Intervenciones para el tratamiento de la gangrena gaseosa
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010577.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 03 diciembre 2015see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Heridas
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Siyan Zhan: conceived, designed and co‐ordinated the review; completed the first draft of the review and performed part of writing and editing the review; approved the final version prior to submission; advised on the review; secured funding; performed previous work that was the foundation of the review; and is the guarantor of the review.
Zhirong Yang: conceived and designed the review; extracted data and undertook quality assessment; performed part of writing and editing the review; approved the final version prior to submission; advised on the review; and performed previous work that was the foundation of the current review.
Jing Hu: co‐ordinated the review; extracted data and undertook quality assessment; performed part of writing and editing the review; and approved the final version prior to submission.
Yanji Qu: co‐ordinated the review; checked quality of data extraction; checked quality assessment; performed part of writing and editing the review; and approved the final version prior to submission.
Feng Sun: co‐ordinated the review; checked quality of data extraction, analysed and interpreted data and checked quality assessment; performed statistical analysis and checked quality of statistical analysis; performed part of writing and editing the review; and approved the final version prior to submission.
Xisheng Leng: designed the review; analysed and interpreted data; performed part of writing and editing the review; advised on the review; and approved the final version prior to submission.
Hang Li: designed the review; analysed and interpreted data; performed part of writing and editing the review; advised on the review; and approved the final version prior to submission.
Contributions of editorial base
Nicky Cullum: edited the protocol; advised on methodology, interpretation and protocol content.
Joan Webster: edited the review; advised on methodology, interpretation and review content.
Sally Bell‐Syer: edited the protocol and the review; advised on methodology, interpretation and content;
Gill Rizzello: co‐ordinated the editorial process; edited the review.
Ruth Foxlee: designed the search strategy
Reetu Child edited the search methods section.
Sources of support
Internal sources
-
Peking University, China.
-
University of Cambridge, UK.
External sources
-
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Wounds. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health, UK.
Declarations of interest
Zhirong Yang: none known.
Jing Hu: none known.
Yanji Qu: none known.
Feng Sun: none known.
Xisheng Leng: none known.
Hang Li: none known.
Siyan Zhan: none known.
Acknowledgements
Many thanks also to peer referees (Elizabeth McInnes, Mark Rodgers, Marialena Trivella, Dirk Ubbink, Uwe Wollina, Gillian Ray‐Barruel, and Durhane Wong‐Rieger, Bestun Ahmed) and copy‐editor Elizabeth Royle.
Version history
| Published | Title | Stage | Authors | Version |
| 2015 Dec 03 | Interventions for treating gas gangrene | Review | Zhirong Yang, Jing Hu, Yanji Qu, Feng Sun, Xisheng Leng, Hang Li, Siyan Zhan | |
| 2013 Jun 06 | Interventions for treating gas gangrene | Protocol | Zhirong Yang, Jing Hu, Yanji Qu, Feng Sun, Xisheng Leng, Hang Li, Siyan Zhan | |
Differences between protocol and review
We did not perform the quantitative synthesis we planned in our protocol due to the small number of included studies and the substantial clinical heterogeneity between them. We assessed quality of the evidence using GRADE system, which was not planned in our protocol.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram
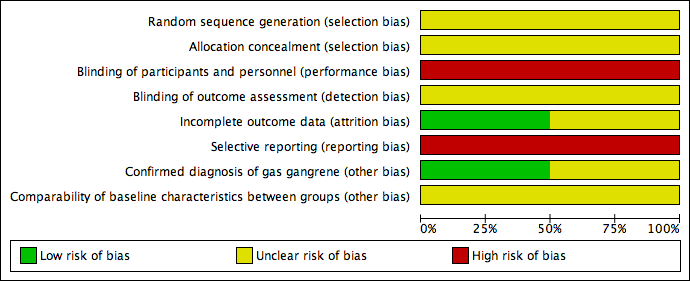
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
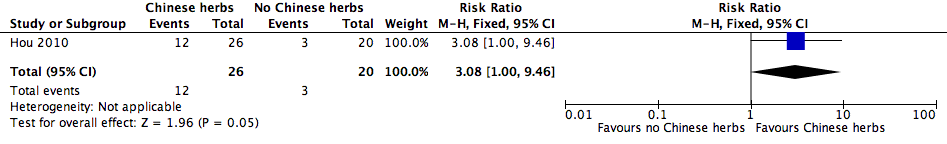
Forest plot of comparison: 1 Additional Chinese herbs versus no additional Chinese herbs, outcome: 1.1 Cure rate.

Forest plot of comparison: 2 Additional topical HBOT versus additional systemic HBOT, outcome: 2.1 Cure rate.
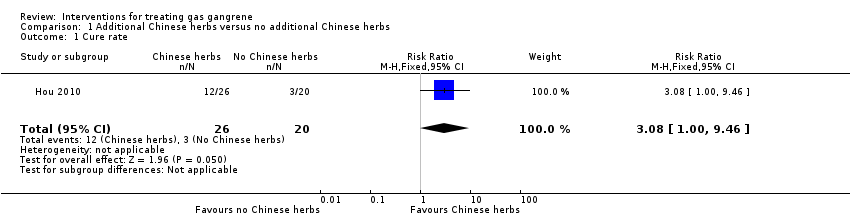
Comparison 1 Additional Chinese herbs versus no additional Chinese herbs, Outcome 1 Cure rate.
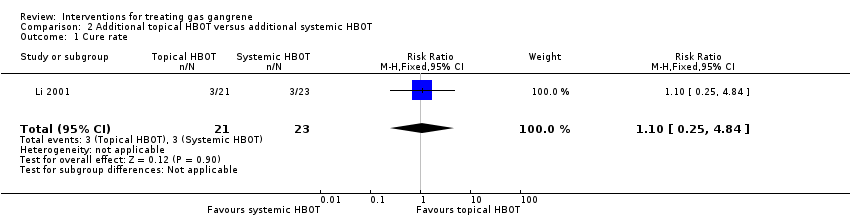
Comparison 2 Additional topical HBOT versus additional systemic HBOT, Outcome 1 Cure rate.
| Additional Chinese herbs compared with no additional Chinese herbs for treating gas gangrene | ||||||
| Patient or population: patients with gas gangrene | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no Chinese herbs | Risk with Chinese herbs | |||||
| Cure rate | Study population | RR 3.08 | 46 | ⊕⊝⊝⊝ | ||
| 150 per 1000 | 462 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels due to limitations in design; high risk of performance and reporting bias, and unclear risk of bias in other bias sources. 2 Downgraded two levels due to imprecision; only one trial with small sample size and very wide confidence interval that included the possibility of an effect in either direction (crosses line of no effect). | ||||||
| Additional topical HBOT compared with additional systemic HBOT for treating gas gangrene | ||||||
| Patient or population: patients with gas gangrene | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with systemic HBOT | Risk with Topical HBOT | |||||
| Cure rate | Study population | RR 1.10 | 44 | ⊕⊝⊝⊝ | ||
| 130 per 1000 | 143 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels due to limitations in design; high risk of performance and reporting bias, and unclear risk of bias in selection and detection bias. 2 Downgraded two levels for imprecision; only one trial with small sample size and very wide confidence interval that included the possibility of an effect in either direction (crosses line of no effect). | ||||||
| Search strategies |
| China Biological Medicine Database (CBM‐disc) |
| #1 "气性坏疽"[常用字段:智能] |
| China National Knowledge Infrastructure (CNKI) |
| (主题="气性坏疽") OR ((主题="梭菌"+"梭状") AND (主题="肌坏死"+"肌炎")) |
| Chinese scientific periodical database of VIP INFORMATION (VIP) |
| ((题名或关键词=肌坏死 或 文摘=肌坏死 或 题名或关键词=肌炎 或 文摘=肌炎 与 专业=经济管理+图书情报+教育科学+自然科学+农业科学+医药卫生+工程技术+社会科学 与 范围=全部期刊) 与 (题名或关键词=梭状 或 文摘=梭状 或 题名或关键词=梭菌 或 文摘=梭菌 与 专业=经济管理+图书情报+教育科学+自然科学+农业科学+医药卫生+工程技术+社会科学 与 范围=全部期刊)) 或者 (题名或关键词=气性坏疽 或 文摘=气性坏疽 与 专业=经济管理+图书情报+教育科学+自然科学+农业科学+医药卫生+工程技术+社会科学 与 范围=全部期刊) |
| Science Citation Index |
| Gas gangrene or clostridi* myonecrosis |
| ClinicalTrials.gov (www.clinicaltrials.gov) |
| "Gas Gangrene"(By topics) |
| Current Controlled Trials (www.controlled‐trials.com) |
| "gas gangrene" or "myonecrosis" |
| WHO International Clinical Trials Registry Platform (www.who.int/trialsearch) |
| gas gangrene or clostridi* myonecrosis or non‐clostridi* myonecrosis or nonclostridi* myonecrosis |
| Australian New Zealand Clinical Trials Registry (www.anzctr.org.au) |
| "gas gangrene" or "clostridial myonecrosis" or "non‐clostridial myonecrosis" or "nonclostridial myonecrosis" |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cure rate Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [1.00, 9.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cure rate Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.25, 4.84] |

