Cribado para la infección por clamidia genital
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: Setting: population‐based screening programme in Aarhus county, Denmark Study duration: 1 year from screening test offer for PID and epididymitis; 9 years from screening offer for ectopic pregnancy and infertility | |
| Participants | Young adult population (aged 21‐24 years) 30,439 eligible individuals (15,459 women, 14,890 men) Inclusion criteria
Exclusion criteria:
| |
| Interventions | Enrolment: through population registry Intervention group:invitation for CT testing, N = 9000 (4000 women, 5000 men) All participants in the intervention group received an invitation by direct mail to be tested for C. trachomatis by taking a sample at home and mailing it directly to the diagnostic laboratory. The intervention group was further subdivided into 2 randomly assigned groups (group 1 and group 2), each containing 2000 women and 2500 men. The difference between intervention groups 1 and 2 was that group 1 participants received the test package together with the invitation, whereas group 2 participants had to return a franked, preaddressed reply card to the study centre to receive the test package. (For the purpose of current analysis, we merged the data for the 2 types of approach strategies.) Co‐interventions: Infected individuals received instructions to contact a general practitioner (GP) for medical treatment and partner notification. People in the intervention groups also had the opportunity of receiving usual care, which consisted of swab samples obtained at a physician's office. All C. trachomatis positive individuals also received a second offer to be tested for the infection by the use of a mail‐in home‐obtained sample 24 weeks after the initial test. Control group: usual care, N = 21,439 (11,459 women, 9980 men) No contact during the study period. Individuals in the intervention groups as well as those in the control group had the opportunity of usual care consisting of an endocervical and/or urethral swab sample taken by a physician in office. Free testing is available in Denmark. At 3 months 9.4% of women in the control group and 9.0% of women in the intervention group had been tested as part of usual care. For men, the corresponding figures were 1.4% and 1.5% for the 2 groups, respectively. Co‐interventions: There are no recommendations with regard to repeated testing in any age group, but, as a general rule, samples are taken because of symptoms or intrauterine procedures such as induced abortion or insertion of an intrauterine device (Andersen 2002). | |
| Outcomes | Primary outcomes
Secondary outcomes
Investigators followed the entire study population (comprising individuals who accepted the test offer, those who did not and the control group) using central governmental registers during the first year after the test offer to assess the rates of PID (women) or epididymitis (men) diagnosed according to the Danish versions of the International Classification of Disease Codes (ICD‐10). | |
| Notes | The study was approved by the local ethical committee in the county of Aarhus and by the Danish Data Protection Agency. Trial registration: www.clinicaltrials.gov NCT00827970. This study received financial support from the Danish Medical Research Council (grant no 22‐02‐0540), the NOVO Foundation and the Research Foundation in Aarhus County. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer‐based randomisation |
| Allocation concealment (selection bias) | Low risk | Comment: Individuals selected for screening invitation did not know there was a control group, so unlikely to have affected decision to take part or not; control group did not know they were in a trial. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Blinding was not used. Participants in intervention group might receive different advice about risks of upper genital tract infection and about what to do if they have symptoms. Control group did not receive any information. |
| Blinding of outcome assessment (detection bias) | Low risk | Comments: Data on blinding of outcome assessment was not provided. The review authors judge that the outcome is not likely to be influenced by lack of blinding. The same applies for both primary outcomes: PID and epididymitis. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: The same applies for both primary outcomes: PID and epididymitis |
| Selective reporting (reporting bias) | Low risk | Comment: The study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon) |
| Other bias | Unclear risk | Contamination is a risk, if women in the control group continued to be tested at the same rate as during the study period, the percentage tested by the time the outcome PID was measured might have been higher. This could reduce the size of any difference between intervention and control groups. Not enough information to know what proportion of control group was tested during the follow‐up period. |
| Methods | Study design: cluster‐randomised trial of a multicomponent intervention for the prevention of sexually transmitted disease in female sex workers and the general population Setting: urban communities in Peru Study duration: 4 years | |
| Participants | Female sex workers (FSW). 24 cities assessed for eligibility. 20 cities cluster‐randomised in 10 pairs, 4483 FSW (range 75‐209 per city enrolled, 4465 provided samples, 4413 completed survey) Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Enrolment: All eligible cities randomised. Sex venues "includ[ing] brothels, bars, nightclubs, street‐based venues and truck stops" were mapped and visited by mobile teams (in all 24 cities assessed for eligibility). Baseline survey participants were non‐randomly sampled, consecutive FSW from all venues in a city or until 200 FSW per city sampled (p. 1121). In intervention cities only, mobile teams approached FSWs in sex venues in spaces varying from private bedrooms to small side rooms at bars. The baseline survey took place from November 2002 to April 2003, outcome surveys and sampling took place from September to December in both 2005 and 2006. Intervention group (median population 190,102, range 54,148‐272,231) Quote: "We created mobile teams and laboratory support systems in intervention cities to deliver clinical and preventive services to FSWs from July, 2003, to December, 2006. Each mobile team was made up of a nurse or midwife and an FSW peer educator. Mobile teams' activities included two visits to each sex venue in each of 20 cycles of 8 weeks to provide periodic presumptive treatment with metronidazole for trichomoniasis and bacterial vaginosis to FSWs who were not pregnant or breastfeeding, and willing to forego alcohol consumption for 72 h. Self obtained vaginal swabs were collected for local T vaginalis culture and for nucleic acid amplification in Lima for N gonorrhoea and C trachomatis. The teams returned 1 week later, providing test results and treatment for specific infections identified (ciprofloxacin for gonorrhoea, azithromycin for chlamydia, and metronidazole for positive T vaginalis cultures not treated a week earlier). FSWs were encouraged to visit local government clinics for periodic syphilis and HIV testing, and for interim STI symptoms. Laboratory technicians joined mobile teams from February, 2005, to December, 2006, and did rapid syphilis testing." Co‐interventions: "Mobile teams also provided motivational interviewing to promote condom use by sex workers, and gave up to 15 condoms to each FSW in each 8 week cycle in the first 1.5 years, then increased to 50 condoms per cycle. For the general population, the local non‐governmental organisation APROPO implemented social marketing of a low‐cost condom, the OK condom, through pharmacies in intervention cities only, from October, 2003, to October, 2004, then more widely." Control group: usual care:10 cities (median population 135,187, range 50,183‐291,408) Usual care: "status quo services", no other description reported | |
| Outcomes | Primary outcomes
Secondary outcomes
Each sex venue visited during 20 cycles lasting 8 weeks each. Continuous mapping to record closed down and new venues.For evaluation surveys, FSW surveyed by quota sampling individuals at randomly selected venues and times. | |
| Notes | Institutional review boards at the University of Washington, Universidad Peruana Cayetano Heredia, and US Naval Medical Research Center Detachment approved the protocol, consent forms, and instruments. Eligible FSWs older than 14 years and survey participants provided verbal consent. Trial registration: ISRCTN43722548. This research was supported by the Wellcome Trust‐Burroughs Wellcome Fund Infectious Disease Initiative 059131/Z/99/Z, 078835/Z/05/Z, and 078835/Z/05/B; National Institutes of Health NIAID STD Cooperative Research Center AI31448, Center for AIDS Research AI27757, and CIPRA U19 AI053218; and USAID‐Peru. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer‐generated randomisation sequence (see below) |
| Allocation concealment (selection bias) | Low risk | Quote: "Within each pair, one city was randomly assigned to an intervention with an S‐PLUS (version 3·1) program written by JPH; the other city was assigned to standard care." (p. 1121), Comment: No chance to know allocation in advance or to change once allocated |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "US and UK investigators (except JPH) were masked to identities of intervention cities until completion of all surveys and laboratory testing." "Fieldworkers and the Peruvian study team could not be masked." (p. 1121) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Laboratory personnel and the data analyst (KKT) were masked to assignments until final analysis." (p. 1121) |
| Incomplete outcome data (attrition bias) | Low risk | Comment: very high participation in cross‐sectional surveys |
| Selective reporting (reporting bias) | Low risk | Comment: primary outcome same as in protocol and in the trial registration |
| Other bias | Low risk | — |
| Methods | Study design: Individually randomised controlled trial comparing immediate with deferred screening Setting: common rooms, lecture theatres, and student bars at universities and further education colleges in London.. Study period: 1 year from acceptance of offer of chlamdia testing | |
| Participants | Sexually active female students 16‐27 years (N = 2563) Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Enrolment: Investigators personally recruited women in bars, common rooms and lecture theatres at 20 London universities and further education colleges, randomising them between September 2004 and October 2006. Intervention group: screening:1273 women randomised (but 14 excluded = 1259 included) Vaginal swab samples were obtained (at nearest lavatory) and analysed for C. trachomatis. In case of infection the woman was contacted and urged to contact a physician for treatment and partner notification. Control group:deferred screening:1290 randomised (but 20 excluded = 1270 included) Samples were obtained (at nearest lavatory) but stored for 12 months. Women were obliged to seek a health care provider if they considered themselves at risk or if they had symptoms (= standard care). | |
| Outcomes | Primary outcomes
Secondary outcomes: none reported Outcomes measured via questionnaires, answered by participants by e‐mail, postal questionnaires or telephone calls. Non‐responders were followed up by contacting a GP. | |
| Notes | The study was approved by Wandsworth research ethics committee (reference 03.0012). Trial registration number: NCT00115388. This study was supported by the BUPA Foundation (grant No 684/GB14B). TMA sample collecting kits were provided by Gen‐Probe (San Diego, CA). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: random number tables were used (p. 2). |
| Allocation concealment (selection bias) | Low risk | Comment: Sealed sample packs, which contained the completed, unopened questionnaires and consent forms were allocated (blinded procedure) (p. 2). |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: Participants were blind to group allocation except for those in the interventions group with baseline samples that tested positive for chlamydia and who were referred for treatment and 38 women with indeterminate test results who were asked to post a repeat sample (masking p. 2). Samples were obtained before allocation and therefore the recruiting personnel could not be aware of allocation. Not clear what happened with indeterminate results in control group if tested after 12 months. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: A panel of 3 genitourinary medicine physicians assessed patient questionnaires and medical records using standardised criteria; they were blinded to trial group (p. 2). |
| Incomplete outcome data (attrition bias) | Low risk | Comment: In the intervention group, 68 were lost to follow‐up; in the control group 84 were lost to follow‐up. These numbers are very low and unlikely to influence the results. |
| Selective reporting (reporting bias) | Low risk | Comment: The single primary aim from the protocol is reported in the publication. 2 primary aims from the introduction section are reported in the results. The protocol is available. |
| Other bias | High risk | Contamination of intervention: possibility of independent testing in the year of the study, which would reduce differences between the intervention and control group (about 22% in each group were tested); Received funding of diagnostic tests from manufacturer. |
| Methods | Study design: cluster‐randomised controlled trial Setting: 17 high schools in Aarhus county, Denmark Study duration: 1 year after the offer of screening | |
| Participants | High school female students in Aarhus region aged 15‐19+ years (N = 5487 randomised, N = 1700 provided follow‐up data) Inclusion criteria:
Exclusion criteria: not mentioned | |
| Interventions | Enrolment: The report does not include a description about how schools were approached or how home sampling kits/instructions for control arm were given. Possibly, investigators may have used the baseline questionnaire to identify sexually experienced students ("eligible responders"), telling these to return specimen. Enrolment took place between January and April 1997. Intervention group: home sampling:2603 women in 8 schools Quote: "[H]ome sampling kits given to the students at the end of gatherings at which information about the C. trachomatis diseases and the study was given. The home sampling kit consisted of a vaginal pipette (containing 5 mL sterile sodium chloride) for obtaining vaginal flush samples (women) and a urine sample (men), a questionnaire, written instructions on how to obtain the sample, and a self‐addressed, stamped envelope. Students were instructed to administer the vaginal pipette for sampling on receipt. The samples obtained at home were mailed by the students directly to the Department of Clinical Microbiology, where they were analyzed. The students also provided the address where the test results were to be sent. Students with positive test results were requested in writing to visit a doctor for treatment and partner tracing and to take a letter to the doctor." (p. 952). Control group:usual care:2884 women in 9 schools Quote: "The control group received the same information and questionnaire as the home sampling group, but they were not supplied with the home sampling kit. Instead, they were offered a free testing at the local clinic for sexually transmitted diseases (STDs) or at the office of any other physician, including that of their general practitioner." | |
| Outcomes | Primary outcomes
Secondary outcomes
A questionnaire was sent to participants asking for "information about treatment for PID and admittance to a hospital for PID during the year of follow‐up. In an attempt to verify that treatment for PID had been given, every student who reported treatment for PID was sought among all records of antimicrobial prescriptions at the central Danish register for prescriptions (Lægemiddelstyrelsen)." | |
| Notes | The study was funded by the Danish National Board of Health (grant No 210 i 1997), Løvens Kemiske Fabriks Research Foundation, Nycomed DAK, Pfizer, and Chairman Jacob Madsen and Hustru Olga Madsen's foundation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All 17 high schools in the county of Aarhus, Denmark, were cluster‐randomised 1:1 by simple redeeming (drawing lots from a hat) into an intervention (home sampling) group consisting of 8 high schools composed 2603 women and 1733 men, and a control group consisting of 9 high schools composed 2884 women and 1689 men." |
| Allocation concealment (selection bias) | High risk | Comment: not described, but randomisation was done before asking for consent. More students in the intervention arm (48%, 1254/2603) than the control arm (38%, 1097/2884) agreed to take part. At a subsequent stage, women were asked to consent to be followed up for the outcome PID. A lower proportion of the sexually experienced women in the intervention arm (93%, 867/928) than the control arm (100%, 833/833) agreed to follow up. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: no blinding |
| Blinding of outcome assessment (detection bias) | High risk | Comment: Participants self assessed PID. Possibility of detection bias. Investigators checked prescription records, and there was no statement about blinding. |
| Incomplete outcome data (attrition bias) | High risk | Comment: Almost 50% in both groups lost to follow‐up (intervention, 51%, 43/867; control 58%, 487/833). |
| Selective reporting (reporting bias) | Unclear risk | Comment: testing in follow‐up period not reported, which might influence primary outcome and determine PID outcome. No protocol was available, so the risk of reporting bias is unclear. |
| Other bias | Unclear risk | Received funding from pharmaceutical company |
| Methods | Study design: Randomisation before assessment of eligibility or obtaining consent for participation Setting: staff‐model health maintenance organization (HMO) located in western Washington State, USA Study duration: 1 year afer offer of screening to intervention group | |
| Participants | Enrolment: Women aged 18‐34 years enrolled in a health maintenance organization (HMO) on 1 October 1990 (N = 36,547 received an initial questionnaire on eligibility; N = 2607 randomised). Duplicate surveys were mailed to non‐responders. Telephone calls were made to some of the non‐responders, focusing on those in the intervention group. Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Intervention group: screening:1009 women enrolled Invitation to be tested for C. trachomatis by use of 2 cervical samples that were analysed by ELISA and culture, respectively. All women with a positive test result were treated for chlamydia infection by their primary care provider. Control group: usual care:1598 women enrolled No intervention; women in the usual care group saw their health care providers as needed. | |
| Outcomes | Primary outcomes
Secondary outcomes:none reported | |
| Notes | All study procedures were reviewed and approved by the institutional review unit at the HMO. It is not clear to what extent this includes ethical approval. Supported in part by a grant (A1‐24756) from the National Institute of Allergy and Infectious Diseases and by a grant from Bristol‐Myers Squibb. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method not stated. Quote: "The women were randomly assigned to either the screening group or the usual‐care group at the time the original sample was selected in October 1990." |
| Allocation concealment (selection bias) | High risk | Comment: Investigators made special efforts in the intervention group to increase participation rate. As a result, the intention was a 1:2 randomisation but the study ended up with 1:1.5. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: no blinding. The lack of participant blinding and the outcome evaluation partly based on questionnaire data may have influenced the results, making women in intervention group more aware of PID symptoms and therefore causing an under‐ or overestimation of the intervention effect. No information about if the 76% completing the questionnaire were from intervention or control group. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "The abstracters were unaware of the study group assignments" (p. 1363). |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Several databases were searched (blinded) for outcome data, and these data represent a low risk of bias as missingness will probably be evenly distributed between groups. However, questionnaires were also used and participants between groups may have answered unequally because they were not blinded. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available, so the risk of reporting bias is unclear. The outcomes specified in the methods were all reported in the results. |
| Other bias | Unclear risk | Received funding of diagnostic tests from manufacturer. |
| Methods | Study design: controlled trial with randomised stepped wedge implementation in 3 blocks Setting: population‐based screening in 3 regions of the Netherlands – the urban areas of Amsterdam and Rotterdam and a defined suburban area of South Limburg (Parkstad) Study duration: March 2008 to February 2011 | |
| Participants | Young adults (women and men aged 16‐29 years old, N = 317,304) Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Enrolment: Personalised yearly invitations to be screened for C. trachomatis infection sent to the target population through the Chlamydia Screening Implementation Programme. The letter included the address of the programme website (www.chlamydiatest.nl) and a secure login code through which eligible participants could request a kit for self sampling (urine for men, vaginal swab or urine for women). Chlamydia‐positive participants automatically received a test package 6 months after the first test. Intervention group 1: invited for screening 3 times (block A, N = 55,776, 39 clusters) Yearly chlamydia screening test offered by post 3 times. People were invited to use an internet site to request a kit for self collected samples to be sent to laboratory for testing. Treatment and partner notification were done via GP or STI clinic. A single reminder letter was sent to anyone who did not access the website within 4 weeks, and email reminders were sent to individuals who requested a kit but did not return a specimen within 2 weeks.Test results, with a referral letter for those with positive results, were provided online, with an email or text message reminder after 14 and 28 days and a letter by post after 6 weeks for those who did not access it. Intervention group 2: invited for screening 2 times (block B, N = 213,497, 114 clusters) Yearly chlamydia screening test offered by post 2 times. See intervention group 1 for details. Control group: usual care (block C, N = 48,031, 39 clusters) One sixth of the population were invited a single time for CT testing after the second invitation was sent to blocks A and B. Testing for chlamydia is available from general practitioners and at sexually transmitted infections clinics. There was no specific promotion of chlamydia testing during the trial period. | |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | The study was approved by the Medical Ethics Committee Free University Amsterdam (Identification number 2007/239). The Dutch organisation for Health Research and Development (ZonMW, project number 12.400.001) funded the project. No protocol available but details of study design are in related paper van den Broek 2010 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The order of invitation of clusters was randomised by assigning computer generated random numbers to clusters and then sorting clusters within one block using these numbers (using Microsoft Excel 2002)" (p. 3). |
| Allocation concealment (selection bias) | Unclear risk | Comment: The investigators were blinded to the identity of clusters (allocated to block = comparison groups A, B, C) and did not know whether the intervention effect might differ by risk level or cluster size. The subsequent randomisation of the order of implementation within blocks and addition of a third round of screening in block B would also reduce the risk of bias in the results. Quotes: "Although we stratified the clusters according to community risk level, the intervention and control block were not completely comparable in all 3 regions" (p. 5). "The participation rate in the control block C was not completely comparable to that achieved after the first invitation in the intervention blocks A and B" (p. 5). |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: no blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: no blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | High risk | Comment: primary outcomes only: incomplete data (for positivity and thus prevalence) associated with intervention uptake. For PID, very low reporting (1st invitation, assumed to be baseline with no screening in the previous 12 months,1072/29,831; 2nd invitation, presumed to be 12 months after screening, 2261/20,246; 3rd invitation 2340/16,853) and different proportions responding to questionnaire at each round. Reason for missing outcome data likely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk | All outcomes in study design paper reported in main paper |
| Other bias | Unclear risk | Contamination; quote: "cluster allocation could have reduced, but not eliminated, transmission of chlamydia within clusters. Sexual networks do not strictly follow geographical boundaries and the blocks for implementation were not contiguous" (p. 5). Not enough information to assess whether contamination occurred. Low uptake of the intervention could reduce the size of any difference between intervention and control groups. |
CT: Chlamydia trachomatis; FSW: female sex workers; GP: general practitioner; PID: pelvic inflammatory disease; STI: sexually transmitted infection.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| RCT. CLASP trial. No eligible primary outcome: report uptake of testing and CT prevalence at 6 months only | |
| Not an RCT. Observational study nested within RCT of pregnant women randomised to antibiotic or no treatment for Trichomonas vaginalis or bacterial vaginosis | |
| RCT; no eligible primary outcome; chlamydia testing at 3 months only | |
| RCT in pregnant women; no real difference between groups; both groups screened, report pre‐term birth for treated vs untreated and for infection with any sexually transmitted infection vs no infection | |
| RCT; no eligible primary outcome; main outcome was chlamydia testing uptake | |
| RCT; no eligible primary outcome; routine chlamydia testing only. Outcomes were patient satisfaction, doctor/nurse screening vs self taken sample, number of cases detected, time taken in clinic | |
| Feasibility study of chlamydia screening in pregnant women; no control group | |
| RCT; no eligible primary outcome; routine chlamydia screening only. Immediate results only; no long term outcomes | |
| Register‐based screening cluster‐CCT, repeat testing in schools 5 screening rounds vs usual care. Wrong intervention, cluster‐CCT 'control' schools not enrolled concurrently with intervention | |
| RCT; participants not eligible. Didn't measure prevalence, but screened women with recent sexually transmitted infection | |
| RCT; no eligible primary outcome; main outcome was screening rate after internet offer of home screening vs usual care | |
| RCT; no eligible primary outcome; chlamydia retesting at 3 months only | |
| RCT; no eligible primary outcome; chlamydia retesting at 4‐5 months only after home vs clinic‐based recall | |
| RCT; no eligible primary outcome; chlamydia retesting at 12 months was cross‐sectional, not performed as a follow‐up | |
| RCT; no eligible primary outcome; CT retesting at 3 months only | |
| RCT; no eligible primary outcome; 'prevalence' at 12 months measured differently in intervention and control after cluster‐randomised mass screening | |
| RCT; no eligible primary outcome; reports uptake of testing and uptake of Papanicolau smears | |
| RCT; no primary outcome; reports uptake of testing only | |
| RCT; no eligible primary outcome; main outcome uptake of screening at 6 weeks | |
| RCT in pregnant women; intervention not eligible. All women screened for chlamydia, and only those with bacterial vaginosis randomised to treatment or no treatment for bacterial vaginosis | |
| RCT; no eligible primary outcome; main outcome was uptake of screening | |
| RCT in pregnant women; no eligible primary outcome; screening for bacterial vaginosis, Trichomonas vaginalis and candida only, but not chlamydia | |
| RCT; no eligible primary outcome; study period 3 months only | |
| RCT; pilot study only. No eligible primary outcome; main outcome was uptake of testing | |
| RCT in pregnant women; intervention not eligible | |
| RCT in pregnant women; intervention not eligible. Treatment with erythromycin vs no treatment was not equivalent to screen vs no screening | |
| CCT in pregnant women; comparison not eligible: no comparison of screened vs unscreened | |
| RCT; no eligible primary outcome; main outcome uptake of chlamydia testing | |
| RCT; no eligible primary outcome: main outcome was incidence of discharge | |
| RCT; no eligible primary outcome; report chlamydia positivity at 3 months only | |
| RCT; no eligible primary outcome; report uptake of chlamydia screening in men | |
| RCT; no eligible primary outcome; main outcome was return of screening kits | |
| RCT; no eligible primary outcome; only reports uptake of chlamydia testing | |
| RCT; no eligible primary outcome; chlamydia retesting at 4 months only | |
| RCT; no eligible primary outcome; only reports uptake of testing and chlamydia positivity up to 4 months | |
| RCT; no eligible primary outcome; do not report positivity at 12 months | |
| RCT; no eligible primary outcome; chlamydia retesting at 1‐4 months only | |
| Study in pregnant teenagers; no eligible primary outcome; only measured chlamydia positivity once in a random subset of pregnancy test urine samples; no pregnancy outcomes | |
| RCT; no eligible primary outcome; report chlamydia screening rate in boys | |
| RCT; no eligible primary outcome; report chlamydia screening rate in girls | |
| RCT; no eligible primary outcome; report uptake of chlamydia testing | |
| RCT; no eligible primary outcome; chlamydia retesting at 3 months of treated women |
CT: Chlamydia trachomatis; CCT: controlled clinical trial; RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | ACCEPt Australian Chlamydia Control Effectiveness Pilot: A randomised controlled trial to determine whether an intervention of annual chlamydia testing in general practice for sexually active men and women aged 16 to 29 years can lead to a reduction in chlamydia prevalence |
| Methods | Cluster‐randomised controlled trial |
| Participants | General practice clinics within postcode areas with a population of between 5000 to 30,000. A total of 54 postcodes (towns) will be randomised, and all general practice clinics within the postcode will be invited to participated. All clinics will be eligible for participation. |
| Interventions | Intervention group: annual testing. GPs will be asked to screen sexually active men and women aged 16 to 29 years for chlamydia. The multifaceted intervention to maximise testing includes: a computer alert prompting GPs to test; incentive payments for GPs and payments for employing practice nurses; a recall system to encourage annual testing; partner notification, and information/support with regular feedback on testing performance. Control group: usual care. Clinics in the control group are encouraged to continue their usual practice. |
| Outcomes | Primary outcome: change in chlamydia prevalence among a consecutive sample of 80‐100 patients attending participating clinics in each postcode Secondary outcomes: incidence of pelvic inflammatory disease; chlamydia testing rates |
| Starting date | 1 May 2010 |
| Contact information | Dr Jane Hocking, [email protected] |
| Notes | Trial registration number: ACTRN12610000297022 Ethical approval obtained from Ethics Committee of the Royal Australian College of General Practitioners |
| Trial name or title | Sexually Transmitted Infections (STI) in Remote communities: ImproVed & Enhanced primary health care ‐ a randomised community trial to reduce STIs in remote Aboriginal and Torres Strait Islander communities, comparing clinical care enhanced with a Sexual Health Quality Improvement Program with standard clinical care |
| Methods | Stepped wedge community cluster‐randomisation |
| Participants | Sexually active 14‐34 year olds living in remote communities in Australia with a resident population of Aboriginal or Torres Strait Islander people aged 16–34 years. A total of 68 communities were randomised. Inclusion criteria: communities considered remote by the Australian Bureau of Statistics (ABS); with a resident population of at least 100 people of Aboriginal people, Torres Strait Islanders or both, aged 16–34 years; with community and health services willing and able to provide access to de‐identified clinical data; with health services able to sustain data collection, consistent with the trial protocol Exclusion criteria: communities where there is a diverse range of health services within the same area that are accessed by Aboriginal people, Torres Strait Islanders or both. |
| Interventions | Randomisation will occur over a period of 3 years. At the start of each year, 7 of the trial clusters will be randomised to the intervention, the following year a further 7 will be randomised, and in the third year, the final 7 will be randomised such that by the end of the trial, all clusters will have received the intervention. For clusters that are randomised in year 1, the intervention will continue for 3 years. For clusters randomised in year 2, the intervention will continue for 2 years. For clusters randomised in year 3, the intervention will continue for 1 year. Intervention group: The intervention, called the Sexual Health Quality Improvement Program will involve the following components:
Control group: standard clinical care according to clinical guidelines which include screening, assessment, treatment, management, prevention and reporting recommendations. Clinicians are recommended to follow these guidelines on a case‐by‐case basis. |
| Outcomes | Primary outcome: Prevalence of chlamydia infection in women and men, measured annually and at the end of the trial Secondary outcome: Proportion of participants receiving the intervention (= uptake of screening), measured annually and at the end of the trial |
| Starting date | 1 September 2010 |
| Contact information | J Kaldor, [email protected] |
| Notes | Ethical approval obtained from Western Australian Aboriginal Health Information Ethics Committee; Cairns Base Hospital Ethics Committee; Central Australian Human Research Ethics Committee; Human Research Ethics Committee of Northern Territory Department of Health and Families and Menzies School of Health Research; Western Australian Country Health Service Board Research Ethics Committee; University of New South Wales Human Research Ethics Committee (B) |
| Trial name or title | Characteristics of a randomised Chlamydia screening trial |
| Methods | Community cluster‐randomised trial |
| Participants | Women born in 1992–1995 living in 44 communities in Finland (33 screened, 11 unscreened) 15,000 women invited for screening per year. The invitation contains information on C. trachomatis and its treatment and about an FVU‐sampling kit, which is available through a website (www.rokotiitus.net). A consent form is included to be mailed/donated together with the FVU‐sample. |
| Interventions | Communities will be divided into 4 groups for biannual, quadrennial or a single screening round at the end of the study. Target number of women born 1992‐1995, N = 60,000, approximately 15,000 per arm. Intervention group 1: biannual screening at the ages of 18.5, 20 and 22 Intervention group 2: biannual screening at the ages of 18.5, 20 and 22 Intervention group 3: quadrennial screening at the ages of 18.5 and 22 Control group: 11 unscreened communities (no offer of screening until age 22) |
| Outcomes | Primary outcome: prevalence of chlamydia infection in women at age 22 (3.5 years after start of study); ITT analysis of groups 1 + 2 vs control (screened 3 times vs screened 1 x only at end of study); prevalence of chlamydia infection in women at age 22 (3.5 years after start of study) groups 1 + 2 vs groups 3 + control, ITT analysis (screened 3 times vs screened 2 x or 1 x only at end of study) Secondary outcome: proportion of participants receiving the intervention (= uptake of screening) at baseline (other time points not reported) |
| Starting date | Autumn 2010 |
| Contact information | M Lehtinen, University of Tampere, Finland |
| Notes | Permission for the trial was obtained from the ethical review board of the North Ostrobotnia Hospital District, Oulu, Finland. Performed as part of an HPV vaccination trial. |
| Trial name or title | Project AWARE: using the ED to prevent STIs in youth |
| Methods | Randomised controlled trial |
| Participants | Sexually experienced adolescents aged 14 to 21 in a large, inner‐city ED in the Bronx (NY) Estimated enrolment: 690 |
| Interventions | Intervention group 1: combined HIV/STI screening. Current standard of care with video to obtain informed consent for rapid on‐site HIV testing, with additional information in video about other STIs and added gonorrhoea and chlamydia screening of a urine sample Intervention group 2: combined HIV/STI screening with theory‐based risk reduction video counselling. As intervention 1 with additional behavioural video to encourage safer sex Control group: HIV testing. Current standard of care with video to obtain informed consent for rapid on‐site HIV testing |
| Outcomes | Primary outcomes: test positivity for chlamydia or gonorrhoea 4 months postintervention Secondary outcomes: test positivity for chlamydia or gonorrhoea 8 months and 12 months postintervention; intentions for condom use immediately after as well as 4, 8 and 12 months postintervention |
| Starting date | December 2011 |
| Contact information | Dr Yvette Calderon, Jacobi Medical Center, North Bronx Healthcare Network, New York, United States, 10461 |
| Notes | Trial registration number: NCT01195220 |
ED: emergency department; FVU: first void urine; ITT: intention‐to‐treat; STI: sexually transmitted infection.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prevalence of chlamydia infection (positivity) measured in the whole study population at least 12 months after start of screening Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Offer of chlamydia screening vs usual care (inactive control), Outcome 1 Prevalence of chlamydia infection (positivity) measured in the whole study population at least 12 months after start of screening. | ||||
| 1.1 3rd invitation vs control | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 2nd invitation vs control | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 1st invitation vs control | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Screening offer in high school students | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Incidence of PID at 12 months (intention‐to‐treat) Show forest plot | 4 | 21080 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.49, 0.94] |
| Analysis 1.2  Comparison 1 Offer of chlamydia screening vs usual care (inactive control), Outcome 2 Incidence of PID at 12 months (intention‐to‐treat). | ||||
| 2.1 Low risk of detection bias | 2 | 18022 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.55, 1.17] |
| 2.2 High risk of detection bias | 2 | 3058 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.22, 0.83] |
| 3 Incidence of PID at 12 months (per protocol analysis) Show forest plot | 2 | 2749 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.35, 1.10] |
| Analysis 1.3  Comparison 1 Offer of chlamydia screening vs usual care (inactive control), Outcome 3 Incidence of PID at 12 months (per protocol analysis). | ||||
| 3.1 Low risk of detection bias | 1 | 2377 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.34, 1.24] |
| 3.2 High risk of detection bias | 1 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.17, 1.80] |
| 4 Incidence of epididymitis in men at 12 months (intention to screen) Show forest plot | 1 | 14980 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.45, 1.42] |
| Analysis 1.4  Comparison 1 Offer of chlamydia screening vs usual care (inactive control), Outcome 4 Incidence of epididymitis in men at 12 months (intention to screen). | ||||
| 5 Secondary outcomes for reproductive tract morbidity Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 Offer of chlamydia screening vs usual care (inactive control), Outcome 5 Secondary outcomes for reproductive tract morbidity. | ||||
| 5.1 Female infertility | 1 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Ectopic pregnancy | 1 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

#Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
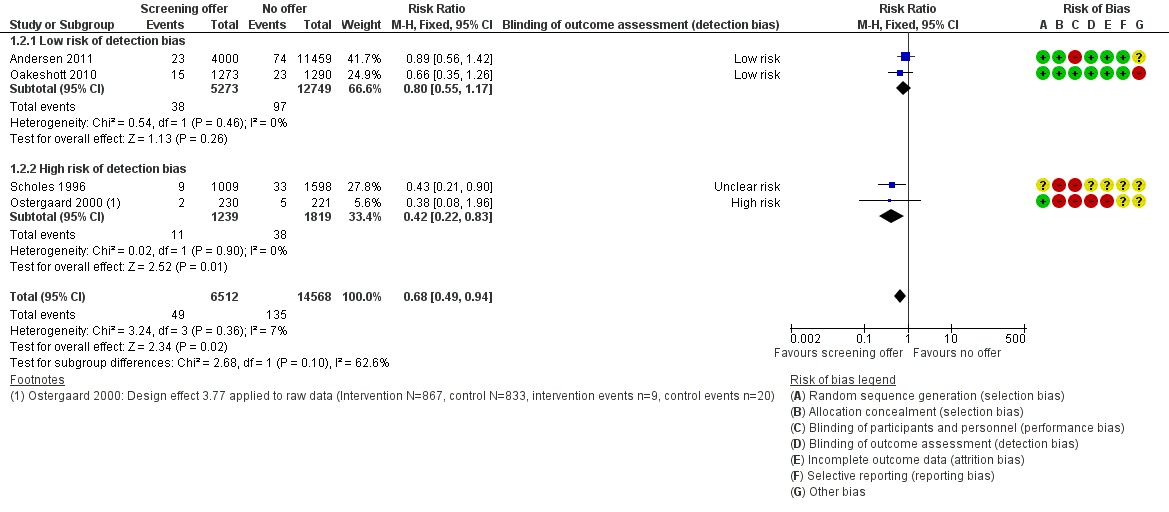
Forest plot of comparison: 1 Offer of chlamydia screening vs usual care (inactive control), outcome: 1.2 Incidence of PID at 12 months (intention‐to‐treat).
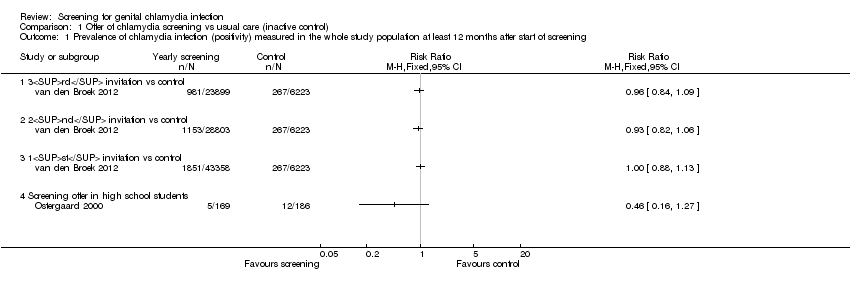
Comparison 1 Offer of chlamydia screening vs usual care (inactive control), Outcome 1 Prevalence of chlamydia infection (positivity) measured in the whole study population at least 12 months after start of screening.

Comparison 1 Offer of chlamydia screening vs usual care (inactive control), Outcome 2 Incidence of PID at 12 months (intention‐to‐treat).
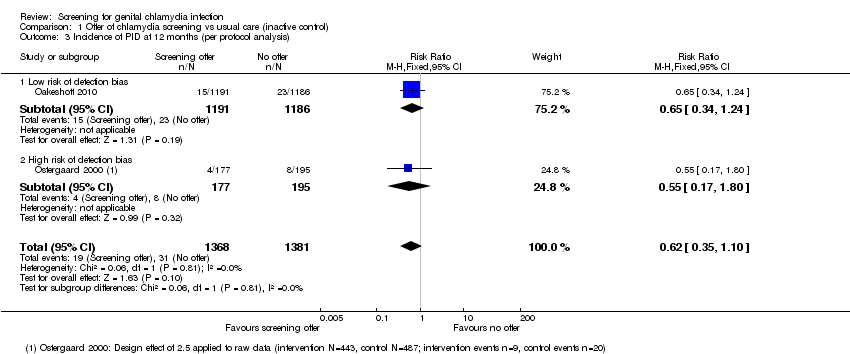
Comparison 1 Offer of chlamydia screening vs usual care (inactive control), Outcome 3 Incidence of PID at 12 months (per protocol analysis).

Comparison 1 Offer of chlamydia screening vs usual care (inactive control), Outcome 4 Incidence of epididymitis in men at 12 months (intention to screen).

Comparison 1 Offer of chlamydia screening vs usual care (inactive control), Outcome 5 Secondary outcomes for reproductive tract morbidity.
| Chlamydia screening compared with usual care for the prevention of C. trachomatis transmission and reproductive tract morbidity | |||||
| Patient or population: healthy adults Settings: general population, high schools or colleges Intervention: chlamydia screening Comparison: usual care | |||||
| Outcomes | Absolute effect | Relative effect | No of Participants | Quality of the evidence | Comments |
| Chlamydia prevalence (general population) Outcome was chlamydia test positivity after 3 yearly invitations in intervention clusters vs 1 invitation in control areas. Uptake was too low for chlamydia positivity to be considered an unbiased estimate of prevalence. | RD 0.0% (‐0‐01, +0.01%) | RR 0.96 (0.84 to 1.09) | 30,122 (1 study) | ⊕⊕⊝⊝1,2 | |
| Chlamydia prevalence (high risk population) Outcome was prevalence of positive chlamydia tests in repeated cross‐sectional surveys of women tested at sex venues after 4 years of intervention. | RD ‐3.7% | RR 0.72 (0.54 to 0.98) | 4156 (1 study) | ⊕⊕⊝⊝3 | |
| Incidence of pelvic inflammatory disease (PID) at 12 months (intention‐to‐treat) Outcome was clinically diagnosed PID reported by the participant or extracted from medical records, pharmacy records or hospital discharge coding. Outcome very likely to be affected by risk of detection bias. | RD 0.0% (0‐0, 0.0%) | RR 0.68 (0.49 to 0.94) | 21,686 (4 studies) | ⊕⊕⊕⊝4 | |
| Incidence of epididymitis in men at 12 months (intention‐to‐treat) Outcome was epididymitis diagnosed in hospital and abstracted from hospital discharge coding. | RD 0.0% (0.0, 0.0%) | RR 0.80 (0.45 to 1.42) | 14,980 (1 study) | ⊕⊝⊝⊝5,6 | |
| GRADE Working Group grades of evidence CI: confidence interval; PID: pelvic inflammatory disease; RR: risk ratio. | |||||
| 1. Selection, attrition and other bias 2. One large non‐randomized cluster‐controlled trial. 3. Single large trial in female sex workers and uncertainty about generalisability to other screening interventions and populations. 4. Selection bias might have overestimated intervention effect. 5. Low uptake of the screening intervention with an imprecise effect estimate and uncertainty about estimated effect of screening interventions with higher sustained levels of uptake. 6. Performance bias | |||||
| Trial | Study population | Baseline | Follow‐up, 12 months | Reported effect (95% CI) | Follow‐up, subsequent | Reported effect (95% CI) | |||
| Intervention | Control | Intervention | Control | Intervention | Control | ||||
| High school students, Denmark | 43/867a | Not measured | 13/443a | 32/487 | RD − 5.5% (− 10 to 0.95%)a | — | — | — | |
| General population, Netherlands | 1851/43358 | 267/6223 | 1153/28803 | Not measured | OR 0.93 (0.81 to 1.07)b | 981/23899 | Not measured | OR 0.96 (0.83 to 1.10)b | |
| Female sex workers, Peru | 13.8% | 15.5% | — | — | — | 9.9% | 14.5% | RR 0.66 (0.47 to 0.94)c | |
| CI: confidence interval; OR: odds ratio; RD: risk difference; RR: risk ratio. | |||||||||
| Trial | Eligibility (ratio intervention: control) | Group | Uptake in intervention | Uptake in control | Comment |
| Selected at random from register (1:4) Intervention: invited for home‐sampling. Assessed after 3 months Control: not contacted. Tests at GP and STI clinics assessed after 3 months | Women | 4000 invited; 1175 (29.4%) sent home‐sample | 11,459 not invited; 1076 (9.4%) opportunistic tests | Control group not aware of trial. Assume routine health‐seeking behaviour over 3 months. If control group testing behaviour continued at the same level over 12 months, the proportion tested by the time the outcome PID was measured could have been higher. | |
| Men | 5000 invited; 1033 (20.7%) sent home‐sample | 9980 not invited; 140 (1.4%) opportunistic tests | |||
| Sex work venues identified and visited by mobile teams | Women | Could not be calculated | Could not be calculated | Not designed to measure uptake; no denominator | |
| Approached in colleges; all women enrolled were tested, randomised (1:1) | Women | 1259 (100%) immediate screening; 269 (21%) opportunistic tests | 1270 (100%) deferred screening; 258 (20%) opportunistic tests | Not designed to measure uptake | |
| Schools randomised (1:1) Intervention: allocated to home‐sampling Control: allocated to offer of GP testing Sexually active respondents eligible. Assessed after 4 months | Women | 2603 allocated; 928 eligible responders; 867 (93.4%) sent home‐sample | 2884 allocated; 833 eligible responders; 63 (7.6%) opportunistic tests | All students in school were allocated to intervention or control groups and asked if they would take part. Of the responders, only those who had ever had sex were eligible. The denominator of of all who had ever had sex was not known. Intervention group given home‐sampling kits | |
| Men | 1733 allocated; 442 eligible responders; 430 (97.3%) sent home sample | 1689 allocated; 246 eligible responders; 4 (1.6%) | — | ||
| Individuals randomised (1:2) Respondents fulfilling criteria for high risk of chlamydia eligible | Women | 36,457 randomised; 20,836 responded; | Numbers allocated to intervention and control not reported. Intervention group actively contacted | ||
| 1009 invited 645 (64%) tested | 1598 not invited; % tested not known | ||||
| Postal areas allocated (5:1) Intervention: allocated to yearly invitation x3 Control: allocated to single invitation | Women 1st 2nd 3rd | 142,419 invited; 141,078 invited; 131,010 invited; | 24,172 invited; | Postal invitation contained secure login code. Recipients had to register on website to request home‐sampling kit. One reminder letter | |
| Men 1st 2nd 3rd | 129,462 invited; 128,299 invited; 121,156 invited; | 23,884 invited | |||
| All 1st 2nd 3rd | 269,273 invited; 265,979 invited; 251,688 invited; | 48,031 invited | |||
| GP: general practitioner; PID: pelvic inflammatory disease; STI: sexually transmitted infection. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prevalence of chlamydia infection (positivity) measured in the whole study population at least 12 months after start of screening Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 3rd invitation vs control | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 2nd invitation vs control | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 1st invitation vs control | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Screening offer in high school students | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Incidence of PID at 12 months (intention‐to‐treat) Show forest plot | 4 | 21080 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.49, 0.94] |
| 2.1 Low risk of detection bias | 2 | 18022 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.55, 1.17] |
| 2.2 High risk of detection bias | 2 | 3058 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.22, 0.83] |
| 3 Incidence of PID at 12 months (per protocol analysis) Show forest plot | 2 | 2749 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.35, 1.10] |
| 3.1 Low risk of detection bias | 1 | 2377 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.34, 1.24] |
| 3.2 High risk of detection bias | 1 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.17, 1.80] |
| 4 Incidence of epididymitis in men at 12 months (intention to screen) Show forest plot | 1 | 14980 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.45, 1.42] |
| 5 Secondary outcomes for reproductive tract morbidity Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Female infertility | 1 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Ectopic pregnancy | 1 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

