Cirugía de mama por cáncer de mama metastásico
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
| |
| Participants | Inclusion criteria:
Exclusion criteria:
| |
| Interventions |
| |
| Outcomes | Primary outcome:
Secondary outcomes:
| |
| Notes | ClinicalTrials.gov register number: NCT00193778 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computerized randomisation will be carried out at the central office after confirmation of eligibility and obtaining informed written consent. Randomisation will be stratified by
Source: ClinicalTrials.gov, NCT00193778, tabular view, last update 18 October 2016. The method of randomisation was appropriate. |
| Allocation concealment (selection bias) | Low risk | "computer‐generated randomisation sequence and a telephone call to the central research office" Allocation concealment was assured by a central office. |
| Blinding of participants and personnel (performance bias) ‐ OS | Low risk | No blinding because it is a surgical intervention. The review authors judge that the outcome overall survival is not likely to be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) ‐ Local PFS | High risk | No blinding because it is a surgical intervention. The review authors judge that this outcome is likely to be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) ‐ Distant PFS | High risk | No blinding because it is a surgical intervention. The review authors judge that this outcome is likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) ‐ OS | Low risk | No blinding because it is a surgical intervention. The review authors judge that the outcome overall survival is not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) ‐ Local PFS | High risk | No blinding because it is a surgical intervention. The review authors judge that this outcome is likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) ‐ Distant PFS | High risk | No blinding because it is a surgical intervention. The review authors judge that this outcome is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) ‐ OS | Unclear risk | There is no information about missing/censored data. |
| Incomplete outcome data (attrition bias) ‐ Local PFS | Unclear risk | There is no information about missing/censored data. |
| Incomplete outcome data (attrition bias) ‐ Distant PFS | Unclear risk | There is no information about missing/censored data. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available, and all of the study’s prespecified primary outcomes that are of interest in the review were reported in the prespecified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods |
| |
| Participants | Inclusion criteria:
Exclusion criteria:
| |
| Interventions |
| |
| Outcomes | Primary:
Secondary:
| |
| Notes | ClinicalTrials.gov register number: NCT00557986 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | As described in the text: "... is a phase III randomized controlled trial ..." Probably done, but we did not find accurate information in the text. We emailed the author but were unable to obtain a precise answer on random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No detailed information in the published text. We emailed the author but were unable to obtain a precise answer on allocation concealment. Insufficient information to allow judgement |
| Blinding of participants and personnel (performance bias) ‐ OS | Low risk | No blinding because it is a surgical intervention. The review authors judge that the outcome overall survival is not likely to be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) ‐ Local PFS | High risk | No blinding because it is a surgical intervention. The review authors judge that this outcome is likely to be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) ‐ Distant PFS | High risk | No blinding because it is a surgical intervention. The review authors judge that this outcome is likely to be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) ‐ Toxicity | Low risk | Toxicity was evaluated by 30‐day mortality. The review authors judge that this outcome is not influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) ‐ OS | Low risk | No blinding because it is a surgical intervention. The review authors judge that the outcome overall survival is not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) ‐ Local PFS | High risk | No blinding because it is a surgical intervention. The review authors judge that this outcome is likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) ‐ Distant PFS | High risk | No blinding because it is a surgical intervention. The review authors judge that this outcome is likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) ‐ Toxicity | Low risk | Toxicity was evaluated by 30‐day mortality. The review authors judge that this outcome is not influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) ‐ OS | Unclear risk | There is no information about missing/censored data. |
| Incomplete outcome data (attrition bias) ‐ Local PFS | Unclear risk | There is no information about missing/censored data. |
| Incomplete outcome data (attrition bias) ‐ Distant PFS | Unclear risk | There is no information about missing/censored data. |
| Incomplete outcome data (attrition bias) ‐ Toxicity | Unclear risk | There is no information about missing/censored data. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available, and all of the study’s prespecified primary outcomes that are of interest in the review were reported in the prespecified way. |
| Other bias | High risk | There was an imbalance between groups at baseline. There was a higher proportion of women who were younger than 55 years of age, had ER‐positive and HER2‐negative tumours, and had single bone metastases in the group undergoing breast surgery. It is likely that these differences between the two groups could have influenced the result. |
ER: oestrogen receptor
OS: overall survival
PFS: progression‐free survival
PR: progesterone receptor
SGOT: serum glutamic oxaloacetic transaminase
SGPT: serum glutamic pyruvic transaminase
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| This study was terminated prior to enrolment due to lack of accrual and no further funding. | |
| This study was terminated prior to enrolment due to low accrual rate. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Primary Operation in SYnchronous meTastasized InVasivE Breast Cancer (POSYTIVE) |
| Methods |
|
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Local therapy consists of lumpectomy or mastectomy with or without radiotherapy (according to centre tumour board decision) with a resection‐free margin of at least 1 mm or more demonstrated on paraffin embedded histological sections. Intraoperative frozen sections are allowed but not definitive for margin assessment. Sentinel node biopsy may be performed and must always be followed by axillary dissection of level I and II (axillary surgery level I and II is mandatory). |
| Outcomes | Primary outcome measure:
Secondary outcome measures:
|
| Starting date | May 2010 |
| Contact information | |
| Notes |
| Trial name or title | Early surgery or standard palliative therapy in treating patients with stage IV breast cancer |
| Methods |
|
| Participants | Disease characteristics:
Patient characteristics:
|
| Interventions | Patients undergo surgery comprising BCT or total mastectomy according to patient and treating physician preference. Surgery is to occur no later than 10 weeks after completion of 32 weeks of systemic therapy. Free surgical margins must be achieved with re‐excision or mastectomy for patients undergoing BCT. After completion of BCT, patients undergo radiotherapy once a day, 5 days per week. Patients who had mastectomy undergo radiotherapy at the discretion of treating physician. |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | February 2011 |
| Contact information | Seema Khan ‐ [email protected] |
| Notes |
| Trial name or title | The effect of primary surgery in patients with stage IV breast cancer with bone metastasis only |
| Methods |
|
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Surgery to primary tumour: mastectomy, lumpectomy |
| Outcomes |
|
| Starting date | April 2014 |
| Contact information | |
| Notes |
| Trial name or title | A randomized controlled trial comparing primary tumour resection plus systemic therapy with systemic therapy alone in metastatic breast cancer (PRIM‐BC): Japan Clinical Oncology Group Study JCOG1017 |
| Methods |
|
| Participants | Inclusion criteria:
First registration
Second registration (after primary chemotherapy)
Exclusion criteria: First registration (no exclusion criteria at second registration)
|
| Interventions | Primary tumour resection plus systemic therapy |
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Starting date | November 2011 |
| Contact information | hiwata@aichi‐cc.jp |
| Notes | doi: 10.1093/jjco/hys120; protocol ID UMIN000005586 |
ACOSOG: American College of Surgeons Oncology Group
BCT: breast‐conserving therapy
CEA: carcinoembryonic antigen
CT: computed tomography
DCIS: ductal carcinoma in situ
ECOG: Eastern Cooperative Oncology Group
FISH: fluorescent in situ hybridisation
IHC: immunohistochemistry
MRI: magnetic resonance imaging
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 2 | 624 | Hazard Ratio (Random, 95% CI) | 0.83 [0.53, 1.31] |
| Analysis 1.1  Comparison 1 Systemic treatment plus surgery versus systemic treatment, Outcome 1 Overall survival. | ||||
| 2 Overall survival ‐ HER2 status Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Systemic treatment plus surgery versus systemic treatment, Outcome 2 Overall survival ‐ HER2 status. | ||||
| 2.1 HER2‐positive | 2 | 192 | Hazard Ratio (Random, 95% CI) | 0.85 [0.48, 1.50] |
| 2.2 HER2‐negative | 2 | 421 | Hazard Ratio (Random, 95% CI) | 0.84 [0.50, 1.40] |
| 3 Overall survival ‐ ER status Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Systemic treatment plus surgery versus systemic treatment, Outcome 3 Overall survival ‐ ER status. | ||||
| 3.1 ER‐positive | 2 | 426 | Hazard Ratio (Random, 95% CI) | 0.83 [0.48, 1.42] |
| 3.2 ER‐negative | 2 | 200 | Hazard Ratio (Random, 95% CI) | 1.04 [0.70, 1.55] |
| 4 Overall survival ‐ only bone metastasis Show forest plot | 2 | 226 | Hazard Ratio (Random, 95% CI) | 0.91 [0.49, 1.69] |
| Analysis 1.4  Comparison 1 Systemic treatment plus surgery versus systemic treatment, Outcome 4 Overall survival ‐ only bone metastasis. | ||||
| 5 Progression‐free survival Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Systemic treatment plus surgery versus systemic treatment, Outcome 5 Progression‐free survival. | ||||
| 5.1 Local progression‐free survival | 2 | Hazard Ratio (Random, 95% CI) | 0.22 [0.08, 0.57] | |
| 5.2 Distant progression‐free survival | 1 | Hazard Ratio (Random, 95% CI) | 1.42 [1.08, 1.86] | |

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Systemic treatment plus surgery versus systemic treatment, outcome: 1.1 Overall survival.
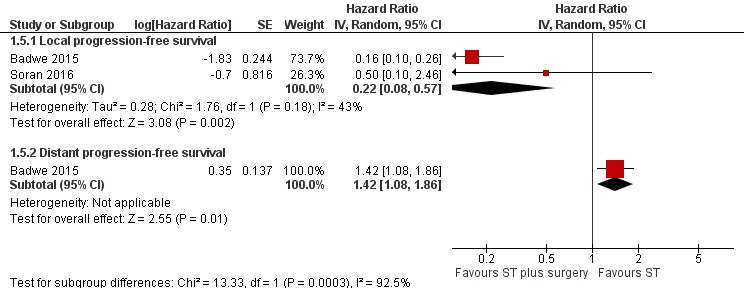
Forest plot of comparison: 1 Systemic treatment plus surgery versus systemic treatment, outcome: 1.5 Progression‐free survival.

Comparison 1 Systemic treatment plus surgery versus systemic treatment, Outcome 1 Overall survival.
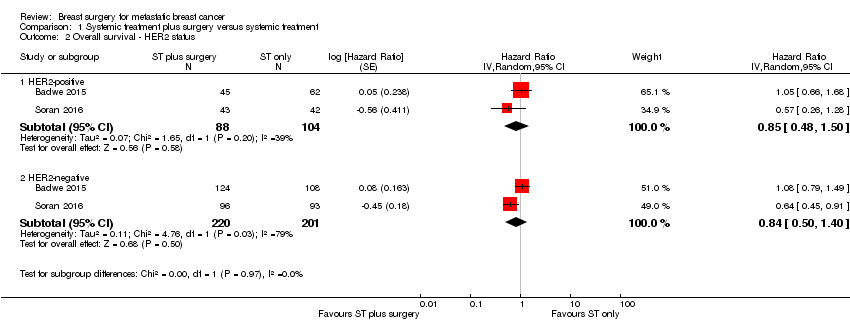
Comparison 1 Systemic treatment plus surgery versus systemic treatment, Outcome 2 Overall survival ‐ HER2 status.

Comparison 1 Systemic treatment plus surgery versus systemic treatment, Outcome 3 Overall survival ‐ ER status.

Comparison 1 Systemic treatment plus surgery versus systemic treatment, Outcome 4 Overall survival ‐ only bone metastasis.

Comparison 1 Systemic treatment plus surgery versus systemic treatment, Outcome 5 Progression‐free survival.
| Breast surgery plus systemic treatment compared to systemic treatment for metastatic breast cancer | ||||||
| Patient or population: metastatic breast cancer | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with systemic treatment | Risk with breast surgery plus systemic treatment | |||||
| Overall survival at 2 years Follow‐up: range 23 months to 40 months | Study population | HR 0.83 | 624 | ⊕⊝⊝⊝ | The estimates for the control group are based upon an average of the estimates from Badwe 2015 and Soran 2016. | |
| 511 per 1000 | 448 per 1000 | |||||
| Quality of life | Not reported | Not reported | ‐ | ‐ | ‐ | |
| Local PFS at 2 years Follow‐up: range 23 months to 40 months | Study population | HR 0.22 | 607 | ⊕⊕⊝⊝ | The estimates for the control group are based upon an average of the estimates from Badwe 2015 and Soran 2016. | |
| 500 per 1000 | 141 per 1000 | |||||
| Distant PFS at 2 years Follow‐up: 23 months | Study population | HR 1.42 | 350 | ⊕⊕⊕⊝ | The estimates for the control group are based upon the estimates from Badwe 2015. | |
| 548 per 1000 | 676 per 1000 | |||||
| Breast cancer‐specific survival | Not reported | Not reported | ‐ | ‐ | ‐ | |
| Toxicity from local therapy Follow‐up: 40 months | Study population | RR 0.99 | 274 | ⊕⊕⊝⊝ | The estimates for the control group are based upon the estimates from Soran 2016. | |
| 15 per 1000 | 15 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1In Soran 2016, trial random sequence generation and allocation concealment were unclear. Downgraded one level. | ||||||
| Overall survival subgroup analysis | Number of studies | N | HR | Lower CI | Upper CI | P value |
| HER2‐positive | 2 | 192 | 0.90 | 0.60 | 1.35 | NS |
| HER2‐negative | 2 | 421 | 0.85 | 0.67 | 1.08 | NS |
| ER positive | 2 | 426 | 0.79 | 0.61 | 1.03 | NS |
| ER negative | 2 | 200 | 1.01 | 0.73 | 1.40 | NS |
| Bone‐only metastasis | 2 | 226 | 0.91 | 0.49 | 1.69 | NS |
| CI: confidence interval | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 2 | 624 | Hazard Ratio (Random, 95% CI) | 0.83 [0.53, 1.31] |
| 2 Overall survival ‐ HER2 status Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 HER2‐positive | 2 | 192 | Hazard Ratio (Random, 95% CI) | 0.85 [0.48, 1.50] |
| 2.2 HER2‐negative | 2 | 421 | Hazard Ratio (Random, 95% CI) | 0.84 [0.50, 1.40] |
| 3 Overall survival ‐ ER status Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 ER‐positive | 2 | 426 | Hazard Ratio (Random, 95% CI) | 0.83 [0.48, 1.42] |
| 3.2 ER‐negative | 2 | 200 | Hazard Ratio (Random, 95% CI) | 1.04 [0.70, 1.55] |
| 4 Overall survival ‐ only bone metastasis Show forest plot | 2 | 226 | Hazard Ratio (Random, 95% CI) | 0.91 [0.49, 1.69] |
| 5 Progression‐free survival Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 5.1 Local progression‐free survival | 2 | Hazard Ratio (Random, 95% CI) | 0.22 [0.08, 0.57] | |
| 5.2 Distant progression‐free survival | 1 | Hazard Ratio (Random, 95% CI) | 1.42 [1.08, 1.86] | |

