Sulfato de magnesio intravenoso para el tratamiento de niños con asma aguda en el servicio de urgencias
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Design: Randomised, double‐blind, parallel‐group, placebo‐controlled trial Urban paediatric emergency department in Boston, USA Conducted from 20 September 1993 to 20 December 1994 | |
| Participants | Participants: 31 participants were randomised to IV MgSO4 (15) and placebo (16) Inclusion criteria: Children aged 6 to 18 years who were being treated for an acute asthma exacerbation with PEFR less than 60% of the predicted value after receiving 3 beta‐2 adrenergic nebuliser treatments Exclusion criteria: Body temperature greater than 38.5 °C; systolic blood pressure at less than the 25th percentile for age; recent use of theophylline; history of cardiac, renal, or pulmonary disease; and pregnancy | |
| Interventions | Treatments: 1. IV MgSO4 25 mg/kg over 20 minutes 2. Saline infusion over 20 minutes Comedications: 3 beta‐2 adrenergic nebuliser treatments; intravenous methylprednisolone infusion (2 mg/kg) was given to children who had not received corticosteroids Timing of intervention: If PEFR was less than 60% of the predicted value after 3 nebulised beta‐2 adrenergic agents, and if the medical team caring for the child concluded that intravenous access was necessary for further medical management, placebo or MgSO4 was then administered | |
| Outcomes | Vital signs, O2 saturations, PEFR, FVC, FEV1 Main follow‐up 110 minutes after start of infusion | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed in blocks of 10 by the pharmacy department, using a random‐number table |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | Low risk | "assigned to receive magnesium sulfate (25 mg/kg; maximum, 2 gm in 100 ml of normal saline solution) or an equivalent volume of normal saline solution (placebo) in a double‐blind fashion". The magnesium and placebo solutions were prepackaged by the hospital pharmacy in identical containers that were coded according to a randomised sequence. The magnesium solution was given in 100 ml of normal saline solution to prevent the warm sensation at the intravenous line site described when magnesium sulfate is infused undiluted, thus maintaining the masked protocol |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear if those taking measurements were blind. All children had arrangements for admission instituted at the time of enrolment into the study, as decided by their physicians, independent of the study. "In this setting the discharge rate from the emergency department was the rate at which decisions to admit were reversed." "Criteria for discharge from the emergency department included (1) SaO2 greater than 94%, (2) no evidence of respiratory distress such as tachypnoea, flaring, or retractions, (3) minimal to no wheezes on auscultation, (4) PEFR greater than 70% of the predicted value, and (5) normal cerebral function ‐ all maintained for 3 h after a nebulization" |
| Incomplete outcome data (attrition bias) | Unclear risk | No mention of non‐completers |
| Selective reporting (reporting bias) | High risk | Only significant results were reported for FEV1, PEFR, FVC. No data reported for oxygen saturations, respiratory rates, length of hospital stay, or blood pressure. Full results only graphical. PICU admission data not reported |
| Other bias | High risk | The study was terminated before the specified sample was reached because of a change in ED practice (intravenous access was used less frequently in the care of status asthmaticus), which impaired the rate at which eligible patients were enrolled "magnesium group started with a lower FEV1 gave this group more room for improvement, potentially magnifying differences in the rates of improvement in FEV1 between the two groups and overestimating the effect of magnesium in our study population." |
| Methods | Design: Randomised, double‐blind, parallel‐group, placebo‐controlled trial 2 urban tertiary care paediatric emergency departments in the USA Recruited from September 1996 to August 1997 | |
| Participants | Participants: 30 participants were randomised to IV MgSO4 (16) and placebo (14) Inclusion criteria: Children aged 6 to 17.9 who required 3 nebulised bronchodilating treatments (albuterol or ipratropium bromide or a combination of the 2); PEFR less than 70% Exclusion criteria: Body temperature greater than 38.5 °C, use of theophylline within the previous week, and a history of cardiac, renal, or pulmonary disease other than asthma | |
| Interventions | Treatments: 1. IV MgSO4 40 mg/kg (maximum 2 g) over 20 minutes 2. Saline infusion over 20 minutes Comedications: 3 nebulised bronchodilating treatments (albuterol or ipratropium bromide or a combination of the 2). IV methylprednisolone (2 mg/kg) was administered to children who had not yet received corticosteroids Timing of treatment: If PEFR was less than 70% of the predicted value after 3 nebulised bronchodilators, and if the medical team caring for the child perceived them to be resistant to nebuliser, they received the placebo or MgSO4 | |
| Outcomes | Change in PEFR, FEV1, and FVC; ED disposition; serial clinical asthma scores; BP; deep tendon reflexes Main follow‐up 105 minutes after start of infusion | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was blocked in groups of 10 |
| Allocation concealment (selection bias) | Low risk | Randomly assigned by the investigational drug pharmacist |
| Blinding of participants and personnel (performance bias) | Low risk | The magnesium and placebo solutions were prepared by the hospital pharmacy |
| Blinding of outcome assessment (detection bias) | Low risk | The physicians on the medical team acted independently from the study physicians and were blinded to the child's magnesium treatment status |
| Incomplete outcome data (attrition bias) | Unclear risk | No mention of non‐completers. 8 were excluded because of unacceptable spirometry efforts (unclear which group, assumed pre‐randomisation) |
| Selective reporting (reporting bias) | High risk | Lung function parameters were only reported graphically or with no variance or inexact P values in the text |
| Other bias | Low risk | None detected |
| Methods | Design: Randomised, double‐blind, parallel‐group, placebo‐controlled trial Pediatric Emergency Department of teaching hospital in India Recruited from January 1994 to January 1995 | |
| Participants | Participants: 47 participants were randomised to IV MgSO4 (24) and placebo (23) Inclusion criteria: Children aged 1 to 12 with inadequate or poor response to initial 3 doses of nebulised salbutamol given at an interval of 20 minutes over a period of 1 hour, and (ii) where a written consent could be obtained from the parents accompanying the child Exclusion criteria: Children with axillary temperature greater than 38 °C, and (ii) blood pressure less than 50th percentile for age | |
| Interventions | Treatments: 1. IV MgSO4 0.2 ml of 50% over 35 minutes 2. Saline infusion over 35 minutes Comedications: All the children received oxygen, nebulised salbutamol, IV aminophylline, and corticosteroids Timing of treatment: Placebo or MgSO4 was given after 60 minutes from entry to the ED and 3 nebulised bronchodilator treatments | |
| Outcomes | Respiratory and heart rates, pulsus paradoxus (measured using a stethoscope as the difference in systolic blood pressure between the pressure at which the first sporadic, faint pulse sounds were heard and the pressure at which all sounds were heard), accessory muscle usage, dyspnoea, colour, wheeze, PEFR in children 5 years of age or older, and SaO2 Main follow‐up time unclear | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised. No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind. "Decoding was done at the completion of the study. Magnesium sulfate and placebo solutions (normal saline) were prepared in the hospital pharmacy, coded and dispensed in identical vials." |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear if those taking measurements were blind. Predetermined discharge criteria used for sending children home from the ED (primary outcome) |
| Incomplete outcome data (attrition bias) | Low risk | 2 children were excluded during the study period as they became febrile (unclear which group). 2/49 only 4% |
| Selective reporting (reporting bias) | Low risk | SaO2 and %PEFR in graph format only |
| Other bias | Low risk | None detected |
| Methods | Design: Randomised, double‐blind, parallel‐group, placebo‐controlled trial Emergency department in Dicle University Hospital, Turkey | |
| Participants | Participants: 20 participants were randomised to IV MgSO4 (10) and placebo (10) Inclusion criteria: Children aged 6 to 16 with moderate to severe acute asthma exacerbation admitted to the ED; PEFR less than 60% of the predicted value after receiving 3 beta‐2 adrenergic nebuliser treatments Exclusion criteria: fever, systolic BP at less than 25th percentile for age, recent use of theophylline, and history of cardiac, renal, or pulmonary disease | |
| Interventions | Treatments: 1. IV MgSO4 40 mg/kg (maximum 2 g), 20 minutes 2. Saline infusion, 20 minutes Comedications: 3 beta‐2 adrenergic nebuliser treatments Timing of treatment: 3 beta‐2 adrenergic agents at 20‐minute intervals, then if PEFR was less than 60% of the predicted value placebo or MgSO4 was administered | |
| Outcomes | Clinical asthma scores, PEFR, side effects Main follow‐up 90 minutes after start of infusion | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised. No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | Low risk | "The investigators performing the study were completely blinded to the treatment offered" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear if those taking measurements were blind |
| Incomplete outcome data (attrition bias) | Unclear risk | No mention of non‐completers |
| Selective reporting (reporting bias) | Low risk | 30‐ and 90‐minute data provided for named outcomes |
| Other bias | Low risk | None detected |
| Methods | Design: Randomised, double‐blind, parallel‐group, placebo‐controlled trial 3 emergency departments in Philadelphia, USA | |
| Participants | Participants: 54 participants were randomised to IV MgSO4 (24) and placebo (30) Inclusion criteria: Children aged 1 to 18 years with a past history of at least 1 episode of wheezing who presented to the ED with a moderate to severe asthma exacerbation (defined as a pulmonary index score of 8 to 13). To avoid enrolling young children with bronchiolitis, the lower age limit for study inclusion was raised to 2 years from 15 November through 30 March. Exclusion criteria: More mild (pulmonary index score less than or equal to 7) or severe (pulmonary index score greater than or equal to 14) asthma exacerbation, children who had used corticosteroids within the preceding 72 hours, had concurrent bronchiolitis, lobar pneumonia, croup, or suspected foreign body aspiration, a history of cystic fibrosis, bronchopulmonary dysplasia, congenital heart disease, liver or renal disease, sickle cell anaemia, or who were pregnant | |
| Interventions | Treatments: 1. IV MgSO4 75 mg/kg over 20 minutes 2. Saline infusion over 20 minutes Comedications: nebulised albuterol and methylprednisolone, oxygen Timing of treatment: After completion of a second nebulised dose of albuterol (run immediately after first), study drug or placebo was given | |
| Outcomes | Improvement on the pulmonary index, hospitalisation rate, time required to discharge Main follow‐up 120 minutes after start of infusion | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no details given |
| Allocation concealment (selection bias) | Low risk | Quote from study: "hospital pharmacists ... created and concealed the allocation schedule, broken only at study’s end" |
| Blinding of participants and personnel (performance bias) | Low risk | The magnesium and placebo were identical in appearance and prepared by hospital pharmacists who also created and concealed the allocation schedule, broken only at study’s end |
| Blinding of outcome assessment (detection bias) | Low risk | In the absence of the study investigator, either study drug was administered by a nurse not involved with study measurements. Children remained in the study for 150 minutes, at which time the blinded investigator decided patient disposition, independent of the emergency physician’s disposition. Guidelines for admission (saturations < 92%). Discharge criteria included sustained good aeration, absent or minimal wheezing, minimal work of breathing, and oxygen saturation greater than 95% in room air |
| Incomplete outcome data (attrition bias) | Low risk | "One child in the placebo group required more aggressive asthma therapy than allowed for by the protocol after 95 minutes. Another child in the magnesium group was mistakenly given an inadequate dose of magnesium. Importantly, there were no changes in outcome measures when a secondary analysis was performed excluding these 2 children |
| Selective reporting (reporting bias) | Low risk | Named outcomes well reported |
| Other bias | Low risk | None detected |
BP = blood pressure
ED = emergency department
FEV1 = forced expiratory volume in one second
FVC = forced vital capacity
IV = intravenous
MgSO4 = magnesium sulfate
O2 = oxygen
PEFR = peak expiratory flow rate
PICU = paediatric intensive care unit
SaO2 = oxygen saturation
h = hours
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Participants were 15 years and above. Included in adult review. Authors cannot provide disaggregated data | |
| Participants were 6 years and above, but only 10/81 participants were under 18 and the mean age was 36 (+/‐ 13.4). Included in adult review | |
| Participants were 15 years and above. Included in adult review. Authors cannot provide disaggregated data | |
| Included in adult review, no data | |
| Children with status asthmaticus and not a placebo comparison | |
| Population did not have asthma | |
| Half of the participants included in the study were inpatients and could not be separated out from the patient sample | |
| Children were all admitted to the Special Pediatric Care Unit prior to commencement of therapy (i.e. not managed in the emergency department) | |
| No placebo group | |
| Adults | |
| Compared with usual care, not placebo | |
| Nebulised vs intravenous with no placebo group |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | "Comparative study" |
| Participants | People with bronchial asthma |
| Interventions | Salbutamol, ipratropium bromide, and magnesium sulfate |
| Outcomes | Ventilatory, cardiovascular, and metabolic responses |
| Notes | Numerous attempts made to locate paper, but no library holdings found |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A pilot study of magnesium infusions (drips) for moderate‐to‐severe pediatric asthma exacerbations |
| Methods | Prospective randomised pilot study that seeks to address the research question: In children with moderate to severe asthma, do intravenous magnesium infusions added to standard PICU‐level asthma care significantly decrease time from patient presentation until PICU discharge? |
| Participants | Male and female children and adolescents aged 2 to 20 years |
| Interventions | Drug: magnesium sulfate continuous magnesium drip, titrated to effect until patient's symptoms improve Placebo: Simple saline drip, without active drug |
| Outcomes | Time to discharge, beta receptor haplotype |
| Starting date | January 2012 |
| Contact information | Keith Cross, MD, 502‐689‐2457, [email protected] Kendra Sikes, 502‐629‐7212 |
| Notes | Updated as "Still recruiting" in February 2016 at http://www.trialdetails.com/detail/NCT01522040/Pilot‐Study‐of‐Magnesium‐Infusions‐in‐Pediatric‐Asthma |
PICU = paediatric intensive care unit
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Hospital admissions Show forest plot | 3 | 115 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.14, 0.74] |
| Analysis 1.1 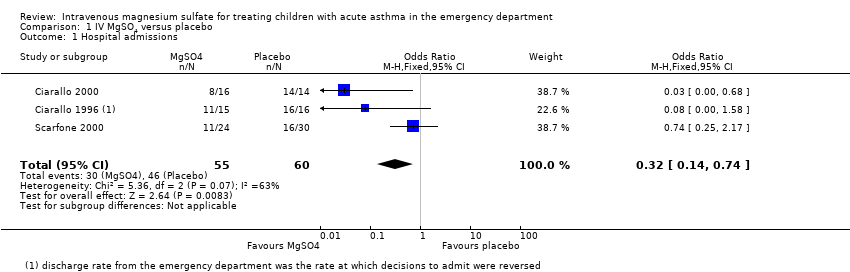 Comparison 1 IV MgSO4 versus placebo, Outcome 1 Hospital admissions. | ||||
| 2 ED treatment time (minutes) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 IV MgSO4 versus placebo, Outcome 2 ED treatment time (minutes). | ||||
| 3 Return to ED within 48 hours Show forest plot | 2 | 85 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.02, 10.30] |
| Analysis 1.3  Comparison 1 IV MgSO4 versus placebo, Outcome 3 Return to ED within 48 hours. | ||||
| 4 Hospital length of stay (hours) Show forest plot | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐5.30 [‐9.46, ‐1.14] |
| Analysis 1.4  Comparison 1 IV MgSO4 versus placebo, Outcome 4 Hospital length of stay (hours). | ||||
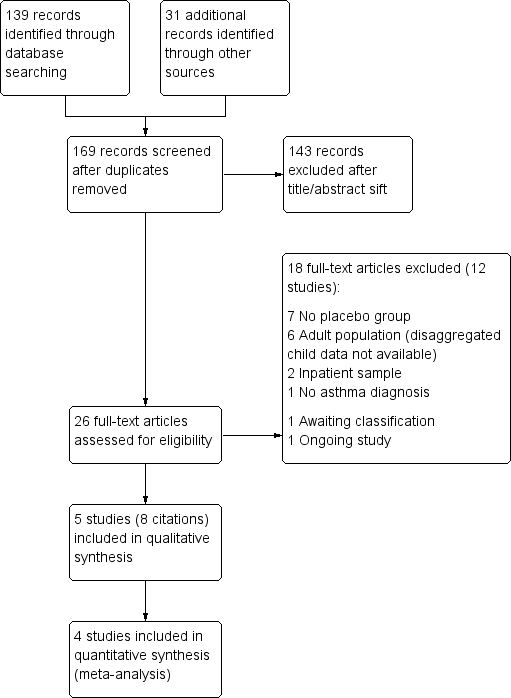
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
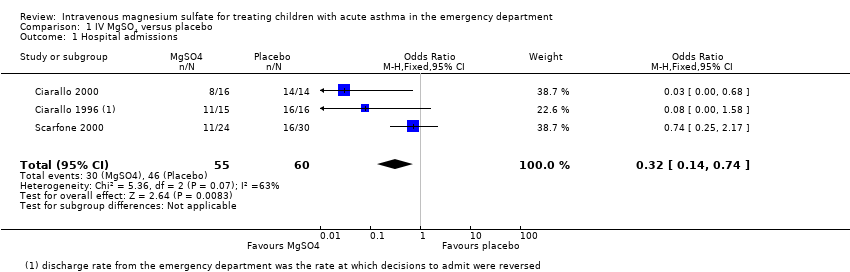
Comparison 1 IV MgSO4 versus placebo, Outcome 1 Hospital admissions.
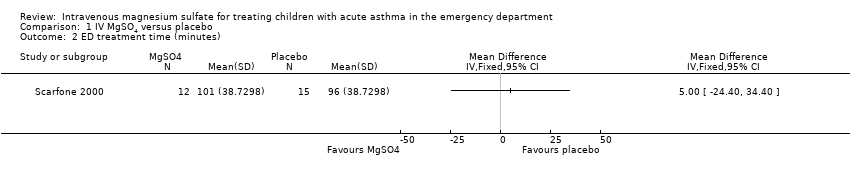
Comparison 1 IV MgSO4 versus placebo, Outcome 2 ED treatment time (minutes).

Comparison 1 IV MgSO4 versus placebo, Outcome 3 Return to ED within 48 hours.

Comparison 1 IV MgSO4 versus placebo, Outcome 4 Hospital length of stay (hours).
| MgSO4 compared to placebo for treating children with acute asthma in the emergency department | ||||||
| Patient or population: children with acute asthma in the emergency department | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | MgSO4 | |||||
| Hospital admissions | 767 per 1000 | 513 per 1000 | OR 0.32 | 115 | ⊕⊕⊝⊝ | MgSO4 reduced hospital admissions, but low confidence due to inconsistency and small numbers. Random‐effects sensitivity analysis: OR 0.18, 95% CI 0.02 to 1.59 |
| ED treatment time (minutes) | The mean ED treatment time in the placebo group was 96 minutes | The mean ED treatment time in the intervention group was (24 less to 34 more) | ‐ | 27 | ⊕⊕⊝⊝ low3 | No clear benefit of MgSO4. Based on the subset of children who were discharged home, not those who were admitted |
| Return to ED within 48 hours | 22 per 1000 | 9 per 1000 | OR 0.4 | 85 | ⊕⊕⊝⊝ | No clear benefit of MgSO4 |
| Hospital length of stay (hours) | The mean hospital length of stay (hours) in the placebo group was | The mean hospital length of stay (hours) in the intervention group was | ‐ | 47 | ⊕⊕⊝⊝ | Possible benefit of MgSO4 but based on 1 small study |
| 4 of the planned outcomes were not reported in a way that could be meta‐analysed in any of the included studies (intensive care admissions, vital signs, spirometry, validated paediatric symptom scores, and adverse events) | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Test for heterogeneity P = 0.07, I2 = 63% (‐1 inconsistency). | ||||||
| Study ID | Country (centres) | Total N | Study design | Age range (yrs) | Dose (infusion) | Comedications |
| USA (2) | 30 | R, DB, PC | 6 to 18 | 25 mg/kg 20 minutes | 3 nebulised bronchodilators (albuterol, ipratropium bromide, or both) | |
| USA (1) | 31 | R, DB, PC | 6 to 18 | 40 mg/kg 20 minutes | 3 nebulised beta‐2 adrenergic treatments IV methylprednisolone (2 mg/kg) if not yet given corticosteroids | |
| India (1) | 47 | R, DB, PC | 1 to 12 | 0.2 ml of 50% 35 minutes | Nebulised salbutamol Oxygen, IV aminophylline, corticosteroids | |
| Turkey (1) | 20 | R, DB, PC | 6 to 16 | 40 mg/kg 20 minutes | 3 beta‐2 adrenergic nebuliser treatments | |
| USA (3) | 54 | R, DB, PC | 1 to 18 | 75 mg/kg 20 minutes | Nebulised albuterol Oxygen, methylprednisolone | |
| R = randomised; DB = double‐blind; PC = placebo‐controlled | ||||||
| Study ID | Inclusion | Group | Age (SD) | % Male | % PEF | FEV1 | Other | Classification |
| PEF < 60% predicted (after 3 beta‐2 adrenergic nebuliser treatments) | MgSO4 | 10.8 | 46.7 | 43.8 | 33.1 | RR = 35 BP = 120 SaO2 = 92 | Moderate | |
| Placebo | 11.9 | 43.8 | 43.0 | 45.1 | RR = 30 BP = 123 SaO2 = 94 | |||
| PEF < 70% predicted (after 3 nebulised bronchodilating treatments) | MgSO4 | 10.9 | 68.8 | 29.9 | 28.9 | BP = 120, SaO2 = 92 | Severe | |
| Placebo | 12.0 | 50.0 | 33.1 | 31.3 | BP = 114, SaO2 = 92 | |||
| "Inadequate or poor response to 3 doses of nebulized salbutamol" | MgSO4 | 6.7 | 79.2 | 30.1 | NR | HR = 142 | Severe | |
| Placebo | 6.8 | 73.9 | 27.1 | NR | HR = 138 | |||
| PEF < 60% predicted (after 3 beta‐2 adrenergic nebuliser treatments) | MgSO4 | 10.4 | 60 | 46.8 | NR | HR = 118 BP = 118 SaO2 = 91.8 | Moderate | |
| Placebo | 11.2 | 50 | 46.2 | NR | HR = 120 BP = 116 SaO2 = 91.4 | |||
| "moderate to severe asthma exacerbation" | MgSO4 | 6.8 | 58 | NR | NR | SaO2 = 93.9 | Moderate | |
| Placebo | 4.8 | 47 | NR | NR | SaO2 = 94.1 | |||
| SD = standard deviation; % PEF = percentage predicted peak expiratory flow; FEV1 = forced expiratory volume in one second; HR = heart rate; RR = respiration rate; BP = systolic blood pressure; SaO2 = oxygen saturation; NR: not reported | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Hospital admissions Show forest plot | 3 | 115 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.14, 0.74] |
| 2 ED treatment time (minutes) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Return to ED within 48 hours Show forest plot | 2 | 85 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.02, 10.30] |
| 4 Hospital length of stay (hours) Show forest plot | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐5.30 [‐9.46, ‐1.14] |

