D‐dimer test for excluding the diagnosis of pulmonary embolism
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010864.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 05 agosto 2016see what's new
- Tipo:
-
- Diagnostic
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Vascular
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
The whole review team has contributed to this review. KW developed the search strategy. FC and AA applied eligibility criteria, extracted data from studies, performed an assessment of study quality and entered data into Review Manager. FMC provided expert statistical and KS and DK expert clinical advice.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Programme Grant funding to Cochrane Vascular (10/4001/14). The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
-
Chief Scientist Office, Scottish Government Health Directorates, Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
FC: none known.
AA: none known.
KW: none known.
KS: Dr Sheares is a member of the National Institute for Health and Care Excellence Venous Thromboembolic Diseases Guidelines Committee and of the British Thoracic Society Outpatient Management of Pulmonary Embolism Guidelines Committee. Dr Sheares has received support from Actelion, Bayer, GSK, Pfizer and United Therapeutics to attend educational meetings/conferences.
DK reports that he received consultancy fees for advisory board roles for Pfizer, Daiichi‐Sankyo, Boehringer Ingelheim, Sobi, Baxalta and Octapharma; lecture fees from NovoNordisk, Boehringer Ingelheim, Bayer and Pfizer; and meeting expenses from Bayer and CSL to attend ISTH and EAHAD meetings.
FMC: none known.
This review forms part of work funded by a National Institute of Health Research (NIHR) Cochrane programme grant. The review was conducted independently of our funders, the NIHR. The NIHR had no input on the conduct or results of the review.
Acknowledgements
This review forms part of work funded by a National Institute of Health Research (NIHR) Cochrane Programme Grant.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Aug 05 | D‐dimer test for excluding the diagnosis of pulmonary embolism | Review | Fay Crawford, Alina Andras, Karen Welch, Karen Sheares, David Keeling, Francesca M Chappell | |
| 2013 Dec 11 | D‐dimer test for excluding the diagnosis of pulmonary embolism | Protocol | Fay Crawford, Alina Andras, Karen Welch, Karen Sheares, David Keeling, Francesca M Chappell | |
Differences between protocol and review
We have added accident and emergency (A&E) room to the study setting to reflect conventional reporting that we encountered in studies considered for inclusion in this review. We were unable to carry out a search of the Cochrane DTA Register.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Humans;

Clinical pathway.
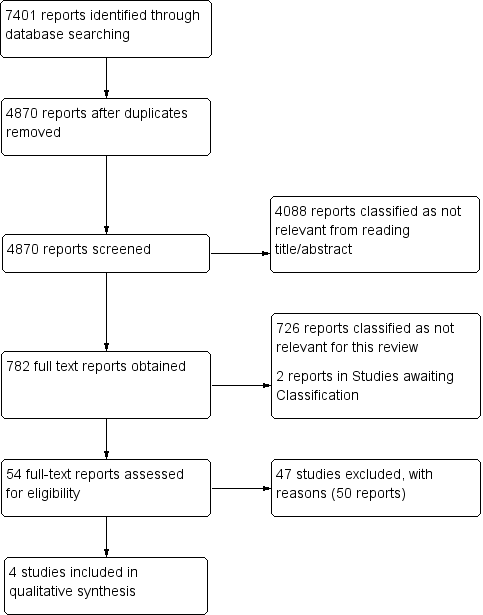
Study flow diagram (see table of Excluded studies for reasons for full‐text exclusions).

Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies.
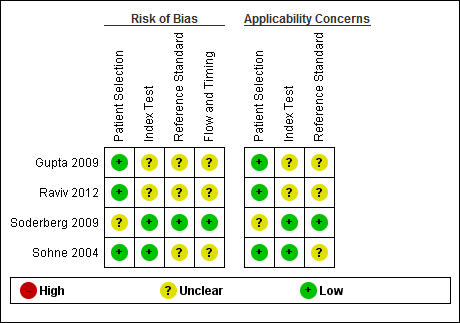
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.
| D‐dimer test for excluding the diagnosis of pulmonary embolism Population: people suspected of having a pulmonary embolism Index test: D‐dimer test Target condition: pulmonary embolism Reference standard: MRPA, pulmonary angiography, V/Q scintigraphy and CTPA Study design: cross‐sectional studies | |||||||
| Study ID | D‐dimer assay | Threshold | Mean age (SD or range) | CPR (cutoff) | Accuracy estimates | Numbers of patients | QUADAS‐2 risk of bias |
| Advanced D‐dimer™ Assay (Dade Behring, Inc, Deerfield, Illinois, USA) | ≥ 1.2 mg/L | 46.9 years (range 15 to 94) | Geneva low PTP: 0 to 3 | Low: sensitivity 100% (95% CI 61% to 100%) specificity 25% (95% CI 20% to 31%) TP = 6 FN = 0 TN = 69 FP = 206 | 281 (prevalence = 2%) 330 (prevalence = 5%) 16 (prevalence = 31%) | Low/Unclear risk of bias | |
| Geneva intermediate PTP: 4 to 10 | Intermediate: sensitivity 100% (95% CI 82% to 100%) specificity 33% (95% CI 28% to 38%) TP = 17 FN = 0 TN = 103 FP = 210 | ||||||
| Geneva high PTP: 11 or more points | High: sensitivity 80% (95% CI 38% to 96%) specificity 33% (95% CI 15% to 65%) TP = 4 FN = 1 TN = 4 FP = 7 | ||||||
| LIA test D‐di (Stago‐Diagnostica, Asnieres‐sur‐Seine, France) | Between 1000 mg/L and 800 mg/L | Females 54.38 ± 19.6 Males 53.7 ± 17.60 | Modified Wells low risk: ≤ 1 unlikely moderate risk: > 1 likely | At 900 mg/L sensitivity 94.4% specificity 49.1% In those younger than 40 years of age sensitivity 100% specificity 54.9% TP = unavailable FN = unavailable TN = unavailable FP = unavailable | 300 (prevalence not available) | Low/Unclear risk of bias | |
| Rapid latex agglutination assay (Tinaquant®, Roche, Basel, Switzerland) | < 0.5 mg/L | 57 years (range 27 to 80) | Wells score > 4.0 high‐risk | sensitivity 91% (95% CI 81% to 97%) specificity 63.0% (95% CI 52% to 73%) TP = 43 FN = 4 TN = 46 FP = 27 | 120 (prevalence = 39%) | Low/Unclear risk of bias | |
| Quantitative rapid immunoturbidimetric D‐dimer assay (Tinaquant D‐dimer® Roche Diagnostica, Mannheim, Germany) | < 0.5 mg/L | People with PE 62 years (range 14 to 95) People without PE 52 years (range 17 to 92) | Wells score ≤ 4 non‐high probability | < 65 years sensitivity 100% (95% CI 97% to 100%) specificity 50% (95% CI 45% to 55%) TP = 34 FN = 302 TN = 34 FP = 34 65 to 75 years sensitivity 100% (95% CI 85% to 100%) specificity 31 % (95% CI 20% to 44%) TP = 6 FN = 50 TN = 6 FP =12 > 75 years sensitivity 100% (95% CI 86% to 100%) specificity 23% (95% CI 12% to 38%) TP = 4 FN = 39 TN = 4 FP =15 | 404 (prevalence = 85%) 74 (prevalence = 76%) 62 (prevalence = 69%) | Low/Unclear risk of bias | |
| CI: confidence interval | |||||||
| CPR | Predictive elements and scoring system |
| Three‐level Wells score | Predictive elements of this CPR include clinical signs and symptoms of DVT (3 points), alternative diagnosis less likely than PE (3 points), heart rate > 100 beats per minute (1.5 points), immobilisation for longer than 3 days or recent (< 4 weeks) surgery (1.5 points), previous VTE (1.5 points), haemoptysis (1 point), cancer treatment in the previous 6 months or palliative care (1 point) Low probability ‐ less than 2; intermediate probability ‐ 2 to 6; high probability ‐ more than 6 |
| Two‐level Wells score | Predictive elements for the 2‐level Wells score are the same as for the 3‐level Wells score, but patients are categorised into 2 as opposed to 3 categories, PE likely or PE unlikely based on a score of more than 4 or 4 or fewer points, respectively |
| Simplified Wells score | Same predictive elements are used as for the 3‐level Wells score, but the point scoring has been simplified ‐ each item now scores 1 point. Patients are regarded as low risk if they have 1 point or less, and as high risk if they score more than 1 |
| Geneva score | Predictive elements of the Geneva score include recent surgery (3 points), previous history of PE or DVT (2 points), heart rate > 100 beats per minute (1 point), 60 to 79 years old (1 point), 80 years old or older (2 points), chest radiograph showing atelectasis (1 point), chest radiograph showing elevated hemidiaphragm (1 point), partial pressure of oxygen (PaO2) < 49 mm Hg (4 points), PaO2 49 to 59 mm Hg (3 points), PaO2 60 to 71 mm Hg (2 points), PaO2 72 to 82 mm Hg (1 point) and partial pressure of carbon dioxide (PaCO2) < 36 mm Hg (2 points), PaCO2 36 to 38.9 mm Hg (1 point) Risk of PE is scored low (0 to 4 points), intermediate (5 to 8 points) or high (9 or more points) |
| Revised Geneva score | Predictive elements of the revised Geneva score include age > 65 years (1 point), previous history of PE or DVT (3 points), surgery with general anaesthesia or fracture within 1 month of symptoms arising (2 points), active malignancy (2 points), heart rate 75 to 94 beats per minute (3 points), heart rate > 94 beats per minute (5 points), pain on leg venous palpation and unilateral oedema (4 points), haemoptysis (2 points) and unilateral leg pain (3 points) This CPR is scored low risk (0 to 3 points), intermediate risk (4 to 10 points) or high risk (11 or more points) |
| Simplified revised Geneva score | Same predictive elements are used as for the revised Geneva score, but point scoring has been simplified. Each item now scores 1 point Risk of PE is scored low (0 to 1 point), intermediate (2 to 4 points) or high (5 or more points) |
| Charlotte rule | Elements of the Charlotte rule include > 50 years old, heart rate higher than systolic blood pressure, unexplained hypoxaemia (O2 < 95%), recent surgery (previous 4 weeks), haemoptysis and unilateral leg swelling Risk score from the Charlotte rule is classified as safe (all predictive elements absent) or unsafe (any of the predictive elements present) |
| CPR: clinical prediction rule | |

