Probióticos para la prevención de la infección urinaria en pacientes con vejiga neuropática
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Stated that patients were blinded. It was stated that nurse administering the bladder instillation was not blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Stated clearly that evaluator was blinded to randomisation group |
| Incomplete outcome data (attrition bias) | High risk | All patients accounted for up to 1 year follow‐up. For patients randomised into the treatment group, if they failed inoculation, they were then combined with the patients in the placebo group for statistical comparisons. High risk of bias as it not fully intention to treat |
| Selective reporting (reporting bias) | High risk | Only adverse event reported was one participant had autonomic dysreflexia. Generally stated that there was lack of evidence of septicaemia and UTI attributable to E. coli 83972 |
| Other bias | Low risk | Study appears free of other biases |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Clearly stated randomisation was computer generated for each centre |
| Allocation concealment (selection bias) | Low risk | Stated that placebo inoculum visually identical to bacterial inoculum |
| Blinding of participants and personnel (performance bias) | Low risk | Clearly stated patients and investigators were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Clearly stated outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | High risk | Clinical characteristics of the 27 patients who were followed up till 1 year was similar. However, no characteristics of the 32 patients who had initial bladder inoculation but dropped out was mentioned. There were more drop outs in the treatment than placebo group 28 versus 4. Authors did mention that patient compliance with inoculation protocol was low to account for the reason that losses to follow‐up was higher in the treatment group Although 59 patients received bladder inoculation, only 27 patients were evaluated and analysed up to the 1 year follow‐up period. The survival analysis were only based on patients who completed the one year follow‐up period and did not account for the loss to follow‐ups; high risk of bias as not fully intention to treat |
| Selective reporting (reporting bias) | High risk | No reporting of adverse events except that female gender was associated with bladder colonisation failure |
| Other bias | Low risk | Study appears free of other biases |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Wullt corresponded in 2015 that both active and placebo inoculum have the same appearance, a clear fluid that could not be differentiated by a non‐informed observer |
| Blinding of participants and personnel (performance bias) | Low risk | Patient were blinded as to the nature of inoculum; investigators were aware of whether patients were in intervention or control arm. |
| Blinding of outcome assessment (detection bias) | Low risk | Wullt corresponded in 2015 that study team was unaware of urine culture results but were aware of patient's symptoms in view of outcome being self‐reported UTI symptoms. Wullt corresponded in 2015 that the investigator who surveyed the urine culture was aware of type of inoculum |
| Incomplete outcome data (attrition bias) | High risk | Patients with unsuccessful colonisation post inoculation were excluded from analysis ‐ not fully intention to treat ‐ only analysed 20/26 patients. Wullt corresponded in 2015 that an intention‐to‐treat analysis was not conducted due to the small number of patients enrolled |
| Selective reporting (reporting bias) | High risk | Only reported that it is safe due to absence of pyelonephritis. In a later article published in 2014, the authors reported symptomatic UTI in 2 patients who were successfully inoculated with E. coli 83972 |
| Other bias | Low risk | Study appears free of other biases |
CFU ‐ colony‐forming units; DM ‐ diabetes mellitus; HPF ‐ high powered field; RCT ‐ randomised controlled trial; UTI ‐ urinary tract infection; VUR ‐ vesicoureteric reflux; WBC ‐ white blood cells
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Wrong population: young premenopausal women with no urologic abnormalities; authors also state study is not powered to evaluate the effect of probiotics on the rate of UTI recurrence | |
| Wrong population: only 5/150 participants had urinary tract abnormality | |
| Wrong intervention: probiotics versus antibiotics, beyond scope of protocol | |
| Wrong intervention: probiotics + antibiotics combination versus antibiotics, beyond scope of the review | |
| Wrong intervention: probiotics versus antibiotics; study did enrol patients with neuropathic bladder | |
| Study withdrawn prior to participant enrolment; no reason provided | |
| Study withdrawn prior to participant enrolment; no reason provided | |
| Wrong intervention: probiotics versus antibiotics and no mention of patients with neuropathic bladders although excluded patients with urinary tract complications |
UTI ‐ urinary tract infection
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Probiotics prophylaxis of Spinal Cord Injury Urinary Tract Infection Therapeutic Trial |
| Methods | Randomised, placebo‐controlled, double‐blind study with factorial design |
| Participants | Participants with known neuropathic bladder with stable spinal cord injuries or stable known demyelinating lesion |
| Interventions | All patients randomised into 1 of 4 groups
|
| Outcomes | Time from randomisation to first occurrence of symptomatic UTI or those with no UTI, until 6 months post randomisation |
| Starting date | April 2011 |
| Contact information | |
| Notes |
LGG ‐ Lactobacillus rhamnose GG; RC14 ‐ Lactobacillus reuteri RC‐14; UTI ‐ urinary tract infection
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic UTI Show forest plot | 3 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.08, 1.19] |
| Analysis 1.1 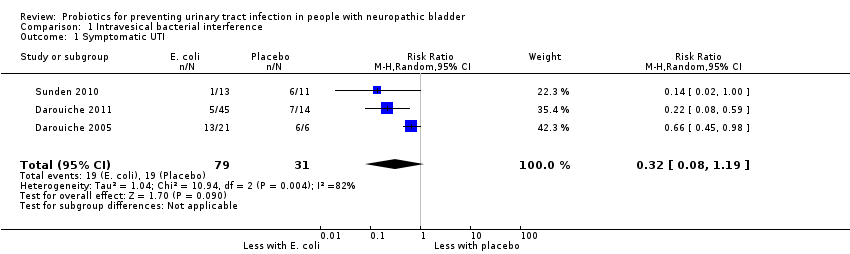 Comparison 1 Intravesical bacterial interference, Outcome 1 Symptomatic UTI. | ||||
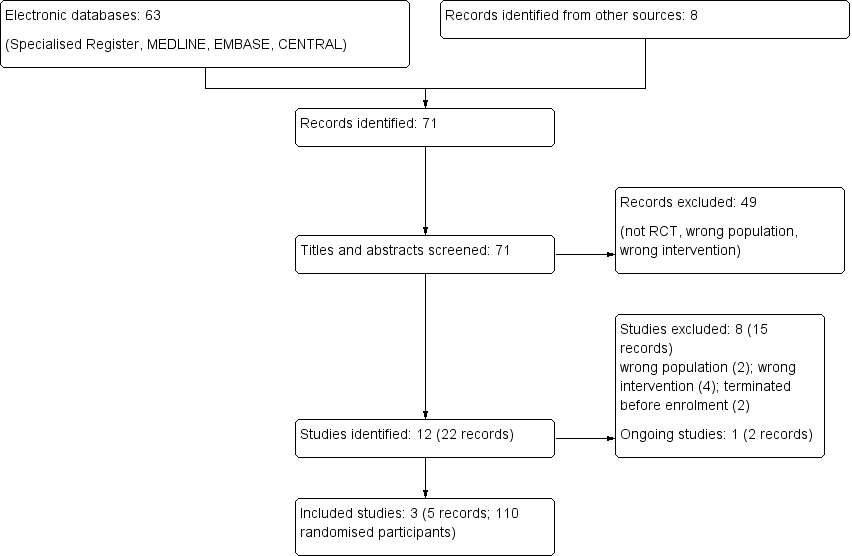
Study flow diagram.
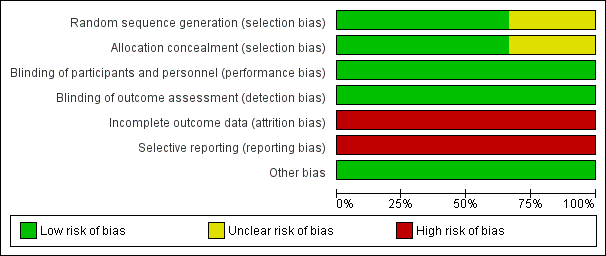
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
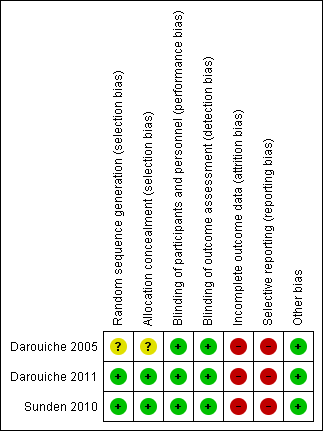
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
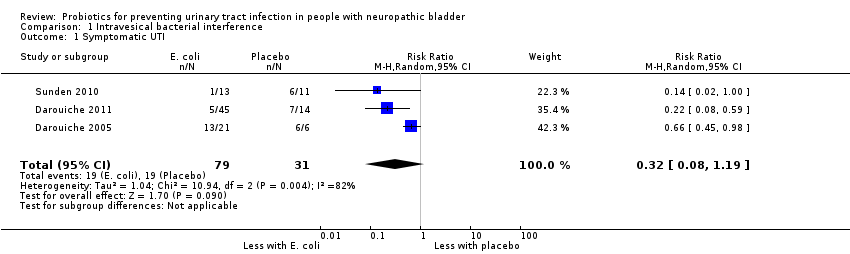
Comparison 1 Intravesical bacterial interference, Outcome 1 Symptomatic UTI.
| Intravesical bacterial interference versus placebo for preventing urinary tract infection in people with neuropathic bladder | |||||
| Patient or population: people with neuropathic bladder | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants (studies) | Quality of the evidence | |
| Risk with placebo | Risk with intravesical bacterial interference | ||||
| Symptomatic UTI | 613 per 1,000 | 196 per 1,000 | RR 0.32 | 110 (3) | ⊕⊝⊝⊝1 |
| GRADE Working Group grades of evidence | |||||
| 1Risk of bias was assessed at high in most domains, with heterogeneity and small studies, suggesting that results overestimate intravesical bacterial interference versus placebo | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic UTI Show forest plot | 3 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.08, 1.19] |

