Prueba del dímero D para la exclusión del diagnóstico de embolia pulmonar
Appendices
Appendix 1. MEDLINE search strategy
Database: Ovid MEDLINE(R) <1946 to November Week 3 2013>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp Pulmonary Embolism/ (31347)
2 (pulmonary adj embol$).ti,ab. (25289)
3 (pulmonary adj thrombo$).ti,ab. (3289)
4 (lung adj embol$).ti,ab. (377)
5 (lung adj thrombo$).ti,ab. (59)
6 (PE or PTE).ti,ab. (23195)
7 or/1‐6 (59563)
8 Fibrin Fibrinogen Degradation Products/an, me [Analysis, Metabolism] (5667)
9 Biological Markers/an, bl, me [Analysis, Blood, Metabolism] (120309)
10 Enzyme‐Linked Immunosorbent Assay/ (130387)
11 "Nephelometry and Turbidimetry"/ (6377)
12 d‐dimer.ti,ab. (5726)
13 (fibrin adj2 d).ti,ab. (532)
14 dimeri?ed plasmin.ti,ab. (6)
15 elisa?.ti,ab. (112004)
16 elfa?.ti,ab. (120)
17 enzyme linked.ti,ab. (70920)
18 latex agglutination.ti,ab. (3168)
19 (latex adj3 assay?).ti,ab. (621)
20 blood agglutination.ti,ab. (40)
21 Immunoturbidimetr$.ti,ab. (874)
22 turbidimetr$.ti,ab. (2576)
23 SimpliRed.ti,ab. (76)
24 Minutex.ti,ab. (6)
25 NycoCard.ti,ab. (45)
26 "Instant I.A".ti,ab. (7)
27 Vidas.ti,ab. (501)
28 LIATEST.ti,ab. (47)
29 ("IL test" or IL‐DD).ti,ab. (29)
30 Turbiquant.ti,ab. (5)
31 Asserachrom.ti,ab. (52)
32 Enzygnost.ti,ab. (200)
33 Fibrinostika.ti,ab. (6)
34 "BC DD".ti,ab. (1)
35 (Tinaquant or Tina‐quant).ti,ab. (94)
36 TriniLIZE.ti,ab. (0)
37 biopool.ti,ab. (31)
38 TintElize.ti,ab. (5)
39 HemosIL.ti,ab. (42)
40 Innovance‐DD.ti,ab. (1)
41 stratus.ti,ab. (812)
42 FDP.ti,ab. (2331)
43 Dimertest.ti,ab. (25)
44 (LPIA or EIA).ti,ab. (8470)
45 or/8‐44 (343616)
46 7 and 45 (2690)
Appendix 2. EMBASE search strategy
Database: Embase <1980 to 2013 Week 49>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 lung embolism/ (58459)
2 (pulmonary adj embol$).ti,ab. (34552)
3 (pulmonary adj thrombo$).ti,ab. (4366)
4 (lung adj embol$).ti,ab. (598)
5 (lung adj thrombo$).ti,ab. (76)
6 (PE or PTE).ti,ab. (33967)
7 or/1‐6 (92164)
8 fibrin degradation product/cr [Drug Concentration] (1)
9 biological marker/cr [Drug Concentration] (14)
10 D dimer/cr [Drug Concentration] (13)
11 enzyme linked immunosorbent assay/ (205450)
12 turbidimetry/ (2792)
13 d‐dimer.ti,ab. (8597)
14 (fibrin adj2 d).ti,ab. (652)
15 dimeri?ed plasmin.ti,ab. (5)
16 elisa?.ti,ab. (155486)
17 elfa?.ti,ab. (186)
18 enzyme linked.ti,ab. (78011)
19 Immunoturbidimetr$.ti,ab. (1361)
20 turbidimetr$.ti,ab. (3317)
21 latex agglutination.ti,ab. (3477)
22 (latex adj3 assay?).ti,ab. (735)
23 blood agglutination.ti,ab. (41)
24 SimpliRed.ti,ab. (89)
25 Minutex.ti,ab. (7)
26 NycoCard.ti,ab. (72)
27 "Instant I.A".ti,ab. (8)
28 Vidas.ti,ab. (729)
29 LIATEST.ti,ab. (114)
30 ("IL test" or IL‐DD).ti,ab. (88)
31 Turbiquant.ti,ab. (8)
32 Asserachrom.ti,ab. (130)
33 Enzygnost.ti,ab. (252)
34 Fibrinostika.ti,ab. (7)
35 "BC DD".ti,ab. (1)
36 (Tinaquant or Tina‐quant).ti,ab. (167)
37 TriniLIZE.ti,ab. (2)
38 biopool.ti,ab. (49)
39 TintElize.ti,ab. (9)
40 (HemosIL‐DD or HemosIL‐DDHS).ti,ab. (5)
41 Innovance‐DD.ti,ab. (2)
42 stratus.ti,ab. (1030)
43 FDP.ti,ab. (2583)
44 Dimertest.ti,ab. (27)
45 (LPIA or EIA).ti,ab. (10588)
46 or/8‐45 (284160)
47 7 and 46 (3250)
Appendix 3. CINAHL search strategy
| Interface ‐ EBSCOhost Research Databases Search Screen ‐ Advanced Search Database ‐ CINAHL Plus 19 December 2013 | ||
| S47 | S7 AND S46 | 711 |
| S46 | S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 | 37,766 |
| S45 | TX LPIA or EIA | 381 |
| S44 | TX Dimertest | 2 |
| S43 | TX FDP | 103 |
| S42 | TX stratus | 50 |
| S41 | TX Innovance‐DD | 0 |
| S40 | TX HemosIL | 6 |
| S39 | TX TintElize | 0 |
| S38 | TX biopool | 4 |
| S37 | TX TriniLIZE | 0 |
| S36 | TX Tinaquant or Tina‐quant | 14 |
| S35 | TX BC DD | 2 |
| S34 | TX Fibrinostika | 0 |
| S33 | TX Enzygnost | 6 |
| S32 | TX Asserachrom | 1 |
| S31 | TX Turbiquant | 0 |
| S30 | TX IL test or IL‐DD | 82 |
| S29 | TX LIATEST | 5 |
| S28 | TX Vidas | 25 |
| S27 | TX Instant I.A | 0 |
| S26 | TX NycoCard | 8 |
| S25 | TX Minutex | 0 |
| S24 | TX SimpliRed | 18 |
| S23 | TX turbidimetr* | 270 |
| S22 | TX Immunoturbidimetr* | 71 |
| S21 | TX blood agglutination | 239 |
| S20 | TX latex N3 assay? | 6 |
| S19 | TX latex agglutination | 107 |
| S18 | TX enzyme linked | 12,863 |
| S17 | TX elfa? | 10 |
| S16 | TX elisa? | 249 |
| S15 | TX dimeri?ed plasmin | 1 |
| S14 | TX fibrin N2 d | 60 |
| S13 | TX d‐dimer | 808 |
| S12 | (MH "Nephelometry and Turbidimetry") | 215 |
| S11 | (MH "Nephelometry and Turbidimetry") | 215 |
| S10 | (MH "Enzyme‐Linked Immunosorbent Assay") | 10,786 |
| S9 | (MH "Fibrin Fibrinogen Degradation Products/AN/BL/ME") | 540 |
| S8 | (MH "Biological Markers+/AN/BL/ME") | 24,146 |
| S7 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 | 15,735 |
| S6 | TX PE or PTE | 9,671 |
| S5 | TX lung N3 thrombo* | 38 |
| S4 | TX lung N3 embol* | 66 |
| S3 | TX pulmonary N3 thrombo* | 1,347 |
| S2 | TX pulmonary N3 embol* | 6,589 |
| S1 | (MH "Pulmonary Embolism") | 5,253 |
Appendix 4. LILACS search strategy
| Database : | LILACS 12 December 2013 |
| Search on : | (Fibrin Fibrinogen Degradation Products or Biological Markers or Enzyme‐Linked Immunosorbent Assay) [Subject descriptor] or (d‐dimer or fibrin or (dimerised and plasmin) or elisa or elfa or (enzyme and linked) or (latex and agglutination) or (latex and assay) or (blood and agglutination) or Immunoturbidimetr$ or turbidimetr$ or SimpliRed or Minutex or NycoCard or (Instant and I.A) or Vidas or LIATEST or (IL and test) or IL‐DD or Turbiquant or Asserachrom or Enzygnost or Fibrinostika or (BC and DD) or Tinaquant or Tina‐quant) [Words] and (Pulmonary Embolism [Subject descriptor]) or ((pulmonary and embol$) or (pulmonary and thrombo$) or (lung and embol$) or (lung and thrombo$) or PE or PTE) [Words] |
| References found : | 62 [refine] |
Appendix 5. DARE (Database of Abstracts of Reviews of Effects) and Health Technology Assessment Database (HTA) search strategy
| Issue 11 2013 | ||
| #1 | MeSH descriptor: [Pulmonary Embolism] explode all trees | 874 |
| #2 | pulmonary near/3 embol*:ti,ab,kw (Word variations have been searched) | 1679 |
| #3 | pulmonary near/3 thromb*:ti,ab,kw (Word variations have been searched) | 515 |
| #4 | lung near/3 embol*:ti,ab,kw (Word variations have been searched) | 165 |
| #5 | lung near/3 thromb* | 64 |
| #6 | PE or PTE:ti,ab,kw (Word variations have been searched) | 1320 |
| #7 | #1 or #2 or #3 or #4 or #5 or #6 | 2928 |
| #8 | MeSH descriptor: [Fibrin Fibrinogen Degradation Products] explode all trees and with qualifier(s): [Analysis ‐ AN, Metabolism ‐ ME] | 389 |
| #9 | MeSH descriptor: [Biological Markers] explode all trees and with qualifier(s): [Analysis ‐ AN, Blood ‐ BL, Metabolism ‐ ME] | 9822 |
| #10 | MeSH descriptor: [Enzyme‐Linked Immunosorbent Assay] explode all trees | 1977 |
| #11 | MeSH descriptor: [Nephelometry and Turbidimetry] explode all trees | 64 |
| #12 | d‐dimer or (fibrin near/2 d) or (dimeri* near/2 plasmin) or elisa or elfa or "enzyme linked" or "latex agglutination" or (latex near/j3 assay) or "blood agglutination" or Immunoturbidimetr* or turbidimetr* or SimpliRed or Minutex or NycoCard or "Instant I.A" or Vidas or LIATEST or "IL test" or "IL‐DD" or Turbiquant or Asserachrom or Enzygnost or Fibrinostika or "BC DD" or Tinaquant or "Tina‐quant" or TriniLIZE or biopool or TintElize or HemosIL or "Innovance‐DD" or stratus or FDP or Dimertest or LPIA or EIA:ti,ab,kw (Word variations have been searched) | 5391 |
| #13 | #8 or #9 or #10 or #11 or #12 | 14492 |
| #14 | #7 and #13 | 110 |
| All Results (110) Cochrane Reviews (1) AllReviewProtocol Other Reviews (17) Trials (84) Methods Studies (0) Technology Assessments (1) Economic Evaluations (7) Cochrane Groups (0) |
Appendix 6. ISI Conference Proceedings Citation Index ‐ Science search strategy
18 December 2013
Topic=(d‐dimer) AND Topic=(pulmonary embolism or Thromboembolism or VTE)
Timespan=All years. Databases=CPCI‐S, CCR‐EXPANDED, IC
216
Appendix 7. British Library Zetoc search strategy
18 December 2013
16 for: conference: d‐dimer and embolism
69 for: conference: d‐dimer and thrombo*
Appendix 8. MEDION search strategy
19 December 2013
d‐dimer: 9 results
Appendix 9. World Health Organization International Clinical Trials Registry search strategy
18 December 2013
21 records for 19 trials found for: d‐dimer and embolism
Appendix 10. ClinicalTrials.gov search strategy
18 December 2013
65 studies found for: d‐dimer and embolism
Appendix 11. Current Controlled Trials search strategy
18 December 2013
4 studies found for: d‐dimer and embolism
Appendix 12. QUADAS‐2
| Domains, signalling questions (SQ) and applicability | Rating criteria |
| Domain 1: Patient selection | |
| A. Risk of bias | Describe the methods of patients' selection given in the paper: |
| SQ1: Was a consecutive or random sample of patients enrolled? | Yes: It is stated that the sample was consecutive or a random sample No: It is stated that the sample was not consecutive or a random sample Unclear: The method of sampling is ambiguous |
| SQ2: Did the study avoid inappropriate exclusions? | Yes: The study excluded patients without CPR scores No: The study excluded patients who had received a PTP score using CPRs Unclear: The test history of the patients in the study is not revealed in the report |
| SQ3: Did the study avoid inappropriate inclusions? | Yes: The study included only outpatients who had received a PTP score for PE using a CPR No: The study included some inappropriate patients, for example, those without a PTP score from a CPR, or included inpatients Unclear: The study's inclusion criteria allow for inappropriate inclusions |
| Applicability Question 1: Are there concerns that the included patients and setting do not match the review question? | High: The study population meets the eligibility criteria Low: The patient population is skewed in some way, for example the study includes mainly younger patients Unclear: Not enough information is given about the study population |
| B. Concerns regarding applicability | Give the paper's description of the inclusion/exclusion criteria, including setting, prior tests, symptoms here |
| Domain 2: Index test | |
| A. Risk of bias | Give the paper's description of the D‐dimer assay, how it was conducted and interpreted including the training of the individual of those carrying out the test |
| SQ1: If a threshold was used was it prespecified? | Yes: Plasma D‐dimer levels are prespecified in the study methods section as a positive test result No: The threshold for a positive test result is not prespecified Unclear: It is unclear if a threshold was used |
| B. Concerns regarding applicability | |
| AQ2: Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Yes: The plasma D‐Dimer test did not use standard methods and is unvalidated No: The presence of plasma D‐dimer was detected using standard D‐dimer test methods previously validated Unclear: The basis of the outcome is unclear |
| Domain 3: Reference standard | |
| A. Risk of bias | Give the paper's description of the pulmonary angiography, scintigraphy, computed tomography PA and follow‐up and how they were conducted and interpreted including the training of the individual of those carrying out the test |
| SQ1: Is the reference standard likely to correctly classify the target condition? | Yes: The reference standard(s) was either pulmonary angiography, CTPA, MRPA, or V/Q scanning No: The reference standard(s) was not any of the above Unclear: Information regarding the conduct of the reference standard is insufficient |
| SQ2: Were the reference standard test results interpreted without knowledge of the index test results? | Yes: The person classifying the RS test results was unaware of the D‐dimer test results No: The person classifying the RS test results was aware of the D‐dimer test results Unclear: No information is available regarding the blinding of test results |
| SQ3: Did the person conducting the pulmonary angiography, V/Q scanning, CTPA, or MRPA have expertise comparable to a radiologist? | Yes: It is stated that a radiologist or similar (e.g. vascular specialist with an interest in VTE) read the test results No: The person conducting the pulmonary angiography, V/Q scanning, CTPA, or MRPA was not a radiologist or similar Unclear: The expertise and background discipline of the reader is not made clear |
| Applicability: Could the reference standard, its conduct, or its interpretation have introduced bias? | High: The RS tests were performed by a person with expertise and were interpreted blind Low: The RS tests were not performed by a person with expertise or were not interpreted blind Unclear: No information about the persons conducting the tests, or interpreting the results is given |
| Domain 4: Flow and timing | |
| A. Risk of bias | Describe the reasons why any patient recruited into the study did not contribute to the 2 x 2 table (i.e. patients who did not undergo the RS tests) referring to the flow diagram |
| SQ1: was there an appropriate interval between the index test and the reference standard? | Yes: The index and reference standard tests were all conducted within 7 days of each other No: Some of the reference standard test results were obtained after more than 7 days Unclear: No information about the relative timing of the tests is provided |
| SQ2: Did all the patients receive the same reference standard? | Yes: A complete set of RS test results are available for all study patients No: The RS results are not available for all patients, or some patients had follow‐up only Unclear: It is not clear whether all patients received an acceptable reference standard |
| SQ3: Were all patients included in the final analysis? | Yes: Data for all study patients are reported No: Data for all study patients are not reported Unclear: It is not clear whether there were patients recruited but not included in the 2 x 2 table |

Clinical pathway.
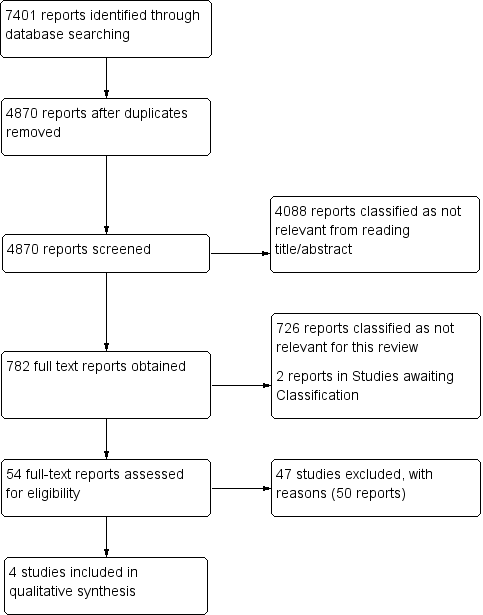
Study flow diagram (see table of Excluded studies for reasons for full‐text exclusions).

Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies.
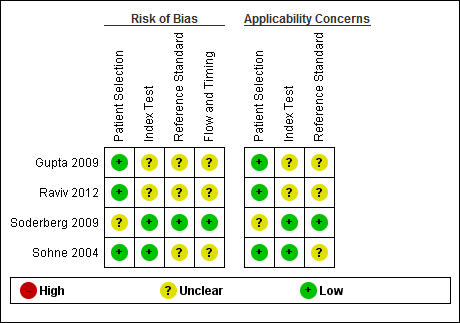
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.
| D‐dimer test for excluding the diagnosis of pulmonary embolism Population: people suspected of having a pulmonary embolism Index test: D‐dimer test Target condition: pulmonary embolism Reference standard: MRPA, pulmonary angiography, V/Q scintigraphy and CTPA Study design: cross‐sectional studies | |||||||
| Study ID | D‐dimer assay | Threshold | Mean age (SD or range) | CPR (cutoff) | Accuracy estimates | Numbers of patients | QUADAS‐2 risk of bias |
| Advanced D‐dimer™ Assay (Dade Behring, Inc, Deerfield, Illinois, USA) | ≥ 1.2 mg/L | 46.9 years (range 15 to 94) | Geneva low PTP: 0 to 3 | Low: sensitivity 100% (95% CI 61% to 100%) specificity 25% (95% CI 20% to 31%) TP = 6 FN = 0 TN = 69 FP = 206 | 281 (prevalence = 2%) 330 (prevalence = 5%) 16 (prevalence = 31%) | Low/Unclear risk of bias | |
| Geneva intermediate PTP: 4 to 10 | Intermediate: sensitivity 100% (95% CI 82% to 100%) specificity 33% (95% CI 28% to 38%) TP = 17 FN = 0 TN = 103 FP = 210 | ||||||
| Geneva high PTP: 11 or more points | High: sensitivity 80% (95% CI 38% to 96%) specificity 33% (95% CI 15% to 65%) TP = 4 FN = 1 TN = 4 FP = 7 | ||||||
| LIA test D‐di (Stago‐Diagnostica, Asnieres‐sur‐Seine, France) | Between 1000 mg/L and 800 mg/L | Females 54.38 ± 19.6 Males 53.7 ± 17.60 | Modified Wells low risk: ≤ 1 unlikely moderate risk: > 1 likely | At 900 mg/L sensitivity 94.4% specificity 49.1% In those younger than 40 years of age sensitivity 100% specificity 54.9% TP = unavailable FN = unavailable TN = unavailable FP = unavailable | 300 (prevalence not available) | Low/Unclear risk of bias | |
| Rapid latex agglutination assay (Tinaquant®, Roche, Basel, Switzerland) | < 0.5 mg/L | 57 years (range 27 to 80) | Wells score > 4.0 high‐risk | sensitivity 91% (95% CI 81% to 97%) specificity 63.0% (95% CI 52% to 73%) TP = 43 FN = 4 TN = 46 FP = 27 | 120 (prevalence = 39%) | Low/Unclear risk of bias | |
| Quantitative rapid immunoturbidimetric D‐dimer assay (Tinaquant D‐dimer® Roche Diagnostica, Mannheim, Germany) | < 0.5 mg/L | People with PE 62 years (range 14 to 95) People without PE 52 years (range 17 to 92) | Wells score ≤ 4 non‐high probability | < 65 years sensitivity 100% (95% CI 97% to 100%) specificity 50% (95% CI 45% to 55%) TP = 34 FN = 302 TN = 34 FP = 34 65 to 75 years sensitivity 100% (95% CI 85% to 100%) specificity 31 % (95% CI 20% to 44%) TP = 6 FN = 50 TN = 6 FP =12 > 75 years sensitivity 100% (95% CI 86% to 100%) specificity 23% (95% CI 12% to 38%) TP = 4 FN = 39 TN = 4 FP =15 | 404 (prevalence = 85%) 74 (prevalence = 76%) 62 (prevalence = 69%) | Low/Unclear risk of bias | |
| CI: confidence interval | |||||||
| CPR | Predictive elements and scoring system |
| Three‐level Wells score | Predictive elements of this CPR include clinical signs and symptoms of DVT (3 points), alternative diagnosis less likely than PE (3 points), heart rate > 100 beats per minute (1.5 points), immobilisation for longer than 3 days or recent (< 4 weeks) surgery (1.5 points), previous VTE (1.5 points), haemoptysis (1 point), cancer treatment in the previous 6 months or palliative care (1 point) Low probability ‐ less than 2; intermediate probability ‐ 2 to 6; high probability ‐ more than 6 |
| Two‐level Wells score | Predictive elements for the 2‐level Wells score are the same as for the 3‐level Wells score, but patients are categorised into 2 as opposed to 3 categories, PE likely or PE unlikely based on a score of more than 4 or 4 or fewer points, respectively |
| Simplified Wells score | Same predictive elements are used as for the 3‐level Wells score, but the point scoring has been simplified ‐ each item now scores 1 point. Patients are regarded as low risk if they have 1 point or less, and as high risk if they score more than 1 |
| Geneva score | Predictive elements of the Geneva score include recent surgery (3 points), previous history of PE or DVT (2 points), heart rate > 100 beats per minute (1 point), 60 to 79 years old (1 point), 80 years old or older (2 points), chest radiograph showing atelectasis (1 point), chest radiograph showing elevated hemidiaphragm (1 point), partial pressure of oxygen (PaO2) < 49 mm Hg (4 points), PaO2 49 to 59 mm Hg (3 points), PaO2 60 to 71 mm Hg (2 points), PaO2 72 to 82 mm Hg (1 point) and partial pressure of carbon dioxide (PaCO2) < 36 mm Hg (2 points), PaCO2 36 to 38.9 mm Hg (1 point) Risk of PE is scored low (0 to 4 points), intermediate (5 to 8 points) or high (9 or more points) |
| Revised Geneva score | Predictive elements of the revised Geneva score include age > 65 years (1 point), previous history of PE or DVT (3 points), surgery with general anaesthesia or fracture within 1 month of symptoms arising (2 points), active malignancy (2 points), heart rate 75 to 94 beats per minute (3 points), heart rate > 94 beats per minute (5 points), pain on leg venous palpation and unilateral oedema (4 points), haemoptysis (2 points) and unilateral leg pain (3 points) This CPR is scored low risk (0 to 3 points), intermediate risk (4 to 10 points) or high risk (11 or more points) |
| Simplified revised Geneva score | Same predictive elements are used as for the revised Geneva score, but point scoring has been simplified. Each item now scores 1 point Risk of PE is scored low (0 to 1 point), intermediate (2 to 4 points) or high (5 or more points) |
| Charlotte rule | Elements of the Charlotte rule include > 50 years old, heart rate higher than systolic blood pressure, unexplained hypoxaemia (O2 < 95%), recent surgery (previous 4 weeks), haemoptysis and unilateral leg swelling Risk score from the Charlotte rule is classified as safe (all predictive elements absent) or unsafe (any of the predictive elements present) |
| CPR: clinical prediction rule | |

