Prueba del dímero D para la exclusión del diagnóstico de embolia pulmonar
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Study characteristics | |||
| Patient sampling | Cross‐sectional study | ||
| Patient characteristics and setting | 627 patients; men n = 213 (34%); women n = 414 (66%). Mean age was 46.9 years (range 15 to 94). The study was conducted at a 500‐bed community teaching hospital Inclusion criteria: arrival to the emergency department with clinically suspected PE; acute onset of new or worsening dyspnoea or chest pain without another obvious cause. D‐dimer assay and pulmonary CTA Exclusion criteria Patients were excluded from the study if they had renal insufficiency, were pregnant or chose not to undergo pulmonary CT Patients had their pre‐test probability calculated with the Geneva CPR as follows: low clinical probability: 0 to 3 points; intermediate clinical probability: 4 to 10 points; high clinical probability: 11 or more points | ||
| Index tests | The index test was a quantitative D‐dimer assay (Advanced D‐dimer™, Dade Behring, Inc, Deerfield, Illinois, USA), an automated latex enhanced immunoturbidimetric assay. The assay was performed with a Sysmex CA‐1500 instrument (Sysmex America). 1.2 mg/L was the NVP cutoff for VTE and PE. The threshold was 1.2 mg/L ‐ the standard threshold at the study authors' institution. Patients received 100 mL/s of iopamidol (Isovue 370, Bracco) at a rate of 4 L/s IV. 50 millilitres of normal saline solution was flushed IV after contrast administration | ||
| Target condition and reference standard(s) | The target condition was clinically suspected PE. The reference standard was pulmonary computerised tomography. Pulmonary CTA was performed with 16 MDCT scanner. All scans were acquired at 1‐mm section thickness. Imaging was performed approximately 15 to 20 seconds after contrast IV. Determined with precise contrast tracking system (SureStart Toshiba Medical Systems). All readings of pulmonary CTA scans were rendered by a board–certified radiologist with 2 to 20 years' experience | ||
| Flow and timing | Timing between the index test and the reference standard test was not reported | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Retrospective cross‐sectional study conducted between 01/01/2010 and 30/10/2010 | ||
| Patient characteristics and setting | 300 patients; males n = 112 (37.34%), females n = 188 (62.66%). Mean age of females was 54.38 ± 19.6 years and of males 53.7 ± 17.60 years Inclusion criteria: patients with suspected clinical presentation of PE and with low or intermediate pre‐test clinical probability of PE calculated with a modified Wells CPR Exclusion criteria: Patients with a high probability based on the Wells score were drawn out of the study, as they were not candidates for D‐dimer testing according to the guidelines. Patients for whom evaluation was incomplete or for whom any required data were insufficient were also excluded Patients with suspected clinical presentation of PE were recruited from the emergency room of BamBam Medical Centre, Northern Israel | ||
| Index tests | LIA test D‐di (Stago Diagnostica, Asnieres‐sur‐Seine, France) thresholds between 800 ng/mL and 1000 ng/mL were used to determine the most appropriate D‐dimer value that study authors regarded as "standard" | ||
| Target condition and reference standard(s) | The reference standard was reported as "imaging studies ‐ default angiograms" | ||
| Flow and timing | Timing between index and reference standard tests was not reported | ||
| Comparative | |||
| Notes | Patients were stratified according to age as follows: 65 years and older, 40 to 65 years old, younger than 40 years. A linear relationship was noted between patient age and D‐dimer values, and a statistically significant difference in D‐dimer levels was observed between the older patient group and each of the other groups | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 120 patients; n = 43 men, n = 77 women. Mean age of people with PE was 57 years (range 27 to 80), mean age of people without PE 57 years (range 20 to 80). Clinical signs and symptoms of PE, high clinical suspicion of PE, Wells pre‐test probability score calculated from patient medical notes retrospectively, with 4.0 or more points considered high risk. Data for scores of 3 and 6 were also analysed Inclusion criteria: high clinical suspicion of PE and clinical signs and symptoms of PE, PA or CTPA that could be performed within 48 hours Exclusion criteria: (1) age younger than 18 years or older than 80 years, (2) advanced psychiatric disease, (3) severe malnutrition or expected survival time less than 6 months, (4) signs of massive unstable PE or 2 or more PEs or DVTs, (5) ongoing anticoagulant therapy, (6) thrombocytes < 70 × 109 L‐1 or prolonged activated thromboplasmin time > 40 seconds, (7) known HIV or hepatitis C infection, (8) pregnancy, (9) acute myocardial infarction, (10) serum creatinine > 150 µmol L‐1, (11) ongoing treatment with metformin, (12) contraindication to the use of contrast media Patients with high clinical suspicion of PE were recruited from the emergency departments of 2 hospitals in Stockholm, Sweden | ||
| Index tests | Rapid latex agglutination procedure (Tinaquant®, Roche), quantitative test, cutoff level < 0.5 mg/L stated but not justified. D‐dimer test was performed on a whole plasma sample | ||
| Target condition and reference standard(s) | Acute PE was confirmed with computerised tomography pulmonary angiography (CTPA) or pulmonary angiography (PA), or both, as the reference standard | ||
| Flow and timing | The reference standard tests were conducted within 48 hours of D‐dimer testing | ||
| Comparative | |||
| Notes | Source of funding: Swedish Heart and Lung Foundation, Stockholm County Council, Karolinska Institutet and Swedish Medical Council | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 538 patients (72% of 747); 72% of study participants (people at low risk of PE) were outpatients. Mean age of people with PE was 62 years (range 14 to 95), those without PE had a mean age of 52 years (17 to 92) Inclusion criteria: a consecutive sample of patients recruited from the Amsterdam Medical Centre (AMC) with clinical suspicion of PE, but non‐high clinical probability Exclusion criteria: younger than 18 years of age, pregnant, had received vitamin K antagonists or heparin at a therapeutic dose for longer than 24 hours, had already undergone objective testing for venous thromboembolism, had an indication for thromboembolism, written informed consent could not be obtained CPR used to calculate a pre‐test probability was Wells, and a score ≤ 4 was regarded as a non‐high probability of PE | ||
| Index tests | Plasma D‐dimer concentration was measured by a quantitative rapid immunoturbidimetric D‐dimer assay (Tinaquant D‐dimer®, Roche Diagnositica, Mannheim, Germany). The cutoff value for a positive test result was 0.5 mg/L, which was stated but was not justified | ||
| Target condition and reference standard(s) | The reference standard was V/Q scanning in combination with compression ultrasound or pulmonary angiography | ||
| Flow and timing | Timing between index and reference standard tests was not reported | ||
| Comparative | |||
| Notes | Study authors reported 3‐month follow‐up of all patients to document the accuracy and safety of the use of D‐dimer in a low probability group | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
CTPA: computerised tomography pulmonary angiography
CPR: clinical prediction rule
CT: computerised tomography
CTA: computerised tomography angiography
DVT: deep vein thrombosis
HIV: human immunodeficiency virus
IV: intravenous
MDCT: Multiple detector computerised tomography
NVP: negative predictive values
PA: pulmonary angiography
PE: pulmonary embolism
V/Q: ventilation/perfusion
VTE: venous thromboembolism
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| No 2 × 2 data; only 6/20 patients had pulmonary embolism | |
| No CPR was used to assess patient pre‐test probability | |
| No CPR that met eligibility criteria was used to assess patient pre‐test probability | |
| Deep venous thrombosis, not PE | |
| Not a diagnostic test accuracy study ‐ a therapeutic impact study | |
| Patients with ‐ve D‐dimer results did not receive reference standard imaging tests | |
| No CPR was used to assess pre‐test probability | |
| Patients with ‐ve D‐dimer results did not receive reference standard imaging tests | |
| No CPR was used to assess pre‐test probability | |
| Patients with ‐ve D‐dimer results did not receive reference standard imaging tests | |
| Not all members of population were treated in an outpatient setting, and data were not presented separately | |
| No 2 × 2 data, reference standard unclear | |
| No CPR was used to assess pre‐test probability | |
| Data on patients with +ve D‐dimer were not included in the results | |
| No CPR was used to assess pre‐test probability | |
| Not all members of population were treated in an outpatient setting;data for those treated in an outpatient setting were not presented separately | |
| Patients with ‐ve D‐dimer results did not receive reference standard imaging tests | |
| Patients with ‐ve D‐dimer results did not receive reference standard imaging tests | |
| Patients with ‐ve D‐dimer results did not receive reference standard imaging tests | |
| Not all members of population were treated in an outpatient setting; CPR (e.g. Wells) not used, no reference standards | |
| DTA data for D‐dimer not available ‐ presented only for pulmonary angiography computerised tomography (PACT) | |
| No 2 × 2 data | |
| Reference standard tests included other D‐dimer tests (IL D‐dimer™ (Instrumentation Laboratory, Aragon, Barcelona, Spain) and MDA D‐dimer™ (Organon Teknika BV Boseind, Boxtel, The Netherlands)) | |
| Reference standard PET/CT, incomplete verification: only 183/541 (34%) patients received a reference standard test | |
| No CPR was used to assess pre‐test probability | |
| Not a diagnostic test accuracy study ‐ a prognostic study | |
| Incomplete verification; not all patients received a reference standard | |
| Patients with ‐ve D‐dimer results did not receive reference standard imaging tests | |
| No CPR was used to assess pre‐test probability | |
| CPR used does not meet review eligibility criteria | |
| Incomplete verification; not all patients received a reference standard | |
| Primary care outpatient population from Christopher study; not a diagnostic test accuracy study ‐ a therapeutic impact study | |
| This study is a summary and interpretation based on data published by the AMUSE study ‐ Geersing 2012 | |
| Not all members of population were treated in an outpatient setting; data for those treated in an outpatient setting were not presented separately | |
| Population included hospital inpatients. Contacted study authors for separate outpatient data ‐ no response | |
| No CPR was used to assess pre‐test probability | |
| CPR does not meet review eligibility criteria | |
| No CPR was used to assess pre‐test probability | |
| Management study; lab‐based study by the pharmaceutical industry ‐ no reference standards | |
| Patients with ‐ve D‐dimer results did not receive reference standard imaging tests | |
| Patients with ‐ve D‐dimer results did not receive reference standard imaging tests | |
| Index test not a D‐dimer (Fibrinopeptide A ‐ FpA); no CPR | |
| Incomplete reference standard | |
| No CPR was used to assess pre‐test probability | |
| No pre‐test probability performed ‐ some post‐test probability performed, but numbers of patients who received it not reported | |
| Deep venous thrombosis, not PE | |
| Deep venous thrombosis, not PE |
CPR: clinical prediction rule
CT: computed tomography
DTA: diagnostic test accuracy
PE: pulmonary embolism
PET: positron emission tomography
‐ve: negative
+ve: positive
Characteristics of studies awaiting classification [ordered by study ID]
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | |||
| Index tests | |||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | Unable to obtain report | ||
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | |||
| Index tests | |||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | Unable to obtain report | ||

Clinical pathway.
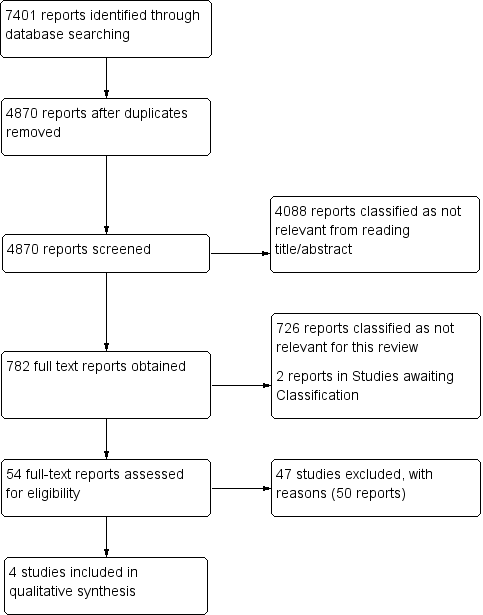
Study flow diagram (see table of Excluded studies for reasons for full‐text exclusions).

Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies.
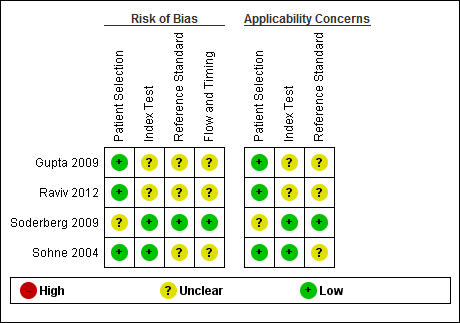
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.
| D‐dimer test for excluding the diagnosis of pulmonary embolism Population: people suspected of having a pulmonary embolism Index test: D‐dimer test Target condition: pulmonary embolism Reference standard: MRPA, pulmonary angiography, V/Q scintigraphy and CTPA Study design: cross‐sectional studies | |||||||
| Study ID | D‐dimer assay | Threshold | Mean age (SD or range) | CPR (cutoff) | Accuracy estimates | Numbers of patients | QUADAS‐2 risk of bias |
| Advanced D‐dimer™ Assay (Dade Behring, Inc, Deerfield, Illinois, USA) | ≥ 1.2 mg/L | 46.9 years (range 15 to 94) | Geneva low PTP: 0 to 3 | Low: sensitivity 100% (95% CI 61% to 100%) specificity 25% (95% CI 20% to 31%) TP = 6 FN = 0 TN = 69 FP = 206 | 281 (prevalence = 2%) 330 (prevalence = 5%) 16 (prevalence = 31%) | Low/Unclear risk of bias | |
| Geneva intermediate PTP: 4 to 10 | Intermediate: sensitivity 100% (95% CI 82% to 100%) specificity 33% (95% CI 28% to 38%) TP = 17 FN = 0 TN = 103 FP = 210 | ||||||
| Geneva high PTP: 11 or more points | High: sensitivity 80% (95% CI 38% to 96%) specificity 33% (95% CI 15% to 65%) TP = 4 FN = 1 TN = 4 FP = 7 | ||||||
| LIA test D‐di (Stago‐Diagnostica, Asnieres‐sur‐Seine, France) | Between 1000 mg/L and 800 mg/L | Females 54.38 ± 19.6 Males 53.7 ± 17.60 | Modified Wells low risk: ≤ 1 unlikely moderate risk: > 1 likely | At 900 mg/L sensitivity 94.4% specificity 49.1% In those younger than 40 years of age sensitivity 100% specificity 54.9% TP = unavailable FN = unavailable TN = unavailable FP = unavailable | 300 (prevalence not available) | Low/Unclear risk of bias | |
| Rapid latex agglutination assay (Tinaquant®, Roche, Basel, Switzerland) | < 0.5 mg/L | 57 years (range 27 to 80) | Wells score > 4.0 high‐risk | sensitivity 91% (95% CI 81% to 97%) specificity 63.0% (95% CI 52% to 73%) TP = 43 FN = 4 TN = 46 FP = 27 | 120 (prevalence = 39%) | Low/Unclear risk of bias | |
| Quantitative rapid immunoturbidimetric D‐dimer assay (Tinaquant D‐dimer® Roche Diagnostica, Mannheim, Germany) | < 0.5 mg/L | People with PE 62 years (range 14 to 95) People without PE 52 years (range 17 to 92) | Wells score ≤ 4 non‐high probability | < 65 years sensitivity 100% (95% CI 97% to 100%) specificity 50% (95% CI 45% to 55%) TP = 34 FN = 302 TN = 34 FP = 34 65 to 75 years sensitivity 100% (95% CI 85% to 100%) specificity 31 % (95% CI 20% to 44%) TP = 6 FN = 50 TN = 6 FP =12 > 75 years sensitivity 100% (95% CI 86% to 100%) specificity 23% (95% CI 12% to 38%) TP = 4 FN = 39 TN = 4 FP =15 | 404 (prevalence = 85%) 74 (prevalence = 76%) 62 (prevalence = 69%) | Low/Unclear risk of bias | |
| CI: confidence interval | |||||||
| CPR | Predictive elements and scoring system |
| Three‐level Wells score | Predictive elements of this CPR include clinical signs and symptoms of DVT (3 points), alternative diagnosis less likely than PE (3 points), heart rate > 100 beats per minute (1.5 points), immobilisation for longer than 3 days or recent (< 4 weeks) surgery (1.5 points), previous VTE (1.5 points), haemoptysis (1 point), cancer treatment in the previous 6 months or palliative care (1 point) Low probability ‐ less than 2; intermediate probability ‐ 2 to 6; high probability ‐ more than 6 |
| Two‐level Wells score | Predictive elements for the 2‐level Wells score are the same as for the 3‐level Wells score, but patients are categorised into 2 as opposed to 3 categories, PE likely or PE unlikely based on a score of more than 4 or 4 or fewer points, respectively |
| Simplified Wells score | Same predictive elements are used as for the 3‐level Wells score, but the point scoring has been simplified ‐ each item now scores 1 point. Patients are regarded as low risk if they have 1 point or less, and as high risk if they score more than 1 |
| Geneva score | Predictive elements of the Geneva score include recent surgery (3 points), previous history of PE or DVT (2 points), heart rate > 100 beats per minute (1 point), 60 to 79 years old (1 point), 80 years old or older (2 points), chest radiograph showing atelectasis (1 point), chest radiograph showing elevated hemidiaphragm (1 point), partial pressure of oxygen (PaO2) < 49 mm Hg (4 points), PaO2 49 to 59 mm Hg (3 points), PaO2 60 to 71 mm Hg (2 points), PaO2 72 to 82 mm Hg (1 point) and partial pressure of carbon dioxide (PaCO2) < 36 mm Hg (2 points), PaCO2 36 to 38.9 mm Hg (1 point) Risk of PE is scored low (0 to 4 points), intermediate (5 to 8 points) or high (9 or more points) |
| Revised Geneva score | Predictive elements of the revised Geneva score include age > 65 years (1 point), previous history of PE or DVT (3 points), surgery with general anaesthesia or fracture within 1 month of symptoms arising (2 points), active malignancy (2 points), heart rate 75 to 94 beats per minute (3 points), heart rate > 94 beats per minute (5 points), pain on leg venous palpation and unilateral oedema (4 points), haemoptysis (2 points) and unilateral leg pain (3 points) This CPR is scored low risk (0 to 3 points), intermediate risk (4 to 10 points) or high risk (11 or more points) |
| Simplified revised Geneva score | Same predictive elements are used as for the revised Geneva score, but point scoring has been simplified. Each item now scores 1 point Risk of PE is scored low (0 to 1 point), intermediate (2 to 4 points) or high (5 or more points) |
| Charlotte rule | Elements of the Charlotte rule include > 50 years old, heart rate higher than systolic blood pressure, unexplained hypoxaemia (O2 < 95%), recent surgery (previous 4 weeks), haemoptysis and unilateral leg swelling Risk score from the Charlotte rule is classified as safe (all predictive elements absent) or unsafe (any of the predictive elements present) |
| CPR: clinical prediction rule | |

