular single vision. Stable recovery was defined as normal binocular single vision with a minor asymptomatic abduction defect or a small asymptomatic vertical deviation. Non‐recovery was defined as a persisting esotropia in primary position with diplopia not controllable by normal amplitudes of fusional vergence. Two control participants were excluded due to change of diagnosis and four were lost to follow‐up. One participant from the botulinum toxin group was lost to follow‐up. Follow‐up ranged from 4 to 42 months. Both groups were considered homogenous as gender, age range, aetiology of sixth nerve palsy, duration of symptoms and laterality of palsy were similar across both groups. The mean deviation of control participants was 17.8 prism dioptres (PD); for botulinum toxin participants this was 28.6 PD. The difference in deviation across both groups was significant (P = 0.02). Three of the 22 participants who received botulinum toxin injection had one repeat injection.
Strupp 2003 recruited 18 participants with acquired downbeat nystagmus (nine females and nine males) aged 50 to 85 years. One person was subsequently excluded due to alcohol misuse. The trial was conducted at one site in Germany. This was a double‐masked randomised controlled trial with a cross‐over design, which compared one pharmacological intervention with placebo (3,4‐Diaminopyridine at 20 mg dose versus lactose). Drug 1 was taken for one day, followed by a wash‐out period of more than one week; and Drug 2 was taken for one day (nine‐day study duration in total). The participant group was considered homogenous in that they had both interventions. There was one exclusion (due to alcohol abuse), but no loss to follow‐up. Outcomes included measurement at baseline and follow‐up of 2D video‐oculography and participant reports of oscillopsia and recall of medical side effects. Inclusion and exclusion criteria were specified.
Thiagarajan 2014 randomised 12 participants with mild traumatic brain injury which happened more than one year previously. Participants aged on average 29 ± 3 years. The trial was conducted at one site in the USA. It was a single‐masked randomised controlled trial with a cross‐over design, which compared oculomotor rehabilitation versus sham treatment. Oculomotor treatment involved two sessions per week, each session lasting 60 minutes. Participants were provided with training of version and vergence eye movements and accommodation for 15 minutes each, interspersed with five‐minute rest periods. Sham treatment involved two sessions of basic reading tasks per week, each session lasting 60 minutes. Each treatment was delivered for a period of six weeks, with a one‐week washout period between treatments and after the second treatment. The participant group was considered homogenous in that they had both interventions. There were no exclusions or losses to follow‐up. Outcomes were measured at baseline, after the first treatment block and after the second treatment block. They included infrared eye recording of reading eye movements, measurement of reading rate, saccadic ratio, near point of convergence, binocular accommodative amplitude and use of the convergence insufficiency symptom scale questionnaire. Inclusion and exclusion criteria were specified.
Excluded studies
We excluded 14 studies (Barton 1994; Cifu 2014; Claassen 2013; Clement 2007; Dai 2003; Feil 2013; Gur 1992; Leigh 1991; Leivo 1996; Lorenz 2006; Metz 1988; Sharpe 2005; Strupp 2008; Zampieri 2009). For reasons of exclusion, please see the Characteristics of excluded studies table. Main reasons for exclusion were that the studies included conditions that were listed in our exclusion criteria such as multiple sclerosis and degenerative conditions, or that studies were not RCTs.
Risk of bias in included studies
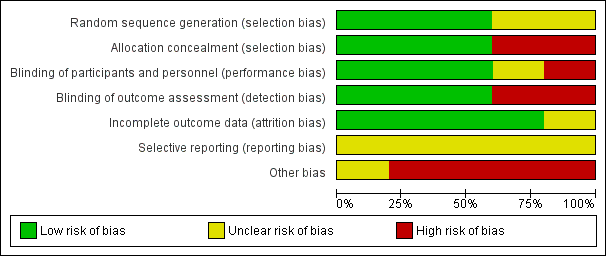
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
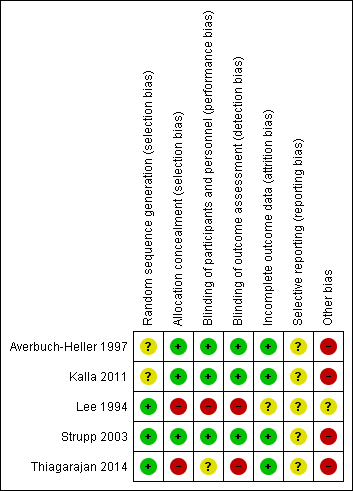
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
Sequence generation was unclear in two trials (Averbuch‐Heller 1997; Kalla 2011). It was evident that randomisation had occurred but the method was not stated. One study (Lee 1994) specified the use of a random number table; another (Strupp 2003) used a computer‐generated randomisation list; and a third (Thiagarajan 2014) used odd/even number randomisation for sequence generation. We considered these three trials to have low risk of bias for this domain.
Adequate prevention of knowledge of the allocated interventions was achieved in three trials (Averbuch‐Heller 1997; Kalla 2011; Strupp 2003). These trials used double‐masked designs, with investigators and participants masked to the allocation of interventions. We judged that allocation concealment did not take place in the other two studies (Lee 1994; Thiagarajan 2014).
Blinding
Masking of participants and personnel:
Adequate prevention of knowledge of the allocated interventions was achieved in three trials (Averbuch‐Heller 1997; Kalla 2011; Strupp 2003). These trials used double‐masked designs, with investigators and participants masked to the allocation of interventions. There was no masking (blinding) of investigators or participants in the other two trials (Lee 1994; Thiagarajan 2014). We judged Lee 1994 to be at high risk of both performance and detection bias. Thiagarajan 2014 was single‐masked (participants were masked to the intervention) but all testing and training was delivered by one investigator. We were less sure as to the extent to which this lack of masking would create performance bias that was independent of the effect of the intervention.
Masking of outcome assessors:
Adequate prevention of knowledge of the allocated interventions was achieved in three trials (Averbuch‐Heller 1997; Kalla 2011; Strupp 2003) in which the outcome assessors were masked to the allocation of interventions. There was no masking of outcome assessors for the remaining studies (Lee 1994; Thiagarajan 2014).
Incomplete outcome data
Incomplete outcome data was adequately addressed in all four trials, for which a low risk of bias was determined. All participants were accounted for throughout each trial, with outcome data provided for participants completing the trials and information provided for any participants lost to follow‐up or excluded. One study (Averbuch‐Heller 1997) reported one person lost to follow‐up and this participant was removed from analysis, with follow‐up equal across groups. One study (Strupp 2003) excluded one participant and removed the related results from analysis, with follow‐up equal across groups. In Lee 1994, follow‐up was clearly described and most participants were followed up (47/54, 87%). However, loss to follow‐up appeared to occur predominantly in the control group. It is unclear whether this could have impacted a comparison between control and intervention groups.
Selective reporting
It was difficult to reliably judge the extent of selective reporting due to lack of published protocols and the fact the trials were unregistered. Complicated analyses may also have been subject to selective reporting.
Other potential sources of bias
We considered the following additional sources of bias for cross‐over studies.
Was the cross‐over design suitable?
We judged the trials of pharmacological interventions possibly suitable for cross‐over design but the oculomotor rehabilitation study (Thiagarajan 2014) we were less sure that this was suitable as the training received at the first phase may influence the second phase.
Was there a carry‐over effect?
Possibly not in the drug trials where there was a washout phase but this was unclear because none of the studies addressed this issue directly.
Was only first period data are available?
Data were not reported fully and so neither first nor second period data were available.
Was the analysis correct?
This was difficult to judge. None of the studies reported appropriate effect estimates and standard error which would have permitted analysis in this review. One study reported findings for the intervention group only (Thiagarajan 2014).
Comparability of results with those from parallel‐group trials
There was no overlap in cross‐over and parallel‐group trials.
Effects of interventions
See: Summary of findings for the main comparison Botulinum toxin versus observation; Summary of findings 2 Pharmacological treatment
Given the differences between studies, it was not possible to provide a meta analysis of results. Each study is discussed individually.
Restitutive interventions
We did not identify any relevant studies of restitutive interventions.
Substitutive interventions
We identified one trial of botulinum toxin compared with observation for acute sixth nerve palsy (Lee 1994); see summary of findings Table for the main comparison.
Improvement in ocular motlity / Achievement of binocular single vision
At four months after entry into the trial, Lee 1994 reported full recovery with reduction in angle of deviation within 10 prism dioptres in 21/22 (95%) participants given botulinum toxin and 20/25 (80%) of control participants (risk ratio 1.19, 95% CI 0.96 to 1.48; number of participants (n) = 47; Table 1). These same participants also achieved binocular single vision. We judged the certainty of evidence as low, downgrading for risk of bias and imprecision. It was not possible to mask investigators or participants to allocation and there was variable follow‐up between groups.
| Study ID | Total participants | Primary: improved ocular motility | Secondary: improved binocular single vision | Secondary: improved symptoms | Secondary: adverse events |
| Lee 1994 | 47, parallel arm RCT 22 ‐ botulinum toxin 25 ‐ observation 6 month follow‐up | 21 (95.5%) ‐ botulinum toxin 20 (80%) ‐ observation | Success: 21 (95.5%) ‐ botulinum toxin 20 (80%) ‐ observation Partial: 3 (12%) ‐ observation Fail: 1 (4.5%) ‐ botulinum toxin 2 (8%) ‐ observation | 21 (95.5%) ‐ botulinum toxin 20 (80%) ‐ observation | 9% ptosis 18% vertical deviation |
RCT: randomised controlled trial
Adverse effects
In the injection group only, there were cases of transient ptosis in 2/22 (9%) of participants, and transient vertical deviation in 4/22 (18%). In total the risk of complications per injection was 24% and the risk of complications per participant was 27%.
Quality of life / Improvement in functional ability
The study did not report on functional ability and quality of life.
Compensatory interventions
We identified one cross‐over trial of a compensatory intervention (Thiagarajan 2014). This trial compared oculomotor rehabilitation with sham training in 12 people with mild traumatic brain injury (Table 2). We have some doubts as to whether a cross‐over study design is appropriate since this is an intervention that may have a permanent effect, i.e. there may be an interaction between phase and effect. The data were not analysed in a way that permitted analysis of phase 1 only. The cross‐over study design appeared to be ignored in the analysis.
| Study ID | Total participants | Primary: improved ocular motility | Secondary: improved functional vision | Secondary: improved symptoms | Secondary: adverse events |
| Thiagarajan 2014 | 12, cross‐over RCT 13‐week follow‐up | Baseline 2.1 saccadic ratio reducing to 1.7, P < 0.05 — OM rehabilitation Control group change not reported | Reading rate: Baseline 142 (10) wpm improving to 177 (14). Reading level: Baseline 4.1 (0.7) grade level improving to 6.3 (1.2), P < 0.01 Fixations per 100 words: Baseline 164 (10) improving to 135 (11), P = 0.02 Regressions per 100 words: Baseline 30 (3) improving to 23 (4) Control group changes not reported [means (SEM)] | Improved for OM rehabilitation. Control group changes not reported | Nil reported |
SEM: standard error mean
OM: oculo motor
RCT: randomised controlled trial
WPM: words per minute
Improvement in ocular motility
With these caveats in mind, the study reported a drop in mean saccade ratio with oculomotor rehabilitation from a mean value of 2.1 to 1.7. This change was reported to be statistically significant (P < 0.05). The saccade ratio was defined as the number of tracking saccades executed divided by the number of test target step displacements, with a ratio of 1 being optimal. The saccade ratio was tested on single and multiple lines. The drop in reading single lines was from 2.7 to 2.2 but this change reported as not statistically significant (no P value reported).
Improvement in functional ability
Reading rate (words per minute) increased with oculomotor rehabilitation from 142 (standard deviation (SD) 10) to 177 (SD 14) (P < 0.01). Reading levels increased by two grades, from 4.1 (SD 0.7) before oculomotor training to 6.3 (SD1.2) after training. There was also a reduced number of fixations per 100 words, from 164 (SD 10) to 135 (SD 11). Binocular accommodative amplitude and near point of convergence improved, alongside a reduction in near fixation symptoms.
Data from the sham training group were not reported but it was stated that the change in parameters was non‐significant.
Achievement of binocular single vision / Quality of life / Adverse effects
This study did not report on binocular single vision, quality of life or adverse effects.
Summary
We considered this evidence to be very low‐certainty. We downgraded by two levels because of the study limitations described above, in particular the limitations of a cross‐over study design for this intervention, and the lack of data reported from the control (no intervention) group. We downgraded by one level for imprecision due to the small number of participants.
Pharmacological interventions
See summary of findings Table 2.
We identified three cross‐over studies of pharmacological interventions (Averbuch‐Heller 1997; Kalla 2011; Strupp 2003).
These studies investigated two classes of pharmacological interventions: GABAergic drugs (gabapentin; baclofen) and aminopyridines (4‐aminopyridines (AP); 3,4‐diaminopyridine (DAP)).
One study compared gabapentin and baclofen (Averbuch‐Heller 1997, n = 21); one study compared 3,4‐DAP with placebo (Strupp 2003, n = 17); and one study compared 4‐AP with 3,4‐DAP (Kalla 2011, n = 8).
The results are summarised in Table 3.
| Study ID | Total participants | Primary: improved ocular motility | Secondary: improved visual acuity | Secondary: improved symptoms | Secondary: adverse events |
| Averbuch‐Heller 1997 | 21, crossover RCT 15 ‐ pendular 6 ‐ jerk 6‐week trial duration | 15 pendular ‐ gabapentin | 15 pendular ‐ gabapentin 1 jerk ‐ gabapentin 1 jerk ‐ baclofen | 6 pendular ‐ gabapentin 1 jerk ‐ gabapentin 1 jerk ‐ baclofen | 1 drug intolerance ‐ gabapentin 4 drug intolerance ‐ baclofen 3 ataxia ‐ gabapentin 2 ataxia ‐ baclofen |
| Kalla 2011 | 8, crossover RCT 8 ‐ downbeat 8‐day trial duration | Baseline ‐6.04; 45 mins ‐1.58; 90 mins ‐1.21 (4‐aminopyridine) Baseline ‐5.68; 45 mins ‐3.29; 90 mins ‐2.96 (3,4‐diaminopyridine) | ‐ | ‐ | All with mild paraesthesia |
| Strupp 2003 | 17, crossover RCT 17 ‐ downbeat 16‐day trial duration | Baseline 7.2 ± 4.2 °/sec reducing to 3.1 ± 2.5 (3,4‐diaminopyridine) Baseline 7.4 ± 4.1 °/sec reducing to 7.3 ± 3.7 (placebo) | ‐ | 10 ‐ reduced symptoms (3,4‐diaminopyridine) 0 ‐ reduced symptoms (placebo) | 3 ‐ mild paraesthesia (3,4‐diaminopyridine) 1 ‐ nausea/headache (3,4‐diaminopyridine) |
RCT: randomised controlled trial
Gabapentin versus baclofen
Improvement in ocular motility
Averbuch‐Heller 1997 included 21 participants but reported results separately for acquired pendular nystagmus (n = 15) and jerk nystagmus (n = 6). It was difficult to extract data on comparative effectiveness from the study report, as the data were only reported in scatter plots separately by intervention and comparator. We have to rely on the authors' statements in the text which were as follows:
Pendular nystagmus
-
"...median eye speed was reduced in all three planes by gabapentin, during viewing of the near or far targets; the effects were similar in darkness. The predominant frequency of oscillation was reduced, on average, by less than 9% by gabapentin, but this reduction was consistent and statistically significant (P < 0.05) in the horizontal and vertical planes during viewing of far and near visual targets."
-
"...median eye speed was reduced significantly (P < 0.005) only in the vertical plane by baclofen. Baclofen produced no significant changes in the predominant frequency of APN."
-
"Neither drug caused any significant change in the gain of saccades, smooth pursuit or the VOR [vestibulo‐ocular reflex], or changes in saccadic velocity, or conjugacy, similar to reported effects in normal subjects. This was the case even in those patients who showed substantial reduction of nystagmus with gabapentin."
Jerk nystagmus
-
"For this group of patients, neither drug produced a significant change in median, slow‐phase eye speed. Changes in individual patients depended on whether they viewed the near or far targets."
As there were fewer participants in this group, it is difficult to know whether the lack of statistical significance was attributable to a different effect in this subgroup or to the different number of participants. Results for individual participants were reported but these are difficult to interpret.
Achievement of binocular single vision
This was not reported but the results of the visual acuity tests were as follows.
Pendular nystagmus
-
"...there was a significant (P < 0.006) improvement of visual acuity with gabapentin; this improvement was greater during testing with the near card."
-
"...baclofen produced no significant change in visual acuity measured at near or at far."
Jerk nystagmus
-
"... neither drug produced a significant change in visual acuity."
Reduction in participant‐reported symptoms
Results for oscillopsia were as follows.
Pendular nystagmus
-
"Twelve patients reported some illusory motion of the visual target before treatment, and gabapentin reduced this oscillopsia in 6."
-
"...Baclofen changed the direction of oscillopsia, without reducing it, in 2 patients."
Jerk nystagmus
-
"Four patients reported some illusory motion of the visual target before treatment; neither drug influenced oscillopsia in 4."
Quality of life
This outcome was not reported but ten participants with pendular nystagmus chose to continue treatment with gabapentin and one chose to continue treatment with baclofen. Two participants with jerk nystagmus chose to continue treatment with gabapentin and one chose to continue with baclofen.
Adverse effects
No significant adverse events were reported. Drug intolerance was reported in one person in the gabapentin group and four participants in the baclofen group. Adverse events of increased ataxia were reported in three participants in the gabapentin group and two participants in the baclofen group.
Improvement in functional ability
This outcome was not reported.
Summary
This study provides very low‐certainty evidence that gabapentin is better than baclofen and that these effects may be different in pendular and jerk nystagmus. The data reported by this study did not allow us to estimate the size of the effect. The lack of a formal subgroup analysis means that it is unclear if the difference between the two types of nystagmus may be a chance finding. The evidence is low‐certainty because of the small numbers of participants (for which we downgraded by two levels for imprecision) and risk of bias (it was a cross‐over study with analysis that did not permit estimation of effect size).
3,4‐diaminopyridine versus placebo
One study (Strupp 2003) compared 3,4‐DAP (20 mg) with placebo (lactose) in 17 people with downbeat nystagmus. Assessments were made 30 minutes after taking the drug. Comparative measures of effect were not reported, and could not be calculated with the information given, but statistical tests accounted for the cross‐over design (by two‐way analysis of variance).
Improvement in ocular motility
In the 3,4‐DAP group there was a reduction in mean peak slow‐phase velocity from 7.2 degrees/second (SD 4.2) to 3.1 degrees/second (SD 2.5), which is considered clinically important. There was no change in the lactose group (7.4 degrees/second (SD 4.1) to 7.3 decrees/second (SD 3.7)). This difference between the groups was reported to be statistically significant (P < 0.001).
Reduction in participant‐reported symptoms
The authors report that in 10 of the 17 participants, mean peak slow‐phase velocity decreased by more than 50%. These 10 people also reported having less oscillopsia.
Adverse effects
No significant adverse events were reported. Nine participants continued treatment. Three participants reported transient side effects of minor perioral/distal paraesthesia.
Outcomes not reported
-
Achievement of binocular single vision
-
Improvement in functional ability
-
Quality of life
Summary
This study provides very low‐certainty evidence that 3,4‐DAP may reduce the mean peak slow‐phase velocity in people with downbeat nystagmus. We judged the evidence to be very low‐certainty because of the small size of the study (for which we downgraded by two levels for imprecision) and risk of bias (it was a cross‐over study with no comparative measure of effect reported).
4‐aminopyridines versus 3,4‐diaminopyridine
One study (Kalla 2011) compared 4‐AP and 3,4‐DAP (both given as a single 10 mg dose) in eight people with downbeat nystagmus. Assessments were made 45 and 90 minutes after drug administration. Comparative measures of effect were not reported, and could not be calculated with the information given, but statistical tests probably accounted for the cross‐over design ("repeated measurement analysis of variances").
Improvement in ocular motility
The authors report the following.
-
Mean slow‐phase velocity decreased from 5.68 degrees/second to: 3.29 degrees/second at 45 minutes, and 2.96 degrees/second at 90 minutes in people who had taken 3,4‐DAP (P < 0.01).
-
Mean slow‐phase velocity decreased from 6.04 degrees/second to: 1.58 degrees/second at 45 minutes, and 1.21 degrees/second at 90 minutes in people who had taken 4‐AP (P < 0.00001).
-
The difference between the two drugs at 45 and 90 minutes was statistically significant (P < 0.05).
Adverse effects
"All 8 patients reported mild paraesthesias from 30 minutes to 2 hours after ingestion of both medications. No other side effects were reported."
Outcomes not reported
-
Achievement of binocular single vision
-
Reduction in participant‐reported symptoms
-
Improvement in functional ability
-
Quality of life
Summary
This study provides low‐certainty evidence that both 3,4‐DAP and 4‐AP may reduce the mean slow‐phase velocity in people with downbeat nystagmus. This effect may be stronger with 4‐AP. We judged the evidence to be very low‐certainty because of the small size of the study (for which we downgraded by two levels for imprecision) and risk of bias (it was a cross‐over study with analysis that did not permit estimation of effect size).
Timing of interventions
Three trials (Averbuch‐Heller 1997; Kalla 2011; Strupp 2003) did not specify the timing at which interventions were provided after onset of the nystagmus. Therefore the timing of intervention cannot be classified as acute or long‐term, and its effect in the treatment of nystagmus cannot be determined. In Lee 1994, participants were recruited at acute presentation to hospital emergency department. Effect of acute intervention can be considered for this trial (see section 2 above). In Thiagarajan 2014, participants were recruited at least one year post‐incident to ensure that any subsequent changes during training were not secondary to any natural recovery period (estimated by the authors as being up to six to nine months post‐incident). Effect of late‐timed intervention can be considered for this trial (see section 3 above).
Discussion
Summary of main results
Following acquired brain injury, a variety of eye movement disorders may occur including: strabismus, ocular cranial nerve palsies, supranuclear and internuclear gaze palsies, nystagmus, saccadic and smooth pursuit palsies. The literature on interventions for these eye movement disorders consists predominantly of retrospective studies, cohort studies and case series, which are useful for describing various treatment options but do not allow the estimation of effect size for recommendations based on clinical and cost effectiveness.
We found five randomised controlled trials (RCTs), with a total of 116 participants, that were eligible for inclusion in this review. These trials included conditions of acquired nystagmus, sixth cranial nerve palsy and ocular motility defects induced by traumatic brain injury. We did not identify any relevant studies of restitutive interventions. In view of the differences between studies, it was not possible to provide a meta analysis of results.
We identified one trial of a substitutive intervention for acute sixth nerve palsy. At four months after entry into the trial, people given botulinum toxin were more likely to make a full recovery (with reduction in angle of deviation within 10 prism dioptres), compared with observation (low‐certainty evidence). These same participants also achieved binocular single vision and had improved visual symptoms. In the injection group only, there were cases of transient ptosis in 2/22 (9%) of participants, and transient vertical deviation in 4/22 (18%); and there was a total complication rate of 24% per injection and 27% per participant. Functional ability and quality of life were not reported. We judged the certainty of evidence as low, downgrading for risk of bias and imprecision.
We identified one cross‐over trial of a compensatory intervention. This study took place in the USA. Oculomotor rehabilitation was compared with sham training in 12 people with mild traumatic brain injury at least one year post‐incident. We judged the evidence from this study to be very low‐certainty. The study was small, data for the sham training group were not fully reported, and it was unclear if a cross‐over study design was appropriate as this is an intervention that may be expected to have a permanent effect.
We identified three cross‐over studies of pharmacological interventions, which took place in Germany and the USA. These studies investigated two classes of pharmacological interventions: GABAergic drugs (gabapentin, baclofen) and aminopyridines (4‐aminopyridines (AP), 3,4‐diaminopyridine (DAP)). We judged the evidence from all three studies as very low‐certainty because of the small numbers of participants (for which we downgraded two levels for imprecision) and risk of bias (they were cross‐over studies with data reported in a way that precluded estimation of effect size). One study compared gabapentin (up to 900 mg/day) and baclofen (up to 30 mg/day) in 21 people with pendular and jerk nystagmus and followed up to two weeks. This study provides very low‐certainty evidence that gabapentin is better than baclofen and that these effects may be different in pendular and jerk nystagmus. There was no formal subgroup analysis, so it is unclear whether the difference between the two types of nystagmus was a chance finding or not. No significant adverse events were reported. Drug intolerance was reported in one person from the gabapentin group and four participants from the baclofen group. Increased ataxia were reported in three participants from the gabapentin group and two participants from the baclofen group. One study compared a single dose of 3,4‐DAP (20mg) with placebo in 17 people with downbeat nystagmus. Assessments were made 30 minutes after taking the drug. This study provides very low‐certainty evidence that 3,4‐DAP may reduce the mean peak slow‐phase velocity in people with downbeat nystagmus and this may be accompanied by less oscillopsia. Three participants reported transient side effects of minor perioral/distal paraesthesia. One study compared a single 10 mg dose of either 4‐AP or 3,4‐DAP, in eight people with downbeat nystagmus. Assessments were made 45 and 90 minutes after drug administration. This study provides very low‐certainty evidence that both 3,4‐DAP and 4‐AP may reduce the mean slow‐phase velocity in people with downbeat nystagmus. This effect may be stronger with 4‐AP.
Overall completeness and applicability of evidence
Overall the evidence on interventions for eye movement disorders due to acquired brain injury is not complete. We identified only five relevant studies. These studies covered a heterogenous group of conditions and interventions. They had limitations in their design which means that it is difficult to draw firm conclusions as to the effects of the treatments studied. Furthermore, no information was obtained on the cost of treatment or on measure of participant or parent satisfaction relating to treatment options and effectiveness. It was, however, possible to describe the outcome of treatment in each trial and ascertain the occurrence of adverse events.
Certainty of the evidence
We judged the certainty of evidence for all outcomes to be very low or low primarily due to risk of bias in the included studies and imprecise effect estimates. Four of the five studies had a cross‐over design but relevant estimates of effect from an appropriate analyses were not reported in these studies. The included studies were small.
Potential biases in the review process
We included five trials in this review. There may be a risk of publication bias regarding other conducted, but not registered or published, trials. We systematically searched the available literature and did not identify further completed trials meeting our inclusion criteria.
We made some modifications to the protocol but none of these were likely to bias the results of the review. The protocol amendments are specified in Differences between protocol and review. The majority of the changes relate to methods that were not implemented because of a lack of data. The main qualitative change we implemented was to consider the different types of interventions separately. It is unlikely that this introduced bias, particularly as the data precluded formal meta‐analysis. Other modifications included preparing a summary of findings table and GRADE assessment, as required by updated Cochrane methods. All outcomes pre‐specified in the protocol were included in the summary of findings table.
Agreements and disagreements with other studies or reviews
Similar results to those in this systematic review were reported in another Cochrane systematic review on interventions for disorders of eye movement in participants with stroke (Pollock 2011). Their review included two trials of pharmacological treatment for nystagmus from which they concluded there was insufficient evidence to reach conclusions about the effectiveness of interventions for participants with eye movement disorders after stroke, and they recommended further high‐quality research. Three of the five trials included in our systematic review were also trials of pharmacological treatments for nystagmus. We also found insufficient high‐quality evidence for the treatment of eye movement disorders due to acquired brain injury.
There have been two recent reviews of the role of botulinum toxin in infantile esotropia (Issaho 2017) and strabismus (Mahan 2017). In both reviews only non‐comparative and non‐randomised comparative studies were included, which would not have been eligible for the current review. Both reviews concluded that the overall success rate of botulinum toxin in these groups of patients was 70% to 80%.
It is important to acknowledge that certain interventions do not warrant randomised controlled trials to prove their effectiveness. For example, Fresnel prisms are an established intervention for correction of double vision (diplopia). These are temporary plastic press‐on prisms that can be applied to spectacles and are used in selected cases of diplopia to correct the misalignment of the eyes that causes this symptom (Gunton 2012; Haller 2014). Although not a restorative treatment, these prisms alleviate the symptom of diplopia and are a clinical and cost‐effective treatment option; and, likely, unethical to withhold as a treatment option. However, for persistent eye movement problems requiring long‐term management, further high‐quality randomised controlled trials are required of the various options for pharmacological and surgical treatment of eye movement disorders.

Study flow diagram
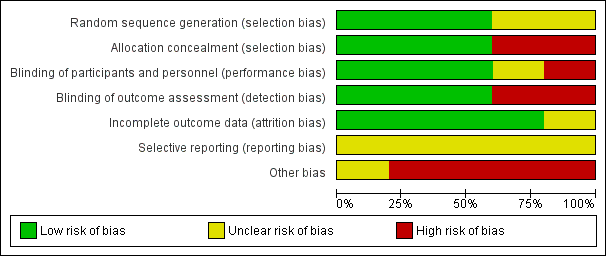
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
| Botulinum toxin versus observation in people with sixth nerve palsy | ||||||
| Participant or population: people with sixth nerve palsy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with observation | Risk with botulinum toxin | |||||
| Improvement in ocular motility (ocular alignment ≤ 10 prism dioptres). Follow‐up to 4 months | 800 per 1,000 | 952 per 1,000 | RR 1.19 | 47 | ⊕⊕⊝⊝ | |
| Achievement of binocular single vision (fusion and stereopsis present). Follow‐up to 4 months | 800 per 1,000 | 952 per 1,000 | RR 1.19 | 47 | ⊕⊕⊝⊝ | |
| Improvement in functional ability | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Adverse events. Follow‐up to 4 months | In the injection group only, there were 2/22 (9%) cases of transient ptosis and 4/22 (18%) with transient vertical deviation, with a total complication rate of 24% per injection and 27% per participant. All adverse events recovered within the follow‐up time period of 6 months with no lasting adverse effects. | 47 (1 RCT) | ⊕⊕⊝⊝ | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for risk of bias (investigators were aware of the randomisation and it was not possible to mask investigators or participants to the allocation and there was variable follow‐up between groups) and downgraded one level for imprecision (confidence intervals include 1, no effect). | ||||||
| Pharmacological treatments (Gabapentin / Baclofen / 3,4‐DAP / 4‐AP) for people with acquired nystagmus | |||
| Participant or population: people with acquired nystagmus | |||
| Comparison | Main findings | № of participants | Certainty of the evidence |
| Gabapentin up to 900 mg/day) versus baclofen (up to 30 mg/day). Follow‐up 2 weeks | Gabapentin may work better than baclofen in improving ocular motility and reducing participant‐reported symptoms (oscillopsia). These effects may be different in pendular and jerk nystagmus but there was no formal subgroup analysis so it is unclear if the difference between the two types of nystagmus was a chance finding. Quality of life was not reported but ten participants with pendular nystagmus chose to continue treatment with gabapentin and one with baclofen. Two participants with jerk nystagmus chose to continue treatment with gabapentin and one with baclofen. Drug intolerance was reported in one person for gabapentin and four participants for baclofen. Increased ataxia was reported in three participants for gabapentin and two participants for baclofen. | 21 | ⊕⊝⊝⊝ |
| 3,4‐DAP (20 mg, single dose) versus placebo. Assessments made 30 minutes after taking the drug or placebo | 3,4‐DAP may reduce the mean peak slow‐phase velocity in people with downbeat nystagmus. In 10 of the 17 participants, mean peak slow‐phase velocity decreased by more than 50% and these 10 people reported having less oscillopsia. No significant adverse events were reported. Nine participants continued treatment. Three participants reported transient side effects of minor perioral/distal paraesthesia. | 17 | ⊕⊝⊝⊝ |
| 4‐AP (10 mg, single dose) versus 3,4‐DAP (10 mg, single dose) Assessments made at 45 and 90 minutes after taking the drug | 3,4 DAP and 4‐AP may reduce mean slow‐phase velocity in people with downbeat nystagmus. This effect may be stronger with 4‐AP. All participants reported mild paraesthesias with both medications. | 8 | ⊕⊝⊝⊝ |
| GRADE Working Group grades of evidence | |||
| 1 Downgraded two levels for imprecision (due to small number of participants) and one level for serious risk of bias (cross‐over study with analysis that did not permit estimation of effect size). | |||
| Study ID | Total participants | Primary: improved ocular motility | Secondary: improved binocular single vision | Secondary: improved symptoms | Secondary: adverse events |
| Lee 1994 | 47, parallel arm RCT 22 ‐ botulinum toxin 25 ‐ observation 6 month follow‐up | 21 (95.5%) ‐ botulinum toxin 20 (80%) ‐ observation | Success: 21 (95.5%) ‐ botulinum toxin 20 (80%) ‐ observation Partial: 3 (12%) ‐ observation Fail: 1 (4.5%) ‐ botulinum toxin 2 (8%) ‐ observation | 21 (95.5%) ‐ botulinum toxin 20 (80%) ‐ observation | 9% ptosis 18% vertical deviation |
| RCT: randomised controlled trial | |||||
| Study ID | Total participants | Primary: improved ocular motility | Secondary: improved functional vision | Secondary: improved symptoms | Secondary: adverse events |
| Thiagarajan 2014 | 12, cross‐over RCT 13‐week follow‐up | Baseline 2.1 saccadic ratio reducing to 1.7, P < 0.05 — OM rehabilitation Control group change not reported | Reading rate: Baseline 142 (10) wpm improving to 177 (14). Reading level: Baseline 4.1 (0.7) grade level improving to 6.3 (1.2), P < 0.01 Fixations per 100 words: Baseline 164 (10) improving to 135 (11), P = 0.02 Regressions per 100 words: Baseline 30 (3) improving to 23 (4) Control group changes not reported [means (SEM)] | Improved for OM rehabilitation. Control group changes not reported | Nil reported |
| SEM: standard error mean | |||||
| Study ID | Total participants | Primary: improved ocular motility | Secondary: improved visual acuity | Secondary: improved symptoms | Secondary: adverse events |
| Averbuch‐Heller 1997 | 21, crossover RCT 15 ‐ pendular 6 ‐ jerk 6‐week trial duration | 15 pendular ‐ gabapentin | 15 pendular ‐ gabapentin 1 jerk ‐ gabapentin 1 jerk ‐ baclofen | 6 pendular ‐ gabapentin 1 jerk ‐ gabapentin 1 jerk ‐ baclofen | 1 drug intolerance ‐ gabapentin 4 drug intolerance ‐ baclofen 3 ataxia ‐ gabapentin 2 ataxia ‐ baclofen |
| Kalla 2011 | 8, crossover RCT 8 ‐ downbeat 8‐day trial duration | Baseline ‐6.04; 45 mins ‐1.58; 90 mins ‐1.21 (4‐aminopyridine) Baseline ‐5.68; 45 mins ‐3.29; 90 mins ‐2.96 (3,4‐diaminopyridine) | ‐ | ‐ | All with mild paraesthesia |
| Strupp 2003 | 17, crossover RCT 17 ‐ downbeat 16‐day trial duration | Baseline 7.2 ± 4.2 °/sec reducing to 3.1 ± 2.5 (3,4‐diaminopyridine) Baseline 7.4 ± 4.1 °/sec reducing to 7.3 ± 3.7 (placebo) | ‐ | 10 ‐ reduced symptoms (3,4‐diaminopyridine) 0 ‐ reduced symptoms (placebo) | 3 ‐ mild paraesthesia (3,4‐diaminopyridine) 1 ‐ nausea/headache (3,4‐diaminopyridine) |
| RCT: randomised controlled trial | |||||

