Cribado para la infección por clamidia genital
Resumen
Antecedentes
Las infecciones genitales causadas por Chlamydia trachomatis son las infecciones bacterianas de transmisión sexual más prevalentes en todo el mundo. El cribado de los adultos jóvenes sexualmente activos para detectar y tratar las infecciones asintomáticas podría reducir la transmisión de clamidia y prevenir las morbilidades del sistema reproductivo, en particular la enfermedad inflamatoria pélvica (EIP) en mujeres, que puede causar infertilidad tubárica y embarazo ectópico.
Objetivos
Evaluar los efectos y la seguridad del cribado para la detección de clamidia versus la atención estándar en la transmisión y las complicaciones de la infección en mujeres embarazadas y no embarazadas y en hombres.
Métodos de búsqueda
Se hicieron búsquedas en las bases de datos electrónicas registro especializado del Grupo Cochrane de Enfermedades de Transmisión Sexual (Cochrane Sexually Transmitted Infections Group ), Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL), MEDLINE, EMBASE, LILACS, CINAHL, DARE, PsycINFO y Web of Science hasta el 14 febrero 2016, además, en el World Health Organization International Clinical Trials Registry (ICTRP) y en ClinicalTrials.gov. También se realizaron búsquedas manuales en actas de congresos, se contactó con los autores de los ensayos y se revisaron las listas de referencias de los estudios recuperados.
Criterios de selección
Ensayos controlados aleatorios (ECA) en mujeres (no embarazadas y embarazadas) y hombres adultos que compararon una intervención para la detección de clamidia con la atención habitual e informaron un resultado primario (prevalencia de C. trachomatis, EIP en mujeres, epididimitis en hombres o incidencia de parto prematuro). Se incluyeron los ensayos clínicos controlados no aleatorios si no hubo ECA para un resultado primario.
Obtención y análisis de los datos
Dos autores de la revisión, evaluaron de forma independiente los ensayos para su inclusión, extrajeron los datos y evaluaron el riesgo de sesgo. Se resolvieron los desacuerdos mediante consenso o adjudicación de un tercer autor de la revisión. Los resultados se describieron mediante diagramas de bosque (forest plots), y cuando fue apropiado se realizaron metanálisis con un modelo de efectos fijos para calcular los cocientes de riesgos (CR con intervalos de confianza del 95%, IC) en los grupos de intervención versus control. Se realizó un análisis de sensibilidad preespecificado del resultado primario, incidencia de EIP, según los riesgos de sesgo de selección y detección.
Resultados principales
Se incluyeron seis ensayos con 359 078 mujeres y hombres adultos. Un ensayo tuvo bajo riesgo de sesgo en los seis dominios específicos evaluados. Dos ensayos examinaron el efecto de rondas múltiples de cribado de clamidia sobre la transmisión de C. trachomatis. Un ensayo controlado grupal en mujeres y hombres de la población general en los Países Bajos no encontró cambios en la positividad de la prueba de clamidia después de tres convocaciones anuales (intervención 4,1% versus control 4,3%, CR 0,96; IC del 95%: 0,84 a 1,09; un ensayo, 317 304 participantes en la primera convocatoria para cribado, pruebas de baja calidad). La aceptación de la intervención fue baja (máxima del 16%). Un ensayo aleatorio grupal con mujeres profesionales del sexo en Perú encontró una reducción en la prevalencia de clamidia después de cuatro años (CR ajustado 0,72; IC del 95%: 0,54 a 0,98; un ensayo, 4465 participantes, pruebas de baja calidad).
Cuatro ECA examinaron el efecto del cribado de clamidia sobre la EIP en las mujeres a los 12 meses después de una oferta única de cribado. En el análisis de cuatro ensayos según el principio de intención de tratar, el riesgo de EIP fue menor en las mujeres de los grupos de intervención con respecto a los grupos control, con pruebas pequeñas de heterogeneidad entre los ensayos (CR 0,68; IC del 95%: 0,49 a 0,94; I2 7%, cuatro ensayos, 21 686 participantes, pruebas de calidad moderada). En un análisis de sensibilidad, la estimación del efecto del cribado de clamidia en dos ECA con bajo riesgo de sesgo de detección (CR 0,80; IC del 95%: 0,55 a 1,17) fue compatible con ningún efecto y fue menor que en dos ECA con riesgo alto o incierto de sesgo de detección (CR 0,42; IC del 95%: 0,22 a 0,83).
El riesgo de epididimitis en los hombres convocados para cribado, a los 12 meses después de una oferta única de cribado, fue 20% menor que el riesgo de epididimitis de los que no fueron convocados; el intervalo de confianza fue amplio y compatible con ningún efecto (CR 0,80; IC del 95%: 0,45 a 1,42; un ensayo, 14 980 participantes, pruebas de muy baja calidad).
No se encontraron ECA de los efectos del cribado de clamidia en el embarazo, y ningún ensayo midió los efectos perjudiciales del cribado de clamidia.
Conclusiones de los autores
Las pruebas acerca de los efectos del cribado en la transmisión de C. trachomatis son de calidad baja debido a la direccionalidad y el riesgo de sesgo. Hay pruebas de calidad moderada de que la detección y el tratamiento de la infección por clamidia pueden reducir el riesgo de EIP en las mujeres a nivel individual. Faltan pruebas de ECA sobre los efectos del cribado de clamidia en el embarazo.
Los ECA futuros de intervenciones para el cribado de clamidia deben determinar los efectos del cribado de clamidia en el embarazo, de las rondas repetidas de cribado en la incidencia de EIP asociada con clamidia y en la reinfección por clamidia en la población general y con alto riesgo.
PICO
Resumen en términos sencillos
Efectos del cribado de la infección de transmisión sexual por clamidia
Pregunta de la revisión
Se examinaron las pruebas acerca de los efectos y la seguridad del cribado para detectar y tratar la infección por clamidia en mujeres y hombres.
Antecedentes
La Chlamydia trachomatis es una infección de transmisión sexual frecuente. En varios países, cerca del 3% al 5% de los adultos sexualmente activos de entre 15 y 25 años de edad presenta clamidia en algún momento. Las infecciones sin tratar pueden provocar complicaciones que incluyen problemas de fertilidad en las mujeres e inflamación testicular en los hombres. El cribado para identificar y tratar a las personas que presentan la infección y no lo saben podría reducir el riesgo de complicaciones y la transmisión a otros.
Características de los estudios
Las pruebas están actualizadas hasta febrero 2016. Se encontraron seis ensayos que incluían a 359 078 mujeres y hombres adultos de Dinamarca, los Países Bajos, Perú, Reino Unido y los Estados Unidos. Dos ensayos examinaron el efecto del cribado de clamidia sobre los niveles de infección por clamidia. En los Países Bajos, los investigadores convocaron cada año, por tres años, a mujeres y hombres con edades de 15 a 29 años a realizarse una prueba de clamidia. En Perú, durante cuatro años equipos móviles visitaron 20 ciudades para ofrecerles a mujeres profesionales del sexo pruebas de clamidia.
Resultados clave
Con respecto al nivel de infección por clamidia, en los Países Bajos no hubo diferencias entre las mujeres y los hombres que se habían convocado a realizarse pruebas anuales para la detección de clamidia en comparación con las mujeres y los hombres que fueron convocados sólo una vez. Sólo el 16% de los convocados a cribado se realizó la prueba en el primer año, y sólo el 10% se realizó la prueba en el tercer año. En Perú, las mujeres profesionales del sexo en las ciudades visitadas con equipos móviles tuvieron niveles inferiores de infección por clamidia que las de las ciudades no visitadas por equipos móviles.
Cuatro ensayos proporcionaron datos comparables sobre la EIP. El riesgo de EIP fue 32% menor en las mujeres convocadas a realizarse una prueba única de detección de clamidia con respecto a las mujeres que no fueron convocadas. Cuando se eliminaron los dos ensayos con pruebas de calidad más baja, disminuyó el efecto protector del cribado de clamidia. No se encontraron efectos sobre la epididimitis en los hombres.
Calidad de la evidencia
El efecto del cribado de clamidia basado en un registro sobre la transmisión de C. trachomatis en adultos jóvenes en la población general no está claro. Se tiene una seguridad moderada de que el cribado de clamidia puede reducir el riesgo de EIP, pero no puede precisarse en qué media por las inquietudes acerca de la calidad de algunos ensayos.
Authors' conclusions
Summary of findings
| Chlamydia screening compared with usual care for the prevention of C. trachomatis transmission and reproductive tract morbidity | |||||
| Patient or population: healthy adults Settings: general population, high schools or colleges Intervention: chlamydia screening Comparison: usual care | |||||
| Outcomes | Absolute effect | Relative effect | No of Participants | Quality of the evidence | Comments |
| Chlamydia prevalence (general population) Outcome was chlamydia test positivity after 3 yearly invitations in intervention clusters vs 1 invitation in control areas. Uptake was too low for chlamydia positivity to be considered an unbiased estimate of prevalence. | RD 0.0% (‐0‐01, +0.01%) | RR 0.96 (0.84 to 1.09) | 30,122 (1 study) | ⊕⊕⊝⊝1,2 | |
| Chlamydia prevalence (high risk population) Outcome was prevalence of positive chlamydia tests in repeated cross‐sectional surveys of women tested at sex venues after 4 years of intervention. | RD ‐3.7% | RR 0.72 (0.54 to 0.98) | 4156 (1 study) | ⊕⊕⊝⊝3 | |
| Incidence of pelvic inflammatory disease (PID) at 12 months (intention‐to‐treat) Outcome was clinically diagnosed PID reported by the participant or extracted from medical records, pharmacy records or hospital discharge coding. Outcome very likely to be affected by risk of detection bias. | RD 0.0% (0‐0, 0.0%) | RR 0.68 (0.49 to 0.94) | 21,686 (4 studies) | ⊕⊕⊕⊝4 | |
| Incidence of epididymitis in men at 12 months (intention‐to‐treat) Outcome was epididymitis diagnosed in hospital and abstracted from hospital discharge coding. | RD 0.0% (0.0, 0.0%) | RR 0.80 (0.45 to 1.42) | 14,980 (1 study) | ⊕⊝⊝⊝5,6 | |
| GRADE Working Group grades of evidence CI: confidence interval; PID: pelvic inflammatory disease; RR: risk ratio. | |||||
| 1. Selection, attrition and other bias 2. One large non‐randomized cluster‐controlled trial. 3. Single large trial in female sex workers and uncertainty about generalisability to other screening interventions and populations. 4. Selection bias might have overestimated intervention effect. 5. Low uptake of the screening intervention with an imprecise effect estimate and uncertainty about estimated effect of screening interventions with higher sustained levels of uptake. 6. Performance bias | |||||
Background
Description of the condition
Genital infections caused by Chlamydia trachomatis serovars D‐K are the most prevalent bacterial sexually transmitted infection worldwide, with an estimated 131 million people being infected in 2012 (Newman 2015). Chlamydia is the most common notifiable infection in the United States, with 1,441,789 infections reported in 2014 compared with 350,062 cases of gonorrhoea, the second most common notifiable condition (CDC 2015). Chlamydia is also the most commonly reported infection in Australia and Europe (DoHA 2016; ECDC 2015), and its prevalence is highest in young, sexually active adults. The prevalence of chlamydia has been estimated to be about 3% to 5% in nationally representative samples of sexually experienced women and men aged 25 years and under in high‐income countries (Redmond 2015). Chlamydia prevalence in adults aged 15 to 44 years is about 2% to 3% in low‐ and lower‐middle‐income countries, 4% to 7% in upper‐middle countries and 2% to 3% in high‐income countries (Newman 2015).
C. trachomatis is a gram negative obligate intracellular bacterium, which infects columnar epithelium in the lower genital tract in women and men and can also infect the rectum, pharynx, conjunctiva and placenta (Rours 2011; Stamm 2008). Chlamydia infection causes complications, most commonly due to its spread from the lower to the upper genital tract. Upper genital tract infection occurs in both sexes, but is more common and has more severe consequences in women (Stamm 2008). In women, chlamydia ascends to the upper genital tract in approximately 10% of cases to cause symptomatic pelvic inflammatory disease (PID) (Herzog 2012; Oakeshott 2010). The resulting tubal damage can then cause ectopic pregnancy, tubal infertility and chronic pelvic pain (Paavonen 2008). Although about 45% of tubal infertility might be attributable to chlamydia infection (Price 2012), the probability of tubal infertility in women who have had chlamydia is estimated to be only 1% to 4% (Kavanagh 2013; Land 2010). Chlamydia infection in pregnancy is associated with preterm labour and can infect the neonate, causing ophthalmia neonatorum and atypical pneumonia (Kohlhoff 2008; Rours 2011). C. trachomatis can cause epididymo‐orchitis in men, but its role in prostatitis and male infertility is not well established (Stamm 2008). Chlamydia can also cause reactive arthritis in men and is a cofactor for HIV infection, increasing both susceptibility and infectiousness (Fleming 1999; Stamm 2008).
Uncomplicated genital chlamydia infections are usually asymptomatic in both women and men (Stamm 2008), and untreated infections last more than a year on average (Althaus 2010). C. trachomatis can be treated with tetracyclines (usually doxycycline) or macrolide antibiotics (usually azithromycin) with short‐term microbiological cure rates of 90% to 95% (Manhart 2013). Immunity after chlamydia infection is incomplete, and repeated chlamydia infection is common (Batteiger 2010a). In studies of women enrolled from primary care and sexual health clinics and followed up prospectively, about 25% of women treated for chlamydia had the infection detected again in the year after treatment (Scott LaMontagne 2007; Walker 2012). There are several reasons for repeated detection of chlamydia. In one prospective study amongst young women in the United States, Batteiger 2010b combined information about sexual behaviour and genotype from 183 women with more than one episode of chlamydia infection to estimate that about 66% of infections were probably acquired from a new partner, 17% were reinfections from untreated or inadequately treated sexual partners, 14% were probable antibiotic treatment failures, and 3% persisted without treatment. There is some evidence to suggest that immunity after natural clearance of chlamydia infection lasts longer than immunity after antibiotic treatment (Geisler 2013).
Description of the intervention
Screening of sexually active young adults is the only way to detect most chlamydia infections because of the lack of symptoms or clinical signs in most infected people. Screening is a process of identifying apparently healthy people who may be at increased risk of a disease or condition. They can then be offered information, further tests and appropriate treatment to reduce their risk and the impact of any complications arising from the disease (UKNSC 2013).
There are two goals of screening for genital chlamydia infection: first, to control the transmission of chlamydia and reduce the prevalence of infection in the population; and second, to reduce the risk of complications, especially reproductive tract complications in women (Meyers 2007; NCSP 2014). Screening is a programme, not a test (Raffle 2007). This means that screening includes the whole system of events needed to reach the endpoint of reducing the risk of disease or complications. For chlamydia infection, screening includes offering a test to diagnose C. trachomatis, treating people with a positive test, partner notification to identify and treat sexual partners and repeated screening to detect and treat newly acquired infection or reinfection.
The target group for chlamydia screening is usually defined by age and sex. For example, recommendations for chlamydia screening in the United States target women aged 25 years and under (USPSTF 2014); in Australia, women under 25 (RACGP 2012); and in the UK, women and men aged 25 years and under (NCSP 2014). Whilst behavioural and demographic factors can be used to identify groups at higher risk of chlamydia infection, risk factors differ between populations, and selective criteria can be difficult to apply in practice (Gotz 2005; Stergachis 1993).
Chlamydia screening can be offered systematically, using a population register to invite people in the target age group (van den Broek 2012). More commonly, screening is recommended as an opportunistic activity to be offered to eligible young adults attending healthcare services (USPSTF 2014; NCSP 2014; RACGP 2012). Some countries recommend repeated screening, given the frequency of repeated chlamydia and the fact that young adults may change sexual partners over time (Scott LaMontagne 2007). In England, the National Chlamydia Screening Programme recommends a screening test every year or after a change of sexual partner. Health professionals may also take advantage of visits for cervical cancer screening in young women to offer chlamydia screening in countries where the target age groups and screening frequency overlap. In the UK, however, cervical cancer screening is only recommended for women over 25 years (NCSP 2014).
How the intervention might work
The way that chlamydia screening might work depends on the goal of screening. To reduce chlamydia prevalence and incidence, the coverage of screening has to be high enough to identify and treat prevalent cases of chlamydia and to interrupt chains of chlamydia transmission in the population. Screening also has to be frequent enough to prevent repeated infections because of the limited immunity after treatment. Mathematical models show that chlamydia screening reduces prevalence over time; in several models, screening of 30% or more of the target population each year is necessary to markedly reduce chlamydia prevalence (Althaus 2012; Regan 2008).
There are two ways in which screening for chlamydia might work to prevent reproductive tract complications (Herzog 2013; Peterman 2009). First, direct prevention of PID occurs if screening detects and treats an endocervical chlamydia infection in an individual woman before the infection ascends into the genital tract to cause PID and subsequent tubal damage. The effectiveness of screening depends on the timing of progression from lower to upper genital tract infection. If PID occurs immediately or shortly after the initial lower genital tract infection, there is no opportunity for screening to work (Herzog 2012; Smith 2007). Randomised controlled trials (RCTs) have shown that the incidence of clinically diagnosed PID is lower in women actively invited for chlamydia screening compared to those receiving usual care (Andersen 2011; Ostergaard 2000; Oakeshott 2010; Scholes 2006). Women infected with chlamydia who are enrolled into trials have persisting prevalent infections with an unknown date of infection. The trial findings and supportive evidence from mathematical modelling studies suggest, therefore, that PID development can occur during the course of infection (Herzog 2012). Second, prevention of chlamydia transmission through screening and treatment has an indirect effect on the risk of PID because it reduces the risk of becoming infected with chlamydia in the first place.
Prevention of PID should lead to a reduction in the incidence of ectopic pregnancy and tubal infertility if tubal scarring is prevented. It is, however, very difficult to measure the impact of chlamydia screening on these outcomes because women in the age groups at highest risk of chlamydia infection are usually using contraception. In one RCT, the incidence of ectopic pregnancy and infertility after 11 years of follow‐up were similar in women who had received a single invitation to be screened for chlamydia and women who received usual care (Andersen 2011).
There are also potential harms of chlamydia screening, for example an increased rate of repeated infection after treatment or the ending of a sexual partnership (Gottlieb 2011; O'Farrell 2013). First, a woman who has been treated for chlamydia becomes susceptible and is at risk of repeated infection and PID. Researchers have suggested that the risk of PID is higher with subsequent chlamydia infections (Hillis 1997), possibly because repeated exposure to C. trachomatis antigens can cause immune‐mediated tubal damage (Brunham 2005). Second, being diagnosed with a sexually transmitted infection can have a negative emotional and psychological impact on the infected person (Gottlieb 2011; Mills 2006). In one study in the United States, sexual partnerships broke down for 33% of women with a positive chlamydia test result compared with 11% of those receiving a negative result (Gottlieb 2011). Third, the experience of screening can cause anxiety. In a study in the UK, however, chlamydia screening did not increase anxiety or depression and did not reduce self esteem (Campbell 2006).
Why it is important to do this review
Screening for chlamydia infection is widely recommended and practised (USPSTF 2014; Low 2012; NCSP 2014; RACGP 2012). Rates of chlamydia testing amongst young adults are high (4000 to 9000 per 100,000 population) in several high‐income countries (Bender 2011). There is a strong rationale for early detection and treatment of chlamydia infection in asymptomatic young adults to reduce both transmission and complications (Low 2013), and these potential benefits should be weighed against the potential harms. There are few data about long‐term trends in chlamydia prevalence in countries that recommend chlamydia screening. In the United States, repeated cross‐sectional studies show that chlamydia prevalence fell between 1999 and 2008 in 14 to 39 year olds as a whole, but not in 15 to 25 year old women, who are the target population for screening (Datta 2012).
It is also important to review the effects of screening programmes from a health policy perspective, as they have implications beyond the application of a diagnostic test and are costly to administer (UKNSC 2013). Economic evaluations about the cost‐effectiveness of chlamydia screening programmes are not consistent (ECDC 2014).
There is a systematic review of the effectiveness of chlamydia screening interventions in studies published up to 2007 (Low 2009). RCTs have found that a one‐off screening invitation could reduce the incidence of PID one year later (Ostergaard 2000; Scholes 1996). Also, we know that there are new completed trials with PID and transmission as endpoints (Andersen 2011; Oakeshott 2010; van den Broek 2012), and there is at least one ongoing trial (Hocking 2012). It is therefore important to develop a Cochrane review about this issue.
Objectives
To assess the effects and safety of chlamydia screening versus standard care for chlamydia transmission and infection complications in pregnant and non‐pregnant women and in men.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised and non‐randomised controlled trials.
We included controlled trials with non‐randomised allocation to intervention and control arms if there were no RCTs addressing a primary outcome of chlamydia screening.
We included trials with cluster allocation as long as investigators collected baseline and outcome data prospectively using the same criteria throughout the trial period.
We analysed results from randomised and non‐randomised study designs separately.
We excluded cohort studies, case‐control studies and interrupted time‐series studies.
Types of participants
We included women (non‐pregnant and pregnant) and men (heterosexual or men who have sex with men) aged over 13 years in any setting. The minimum age was arbitrary but allowed us to include only trials of sexually transmitted chlamydia infections.
Types of interventions
-
Intervention: screening for sexually transmitted genital chlamydia infection, defined as the offer of a test to apparently healthy people to identify those at increased risk of chlamydia infection. This definition was adapted from the UK National Screening Committee (UKNSC 2013). We included any test used to diagnose genital chlamydia infection.
-
Comparison: inactive control (no offer of screening or standard care)
Types of outcome measures
Eligible trials included at least one of the pre‐specified primary outcomes. The primary outcomes were measures of morbidity because one criterion for assessing the effectiveness of a screening programme is that 'there should be evidence from high quality Randomised Controlled Trials that the screening programme is effective in reducing mortality or morbidity' (http://www.screening.nhs.uk/criteria).
Primary outcomes
We included one primary outcome for each goal of chlamydia screening.
-
Outcome for C. trachomatis transmission: prevalence of chlamydia infection in women and men at least 12 months after the start of the screening intervention. Prevalence was estimated as the number of positive chlamydia tests divided by the number of people tested.
-
Outcomes for reproductive tract morbidity: incidence of upper genital tract infection in women and men in the 12 months after the offer of screening. PID (women) or epididymitis (men) were clinical diagnoses made using clinical criteria defined in advance by the authors. Examples include criteria published by USPSTF 2014 or Hager 1983.
-
Outcome for chlamydia infection in pregnancy: incidence of preterm delivery. Preterm delivery was defined as delivery at a gestational age of less than 37 weeks, with subgroups of gestational ages less than 32 weeks and less than 35 weeks (Rours 2011).
Secondary outcomes
-
Outcomes measured in all participants
-
Proportion of participants receiving the intervention, defined as the number tested for chlamydia divided by the number eligible and invited to take part. We did not consider uptake of chlamydia testing as a primary outcome because it is an intermediate outcome of a chlamydia screening intervention, and the relationship between uptake of screening and the primary outcomes has not been quantified.
-
Harms of screening, including psychological distress, partner violence, relationship breakdown, using definitions described by the authors
-
-
Outcomes measured in women who were not pregnant during the trial or in men
-
Prevalence of chronic female pelvic pain, defined as patient‐reported pain in the lower abdomen or pelvis lasting at least six months (Paavonen 2008)
-
Prevalence of female or male infertility, defined using a clinical definition of lack of pregnancy despite unprotected intercourse for 12 months or more (Paavonen 2008)
-
-
Outcomes measured in women who were pregnant during the trial, or in their infants
-
Incidence of C. trachomatis neonatal conjunctivitis, defined as C. trachomatis isolated from the conjunctiva by culture or detected by nucleic acid amplification test (Kohlhoff 2008)
-
Incidence of C. trachomatis neonatal pneumonitis, defined as signs of lower respiratory tract infection presenting between 4 and 12 weeks with C. trachomatis isolated from the nasopharynx by culture or detected by nucleic acid amplification test (Kohlhoff 2008)
-
Search methods for identification of studies
We aimed to identify trials meeting the inclusion criteria irrespective of their language, publication date or publication status (published, unpublished, in press and in progress). We used both electronic searching in bibliographic databases and handsearching, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
We downloaded and managed the results of all searches using Endnote bibliographic software. We deleted duplicate records of the same study.
Electronic searches
The Cochrane Sexually Transmitted Infections (STI) Review Group Specialised Register includes RCTs and controlled clinical trials, from 1944 to 2015, located through electronic searching in the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and EMBASE, and handsearching in journals not indexed in those databases (according to the journals' master list of the STI Cochrane Review Group): Anatolian Journal of Obstetrics & Gynecology, Current Medical Literature Gynecology & Obstetrics, Current Obstetrics and Gynecology Reports, ISRN Obstetrics and Gynecology, Journal of South Asian Federation of Obstetrics & Gynecology, Obstetrics and Gynecology International, Obstetrics Gynaecology and Reproductive Medicine, and Sexual Science: the newsletter of the Society for the Scientific Study of Sexuality and Sexualities.
The Trials Search Coordinator (TSC) of the STI Cochrane Review Group implemented a comprehensive search strategy to capture as many relevant trials as possible in electronic databases. We used a combination of controlled vocabulary (MeSH, Emtree, DeCS, including exploded terms) and free‐text terms (considering spelling variants, plurals, synonyms, acronyms and abbreviations) for 'genital chlamydia infection' and 'screening', with field labels, truncation, and proximity and Boolean operators. We present the electronic search strategies in Appendix 1.
We searched the following electronic databases.
-
The Cochrane Sexually Transmitted Infections (STI) Review Group Specialised Register.
-
CENTRAL, Ovid platform.
-
MEDLINE, Ovid platform (1991 to February 2016).
-
MEDLINE In‐Process & Other Non‐Indexed Citations, Ovid platform (January 1946 to February 2016).
-
MEDLINE Daily Update, Ovid platform (January 1946 to February 2016).
-
EMBASE (1947 to February 2016).
-
LILACS, iAHx interface (1982 to February 2016).
-
CINAHL (inception to February 2016).
-
Database of Abstracts of Reviews of Effects (DARE) (inception to February 2016).
-
PsycINFO (inception to February 2016).
We searched MEDLINE using the Cochrane highly sensitive search strategy for identifying RCTs: sensitivity and precision maximizing version (2008 revision), Ovid format (Higgins 2011a). We combined the LILACS search strategy with the RCT filter of the iAHx interface.
Searching other resources
-
Trial registers
-
WHO International Clinical Trials Registry Platform (ICTRP) portal (http://apps.who.int/trialsearch/) (inception to February 2016)
-
ClinicalTrials.gov (http://clinicaltrials.gov/) (inception to February 2016)
-
-
Web of Science (inception to February 2016)
-
Grey literature in System for Information on Grey Literature in Europe 'OpenGrey' (http://www.opengrey.eu/) (inception to February 2016)
-
Handsearch of conference proceeding abstracts
-
The International Society for Sexually Transmitted Diseases Research ‐ ISSTDR (http://www.isstdr.org/): 2007, 2009 and 2011
-
The British Association for Sexual Health and HIV ‐ BASHH (http://www.bashh.org/): 2004, 2006, 2007 and 2009
-
International Congress on Infectious Diseases ‐ ICID (http://www.isid.org/): 2010 and 2012
-
The International Union against Sexually Transmitted Infections ‐ IUSTI (http://www.iusti.org/): 2011 and 2012
-
International Society for Infectious Diseases ‐ ISID (http://www.isid.org/): 2011
-
International Meeting on Emerging Diseases and Surveillance ‐ IMED (http://www.isid.org/): 2007, 2009 and 2011
-
Interscience Conference on Antimicrobial Agents and Chemotherapy ‐ ICAAC (http://www.icaac.org/): 2011 and 2012
-
The International Federation of Gynecology and Obstetrics ‐ FIGO (http://www.figo2012.org/home/): 2012
-
-
Handsearching previous systematic reviews and other relevant publications on the same topic
-
Handsearching reference lists of all relevant RCTs identified by other methods
Data collection and analysis
Selection of studies
Two review authors (NL, SR) independently reviewed titles and abstracts of articles identified through the search strategy, using a pilot‐tested form to document potential eligibility. We obtained the full‐text records that both review authors agreed were potentially eligible as well as articles about which the authors still disagreed after discussion; we also retrieved records with no abstract if there was insufficient information available from the title or publication type to make a decision.
We used the same criteria for the abstracts of articles identified through searching other resources as for studies identified through electronic database searches.
NL and SR examined full‐text records using a pilot‐tested form to assess eligibility, and we included studies identified by both authors as fulfilling our inclusion criteria. Where there were discrepancies, the authors discussed the article and reached a consensus decision.
We used a flow chart to document the numbers of articles assessed, included and excluded at each stage, with a summary of reasons for exclusion (Figure 1). The flow chart shows the total number of studies included in the review and the total number of articles pertaining to these studies. We briefly record the characteristics of excluded studies in case readers might expect them to have been included.

#Study flow diagram.
Data extraction and management
We used piloted, standardised forms to extract data about:
-
study location and setting;
-
trial design and power calculation;
-
ethical approval;
-
inclusion and exclusion criteria;
-
baseline characteristics of trial participants, including sex, age, sexual orientation, pregnancy status for women, diagnostic test used to detect C. trachomatis;
-
types of intervention: opportunistic or systematic invitation for screening, number of screening rounds, screening interval;
-
types of comparison group: usual care, alternative screening method;
-
types of outcome: primary, secondary;
-
reporting of methodological characteristics (see next section, Assessment of risk of bias in included studies for details).
We extracted numerical data on:
-
number of people assessed for eligibility;
-
numbers randomised to intervention and comparison groups;
-
numbers receiving screening in intervention and comparison groups (at each screening round if multiple rounds);
-
numbers included in analyses in intervention and comparison groups;
-
numbers with outcomes in intervention and comparison groups;
SR extracted data about study characteristics, and NL checked these details for accuracy. They resolved discrepancies by discussion.
Pairs of appropriately qualified review authors (BA and JvB, HG and NL, AU and HW) extracted numerical data independently from each included study into Epidata using a structured form (Epidata). If a reviewer had been an investigator of an included study, they did not extract data from that study. If there were multiple publications relating to the same study, we allowed the extraction of data items from different publications. If there were discrepancies between publications about a data item, we used the data presented in the main trial publication (the publication that includes the results for the primary outcome) or the first chronological publication reporting that data item.
We compared the two files using the validation function available in Epidata, resolving discrepancies in data extraction or data entry by consensus. If there was no agreement, a third independent author adjudicated to make a final decision. We entered the agreed data into Review Manager 5.3 (RevMan) software (RevMan).
If there were insufficient details given to allow the extraction of numerical data, we included the study and described the results narratively.
Assessment of risk of bias in included studies
We assessed the methods that trial authors reported using in the design and execution of all included trials. The assessment determined whether there was a risk of bias that would over‐ or underestimate the effect of the intervention on one or more outcomes (Higgins 2011a). This assessment relied on reports of methods described by trial authors in publications and, where available, trial protocols. For any trial, the findings of the assessment could only say whether there was a risk of biased results, not whether the results themselves were or were not biased.
For both randomised and non‐randomised trials, we assessed the risk of five specific sources of bias: selection bias, performance bias, detection bias, attrition bias and reporting bias, and we also recorded any other biases related to a particular trial.
For RCTs we used the Cochrane 'Risk of bias' tool and criteria in Higgins 2011a (Table 8.5.d) to assess the relevant domains of the reported methods and results (Reeves 2011).
Selection bias was only the domain for which there are important differences in assessing the risk of bias in randomised and non‐randomised controlled trials. For non‐randomised controlled trials, we used the UK National Insitute of Health and Care Excellence (NICE) 'methodology checklist' for cohort studies to assess the risk of selection bias (NICE 2012). The NICE methodology checklist format follows that of the Cochrane tool, with criteria to assess bias in each domain and a choice of low, high or unclear risk of bias. We used the Cochrane 'Risk of bias' tool to assess non‐randomised controlled trials for risk of performance, detection, attrition and reporting biases.
Assessors recorded whether there was a low, high or unclear risk of bias in each domain of each included trial and gave a justification for their decision. Two independent assessors assessed each trial, including at least one expert in trial methodology (NL) and one expert in chlamydia screening (HG). They resolved discrepancies by discussion.
The domains and their source are summarised here.
(1a) Random sequence generation (possible selection bias, Cochrane 'Risk of bias' tool)
Selection bias could occur if investigators are able to predict allocation to intervention or control groups and selectively enrol participants or clusters of participants. The method used to generate the allocation sequence should be unpredictable and should balance prognostic factors, on average, across intervention and comparison groups. We assessed the method as being at:
-
low risk of bias (adequate description of a truly random process, e.g. random number tables, computer‐generated random numbers);
-
high risk of bias (explicit description of an allocation process that is not truly random, e.g. odd or even dates of birth of individuals, clusters of participants selected for implementation of the intervention with subsequent enrolment of comparison groups); or
-
unclear risk of bias (description that does not include enough information to decide whether sequence generation was truly random or not).
(1b) Allocation concealment (possible selection bias, Cochrane 'Risk of bias' tool)
Selection bias can occur if investigators selectively enrol participants or clusters of participants and allocate them to a particular group according to whether their characteristics are associated with the outcome. If the sequence has been randomly generated, selective enrolment can occur if the next assignment is known before allocation. Allocation concealment up to the point of assignment prevents selective assignment to a particular intervention group. We assessed the methods of allocation concealment as having:
-
low risk of bias (adequate description of a process that prevented foreknowledge of allocation up to the point at which assignment was recorded, e.g. telephone or central randomisation);
-
high risk of bias (description of a process that meant that those assigning participants or clusters of participants knew or could predict the allocation in advance); or
-
unclear risk of bias (insufficient details to be able to decide whether the allocation was concealed or not).
(1c) Systematic differences between comparison groups (possible selection bias, NICE 'methodology checklist')
In a non‐randomised trial, selection bias can occur because of the lack of a random allocation sequence and concealment. If the person assigning individuals or clusters to a particular group knows about the distribution of factors associated with the outcome, they might introduce selection bias. We assessed studies as having:
-
low risk of bias (the reason for participant allocation to treatment groups is not expected to affect the outcomes of the study, there were attempts made within the design or analysis to balance the comparison groups for potential confounders, and the groups were comparable at baseline for all known major confounders and prognostic factors);
-
high risk of bias (any of the above conditions are not fulfilled); or
-
unclear risk of bias (insufficient details to be able to decide whether there was a risk of systematic differences between comparison groups).
(2) Blinding of participants and personnel (possible performance bias)
Screening is an intervention that involves systematic differences in the delivery of a health service. Personnel who offer chlamydia screening tests might offer other sexual health information, advice or interventions, such as condoms, that could affect participants' risk of chlamydia infection or another outcome. Such information and interventions could also be considered a part of the screening programme, however. Trial participants or clusters of participants in an inactive 'usual care' control group might also be considered blinded if they do not know that they are part of a trial. For each included trial, we described the intervention. We considered studies as being at low risk of bias if participants were blinded or if the lack of blinding was unlikely to affect results for a particular outcome.
(3) Blinding of outcome assessment (possible detection bias)
For chlamydia screening interventions, adequate descriptions of blinding of those assessing the outcomes are important. We distinguished between outcomes that were objectively assessed (e.g. chlamydia test results obtained from automated diagnostic systems) and those that were subjective (e.g. clinical diagnosis of pelvic inflammatory disease).
The incidence of clinically diagnosed pelvic inflammatory disease is a primary outcome of chlamydia screening interventions. The main symptom is lower abdominal pain, which is common and non‐specific. Knowledge of group assignment could influence the interpretation of symptoms by both trial participants and personnel delivering the intervention in unpredictable ways. For example, healthcare providers who know whether a woman has been screened for chlamydia might be more likely to assign a diagnosis of pelvic inflammatory disease to a woman who presents with abdominal pain because of increased awareness of the complications of chlamydia infection. On the other hand, they might be reassured if the test was negative or if treatment had been given and then interpret abdominal pain with or without accompanying signs as resulting from another cause. Women who have accepted or declined screening might also modify their assessment of symptoms or their health‐seeking behaviour. For subjective outcomes, we assessed methods as having:
-
low risk of bias (adequate description of assessment that reduced the risk of bias, e.g. uniform assessment of all trial participants by an independent assessor blinded to allocation, or assessment of diagnoses by an independent assessment panel blinded to allocation);
-
high risk of bias (assessment of outcomes by personnel who knew the group assignment); or
-
unclear risk of bias (insufficient information to determine whether outcome assessment was blinded or not).
(4) Incomplete outcome data (possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For each outcome or class of outcomes, we described the completeness of data and exclusions from analysis in each included trial. We stated whether analyses were conducted and reported according to intention‐to‐treat or not. Where reported, we stated numbers included in the analysis as a proportion of the totals randomised to intervention and comparison groups, reasons for attrition or exclusion, and whether missing data were balanced across groups or were related to outcomes. Where trials or trial authors provided sufficient information, we re‐included missing data in our analyses. We used a cutoff of 20% to assign trials with missing outcome data as being at low or high risk of bias. In addition, we assessed methods as being at:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; 'per protocol' analysis done with substantial departure of intervention received from that assigned at randomisation); or
-
unclear risk of bias (insufficient information about missing data or exclusions from analysis).
(5) Selective outcome reporting (possible reporting bias)
Where available, we assessed the trial protocol and trial registration documents as well as articles or publications resulting from a trial. We described the documents available for each included study and assessed the methods as having:
-
low risk of bias (adequate description that reports all pre‐specified outcomes and all expected outcomes of interest to the review);
-
high risk of bias (explicit evidence that trials did not report all pre‐specified outcomes or did not pre‐specify one or more reported primary outcomes, that they reported outcomes of interest incompletely so that they cannot be used in the review, or that there are no results for a key outcome that would have been expected to have been reported); or
-
unclear risk of bias (insufficient information to decide whether selective reporting bias is likely or not).
(6) Other biases
For each included trial we described other potential sources of bias. We assessed these sources after data extraction and did not pre‐specify them in the protocol.
We assessed trials that received any funding from pharmaceutical or diagnostic manufacturers as being at unclear risk of bias.
'Contamination' of the intervention could occur if chlamydia testing in the control group increased to approach the levels achieved in the intervention group. This would reduce the size of the effect in cluster‐randomised trials. Contamination could also occur in a cluster‐controlled trial if sexual mixing between individuals in intervention and control groups occurred. We assessed trials as being at:
-
low risk of bias (adequate description that contamination did not occur or was not relevant to the trial design);
-
high risk of bias (explicit evidence that contamination occurred, with documentation of chlamydia testing rates in control group during the intervention period); or
-
unclear risk of bias (contamination was possible but there was insufficient information to assess).
Measures of treatment effect
We compared the treatment effect or harmful effect for each outcome in the intervention versus the control group, expressing the association as a risk ratio (RR) with 95% confidence intervals (CI). An advantage of the RR is that it can be interpreted easily for both high and low event rates. We also calculated the risk difference (RD, 95% CI), the actual difference in the event rate between intervention and control groups.
For the primary outcome of chlamydia prevalence, we reported the overall effect estimate at the level of the cluster, and stated whether the analysis took into account the correlation between individuals within a cluster.
Unit of analysis issues
Cluster‐randomised trials of chlamydia screening interventions might measure the effect of the intervention in a geographic area or a school community. In trials of chlamydia screening, the intervention affects not only individuals who are screened and treated (direct effect), but their sexual partners and members of the same sexual network (indirect effect). The indirect effect of screening can reduce the level of repeated exposure to infection of individuals within a cluster.
If meta‐analysis was appropriate and both individually and cluster‐randomised trials reported the same outcome, we adjusted the size of the trial to an 'effective sample size' (Campbell 2005; Higgins 2011a; Ukoumunne 1999). We then combined the effect sizes in meta‐analyses. The effective sample size took into account the design effect of the cluster, based on the average cluster size and intraclass correlation coefficient (ICC). The design effect was (1 + (average cluster size ‐ 1) * ICC). We calculated an effective sample size, dividing both number of events and number of participants by the design effect (Higgins 2011a).
This issue applied to the outcome PID in (Ostergaard 2000), which had 17 clusters (schools) and an average cluster size of 100. The trial publication did not report the ICC so we used an external source that reported a median ICC of 0.028 for 12‐month follow‐up data from cluster‐randomised trials of adolescent HIV/STI/pregnancy prevention interventions (Glassman 2015). The design effects were 3.77 for the intention‐to‐treat and 2.5 for per protocol data.
If we identified studies with multiple treatment groups we reported all intervention groups in the 'Characteristics of included studies' section and included only those that met the inclusion criteria. We combined relevant groups to create a single pair‐wise comparison; all relevant intervention groups were combined into a single intervention group.
Dealing with missing data
We reported the percentage of observations with missing data in each included trial. We used sensitivity analyses to explore the effect of including or excluding trials with > 20% missing data.
For each outcome we attempted to analyse data according to the intention‐to‐treat principle, with all participants included in the group to which they were randomised and exclusion only of participants with missing outcome data.
Assessment of heterogeneity
We reported statistical heterogeneity in results between studies using I2, Tau2 and Chi2 statistics obtained from analyses in RevMan. We used the I2 statistic to quantify the percentage of variability between the results that is due to heterogeneity rather than sampling error (Higgins 2002). We took into account the fact that I2 values are affected by the number of studies, the magnitude and direction of effects in individual trials, and the strength of evidence of heterogeneity. In general, we considered I2 values below 40% as showing little evidence of statistical heterogeneity.
Assessment of reporting biases
We looked for evidence of publication and other reporting biases using funnel plots that plot the effect size against precision. If there were more than 10 studies in a meta‐analysis we used statistical tests of funnel plot asymmetry for continuous or binary endpoints (Egger 1997; Harbord 2005).
Data synthesis
We described the results of trials where there were too few studies for meta‐analysis or where we considered that meta‐analysis was not clinically meaningful. We used forest plots to display results of trials examining the same outcome.
Where appropriate, we combined data using meta‐analyses conducted in RevMan. If there were trials that examined the same intervention and measured the same underlying effect in similar populations, we used a fixed‐effect model. If there was clinical heterogeneity or evidence of substantial statistical heterogeneity, we used a random‐effects model to estimate the average treatment effect across trials. We presented results as the summary RR (95% CI) with I2 and Tau2 estimates. For meta‐analyses with at least three studies combined using a random‐effects model, we also calculated a prediction interval to examine the range of effect estimates that might be expected in different settings or populations (Riley 2011). We did not combine results from randomised and non‐randomised trials in the same meta‐analysis but compared these in a sensitivity analysis.
Subgroup analysis and investigation of heterogeneity
If there was evidence of substantial heterogeneity (I2 greater than 40%) for the primary outcome measures and if there were enough trials, we used subgroup analyses to explore it. We explored the following subgroups.
-
Sex of the participant.
-
Level of sexual behaviour risk of the study population (high risk, low risk).
-
Uptake of the intervention (greater or less than 50%).
-
Intensity of the intervention (single offer, multiple screening rounds).
For fixed‐effect models based on inverse variance meta‐analysis, we used tests of interaction to examine differences between groups. For random‐effects models and fixed‐effect models using methods other than inverse variance, we inspected confidence intervals for the subgroup estimates.
Sensitivity analysis
We planned to conduct the following sensitivity analyses to investigate the influence of methodological aspects of the review that might influence the results.
-
The treatment effect for pelvic inflammatory disease incidence in RCTs assessed as being at low versus high risk of detection bias (i.e. blinded versus non‐blinded assessment).
-
The treatment effect for each primary outcome in RCTs assessed at being at low versus high risk of selection bias.
-
The treatment effect for chlamydia prevalence in RCTs versus non‐randomised studies.
-
The treatment effect for each primary outcome in intention‐to‐treat versus per protocol study populations.
In view of the small number of included studies, we only did sensitivity analyses for PID as an outcome.
Summary of findings table
We produced a 'Summary of findings' table to present the assessment of the overall level of evidence for each primary outcome (Higgins 2011b). We used the GRADE approach, as incorporated in RevMan. We summarised the quality of evidence as high, moderate, low or very low. We downgraded the overall level from 'high quality' by one level for serious (and by two levels for very serious) methodological limitations (risk of bias), inconsistency of results, indirectness of evidence, imprecision or publication bias.
Results
Description of studies
Results of the search
Our search strategy identified 981 unique records as of 14 February 2016 (Figure 1). Of these, we examined the full text manuscript for 150 records. We found 10 potentially eligible trials (33 records). Of these, four were ongoing trials with no results about the primary outcomes (Characteristics of ongoing studies) (Hocking 2010; Kaldor 2010; Lehtinen 2015; NCT01195220).
Included studies
We included six trials that studied 359,078 adult women and men (Characteristics of included studies). Scholes 1996 took place in the United States, Andersen 2011 and Ostergaard 2000 in Denmark, Oakeshott 2010 in the UK, van den Broek 2012 in the Netherlands and Garcia 2012 in Peru.
Participants
Two trials included both women and men. Andersen 2011 enrolled 30,439 women and men aged 21 to 23 years, selected at random from the county health service register in Aarhus, Denmark. van den Broek 2012 took place in three locations in the Netherlands; investigators invited all women and men aged 16 to 29 years in Amsterdam and Rotterdam, while in South Limburg, participants were women and men aged 16 to 29 years who completed a web‐based questionnaire and had a score indicating a high risk of chlamydia infection (total 317,304). Ostergaard 2000 enrolled both women and men, but for this review, primary outcome data were only from 1700 women aged around 18 years in high schools in Aarhus. Three trials included only women (Garcia 2012; Oakeshott 2010; Scholes 2007, total 9635).
Four trials enrolled participants from the general population, rather than from groups known to be at high risk of chlamydia infection (Andersen 2011; Oakeshott 2010; Ostergaard 2000; van den Broek 2012). Andersen 2011 and van den Broek 2012 invited people from municipal population registers so the eligible participants in these trials could be considered representative of the general population. Ostergaard 2000 invited students in their final year at school. The trial included all schools in one Danish county, so the eligible participants would be representative of the general population. Oakeshott 2010 invited older students at further education colleges in London and only enrolled those agreeing to be tested for chlamydia.
Two trials enrolled participants at increased risk of chlamydia. Scholes 1996 randomised 36,547 women aged 18 to 34 years in a health maintenance organisation in the United States, assessed questionnaire responses from 20,836 and enrolled 2607 who had a score indicating a high risk of chlamydia infection and agreed to take part. Garcia 2012 implemented an intervention for female sex workers (median age 22 years, interquartile range 20 to 26 years) in 20 cities in Peru and assessed 4465 in repeated cross‐sectional surveys.
Trial design
In two RCTs, individuals were first randomised to intervention and control groups and then invited to undergo screening (Andersen 2011; Scholes 1996). In Andersen 2011, individuals in the intervention group were selected at random from the population register and then invited by post to undergo screening. In the trial by Scholes 1996, women in the intervention group fulfilling criteria for being at high risk of chlamydia were invited to a study clinic. In both control groups, individuals could receive usual care but did not receive any further information about the trial.
In one RCT, Oakeshott 2010 invited women to take part and randomised those who agreed to be tested for chlamydia individually to intervention or control groups.
The other three trials used a cluster design (Garcia 2012; Ostergaard 2000; van den Broek 2012). In the RCTs by Garcia 2012 and Ostergaard 2000, the clusters were allocated to intervention or control groups, and investigators compared outcomes at the same time points in both groups. In Ostergaard 2000 the statistical analysis did not account for clustering.
van den Broek 2012 used a stepped wedge design in a controlled clinical trial. Clusters were postal areas, which were allocated to intervention (two groups of postal areas) and control areas (one group of postal areas) according to population size, level of community risk of STI (low, medium or high) and demographic characteristics (proportions of 16 to 29 year olds, African Caribbean minority ethnic groups and low income earners). The screening intervention was rolled out sequentially in a randomly determined order so that each person in the cluster received an invitation for screening at least once. People in the intervention group clusters received three invitations at roughly 10‐month intervals. The control group clusters received only one screening invitation. We included this trial because there were no RCTs in the general population that reported prevalence of chlamydia as a primary outcome. For the outcome of PID, we estimated the design effect for participants in 164 clusters involved in the first two screening rounds.
Screening interventions
Four trials used a register‐based approach to identify and invite potentially eligible participants (Andersen 2011; Ostergaard 2000; Scholes 1996; van den Broek 2012). In Ostergaard 2000, the register included the final classes of high schools, and students in the intervention group received an invitation to be screened by means of a home‐collected specimen. Two trials identified participants from municipal population registers and sent a postal invitation that offered a screening test by means of a home‐collected specimen (Andersen 2011; van den Broek 2012). Andersen 2011 tested two methods for offering the home‐collected specimen. We combined the results for both methods into a single intervention. All four trials used nucleic acid amplification tests to detect C. trachomatis.
In the trial by Scholes 1996, women in the intervention group were invited to a study clinic to have endocervical swabs taken by a physician. C. trachomatis was detected by enzyme‐linked immunoassay.
Two trials used a venue‐based approach. In the trial by Oakeshott 2010, women were approached in colleges. All women gave consent to be screened before enrolment. They provided a self collected vaginal swab specimen. Those in the intervention group received their results and were treated if the chlamydia test result was positive. Women in the control group had their specimens stored and received the result one year later. They were therefore unscreened for one year and underwent deferred screening. Garcia 2012 used mobile teams who visited sex work venues of 8‐week periods in each city. They invited female sex workers opportunistically and offered testing for chlamydia and other STIs. C. trachomatis was detected by nucleic acid amplification test.
All trials made arrangements to follow up and treat participants with positive screening test results. Two trials offered screening on more than one occasion (Garcia 2012; van den Broek 2012).
Primary outcomes
Outcome for C. trachomatis transmission
Three trials measured the proportion of positive chlamydia test results amongst all screened participants in women (in the case of Garcia 2012) or in women and men (in Ostergaard 2000 and van den Broek 2012) at least 12 months after the start of the screening intervention. Garcia 2012 measured the prevalence of positive test results among samples of female sex workers at sex work venues in each city in 2002 and 2003 (baseline) and at follow‐up in 2005 and 2006, after implementing the intervention. van den Broek 2012 measured the prevalence of positive chlamydia test results following each round of postal invitations. They compared the results in postal areas that had received two or three yearly invitations versus those that had received only one invitation (control group). Ostergaard 2000 measured chlamydia prevalence amongst women who agreed to be followed up 12 months after the intervention, but there was no measure of prevalence in the control group at baseline (Table 1).
| Trial | Study population | Baseline | Follow‐up, 12 months | Reported effect (95% CI) | Follow‐up, subsequent | Reported effect (95% CI) | |||
| Intervention | Control | Intervention | Control | Intervention | Control | ||||
| High school students, Denmark | 43/867a | Not measured | 13/443a | 32/487 | RD − 5.5% (− 10 to 0.95%)a | — | — | — | |
| General population, Netherlands | 1851/43358 | 267/6223 | 1153/28803 | Not measured | OR 0.93 (0.81 to 1.07)b | 981/23899 | Not measured | OR 0.96 (0.83 to 1.10)b | |
| Female sex workers, Peru | 13.8% | 15.5% | — | — | — | 9.9% | 14.5% | RR 0.66 (0.47 to 0.94)c | |
CI: confidence interval; OR: odds ratio; RD: risk difference; RR: risk ratio.
aNumbers of infections and people are the numbers reported by the authors. Risk difference, as reported by authors (difference in mean incidence proportions across clusters). Confidence intervals for the difference did not take into account clustering.
bComparison is between intervention group at follow‐up and control group at baseline. Confidence intervals do not take into account intraclass correlation because, in a hierarchical multivariable model, clustering did not affect the results.
cTotal participants at baseline in 2002, 4130; total participants at follow‐up in 2006, 4156; RR adjusted for 2002 prevalence but not for pairing of cities.
Outcomes for reproductive tract morbidity
Five trials measured the incidence of PID in women in the 12 months after the offer of screening (Andersen 2011; Oakeshott 2010; Ostergaard 2000; Scholes 1996; van den Broek 2012). For the cluster‐randomised trial by Ostergaard 2000, we applied a design effect of 3.77 (based on an ICC 0f 0.028) to calculate an effective sample size so that we could combine the results with those of the individually randomised trials (Glassman 2015).
One trial measured the incidence of epididymitis in men in the 12 months after the offer of screening (Andersen 2011).
Outcome for chlamydia infection in pregnancy
We did not find any RCTs that measured the incidence of preterm delivery or secondary outcomes related to chlamydia screening in pregnancy.
Secondary outcomes
Outcomes measured in all participants
-
Proportion of participants receiving the intervention (screening uptake) (Andersen 2011; van den Broek 2012)
-
Harms of screening (not reported in any trial)
Outcomes measured in women who were not pregnant during the trial or in men
-
Prevalence of chronic female pelvic pain (not reported in any trial)
-
Prevalence of female infertility and incidence of ectopic pregnancy (Andersen 2011)
-
Male infertility (not reported in any trial)
We did not find any trials reporting neonatal outcomes of chlamydia screening interventions.
Excluded studies
We assessed and excluded 41 full‐text records. Of these, 31 were RCTs that involved screening for chlamydia but did not report a pre‐specified primary outcome (ISRCTN38526137; Bailey 2013; ISRCTN16261241; Bowden 2008; Brown 2010; Chandeying 1998; Cook 2007; De Barbeyrac 2013; Downing 2013; Gotz 2013; Graseck 2010; Guy 2013; ACTRN12608000499381; Jones 2007; Kersaudy‐Rahib 2013; Klovstad 2013; Lawton 2010; McKee 2011; Meyer 1991; Niza 2014; NCT01654991; Scholes 2006; Scholes 2007; NCT00829517; Senok 2005; Shafer 2002; Smith 2014; Tebb 2005; Tebb 2009; Walker 2010; Xu 2011). We excluded two trials that reported chlamydia prevalence as an outcome. One was a non‐randomised controlled trial with retrospective inclusion of a control group (Cohen 1999). One trial did not measure chlamydia prevalence in all eligible participants in the control group (Hodgins 2002). We assessed and excluded eight studies in pregnant women (Andrews 2006; Banhidy 2011; Kekki 2001; Kiss 2004; Martin 1997; McGregor 1990; McGregor 1995; Stevens‐Simon 2002). None involved chlamydia screening as the intervention.
Risk of bias in included studies
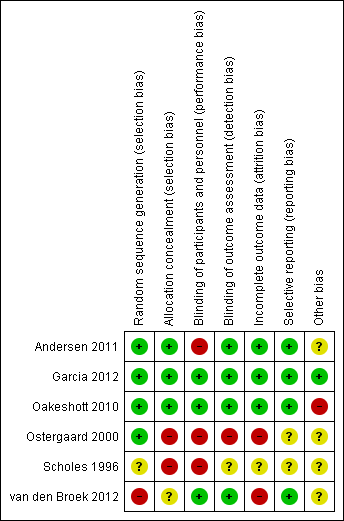
Of the six included trials, we considered one to be at low risk of bias in the six specified domains (Garcia 2012). The other trials had an unclear or high risk of bias in at least one domain (Figure 2; Figure 3).
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Of the five RCTs, four were at low risk of selection bias in the generation of the randomisation sequence. Two used computer‐generated lists (Andersen 2011; Garcia 2012), one used random number tables (Oakeshott 2010), and one allocated schools by drawing lots from a hat (Ostergaard 2000). In one RCT the risk of bias was unclear because the methods only stated that "women were randomly assigned" (Scholes 1996).
In van den Broek 2012, allocation to intervention and control groups did not follow a randomly generated sequence, so we judged it to be at high risk of bias in this domain.
We judged two trials to be at low risk of bias for allocation concealment, as they explicitly stated that neither participants nor staff could predict the allocation (Garcia 2012; Oakeshott 2010). Andersen 2011 did not explicitly describe allocation concealment, but we judged it to be at low risk of bias, as individuals in the intervention group were selected at random from a population register and invited to be screened without knowing that there was a control group.
Unconcealed allocation resulted in a high risk of selection bias in two trials in which randomisation occurred before seeking consent (Ostergaard 2000; Scholes 1996). In both trials the ratio of intervention to control group participants was distorted. In Ostergaard 2000, students randomised to the intervention arm were more likely to agree to take part (48%, 1254/2603) than those randomised to the control arm (38%, 1097/2884). In Scholes 1996, women randomised to the intervention group were actively encouraged to complete the risk assessment questionnaire. The planned ratio of intervention to control was 1:2, while the actual ratio of enrolled women was 1:1.6 (1009 intervention, 1598 control).
The risk of bias in allocation concealment was unclear in van den Broek 2012. The intervention was allocated and implemented in two stages. Allocation was not random, but the investigators who assigned 191 postal areas to intervention and control groups were blinded to their identity. The invitations were sent out in a random order.
Blinding
The two trials reporting the primary outcome of chlamydia prevalence did not explicitly mention that laboratory staff were blinded, but we considered the risk of detection bias to be low because the C. trachomatis test result reporting was automated (Garcia 2012; van den Broek 2012).
The risk of performance bias was high in RCTs that sent invitations or instructions for sample collection only to participants in the intervention group (Andersen 2011; Ostergaard 2000; Scholes 1996). Advice about healthcare‐seeking behaviour for symptoms related to upper genital tract infection might result in differences in management compared with participants in the control groups.
Detection bias was possible for the primary outcome PID, which is a subjective diagnosis based on clinical findings. Assigning a diagnosis of PID can be influenced if the assessor knows whether a person has received screening and treatment for chlamydia or not. We considered Oakeshott 2010 to be at low risk of detection bias because a panel of independent experts used pre‐specified criteria to assign the outcome PID. We also deemed Andersen 2011 to be at low risk of detection bias because outcomes were extracted from hospital discharge and prescription registers. In the trial by Scholes 1996 the risk of detection bias was unclear; people abstracting information from medical records were unaware of group allocation, but investigators did not explicitly describe the blinding of those assigning outcome diagnoses. In Ostergaard 2000 the risk of detection bias was high because outcome assessors were not reported to have been blinded. In the trial by van den Broek 2012 the risk of detection bias was high because investigators only collected data from women who responded to the screening invitation, and the response rate within this group to questions about self reported PID was very low.
Incomplete outcome data
In trials reporting the primary outcome of chlamydia prevalence, attrition was high in van den Broek 2012. Garcia 2012 did not measure attrition from participation in the intervention, but participation in surveys to assess the outcome was high.
In trials that reported the primary outcome of PID, the risk of attrition bias was low in Andersen 2011, Oakeshott 2010 and Scholes 1996 and high in Ostergaard 2000 and van den Broek 2012.
Selective reporting
There was a risk of reporting bias in three RCTs that did not have a protocol (Andersen 2011; Ostergaard 2000; Scholes 1996). The reported results matched the methods, so we assessed the risk of bias as unclear. We judged the remaining three trials to be at low risk of reporting bias.
Other potential sources of bias
There was a risk of contamination of the intervention in three trials (Andersen 2011; Oakeshott 2010; van den Broek 2012). We considered the risk to be high in Oakeshott 2010 and unclear in the other two trials. Three trials reported receiving funding from diagnostic or pharmaceutical companies (Oakeshott 2010; Ostergaard 2000; Scholes 1996). We assessed the risk that this had resulted in biased results to be unclear in all three trials.
Effects of interventions
See: Summary of findings for the main comparison
Primary outcomes
C. trachomatis transmission
The three included trials that evaluated C. trachomatis transmission used different populations, screening interventions, study designs and follow‐up periods (Garcia 2012; Ostergaard 2000; van den Broek 2012). The effect measure differed in each trial. We could not generate a common effect measure because all were cluster trials and none reported an ICC. We describe the results of each trial separately and report results in Table 1.
In the general adult population, van den Broek 2012 found no statistical evidence of a difference in overall chlamydia test positivity in intervention compared with control clusters after the first (baseline, 4.3% vs 4.3%), second (12 months, 4.0% vs 4.3%) and third (24 months, 4.1% vs 4.3%) invitations (numbers invited differed between screening rounds, see Table 2 for details). Analysis 1.1 shows the results as the risk ratio (RR) comparing second and third rounds with the first round. The results for women and men separately were similar. The results from analyses at the individual level, with clustering taken into account, were the same. We graded the quality of the evidence to be low, as data from non‐randomised trials begin at low quality. We would have downgraded this evidence further because there were no other trials and because of uncertainty about the effect of this intervention at higher sustained levels of uptake. However, the trial was very large, at low risk of other biases and it is unclear whether, in practice, the lack of randomisation resulted in biased allocation.
| Trial | Eligibility (ratio intervention: control) | Group | Uptake in intervention | Uptake in control | Comment |
| Selected at random from register (1:4) Intervention: invited for home‐sampling. Assessed after 3 months Control: not contacted. Tests at GP and STI clinics assessed after 3 months | Women | 4000 invited; 1175 (29.4%) sent home‐sample | 11,459 not invited; 1076 (9.4%) opportunistic tests | Control group not aware of trial. Assume routine health‐seeking behaviour over 3 months. If control group testing behaviour continued at the same level over 12 months, the proportion tested by the time the outcome PID was measured could have been higher. | |
| Men | 5000 invited; 1033 (20.7%) sent home‐sample | 9980 not invited; 140 (1.4%) opportunistic tests | |||
| Sex work venues identified and visited by mobile teams | Women | Could not be calculated | Could not be calculated | Not designed to measure uptake; no denominator | |
| Approached in colleges; all women enrolled were tested, randomised (1:1) | Women | 1259 (100%) immediate screening; 269 (21%) opportunistic tests | 1270 (100%) deferred screening; 258 (20%) opportunistic tests | Not designed to measure uptake | |
| Schools randomised (1:1) Intervention: allocated to home‐sampling Control: allocated to offer of GP testing Sexually active respondents eligible. Assessed after 4 months | Women | 2603 allocated; 928 eligible responders; 867 (93.4%) sent home‐sample | 2884 allocated; 833 eligible responders; 63 (7.6%) opportunistic tests | All students in school were allocated to intervention or control groups and asked if they would take part. Of the responders, only those who had ever had sex were eligible. The denominator of of all who had ever had sex was not known. Intervention group given home‐sampling kits | |
| Men | 1733 allocated; 442 eligible responders; 430 (97.3%) sent home sample | 1689 allocated; 246 eligible responders; 4 (1.6%) | — | ||
| Individuals randomised (1:2) Respondents fulfilling criteria for high risk of chlamydia eligible | Women | 36,457 randomised; 20,836 responded; | Numbers allocated to intervention and control not reported. Intervention group actively contacted | ||
| 1009 invited 645 (64%) tested | 1598 not invited; % tested not known | ||||
| Postal areas allocated (5:1) Intervention: allocated to yearly invitation x3 Control: allocated to single invitation | Women 1st 2nd 3rd | 142,419 invited; 141,078 invited; 131,010 invited; | 24,172 invited; | Postal invitation contained secure login code. Recipients had to register on website to request home‐sampling kit. One reminder letter | |
| Men 1st 2nd 3rd | 129,462 invited; 128,299 invited; 121,156 invited; | 23,884 invited | |||
| All 1st 2nd 3rd | 269,273 invited; 265,979 invited; 251,688 invited; | 48,031 invited | |||
GP: general practitioner; PID: pelvic inflammatory disease; STI: sexually transmitted infection.
Garcia 2012 found a lower risk of chlamydia infection in female sex workers in intervention compared with control cities in 2006 (RR 0.72, 95% CI 0.54 to 0.98, 4465 women). Amongst female sex workers tested in 2002 (baseline), prevalence was 13.8% in intervention vs 15.5% in control cities, and in 2006 (at 48 months), it was 9.9% in intervention vs 14.5% in control cities. We adjusted the analysis for differences in prevalence between intervention and control groups in 2002, but not for the pairing of intervention and control cities. We downgraded the quality of the evidence for this outcome to low because directeness and only one trial assessed screening in this population.
Reproductive tract morbidity in women
In five trials that reported the incidence of clinically diagnosed PID, follow‐up data at 12 months were available for 100% of participants either invited to be screened or in the control group in the RCT by Andersen 2011, 94% in Oakeshott 2010, 76% in Scholes 1996, and 55% in Ostergaard 2000. In the cluster‐controlled trial by van den Broek 2012, information was not available from all women invited; from those who responded to the screening invitation, information was available for 3.6% in the control group and 11.2% in the intervention group after the first 12‐month intervention period. We applied a design effect of 1.608 (average cluster size 100) to data extracted from the trial by Ostergaard 2000.
Intention‐to‐treat analysis (Analysis 1.2, Figure 4): We calculated the risk ratio of clinically diagnosed PID in women invited to be screened compared with women who were not invited or who had deferred screening (Andersen 2011; Oakeshott 2010; Ostergaard 2000; Scholes 1996). This analysis assumed that women who were not followed up at 12 months did not develop PID. The risk of PID was lower in women in intervention than control groups, with little evidence of between‐trial heterogeneity (RR 0.68, 95% CI 0.49 to 0.94, I2 7%, 4 trials, 21,686 participants). We found one trial that provided results in women who had a positive chlamydia test result at baseline (Oakeshott 2010). PID incidence was much lower in women who received immediate treatment (1/64) than those who received deferred treatment (7/74, RR 0.17, 95% CI 0.03 to 1.01). In women in the same RCTs with complete data at follow‐up, the risk of PID in the intervention group was 32% lower than in the control group (RR 0.68, 95% CI 0.49 to 0.93, I2 0%).
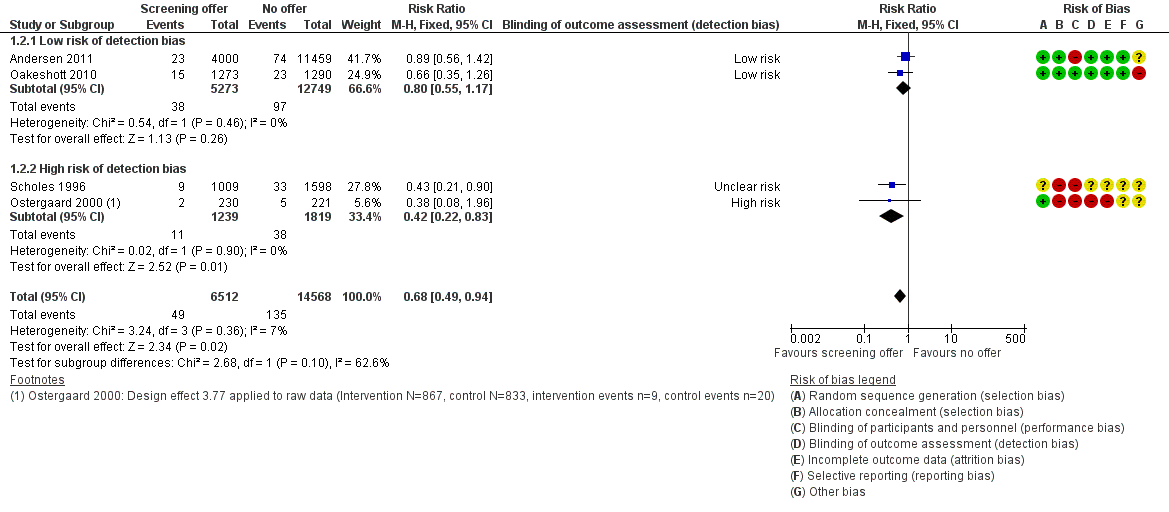
Forest plot of comparison: 1 Offer of chlamydia screening vs usual care (inactive control), outcome: 1.2 Incidence of PID at 12 months (intention‐to‐treat).
Data from the non‐randomised cluster‐controlled trial by van den Broek 2012 were not combined with RCT data. The authors reported results according to screening round; in women who provided information, the incidence in the previous 12 months was 1.8% (19/1072) at the first invitation, 2.1% (47/2261) at the second invitation and 1.9% (44/2340) at the third invitation. Using data from the first two screening rounds, we calculated a design effect of 1.119 (average cluster size 20.3).
For our sensitivity analyses, we analysed the results separately for, on the one hand, Andersen 2011 and Oakeshott 2010, two RCTs at low risk of selection bias and bias for detecting PID, and, on the other, Ostergaard 2000 and Scholes 1996, two trials at high or unclear risk of bias. Within these groups there was no evidence of between trial heterogeneity (I2 0%). The estimated effect of chlamydia screening was less strong in RCTs at low risk of detection bias than in RCTs at high or unclear risk of detection bias (Analysis 1.2, P = 0.10; Analysis 1.3,). In the intention‐to‐treat analysis, the risk of PID in women invited for screening was 20% lower than for those whose were not in RCTs at low risk of detection bias (RR 0.80, 95% CI 0.55 to 1.17, 2 trials, 18,022 participants) and 53% lower in RCTs at high or unclear risk of detection bias (RR 0.47, 95% CI 0.26 to 0.84) (2 trials, 3664 participants).
We downgraded the quality of the evidence to moderate because of statistical evidence that detection bias might have overestimated the intervention effect.
Reproductive tract morbidity in men
The trial by Andersen 2011 was the only one to provide information about epididymitis in men. Men invited for screening had a 20% lower risk for epididymitis than those not invited (RR 0.80, 95% CI 0.45 to 1.42, 1 trial, 14,980 participants). The effect size is similar to that observed for PID for women in the same trial, but the number of events in men was smaller so the confidence interval is wider and includes the possibility of no effect. We downgraded the quality of the evidence to very low because risk of bias and there was only one trial with low uptake of the screening intervention.
Chlamydia infection in pregnancy
We did not find any trials of chlamydia screening in pregnant women.
Secondary outcomes in all participants
Uptake of chlamydia screening
The included trials did not calculate uptake of the chlamydia screening intervention uniformly, owing to differences in trial design. Table 2 shows the data reported in each trial.
Harms of screening
None of the included trials reported any harms of screening.
Secondary outcomes in non‐pregnant women
Female infertility
The trial by Andersen 2011 was the only one to provide information about infertility in women, assessed up to nine years after a single round of the screening intervention. The risk of infertility was 15% higher in women invited to be screened compared with those not invited; the number of events was small so the confidence interval is wide and compatible with no effect (RR 1.15, 95% CI 0.94 to 1.40, 1 trial, 15,459 participants, very low quality evidence).
Ectopic pregnancy
The trial by Andersen 2011 was the only one to provide information about ectopic pregnancy, assessed up to nine years after a single round of the screening intervention. There was no evidence of a difference in the risk of ectopic pregnancy in women invited to be screened compared with those not invited (RR 1.03, 95% CI 0.67 to 1.60, 1 trial, 15,459 participants, very low quality evidence).
Subgroup and sensitivity analyses
There were too few included trials for subgroup analyses or sensitivity analyses other than the one reported above for reproductive tract morbidity in women.
Discussion
Summary of main results
We found six trials that investigated the effect of chlamydia screening interventions on either C. trachomatis transmission (one RCT, one cluster‐controlled trial) or reproductive tract morbidity (three RCTs, one cluster‐RCT). We found no trials assessing the effects of chlamydia screening in pregnancy on obstetric or neonatal outcomes. One trial in the Netherlands found no effect of register‐based yearly invitations in the general population, but uptake of the intervention was low. One trial in Peru found that mobile teams offering periodic testing and treatment for sexually transmitted infections in female sex workers reduced the prevalence of chlamydia infection. Four RCTs found a reduction in the incidence of clinically diagnosed PID in women (RR 0.67, 95% CI 0.49 to 0.92) 12 months after a single offer of chlamydia screening. The quality of this evidence was downgraded from high to moderate because of statistical evidence that the effect of the intervention was less strong in trials with a low risk of detection bias compared with trials at high or unclear risk. One trial found no statistical evidence of an effect of chlamydia screening on epididymitis, female infertility or ectopic pregnancy.
Overall completeness and applicability of evidence
We aimed to assess evidence about the effects of chlamydia screening interventions on transmission of C. trachomatis infection, reproductive tract morbidity and pregnancy and neonatal outcomes. We found a small number of trials assessing outcomes in non‐pregnant women and men and no trials of chlamydia screening in pregnancy (Andersen 2011; Garcia 2012; Oakeshott 2010; Ostergaard 2000; Scholes 1996; van den Broek 2012).
Trials of the effect of screening on C. trachomatis transmission aimed to measure effects on chlamydia prevalence but not on chlamydia incidence. van den Broek 2012 focused on the general population, Ostergaard 2000 on school students and Garcia 2012 on female sex workers, all attempting to measure prevalence as an outcome. Garcia 2012 and van den Broek 2012 implemented the intervention over more than one screening round, which acknowledges screening as an ongoing process. Multiple screening rounds need to be done for infections like chlamydia because people who have been treated become susceptible to further infections from untreated partners or from new infected partners.
van den Broek 2012 found that the uptake of register‐based postal invitations for screening in the Netherlands was very low and there was no reduction in the proportion of positive tests after three rounds of screening. This trial was done in a pragmatic way and these results are applicable to all settings. In contrast, Ostergaard 2000 found that a subset of female school students with very high screening uptake at baseline had a lower prevalence of chlamydia after 12 months than women who had not been screened. The generalisability of this finding is unknown because attrition was high, and there was no long‐term follow‐up of the sustainability of the intervention. Between these extremes, the precise relationship between screening uptake and change in chlamydia prevalence remains unknown. Mathematical modelling studies show that uptake is the strongest determinant of the impact of screening on chlamydia prevalence over time (Althaus 2010; Regan 2008).
Trials of the effects of chlamydia screening interventions on PID mostly assessed the effects of a single round of screening (Andersen 2011; Oakeshott 2010; Ostergaard 2000; Scholes 1996). It is not known whether the effect size stays the same over time. van den Broek 2012 found no change in self reported PID over three rounds of invitations for chlamydia screening. Uptake of screening was low and information about PID was only available for a small minority of eligible participants, so this trial could not address the question.
Evidence about the effects of chlamydia screening on epididymitis and on female infertility and ectopic pregnancy is incomplete. Only one trial examined these outcomes following a single offer of a screening test and found no strong statistical evidence of an effect on any of them (Andersen 2011). Uptake and intensity of the intervention in this trial might have been too low to have a measurable impact on rare and long‐term outcomes.
RCT evidence about the effects of chlamydia screening in pregnancy is lacking.
Quality of the evidence
The quality of evidence about the effects of chlamydia screening interventions is influenced by the small number of trials and the risk of bias. For any trial, we could only assess whether there was a risk of biased results, and not whether the results themselves were or were not biased.
The paucity of trials assessing chlamydia prevalence or incidence as an outcome means that we are very uncertain about the effects of chlamydia screening for preventing transmission of C. trachomatis in high risk individuals and the general population (Garcia 2012; van den Broek 2012). In the general population, the large trial by van den Broek 2012 provides a precise estimate for that particular intervention. Additional trials that evaluate different interventions with higher and sustained coverage might well find different results. The trial by Garcia 2012 in high risk populations was at low risk of bias, but the findings might not be applicable to other settings and interventions.
For the effect of chlamydia screening on reducing the risk of PID, the summary risk ratio might overestimate the effect because of the risks of selection and detection bias in some trials. Whilst the findings of the four RCTs were statistically compatible (I2 7%), the effect sizes in individual trials varied from modest in Andersen 2011 and Oakeshott 2010 to large in Ostergaard 2000 and Scholes 1996. We pre‐specified sensitivity analyses according to the risks of selection and detection bias. Two trials were at high or unclear risk of both of these biases, and these trials showed the largest effects (Ostergaard 2000; Scholes 1996).
Potential biases in the review process
The search strategy was broad and unlikely to have missed RCTs. We followed the protocol reasonably closely and did not undertake any non‐specified subgroup analyses.
Agreements and disagreements with other studies or reviews
This review updates systematic reviews of literature published up to October 2007 and August 2012 (ECDC 2014; Low 2009). The current review identified new studies about the effects of chlamydia screening on both C. trachomatis transmission and reproductive tract morbidity (PID, infertility and ectopic pregnancy in women, epididymitis in men). Narrative reviews have also addressed this research question (Gottlieb 2010; Gottlieb 2013). The main conclusions of these reviews agree with each other.
The modelling study by Herzog 2013 examined the direct effect of identifying and treating asymptomatic infections on the risk of clinically diagnosed PID. It is assumed in this study that a fall in the incidence of PID is the direct result of antimicrobial treatment of a prevalent chlamydia infection. A fall in PID incidence in clinical trials of women who might have been infected with chlamydia many months before enrolment suggests that symptoms can develop throughout the course of a lower genital tract chlamydia infection. This finding contradicts the assumption that PID resulting from ascending chlamydial infection occurs at the beginning of the infectious duration.
Chlamydia screening can also have an indirect effect on the risk of PID (Herzog 2013). If screening and treatment are sustained at high enough levels, the prevalence of chlamydia infection should fall, and exposure to the risk of chlamydia should also decrease. Gottlieb 2013 and colleagues suggest that PID incidence at the population level can fall, even if chlamydia prevalence stays the same. A screening intervention could detect and treat chlamydia infections and shorten the average duration of infection. Women who have received treatment are susceptible to infection again. The rate of incidence of C. trachomatis infections following treatment might then increase so the net effect would be to maintain prevalence. It is not clear what levels of chlamydia screening would result in this apparently paradoxical effect. In the trial by van den Broek 2012, at the low levels of chlamydia screening uptake achieved, neither chlamydia positivity in the tested population nor self reported PID decreased.
The outcome measured in the included trials was PID from all causes. Only the trial by Oakeshott 2010 collected information about PID in women with and without C. trachomatis at baseline. In this subset of women, investigators assumed that PID diagnosed during the trial was caused by untreated chlamydia infection. The risk of chlamydia‐associated PID in screened compared with unscreened women was 83% lower (RR 0.17, 95% CI0.03 to 1.01). C. trachomatis is only one cause of PID, however. The reduction in PID at the population level if chlamydia screening overall population might therefore be modest if the population attributable fraction is small.
Ongoing studies should provide more evidence about the effects of opportunistic chlamydia screening interventions on chlamydia prevalence in young adults in the general population (Hocking 2010; Lehtinen 2015).

#Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Forest plot of comparison: 1 Offer of chlamydia screening vs usual care (inactive control), outcome: 1.2 Incidence of PID at 12 months (intention‐to‐treat).
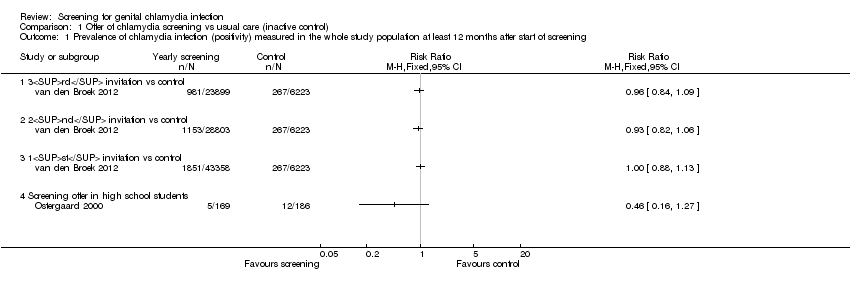
Comparison 1 Offer of chlamydia screening vs usual care (inactive control), Outcome 1 Prevalence of chlamydia infection (positivity) measured in the whole study population at least 12 months after start of screening.

Comparison 1 Offer of chlamydia screening vs usual care (inactive control), Outcome 2 Incidence of PID at 12 months (intention‐to‐treat).
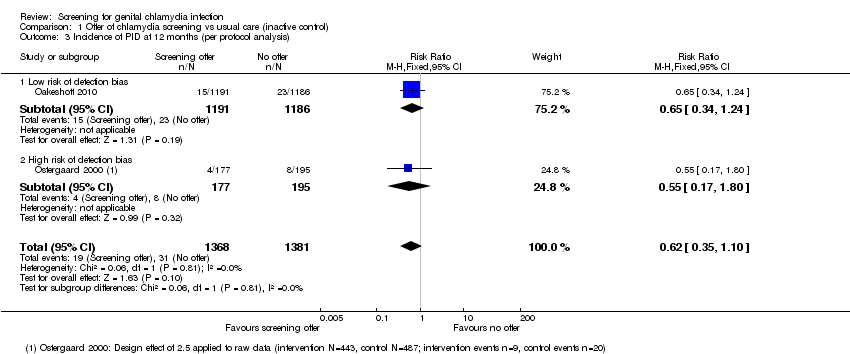
Comparison 1 Offer of chlamydia screening vs usual care (inactive control), Outcome 3 Incidence of PID at 12 months (per protocol analysis).

Comparison 1 Offer of chlamydia screening vs usual care (inactive control), Outcome 4 Incidence of epididymitis in men at 12 months (intention to screen).

Comparison 1 Offer of chlamydia screening vs usual care (inactive control), Outcome 5 Secondary outcomes for reproductive tract morbidity.
| Chlamydia screening compared with usual care for the prevention of C. trachomatis transmission and reproductive tract morbidity | |||||
| Patient or population: healthy adults Settings: general population, high schools or colleges Intervention: chlamydia screening Comparison: usual care | |||||
| Outcomes | Absolute effect | Relative effect | No of Participants | Quality of the evidence | Comments |
| Chlamydia prevalence (general population) Outcome was chlamydia test positivity after 3 yearly invitations in intervention clusters vs 1 invitation in control areas. Uptake was too low for chlamydia positivity to be considered an unbiased estimate of prevalence. | RD 0.0% (‐0‐01, +0.01%) | RR 0.96 (0.84 to 1.09) | 30,122 (1 study) | ⊕⊕⊝⊝1,2 | |
| Chlamydia prevalence (high risk population) Outcome was prevalence of positive chlamydia tests in repeated cross‐sectional surveys of women tested at sex venues after 4 years of intervention. | RD ‐3.7% | RR 0.72 (0.54 to 0.98) | 4156 (1 study) | ⊕⊕⊝⊝3 | |
| Incidence of pelvic inflammatory disease (PID) at 12 months (intention‐to‐treat) Outcome was clinically diagnosed PID reported by the participant or extracted from medical records, pharmacy records or hospital discharge coding. Outcome very likely to be affected by risk of detection bias. | RD 0.0% (0‐0, 0.0%) | RR 0.68 (0.49 to 0.94) | 21,686 (4 studies) | ⊕⊕⊕⊝4 | |
| Incidence of epididymitis in men at 12 months (intention‐to‐treat) Outcome was epididymitis diagnosed in hospital and abstracted from hospital discharge coding. | RD 0.0% (0.0, 0.0%) | RR 0.80 (0.45 to 1.42) | 14,980 (1 study) | ⊕⊝⊝⊝5,6 | |
| GRADE Working Group grades of evidence CI: confidence interval; PID: pelvic inflammatory disease; RR: risk ratio. | |||||
| 1. Selection, attrition and other bias 2. One large non‐randomized cluster‐controlled trial. 3. Single large trial in female sex workers and uncertainty about generalisability to other screening interventions and populations. 4. Selection bias might have overestimated intervention effect. 5. Low uptake of the screening intervention with an imprecise effect estimate and uncertainty about estimated effect of screening interventions with higher sustained levels of uptake. 6. Performance bias | |||||
| Trial | Study population | Baseline | Follow‐up, 12 months | Reported effect (95% CI) | Follow‐up, subsequent | Reported effect (95% CI) | |||
| Intervention | Control | Intervention | Control | Intervention | Control | ||||
| High school students, Denmark | 43/867a | Not measured | 13/443a | 32/487 | RD − 5.5% (− 10 to 0.95%)a | — | — | — | |
| General population, Netherlands | 1851/43358 | 267/6223 | 1153/28803 | Not measured | OR 0.93 (0.81 to 1.07)b | 981/23899 | Not measured | OR 0.96 (0.83 to 1.10)b | |
| Female sex workers, Peru | 13.8% | 15.5% | — | — | — | 9.9% | 14.5% | RR 0.66 (0.47 to 0.94)c | |
| CI: confidence interval; OR: odds ratio; RD: risk difference; RR: risk ratio. | |||||||||
| Trial | Eligibility (ratio intervention: control) | Group | Uptake in intervention | Uptake in control | Comment |
| Selected at random from register (1:4) Intervention: invited for home‐sampling. Assessed after 3 months Control: not contacted. Tests at GP and STI clinics assessed after 3 months | Women | 4000 invited; 1175 (29.4%) sent home‐sample | 11,459 not invited; 1076 (9.4%) opportunistic tests | Control group not aware of trial. Assume routine health‐seeking behaviour over 3 months. If control group testing behaviour continued at the same level over 12 months, the proportion tested by the time the outcome PID was measured could have been higher. | |
| Men | 5000 invited; 1033 (20.7%) sent home‐sample | 9980 not invited; 140 (1.4%) opportunistic tests | |||
| Sex work venues identified and visited by mobile teams | Women | Could not be calculated | Could not be calculated | Not designed to measure uptake; no denominator | |
| Approached in colleges; all women enrolled were tested, randomised (1:1) | Women | 1259 (100%) immediate screening; 269 (21%) opportunistic tests | 1270 (100%) deferred screening; 258 (20%) opportunistic tests | Not designed to measure uptake | |
| Schools randomised (1:1) Intervention: allocated to home‐sampling Control: allocated to offer of GP testing Sexually active respondents eligible. Assessed after 4 months | Women | 2603 allocated; 928 eligible responders; 867 (93.4%) sent home‐sample | 2884 allocated; 833 eligible responders; 63 (7.6%) opportunistic tests | All students in school were allocated to intervention or control groups and asked if they would take part. Of the responders, only those who had ever had sex were eligible. The denominator of of all who had ever had sex was not known. Intervention group given home‐sampling kits | |
| Men | 1733 allocated; 442 eligible responders; 430 (97.3%) sent home sample | 1689 allocated; 246 eligible responders; 4 (1.6%) | — | ||
| Individuals randomised (1:2) Respondents fulfilling criteria for high risk of chlamydia eligible | Women | 36,457 randomised; 20,836 responded; | Numbers allocated to intervention and control not reported. Intervention group actively contacted | ||
| 1009 invited 645 (64%) tested | 1598 not invited; % tested not known | ||||
| Postal areas allocated (5:1) Intervention: allocated to yearly invitation x3 Control: allocated to single invitation | Women 1st 2nd 3rd | 142,419 invited; 141,078 invited; 131,010 invited; | 24,172 invited; | Postal invitation contained secure login code. Recipients had to register on website to request home‐sampling kit. One reminder letter | |
| Men 1st 2nd 3rd | 129,462 invited; 128,299 invited; 121,156 invited; | 23,884 invited | |||
| All 1st 2nd 3rd | 269,273 invited; 265,979 invited; 251,688 invited; | 48,031 invited | |||
| GP: general practitioner; PID: pelvic inflammatory disease; STI: sexually transmitted infection. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prevalence of chlamydia infection (positivity) measured in the whole study population at least 12 months after start of screening Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 3rd invitation vs control | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 2nd invitation vs control | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 1st invitation vs control | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Screening offer in high school students | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Incidence of PID at 12 months (intention‐to‐treat) Show forest plot | 4 | 21080 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.49, 0.94] |
| 2.1 Low risk of detection bias | 2 | 18022 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.55, 1.17] |
| 2.2 High risk of detection bias | 2 | 3058 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.22, 0.83] |
| 3 Incidence of PID at 12 months (per protocol analysis) Show forest plot | 2 | 2749 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.35, 1.10] |
| 3.1 Low risk of detection bias | 1 | 2377 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.34, 1.24] |
| 3.2 High risk of detection bias | 1 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.17, 1.80] |
| 4 Incidence of epididymitis in men at 12 months (intention to screen) Show forest plot | 1 | 14980 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.45, 1.42] |
| 5 Secondary outcomes for reproductive tract morbidity Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Female infertility | 1 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Ectopic pregnancy | 1 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

