Sulfato de magnesio intravenoso para el tratamiento de niños con asma aguda en el servicio de urgencias
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011050.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 29 abril 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Vías respiratorias
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Ben Griffiths wrote the background and managed the clinical implications of the methods. Kayleigh Kew wrote the methods. Review authors extracted the data independently, and constructed the analyses and assessed the evidence together. Both review authors contributed to and approved the final draft.
Sources of support
Internal sources
-
Kayleigh Kew, UK.
St George's, University of London
External sources
-
NIHR, UK.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to the Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Declarations of interest
Benedict Griffiths: None known
Kayleigh Kew: None known
Acknowledgements
Chris Cates was the Editor for this review and commented critically on the review. Elizabeth Stovold designed the search strategy.
We would like to thank Clare Michell and Liza Kirtchuk for contributing to the design of the review question, which was written alongside the adult version of this review.
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Apr 29 | Intravenous magnesium sulfate for treating children with acute asthma in the emergency department | Review | Benedict Griffiths, Kayleigh M Kew | |
| 2014 Apr 25 | Intravenous magnesium sulfate for treating children with acute asthma in the emergency department | Protocol | Benedict Griffiths, Kayleigh M Kew, Clare I Michell, Liza Kirtchuk | |
Differences between protocol and review
We were not able to pool more than 10 studies, and so could not create and examine a funnel plot to explore possible small‐study and publication biases. Since there were only three studies in the primary analysis (and fewer in the secondary), we did not consider the planned subgroup and sensitivity analyses to be justified.
We did not use an independent assessor as planned to classify the study populations as having moderate, severe, and life‐threatening exacerbations because we were not able to perform the associated subgroup analysis. Instead, the classification was made for descriptive purposes only by one of the review authors (BG). If additional studies allow the subgroup analysis to be undertaken in a future update of this review, this will be done by an assessor blinded to the study results.
We did not specify 'Return to the emergency department within 48 hours' as an outcome in the protocol for this systematic review, but chose to present the results because it is related to other named outcomes which were not well reported across studies.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Child; Humans;
PICO
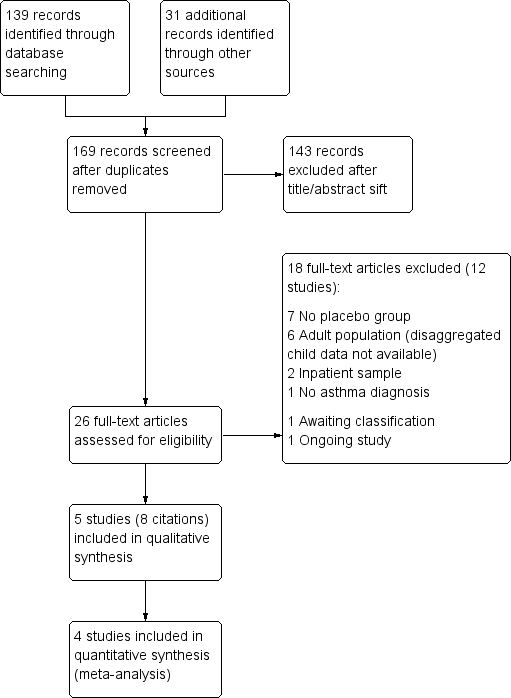
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
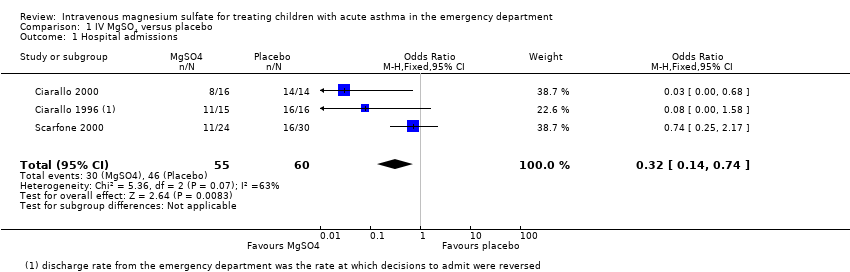
Comparison 1 IV MgSO4 versus placebo, Outcome 1 Hospital admissions.
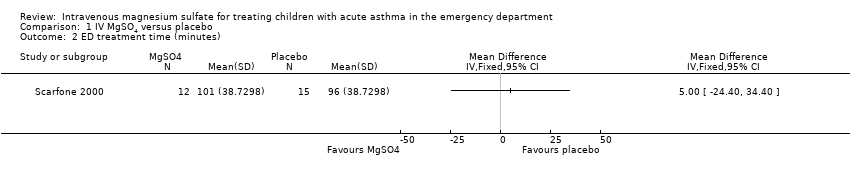
Comparison 1 IV MgSO4 versus placebo, Outcome 2 ED treatment time (minutes).

Comparison 1 IV MgSO4 versus placebo, Outcome 3 Return to ED within 48 hours.

Comparison 1 IV MgSO4 versus placebo, Outcome 4 Hospital length of stay (hours).
| MgSO4 compared to placebo for treating children with acute asthma in the emergency department | ||||||
| Patient or population: children with acute asthma in the emergency department | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | MgSO4 | |||||
| Hospital admissions | 767 per 1000 | 513 per 1000 | OR 0.32 | 115 | ⊕⊕⊝⊝ | MgSO4 reduced hospital admissions, but low confidence due to inconsistency and small numbers. Random‐effects sensitivity analysis: OR 0.18, 95% CI 0.02 to 1.59 |
| ED treatment time (minutes) | The mean ED treatment time in the placebo group was 96 minutes | The mean ED treatment time in the intervention group was (24 less to 34 more) | ‐ | 27 | ⊕⊕⊝⊝ low3 | No clear benefit of MgSO4. Based on the subset of children who were discharged home, not those who were admitted |
| Return to ED within 48 hours | 22 per 1000 | 9 per 1000 | OR 0.4 | 85 | ⊕⊕⊝⊝ | No clear benefit of MgSO4 |
| Hospital length of stay (hours) | The mean hospital length of stay (hours) in the placebo group was | The mean hospital length of stay (hours) in the intervention group was | ‐ | 47 | ⊕⊕⊝⊝ | Possible benefit of MgSO4 but based on 1 small study |
| 4 of the planned outcomes were not reported in a way that could be meta‐analysed in any of the included studies (intensive care admissions, vital signs, spirometry, validated paediatric symptom scores, and adverse events) | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Test for heterogeneity P = 0.07, I2 = 63% (‐1 inconsistency). | ||||||
| Study ID | Country (centres) | Total N | Study design | Age range (yrs) | Dose (infusion) | Comedications |
| USA (2) | 30 | R, DB, PC | 6 to 18 | 25 mg/kg 20 minutes | 3 nebulised bronchodilators (albuterol, ipratropium bromide, or both) | |
| USA (1) | 31 | R, DB, PC | 6 to 18 | 40 mg/kg 20 minutes | 3 nebulised beta‐2 adrenergic treatments IV methylprednisolone (2 mg/kg) if not yet given corticosteroids | |
| India (1) | 47 | R, DB, PC | 1 to 12 | 0.2 ml of 50% 35 minutes | Nebulised salbutamol Oxygen, IV aminophylline, corticosteroids | |
| Turkey (1) | 20 | R, DB, PC | 6 to 16 | 40 mg/kg 20 minutes | 3 beta‐2 adrenergic nebuliser treatments | |
| USA (3) | 54 | R, DB, PC | 1 to 18 | 75 mg/kg 20 minutes | Nebulised albuterol Oxygen, methylprednisolone | |
| R = randomised; DB = double‐blind; PC = placebo‐controlled | ||||||
| Study ID | Inclusion | Group | Age (SD) | % Male | % PEF | FEV1 | Other | Classification |
| PEF < 60% predicted (after 3 beta‐2 adrenergic nebuliser treatments) | MgSO4 | 10.8 | 46.7 | 43.8 | 33.1 | RR = 35 BP = 120 SaO2 = 92 | Moderate | |
| Placebo | 11.9 | 43.8 | 43.0 | 45.1 | RR = 30 BP = 123 SaO2 = 94 | |||
| PEF < 70% predicted (after 3 nebulised bronchodilating treatments) | MgSO4 | 10.9 | 68.8 | 29.9 | 28.9 | BP = 120, SaO2 = 92 | Severe | |
| Placebo | 12.0 | 50.0 | 33.1 | 31.3 | BP = 114, SaO2 = 92 | |||
| "Inadequate or poor response to 3 doses of nebulized salbutamol" | MgSO4 | 6.7 | 79.2 | 30.1 | NR | HR = 142 | Severe | |
| Placebo | 6.8 | 73.9 | 27.1 | NR | HR = 138 | |||
| PEF < 60% predicted (after 3 beta‐2 adrenergic nebuliser treatments) | MgSO4 | 10.4 | 60 | 46.8 | NR | HR = 118 BP = 118 SaO2 = 91.8 | Moderate | |
| Placebo | 11.2 | 50 | 46.2 | NR | HR = 120 BP = 116 SaO2 = 91.4 | |||
| "moderate to severe asthma exacerbation" | MgSO4 | 6.8 | 58 | NR | NR | SaO2 = 93.9 | Moderate | |
| Placebo | 4.8 | 47 | NR | NR | SaO2 = 94.1 | |||
| SD = standard deviation; % PEF = percentage predicted peak expiratory flow; FEV1 = forced expiratory volume in one second; HR = heart rate; RR = respiration rate; BP = systolic blood pressure; SaO2 = oxygen saturation; NR: not reported | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Hospital admissions Show forest plot | 3 | 115 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.14, 0.74] |
| 2 ED treatment time (minutes) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Return to ED within 48 hours Show forest plot | 2 | 85 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.02, 10.30] |
| 4 Hospital length of stay (hours) Show forest plot | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐5.30 [‐9.46, ‐1.14] |

