Intervenciones para el tratamiento de los trastornos gustativos
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias de los estudios en curso
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Title: Efficacy of acupuncture in the treatment of idiopathic taste disorders Year of publication: 2008 Language: German Trial design: randomised controlled trial (single‐blind), two treatment arms Location: university clinic, Dresden, Germany Number of centres: 1 Recruitment period: December 2003 ‐ December 2005 Funding source: German Doctor's association for acupuncture | |
| Participants | Inclusion criteria:
Exclusion criteria:
Baseline taste acuity: not given Baseline taste discrimination: Group A: 11, 7 Group B: 11,9 Standard deviation not given, scale used: 32 taste strips, hypogeusia threshold: < 16 for ages 60 and younger/ < 14 for ages 60 and older Type of test: taste strips Age (standard deviation) at baseline: only given for the two groups combined: mean 63 years, range 25 ‐ 83 (standard deviation not given) Gender: only given for the two groups combined: 25 female and 12 male Any other details of important prognostic factors: disease duration across both groups: mean 19 months (range 1 month to 12 years) Number randomised: 37 Method of randomisation: assigned by lot Number evaluated: 37 | |
| Interventions | Comparison: Group A (n = 17): acupuncture with needles Group B (n = 20): sham acupuncture with deactivated acupuncture laser Duration of treatment: 15 acupuncture treatments (2 patients in the interventions group did not require further acupuncture treatment after 10 treatments), over a course of 8 weeks | |
| Outcomes | Taste discrimination: taste strips (scale used 32 taste strips, hypogeusia threshold: < 16 for ages 60 and younger/ < 14 for ages 60 and older) assessed before and after treatment Quality of life: five questions to be answered via visual analog scale Depressive symptoms: Beck depression inventory Subjective well‐being: Zerssen Mood scale | |
| Notes | Sample size calculation: reported Adverse events: not reported Health‐related quality of life: reported Correspondence required: yes, to get the missing data (Email sent on 27th November 2013) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the report "Assigned by lot" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Single‐blind study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not possible (needle versussham laser acupuncture) |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | All the outcomes mentioned in the methodology section are reported |
| Other bias | Low risk | The characteristics of the two groups before treatment did not differ significantly |
| Methods | Title: Zinc supplementation in chronic renal failure Year of publication: 1982 Language: English Trial design (including number of arms): double‐blind cross‐over design Location: Division of Nephrology, Department of pediatrics, University of Utah School of Medicine, Provo and Salt lake city, USA Number of centres: one Recruitment period: one year Funding source: Thrasher Research Fund | |
| Participants | Total number: 17 Inclusion criteria: pediatric patients, varying degrees of chronic renal failure, not yet on dialysis or in need of a transplant, taste impairment Exclusion criteria: none if they were in paediatric renal clinic (Personal communication)
Sodium chloride (3.0, 5.3, 10 gm/litre; Sucrose 1000, 1750, 2650 mg/dl, hydrogen chloride .07, .16, .33 normality and Urea 460, 860, 1460 mg/dl ‐ Personal communication)
Type of test: Quote from personal communication "see above, Initially we bought the kit with the 12 dropper bottles from Henkin, then our laboratory could make refills. For the 6 mo old, as I recall we were limited to a smile with the sucrose bottle and making a grimace or turning away from the other solutions as being “data”. Age at baseline: mean age of 14 patients who completed the trial was 10 years with a range of 0.5 to 19 years Gender: not mentioned Any other details of important prognostic factors: nil Number randomised: 14 ‐ Personal communication Method of randomisation: quote from personal communication "pharmacy prepared capsules and numbered bottles randomly and kept “the code” until the end of the study. We just gave each enrollee the next set of bottles" Number evaluated: 14 | |
| Interventions | Total number of intervention groups: two Comparison: Zinc sulfate (0.50 to 0.75 mg/Zn/Kg/day) and placebo Group 1 (n = 7*): first they received placebo and then zinc Group 2 (n = 7*): first they received zinc and then placebo Duration of treatment: each sequence lasted for six months (*n = 7 based on personal communication) | |
| Outcomes | Taste acuity: taste detection and recognition improved (P < 0.05) in both groups following zinc supplementation | |
| Notes | Sample size calculation: not done as this was a limited population. We started with 17 patients (seven male and 10 female). Two patients left the area. One patient died leaving 14 patients, unknown what the gender mix was of the 14 Adverse events: none reported Health‐related quality of life: none Key conclusions of the study authors: zinc supplementation increased RBC zinc concentration and taste acuity. In those with less advanced renal failure (serum creatinine < 5.0 mg/dl) it also improved caloric intake Correspondance required: contacted and reply received on 22 December 2013 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It is assumed that the pharmacy did use an acceptable random sequence generation |
| Allocation concealment (selection bias) | Low risk | Pharmacy prepared random numbered capsules – Personal communication |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | High risk | Out of 17, only 14 patients completed the trial (> 10% attrition) |
| Selective reporting (reporting bias) | Low risk | Reasons for dropout: Quote from personal communication "Two patients left the area. One patient died leaving 14 patients" |
| Other bias | High risk | 1. As it is a cross‐over study, possible carry over effect could be there because of "no washout period" 2. Inclusion of six months child (1), three‐year old patient (1), five‐year old patients (2) for assessment of taste acuity is questionable 3. Data on taste acuity was only assessed by smile or grimace in these patients |
| Methods | Title: Zinc Gluconate in the Treatment of Dysgeusia—a Randomized Clinical Trial Year of publication: 2005 Language: English Trial design: randomised controlled trial (Double‐blind), fixed block randomisation Location: Smell and Taste Clinic, Dept. of Otorhinolaryngology, University of Dresden, Germany Number of centres: one Recruitment period (Duration): 1999 to 2001 Funding source: Sander‐Stiftung (No.2001.019.1); Taste strips given by Christian Müller, University of Vienna | |
| Participants | Total number: 50 Inclusion criteria:
Exclusion criteria:
Base line taste acuity (+ standard deviation + scale used) Scales used (before and after treatment):
1. Filter paper strip at baseline: 2. Visual analogue scale 3. Beck depression inventory 4. Mood scale 5. Zinc in serum: placebo 6. Zinc in saliva: placebo Age: placebo = 61.0 ± 8.9; zinc gluconate = 61.1 ± 10.6 Gender: placebo = 2 male, 22 female; zinc gluconate = 5 male, 21 female Any other details of important prognostic factors:
Number randomised: 50 Method of randomisation: special computer software program RANDOM Number evaluated: 50 | |
| Interventions | Total number of intervention groups: two Comparison: placebo: n = 26 zinc gluconate: n = 24 Duration of treatment: three months | |
| Outcomes | Taste acuity: taste strips (an improvement by six points in the taste test can be regarded as substantial) Visual analogue scale (defined as an improvement of more than 5%) | |
| Notes | Sample size calculation: unclear Adverse events: not reported Health‐related quality of life: reported Key conclusions: in conclusion, zinc appears to improve general gustatory function and, consequently, general mood scores in dysgeusia patients Correspondence required: nil | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the report "Blinding and randomisation were performed by an independent individual using a special computer software program (RANDOM by Joern Loetsch, Institute of Clinical Pharmacology, University of Frankfurt, Germany)" |
| Allocation concealment (selection bias) | Low risk | Quotes from the report: "The bottles were sealed and labelled with the study code and the enrolment number. After the initial investigation for the baseline data, each patient was given an enrolment number and the corresponding screw‐top bottle" "The zinc and placebo showed no significant difference in taste" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from the report "Screw‐top bottles were prepared containing either 100 zinc gluconate tablets (140 mg, "Zink Verla"®) or 100 placebo tablets (lactose, "Placebo Lichtenstein 10 mm"). The bottles were sealed and labelled with the study code and the enrolment number" |
| Blinding of outcome assessment (detection bias) | Low risk | Neither patient nor investigator had any knowledge during the study as to whether the patient was being treated with zinc or placebo. When the study was complete, this information was then revealed by the independent individual |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methodology section are reported |
| Other bias | Low risk | The characteristics of the two groups before treatment did not differ significantly |
| Methods | Title: The effect of zinc agent in 219 patients with zinc deficiency‐inductive/Idiopathic taste disorder: A placebo controlled randomized study Year of publication: 2013 Language: Japanese Trial design: randomised controlled trial with two arms (zinc tablets versus placebo) Location: University hospitals, mostly departments of otolaryngology, Japan Number of centres: 32 centres Recruitment period: from November 2008 to January 2010 Funding source: Zeria Pharmaceutical Co, Ltd, Japan | |
| Participants | Inclusion criteria: zinc deficiency‐inducive and idiopathic taste disorder Exclusion criteria: who had unbalanced eating habits identified with meal diary during the screening period Baseline taste acuity: average taste scores of four types of taste less than 4.5 by filter disc method were included in both group A and B. Exact baseline scores and variations were not described in the text Baseline taste discrimination: only average taste scores of 4 types by filter disc method were described in the text Type of test: filter paper disk method by Tomita Average age at baseline: Group A: 43.3 years Group: B 47.1 years (no standard deviation values were indicated in the text) Gender: Group A: Male 48/Female 60 Group B: Male 39/Female 72 Other details of important prognostic factors: Average serum zinc concentration Group A: 71.8 ug/dL, Group B: 73.5 ug/dL Average zinc intake from food Group A: 7.9 mg/day, Group B: 7.9 mg/day (assumed by food frequency questionnaire) Number randomised: 219 subjects Method of randomisation: randomisation method was no described Number evaluated: 219 subjects | |
| Interventions | Comparison: Zinc agents versus placebo Group A (n = 108) Prescribed 17 mg of Zinc containing tablets (Polaprezinc, Promac, Zeria Pharmaceutical Co.Ltd. Japan), twice a day for 12 weeks Group B (n = 111) Prescribed placebo tablets without zinc, twice/day for 12 weeks Duration of treatment: 12 weeks | |
| Outcomes | Main outcome measure was the change of the average four basic taste sensitivity scores by filter paper disk method at 4, 8, 12 weeks from baseline and four weeks after the end of zinc tablets administration Another outcome measure they used was binary measure, Improved/Not improved Patients showing taste acuity equal or less than 3.0 of average 4 taste sensitivity by filter disc method were regarded as improved, or patients showing more than 1.0 of improvement Taste discrimination was not described in the text | |
| Notes | Sample size was not calculated prior to the trial One case of eczema was reported with zinc containing tablets No severe adverse event was reported Correspondence required: Yes, details about random sequence generation and allocation concealment needed. Email sent on 25th November 2013 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided by the translator (foreign language article) |
| Allocation concealment (selection bias) | Unclear risk | No information provided by the translator (foreign language article) |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) | Low risk | 100% of participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the method section are reported adequately |
| Other bias | Low risk | The characteristics of the two groups before treatment did not differ significantly |
| Methods | Title: Improvement of uremic hypogeusia by zinc: a double‐blind study Year of publication: 1980 Language: English Trial design: three arms (placebo group: 25 mg sucrose; study group: 25 mg zinc acetate; control group 20 healthy age and sex matched controls were also studied for taste and plasma zinc determination for comparison) Location: Department of Medicine, Veterans Administration Medical Center, Allen Park, Harper Hospital and Wayne State University School of Medicine, Detroit, Michigan 48101 Number of centres: One Recruitment: 6 to 12 weeks Funding source: supported in part by Grant AM19338 from NIAMDD and BMA Management Research Fund of Boston, Massachusetts | |
| Participants | Total number: 42 (Placebo = 11; zinc acetate = 11; control = 20) Inclusion criteria:
Exclusion criteria: none Baseline taste acuity and taste discrimination: Baseline detection thresholds for sodium chloride correlated well with detection thresholds for sucrose, urea and hydrochloric acid Baseline recognition thresholds for sodium chloride also correlated well with those for sucrose, urea and hydrogen chloride Type of test: three‐drop stimulus technique. Thresholds for taste detection and recognition were determined for one taste quality before proceeding to the next taste quality. Lowest concentration of solute that the patient could consistently distinguish as different from water for each taste quality was called the detection threshold. The lowest concentration of solute that the patient could consistently recognise correctly as salty, sweet, sour or bitter was called the recognition threshold Nerve conduction velocity: placebo ‐ 50.4 ± 1.8; zinc acetate ‐ 47.9 ± 2.6 (normal range is 43 to 56 m/sec) Age (±standard deviation) at baseline: placebo ‐ 55.1 ± 2.8; zinc acetate ‐ 51.3 ± 3.2 Gender: not mentioned Any other details of important prognostic factors: smokers were asked not to smoke at least one to two hours prior to taste testing. Water was allowed up to the time of testing Number randomised: placebo 11, zinc acetate 11 Method of randomisation: the patients were assigned to the treatment or placebo group by the pharmacist by opening the consecutively numbered sealed envelopes which indicated zinc acetate or placebo in equal numbers. As each patient entered the trial, the next sequential envelope was opened and the patient was assigned to the appropriate treatment group. Identical capsules containing either 25 mg zinc acetate or 25 mg sucrose were used. Neither patients nor physicians were aware of the medication being given Number evaluated: 22 | |
| Interventions | Total number of intervention groups: two (zinc acetate and placebo) and control Comparison: Group A (n = 11): (describe intervention including any baseline findings) The treatment group received 25 mg of elemental zinc as zinc acetate and then each patient was tested for taste and his blood samples were drawn for plasma zinc before and at various intervals exceeding six weeks after starting the treatment Test for taste that was used was taste detection and recognition thresholds measured for sodium chloride, sucrose, hydrogen chloride and urea, to monitor the four tastes salt, sweet, sour and bitter Group B (n = 11 ): (describe intervention including any baseline findings) The placebo group received 25 mg of sucrose and then each patient was tested for taste and his blood samples were drawn for plasma zinc before and at various intervals exceeding six weeks after starting the treatment Test for taste that was used was taste detection and recognition thresholds measured for sodium chloride, sucrose, hydrogen chloride and urea, to monitor the four tastes salt, sweet, sour and bitter Duration of treatment: 6 to 12 weeks | |
| Outcomes | Taste detection and recognition: Sodium chloride: placebo (baseline) and zinc acetate (baseline): not statistically significant Baseline (placebo) and end point (placebo): P < 0.01 (for both detection and recognition) Placebo and zinc acetate: P < 0.05 for detection and P < 0.005 for recognition Sucrose: placebo (baseline) and zinc acetate (baseline): not statistically significant Baseline (placebo) and end point (placebo): P < 0.025 Placebo and zinc acetate: P < 0.05 Comparison between placebo and zinc acetate group for detection threshold and recognition threshold:
| |
| Notes | Sample size calculation: not mentioned Adverse events: not reported Health‐related quality of life: not reported Key conclusions of the study authors:
Correspondence required: Yes, for the missing data (co‐author Anand Prasad contacted and received reply on 7th November 2013 and on 17th December 2013) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It is assumed that the pharmacy did use an acceptable random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote from the report "The patients were assigned to the treatment or placebo group by the pharmacist by consecutively numbered sealed envelopes." Pharmacy controlled randomisation, sealed envelopes, identical capsules of zinc acetate and sucrose (placebo) |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from the report "neither patients nor physicians were aware of the medication being given" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from the report "neither patients nor physicians were aware of the medication being given" |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts in the study |
| Selective reporting (reporting bias) | Low risk | All the stated outcomes in the methodology are adequately reported |
| Other bias | Low risk | The timing of end point analysis is not clear. The study says that the post‐treatment evaluation was done after 6 weeks to 12 weeks. This will not affect the outcome of the study clinically |
| Methods | Title: Zinc deficiency: a reversible complication of uremia Year of publication: 1982 Language: English Trial design (including number of arms): double‐blind study Location (including setting e.g. private practice/university detail hospital/etc): Department of Medicine, Veterans administration Medical centre, Allen Part, MI and Harper Hospital, and Wayne State University School of Medicine, Detroid, MI, USA Number of centres: two Recruitment period (duration): six months Funding source: supported in part by a Sickle cell centre Grant from the National Heart, Lung and Blood Institute and a Grant from the United states Department of Agriculture | |
| Participants | Total number: 24 Inclusion criteria:
Exclusion criteria: not mentioned Baseline taste acuity (± standard deviation + scale used): 17 patients had lack of appetite, or taste, or both, for various foods and metallic sensation in the mouth and remaining seven had no symptoms regarding their taste. At baseline testing, all had decreased taste acuity. (Mean and standard deviation not given, no scale given) Baseline taste discrimination: not mentioned Type of test: Henkin's three‐drop stimulus technique Age (± standard deviation) at baseline: Group A (zinc acetate): 46 ± 8 Group B (placebo): 49 ± 12 Gender: All males Any other details of important prognostic factors: none of the patients had an intercurrent illness of gastrointestinal tract disorder. All patients were consuming weight‐maintaining diets consisting of 60 to 80 gm of protein with variable sodium restriction. The etiology of the end stage renal disease was hypertensive nephrosclerosis in 15 patients, chronic glomerulonephritis in eight patients and diabetic glomerulosclerosis in one patient. All patients were receiving phosphate‐binding gels, multivitamins, folic acid and iron Number randomised: 24 (12 ‐ zinc and 12 ‐ placebo) Method of randomisation: not mentioned Number evaluated: 24 | |
| Interventions | Total number of intervention groups: two (zinc acetate and placebo) Comparison: Group A (zinc acetate, n = 12): no other details given Group B (placebo, n =12) Duration of treatment: six months | |
| Outcomes | Taste acuity: in zinc acetate group, significant improvement in their ability to taste various foods occurred in eight of the nine symptomatic patients. Taste detection and recognition thresholds for sodium chloride, sucrose, urea normalised in all patients after six months; but not hydrochloric acid. Improvement in taste acuity was demonstrated as early as 12 weeks in some patients In placebo group, symptoms of abnormal taste persisted in all the eight symptomatic patients and there was no significant improvement in any of the taste modality tested | |
| Notes | Sample size calculation: not mentioned Adverse events: none reported Health‐related quality of life: none reported Key conclusions of the study authors: zinc supplementation is able to improve taste in uremic males and uremia is a zinc deficient state Correspondence required: yes, comparative data is needed. (Co‐author, Anand Prasad contacted and received reply on 7th November 2013 and on 17th December 2013) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It is assumed that the pharmacy did use an acceptable random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote from the report "The patients were assigned to the treatment or placebo groups by opening consecutively numbered, sealed envelopes that indicated zinc acetate or placebo in equal numbers. As each patient entered the trial, the next sequential envelope was opened and the patient was assigned to the appropriate treatment. Identical capsules containing 25 mg of elemental zinc as zinc acetate or 25 mg of sucrose were used" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from the report "Neither the patients nor physicians were aware of the content of the capsules being given" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from the report "Neither the patients nor physicians were aware of the content of the capsules being given" |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts in the study |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the method section are reported adequately |
| Other bias | Low risk | The characteristics of the two groups before treatment did not differ significantly |
| Methods | Title: A zinc‐containing compound, Polaprezinc, is effective for patients with taste disorders: randomized, double‐blind, placebo‐controlled, multi‐center study Year of publication: 2009 Language: English Trial design: four arms ‐ Placebo group and three study groups with different dosage of Polaprezinc 75 mg (17 mg zinc), 150 mg (34 mg zinc) and 300 mg (68 mg zinc) Location: university hospitals, across various places in Japan Number of centres: 22 Recruitment period: 12 weeks Funding source: Polaprezinc and placebo were provided by Zeria Pharmaceutical Co., Ltd. (Personal communication) | |
| Participants | Total number: 109 Inclusion criteria: idiopathic taste disorder, age 20 ‐ 80 years, disease duration of less than 6 months, no underlying illness, not being administered any drugs affecting the disease condition Baseline taste acuity: two scales are used 1. Filter paper disk method 2. Subjective symptoms using questionnaire Baseline taste discrimination: not available Age (standard deviation) at baseline: placebo group: 44.9 ± 15.4; 17 mg zinc group: 47.1 ± 16.5; 34 mg zinc group: 43.7 ± 18.1; 68 mg zinc group: 44.7 ± 15.6 Gender: Placebo: male 12, female 15; 17 mg: male 18, female 9; 34 mg: male 12, female 13; 68 mg: male 9 female 19 Number randomised: placebo: 28, 17 mg: 27, 34 mg: 26, 68 mg: 28 Method of randomisation: permuted block method with a number independent from the drugs and administered to subjects in an ascending order of informed consent (Personal communication) Number evaluated: 107 | |
| Interventions | Total number of intervention groups: 3 (Polaprezinc and placebo) Filter paper disk method: mean values of all four regions taken Filter paper disk scale: normal < 3.5; mild ≥ 3.5 to < 4.5; moderate ≥ 4.5 to < 5.5; severe ≥ 5.5 Subjective symptoms using questionnaire method: Scale used: 1 to 5 scale, 1 ‐ no taste and 5 ‐ normal taste Placebo group (n = 27): Baseline ‐ not available 17 mg group (n = 27): Base line ‐ not available 34 mg group (n = 25): Base line ‐ not available 68 mg group (n = 28): Base line ‐ not available There was no significant imbalance amongst the 4 groups in the data of subjective symptoms prior to administration (Personal communication) | |
| Outcomes | Filter paper disk method: cured, overall mean values were < 3.5; improved, improvement of 1.0 either in the area of chorda tympani or glossopharyngeal nerves; unchanged, neither cured nor improved nor worsened; aggravated, aggravation of ≥1.0 in both chorda tympani and glossopharyngeal nerve areas Overall mean value was calculated by dividing the sum of the score of the disc containing each taste quality that was obtained at four different locations by 16. The number of ‘efficient’ cases was presented as a sum of the ‘cured’ and ‘improved’ cases Subjective symptoms questionnaire: the change in subjective symptoms was defined as the difference of the value obtained before and after the treatment Mean subjective symptoms score in 34 mg and 68 mg groups were improved compared with placebo group (descriptive) | |
| Notes | Sample size calculation: not mentioned Adverse events: seen in all four groups
Key conclusion: polaprezinc is effective in improving the gustatory sensitivity of patients with idiopathic taste disorder with a daily dose of over 150 mg and 300 mg without any serious side effects Correspondence required: (Email sent on 12/11/2013, received reply on 20/11/2013) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block method (personal communication) |
| Allocation concealment (selection bias) | Unclear risk | Quote from personal communication "Drugs were labelled with a number independent from the drugs and administered to subjects in an ascending order of informed consent" This does not clearly say that the concealment of allocation was done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from the report "Double‐blind". No details of the blinding given |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from the report "Double‐blind". No details of the blinding given |
| Incomplete outcome data (attrition bias) | Low risk | Only 2 dropouts out of 109 (less than 10%). One patient was disqualified due to noncompliance with participation criteria and another one patient discontinued the study voluntarily |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methodology are reported |
| Other bias | Unclear risk |
|
| Methods | Title: Double‐blind, placebo‐controlled trial of zinc picolinate for taste disorders. Year of publication: 2002 Language: English Trial design: two arms ‐ placebo and zinc picolinate Location: special outpatient clinic for taste disorders of Nihon University, Itabashi Hospital, Tokyo, Japan Number of centres: one Recruitment period: three months, between July 1991 to May 1994 Funding source: none | |
| Participants | Total number: 89 (only 73 completed the study) Inclusion criteria: main complaint of taste disorder, who were found by the filter paper disk taste test to be suffering from a taste disorder, no underlying illness. Such patients were tested for serum zinc levels. If their serum zinc levels were ≤ 68 μg/dl, zinc deficient taste disorder was diagnosed and if their serum zinc levels were ≥ 70μg/dl, idiopathic taste disorder was diagnosed Exclusion criteria: none Baseline taste acuity (standard deviation + scale used): 48 suffered from idiopathic taste disorders and 25 had zinc deficiency taste disorders Placebo group: severe ‐ 17, moderate ‐ 12 and mild ‐ 7 Zinc picolinate group: severe ‐ 16, moderate ‐ 18 and mild ‐ 3 Type of test (before and after treatment): Subjective symptoms questionnaire: scale of 1 ‐ 5, with 1 ‐ no taste and 5 ‐ normal taste Filter paper disk method: 5 ‐ not recognise any taste and 6 ‐ recognise a taste incorrectly Severity of taste disorder was rated as:
Measurement of serum zinc levels Age (standard deviation) at baseline: 23 to 79 years; mean age 55.2 years for zinc picolinate and 50.4 years for the placebo group, standard deviation not given Gender: placebo ‐ 13 male and 23 female; zinc picolinate ‐ 13 male and 24 female Any other details of important prognostic factors: nil Number randomised: 89 Method of randomisation: Not mentioned Number evaluated: 73 | |
| Interventions | Total number of intervention groups: two (zinc picolinate and Placebo) Comparison: 1. Subjective symptoms questionnaire placebo (n = 35): zinc picolinate (n = 34) 2. Filter paper disk method placebo (n = 36); zinc picolinate (n = 37) 3. Serum zinc levels placebo (n = not available); zinc picolinate (n = not available) Duration of treatment: 3 months | |
| Outcomes | Taste acuity: questionnaire scale of 1 ‐ 5 Filter paper disk method
Serum zinc levels: before and after study, in μg/dl | |
| Notes | Sample size calculation: not reported Adverse events: six patients (16%) reported side effects
Health‐related quality of life: not reported Key conclusions of the study authors: Administration of zinc picolinate was significantly (P < 0.01) more effective than placebo in improving taste function in patients with zinc deficiency or idiopathic taste disorders In addition, serum zinc level was found to increase significantly with three months of zinc picolinate therapy Correspondence required for clarifications on allocation concealment and blinding of participants and assessors, reasons for dropout. Present contact of none of the three authors were available (checked in university Nihon web site on 17th November 2013) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from the report "89 patients were randomly assigned to receive placebo (lactose) capsules or capsules containing 28.9 mg of zinc picolinate plus lactose." No details of random sequence generation reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote from the report "Double‐blind". No details of the blinding given |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote from the report "Double‐blind". No details of the blinding given |
| Incomplete outcome data (attrition bias) | High risk | Out of 89, only 73 (> 10%) patients completed the study for flame photometric detection method and 69 (> 10%) completed the subjective questionnaire. Reasons for dropout not mentioned in the report |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in methodology section are reported adequately |
| Other bias | Low risk | The characteristics of the two groups before treatment did not differ significantly |
| Methods | Title: Zinc supplementation and its effect on taste acuity in children with chronic renal failure Year of publication: 1983 Language: English Trial design (including number of arms): double‐blind cross‐over study, two arms (zinc sulphate and placebo) Location (including setting e.g. private practice/university detail hospital etc): Department of paediatrics, Royal Mancter Children's hospital, Pendlebury, Manchester M271HA and Booth Hall Children's hospital, Blackley, Manchester M9 2AA, UK Number of centres: two Recruitment period (duration): 18 weeks (two 6‐week periods for intervention and 6‐week washout period) Funding source: none declared | |
| Participants | Total number: 25 Inclusion criteria: children with chronic renal failure with hypogeusia Exclusion criteria: none mentioned Baseline taste acuity (+ standard deviation + scale used): Median detection thresholds were salt: 30 mmol/l (range 6 ‐ 500); Sucrose: (range 12 ‐ 800); Urea 300 mmol/l (range 120‐1000) and hydrogen chloride: 6 mmol/l (range 0.8 ‐ 30) Scale used: salt and sucrose: 6, 12, 30, 60, 90, 150, 300, 500, 800, 1000 Hydrogen chloride: 0.5, 0.8, 3, 6, 15, 30, 60, 90, 150, 300, 500, 800, 1000 Urea: 60, 90, 120, 150, 300, 500, 800, 1000, 2000, 5000 Baseline taste discrimination: not done (just mentioned that the study population were unable to distinguish clearly between acid and bitter solutions) Type of test: three‐drop stir technique Age (standard deviation) at baseline: mean age 11.2 (range 7‐17 years) Gender: 7 girls and 13 boys Any other details of important prognostic factors: mean baseline glomerular filtration rate was 28 ml/min/1.73 m2 (range 8‐60 ml/min/1.73 m2). None of the patients had a serum albumin of less than 30 g/l Number randomised: 25 Method of randomisation: not mentioned Number evaluated: 20 | |
| Interventions | Total number of intervention groups: two Comparison: 1. Group A (Zinc sulphate group, n = 9) 15 mg elemental zinc (0.23 mmol) for children under 10 years of age and 50 mg (0.77 mmol) for adolescents Group B (Placebo group, n = 11) Identical lactose placebo capsules 2. Group A (placebo group): n =9 Group B (Zinc sulphate group): n =11 Duration of treatment: 18 weeks | |
| Outcomes | Taste acuity: no significant improvement at the five per cent level in the detection or recognition thresholds for each taste modality during the zinc supplementation period compared to placebo Taste discrimination: not mentioned | |
| Notes | Sample size calculation: not mentioned Adverse events: nausea and vomiting in a patient on 50 mg zinc sulphate Health‐related quality of life: no significant difference in energy, protein and dietary zinc intakes during the zinc supplementation period compared to placebo Key conclusions of the study authors: children with varying degrees of chronic renal failure have variable taste thresholds. Oral zinc therapy did not improve taste acuity in such patients and the study provides no support for the belief that routine zinc supplements are necessary in such children Correspondence required to get the raw data (mean, standard deviation). Present contact details could not be found | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It is assumed that the pharmacy did use an acceptable random sequence generation |
| Allocation concealment (selection bias) | Low risk | Pharmacy controlled randomisation |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote from the report "Double‐blind". No other details available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote from the report "Double‐blind". No other details available |
| Incomplete outcome data (attrition bias) | High risk | 5 out of 25 randomised patients failed to complete the study (20% attrition); dropout details from which group is not mentioned |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methodology are reported |
| Other bias | Unclear risk | Quote from the report "Routine medications were continued throughout the study". These medications could have a role in the causation of dysgeusia |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Included one subject with normal taste acuity | |
| Subjects undergoing chemotherapy and radiotherapy were included in the study | |
| Patients without gustatory disorder were also included in the trial | |
| Subjects were under maintenance‐opiate treatment or intravenous naloxone | |
| Subjects were given zinc sulfate to prevent therapy‐induced taste alterations | |
| Subjects included in the study had dysgeusia secondary to head trauma (14 cases), postoperative (6 cases), encephalitis (2 cases), cerebral vascular accident (1 case), xerostomia (1 case), lingual anaesthesia (1 case) and tic douloureux (1 case). All these were excluded in our study 44 patients were taking various drugs before and during the study period for other disorders | |
| Subjects included in the study were given bethanechol to prevent taste loss | |
| The study included healthy subjects to study the effect of linoleic acid | |
| Patients receiving chemotherapy were also included in the study Patients with mucositis and oral infections secondary to chemotherapy were included in the study | |
| Subjects included in the study are only normal healthy male volunteers | |
| Study aimed at prevention of taste alterations in patients undergoing radiation therapy using zinc sulfate | |
| Trial aimed at prevention of dysgeusia in patients undergoing chemotherapy giving active nutritional support | |
| Subjects with taste disorder were excluded from the study | |
| Study aims at prevention of taste disorders by administering zinc sulphate | |
| Subjects included in the study were diagnosed to have uremic neuropathy and had decreased nerve conduction velocity (neurological problems) i.e. < 43 to 56 m/sec | |
| Subjects included were healthy older European adults | |
| Subjects included in the study were given glutamine to prevent the docetaxel or paclitaxel associated taste alterations | |
| Subjects included in the study were healthy adolescent girls | |
| Included parotid cancer patients having undergone radiation therapy for a minimum of 54 Gy. Xerostomia could have lead to change in taste perception in such patients | |
| Taste disorders due to local organic damage were included in the study |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised, parallel, double‐blind, placebo control study |
| Participants | 20 to 80 years old, both genders Inclusion criteria: 1) Patients who do not fall in any of the "Exclusion Criteria concerning Diagnosis" and are diagnosed to be suffering from zinc deficient and idiopathic taste disorders (1) The total mean value is 3.5 or higher 3) Patients who have been suffering from taste disorder 1 year or less from the time of their recognition of the onset of taste disorder, at the time of obtaining consents from them Exclusion criteria: Exclusion criteria concerning diagnosis Exclusion criteria concerning the characteristics of subjects 2) Patients taking polaprezinc within 28 days immediately before the first examination of the observation period 3) Patients taking other zinc containing drugs within 28 days immediately before the first examination of the observation period or patients who have taken a zinc containing supplement under the guidance of a physician during the same period 4) Patients who take meals only once a day or so, or who clearly limit food intake with a purpose of reducing body weight 5) Patients having serious cardiac diseases or blood diseases 6) Patients having anemia 7) Patients being treated for mental or nervous disorders 8) Patients being treated for malignant tumours 9) Patients whose stomach, duodenum or small intestines have been excised 10) Patients having a history of serious drug allergies 11) Female subjects who are pregnant, lactating or wish to become pregnant 12) Patients who had participated in a study for taste disorder by Z‐103 in the past 13) Patients who are participating in other studies or have participated in other studies within three months before obtaining a consent 14) Patients who are otherwise judged unfit as a subject for this trial by a principal investigator or investigators participating in this trial Target sample: 150 |
| Interventions | Three arms Placebo group: placebo administration group: 2 packs of 75 mg of granular Z‐103 (as a zinc content, 0 mg/day) Intervention group 1: 150 mg administration group: 1 pack of 75 mg granular Z‐103 and 1 pack of 75 mg of placebo granules (as a zinc content, 33.87 mg/day) Intervention group 2: 300 mg administration group: 2 packs of 75 mg granular Z‐103 (as a zinc content, 67.74 mg/day) |
| Outcomes | Primary outcome: final judgement of the effects by filter paper disk method examination Secondary outcome: judgement of effects of each evaluation period by filter paper disk method examination |
| Notes | Unpublished trial Contact author: Akinori Kida and Zeria Pharmaceutical Pvt Ltd. Company person contacted via email on 27th October, 2013 and email bounced. Have contacted another person from same company, Tadahiro‐Ooshiro on 21st November 2013 ‐ Received reply on 26th November 2013: Refused to share any details of the study |
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | No details available. Co‐author Ananda Prasad contacted for the full text on 7th November 2013. Reply received on 17th December and he was unable to find the same |
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | No details available from British Medical Library |
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | No details available from British Medical Library |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Z‐103 Phase III clinical study in patients with taste disorder ‐ A placebo‐controlled superiority study ID ‐ 1 |
| Methods | Randomised, multicentre, double‐blind, placebo‐controlled, parallel‐group study |
| Participants | Sample size: 260 Inclusion criteria: Patients diagnosed with the following three types. Exclusion criteria: 1. Central nervous system disorder |
| Interventions | Z‐103 (Classification name/code of the intervention: 322 (mineral preparations)) and placebo Oral administration of one tablet twice a day after meal |
| Outcomes | Primary outcome: final overall efficacy evaluation Timepoints: filter paper disk method Secondary outcome: efficacy evaluation at each evaluation period Timepoints: filter paper disk method |
| Starting date | 01‐06‐2012 |
| Contact information | Zeria Pharmaceutical Co., Ltd., R&D planning division, Phone: +81‐3‐5644‐7053 |
| Notes | Expected to be complete by 30‐09‐2014 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Taste acuity improvement ‐ Patient reported outcome Show forest plot | 2 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.00, 2.10] |
| Analysis 1.1  Comparison 1 Zinc verses placebo, Outcome 1 Taste acuity improvement ‐ Patient reported outcome. | ||||
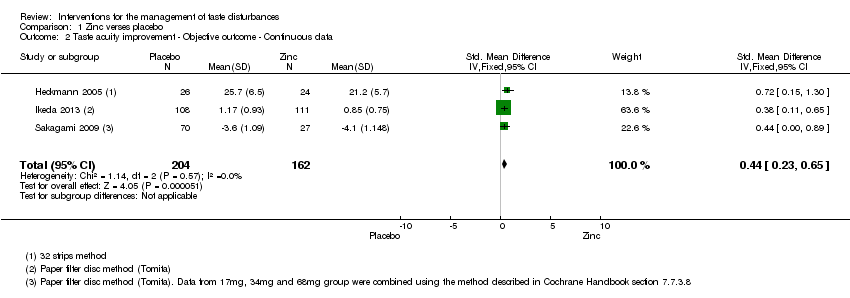
| 2 Taste acuity improvement ‐ Objective outcome ‐ Continuous data Show forest plot | 3 | 366 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.44 [0.23, 0.65] |
| Analysis 1.2  Comparison 1 Zinc verses placebo, Outcome 2 Taste acuity improvement ‐ Objective outcome ‐ Continuous data. | ||||
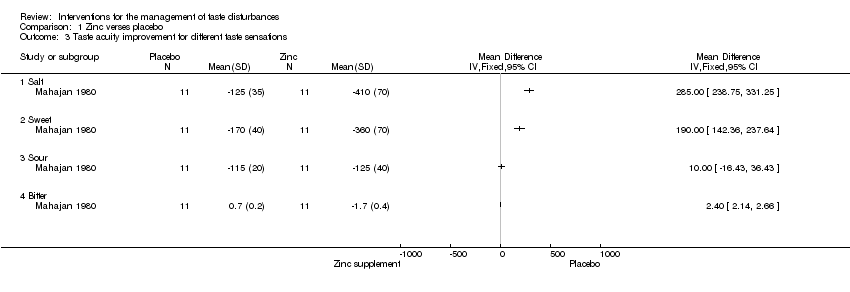
| 3 Taste acuity improvement for different taste sensations Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3 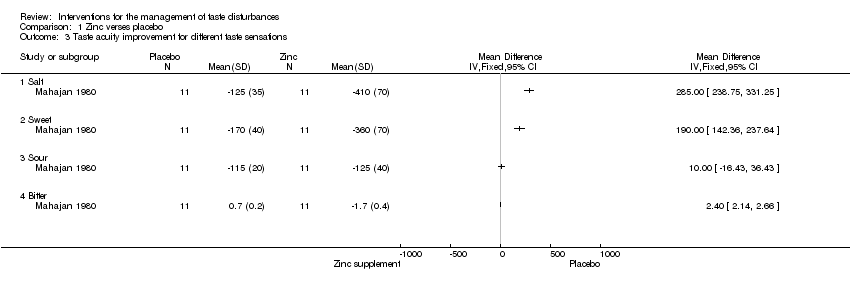 Comparison 1 Zinc verses placebo, Outcome 3 Taste acuity improvement for different taste sensations. | ||||
| 3.1 Salt | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Sweet | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Sour | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Bitter | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Cross‐over study Show forest plot | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 3.00 [0.66, 5.34] |
| Analysis 1.4  Comparison 1 Zinc verses placebo, Outcome 4 Cross‐over study. | ||||
| 5 Taste acuity improvement ‐ Objective outcome ‐ Dichotomous Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Zinc verses placebo, Outcome 5 Taste acuity improvement ‐ Objective outcome ‐ Dichotomous. | ||||
| 5.1 Idiopathic and zinc deficient taste disorders | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [1.13, 2.56] |
| 5.2 Taste disorder secondary to chronic renal failure | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 25.0 [1.65, 379.57] |
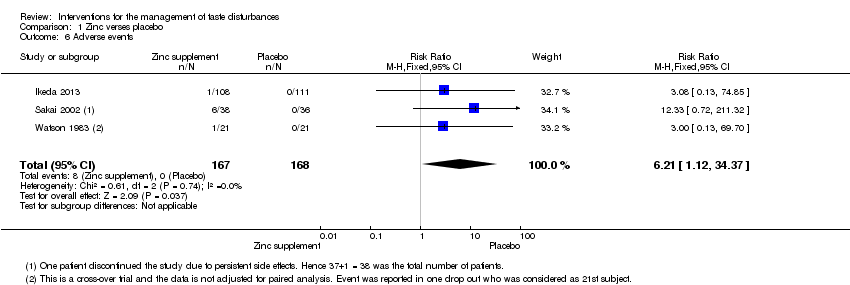
| 6 Adverse events Show forest plot | 3 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.21 [1.12, 34.37] |
| Analysis 1.6 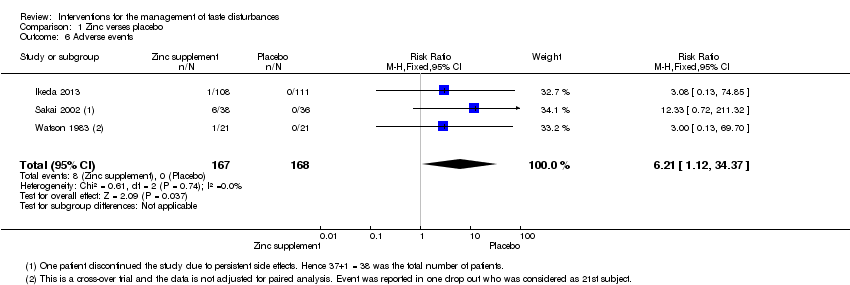 Comparison 1 Zinc verses placebo, Outcome 6 Adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
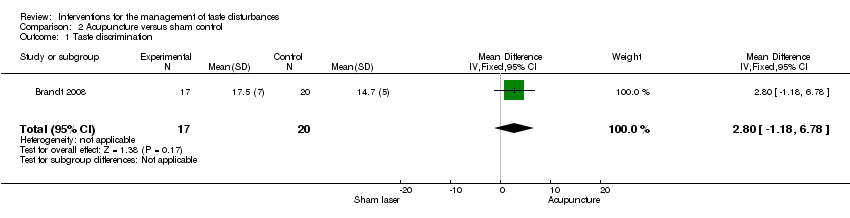
| 1 Taste discrimination Show forest plot | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 2.80 [‐1.18, 6.78] |
| Analysis 2.1  Comparison 2 Acupuncture versus sham control, Outcome 1 Taste discrimination. | ||||
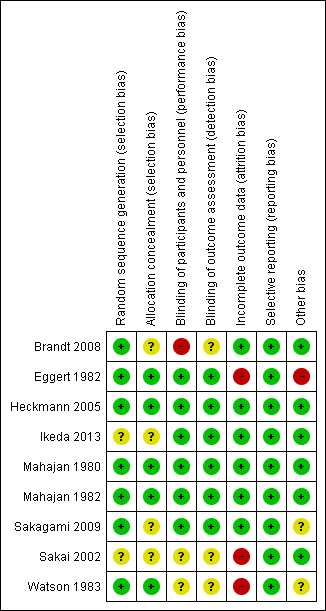
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Study flow diagram.
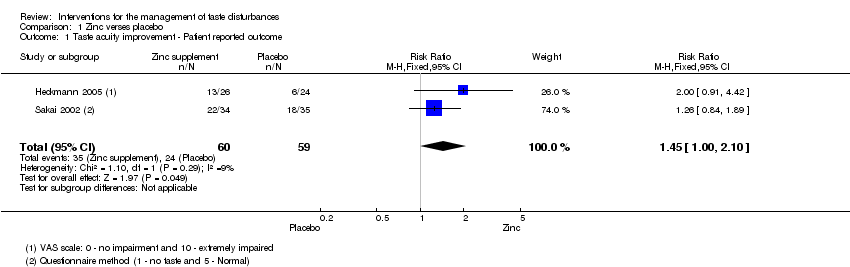
Comparison 1 Zinc verses placebo, Outcome 1 Taste acuity improvement ‐ Patient reported outcome.
Comparison 1 Zinc verses placebo, Outcome 2 Taste acuity improvement ‐ Objective outcome ‐ Continuous data.
Comparison 1 Zinc verses placebo, Outcome 3 Taste acuity improvement for different taste sensations.

Comparison 1 Zinc verses placebo, Outcome 4 Cross‐over study.

Comparison 1 Zinc verses placebo, Outcome 5 Taste acuity improvement ‐ Objective outcome ‐ Dichotomous.
Comparison 1 Zinc verses placebo, Outcome 6 Adverse events.
Comparison 2 Acupuncture versus sham control, Outcome 1 Taste discrimination.
| Zinc compared to placebo for the management of taste disturbances | ||||||
| Patient or population: patients with taste disturbances | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Zinc | |||||
| Taste acuity improvement ‐ Patient reported outcome | Study population | RR 1.45 | 119 | ⊕⊝⊝⊝ | ─ | |
| ˗407 per 1000 | 590 per 1000 | |||||
| Moderate | ||||||
| 382 per 1000 | 554 per 1000 | |||||
| Taste acuity improvement ‐ Objective outcome ‐ Continuous ‐ Overall taste improvement | ─ | The mean taste acuity improvement ‐ objective outcome ‐ continuous ‐ overall taste improvement in the intervention groups was | ─ | 366 | ⊕⊕⊕⊝ | SMD 0.44 (0.23 to 0.65) |
| Taste acuity improvement ‐ Objective outcome ‐ Continuous ‐ Taste recognition | — | The mean taste acuity improvement ‐ objective outcome ‐ continuous ‐ taste recognition in the intervention groups was | ─ | 14 | ⊕⊝⊝⊝ | Standardised mean difference (SMD) 1.26 (0.07 to 2.44) |
| Taste acuity improvement ‐ Objective outcome ‐ Dichotomous ‐ Idiopathic and zinc deficient taste disorders | Study population | RR 1.7 | 73 | ⊕⊕⊝⊝ | ─ | |
| ─444 per 1000 | 756 per 1000 | |||||
| Moderate | ||||||
| 444 per 1000 | 755 per 1000 | |||||
| Adverse events | Study population7 | RR 6.21 | 335 | ─ | ─ | |
| 1 per 1000 | 6 per 1000 | |||||
| Moderate | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: unclear randomisation and blinding and high risk of bias due to attrition in Sakai 2002. Downgraded by 1 level. 7Risk of one per 1000 assumed in placebo group (as it was zero). 8Sakagami 2009 was not included in the grading of evidence as the number of patients reporting adverse events was not reported. | ||||||
| Acupuncture compared to sham control for patients with taste disturbances | ||||||
| Patient or population: patients with taste disturbances | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Sham control | Acupuncture | |||||
| Taste discrimination | ─ | The mean taste discrimination in the intervention groups was | ─ | 37 | ⊕⊕⊝⊝ | ─ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Single‐blinded study. | ||||||
| Outcome | Gp A | Gp B | Time when measured | ||||
| Mean* | S.D. | n= | Mean* | S.D. | n= | ||
| Change of the mean 4 basic taste sensitivity scores from baseline | ‐0.52 | 0.68 | 108 | ‐0.47 | 0.61 | 111 | 4 weeks |
| ‐0.90 | 0.85 | 108 | ‐0.67 | 0.73 | 111 | 8 weeks | |
| ‐1.17 | 0.93 | 108 | ‐0.85 | 0.75 | 111 | 12 weeks | |
| ‐1.28 | 0.94 | 108 | ‐0.97 | 0.76 | 111 | 4 weeks after treatment | |
| *Minus change score means better by filter paper disc method by Tomita | |||||||
| Outcome | Gp A events (Improved) | Gp A total | Gp B events | Gp B total | Time when measured |
| Improved/not improved | 60 | 108 | 48 | 111 | 12 weeks |
| Outcome | Gp A (Placebo) N = 27 | Gp B (17 mg zinc) N = 27 | Gp C (34 mg zinc) N = 25 | Gp D (68 mg zinc) N = 28 | Time when measured | ||||
| Secondary outcome | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | 12 weeks |
| Mean filter paper disk test scores (filter paper disk) | 4.095 | 1.148 | 4.350 | 1.030 | 3.448 | 0.928 | 3.454 | 1.138 | ─ |
| Mean serum zinc level | 1.8 | 12.7 | 5.7 | 13.5 | 11.4 | 16.6 | 20.6 | 21.3 | ─ |
| Gp A | Gp B | Gp C | Gp D | ─ | |||||
| Increase in the average score of subjective symptoms | 0.6 | 0.9 | 1.2 | 1.0 | ─ | ||||
| Primary outcome: quantitative analysis of taste perception using filter paper disk method | Event (success) Cured + improved | No event (fail) Unchanged, neither cured nor improved nor worsened; aggravated | Total |
| Experimental intervention (17 mg Zinc) | SE= 14 | FE= 13 | NE= 27 |
| Control intervention (Placebo) | SC= 17 | FC= 10 | NC= 27 |
| RR = 0.824; OR = 0.634; RD = 0.447 | |||
| Experimental intervention (34 mg Zinc) | SE= 20 | FE= 5 | NE= 25 |
| Control intervention (Placebo) | SC= 17 | FC= 10 | NC= 27 |
| RR = 0.318; OR = 2.353; RD = 0.17 | |||
| Experimental intervention (68 mg Zinc) | SE= 25 | FE= 3 | NE= 28 |
| Control intervention (Placebo) | SC= 17 | FC= 10 | NC= 27 |
| RR = 1.418; OR = 4.902; RD = 0.263 | |||
| RR = risk ratio: risk of event in experimental group/risk of event in control group. OR = odds ratio: odds of event in experimental group/ odds of event in control group. RD = risk difference: risk of event in experimental group – risk of event in control group. | |||
| Filter paper disk method | Event (success) Improved (+cured) | No event (fail) Unchanged | Total (N = 73) |
| Experimental intervention (Zinc picolinate) | SE= 28 | FE= 9 | NE= 37 |
| Control intervention (Placebo) | SC= 16 | FC= 20 | NC= 36 |
| RR = 1.703; OR = 3.889; RD = 0.312 | |||
| Experimental intervention (Zinc picolinate) | SE= 22 | FE= 12 | NE= 34 |
| Control intervention (Placebo) | SC= 18 | FC= 17 | NC= 35 |
| RR = 1.258 ; OR = 1.732; RD = 0.133 | |||
| RR = risk ratio: risk of event in experimental group/risk of event in control group. OR = odds ratio: odds of event in experimental group/ odds of event in control group. RD = risk difference: risk of event in experimental group – risk of event in control group. | |||
| Outcome | Gp A (Zinc treatment) | Gp B (Placebo) | Time when measured | ||||
| Mean | Std Dev | N= | Mean | Std Dev | N= | At the end of 3 months | |
| Primary outcome | ─ | ||||||
| Taste test (32 filter paper strip method by Muller et al 2003) | 25.7 | 6.5 | 26 | 21.2 | 5.7 | 24 | ─ |
| Self rated impairment in % (VAS scale of 10 cm length equivalent to 100%; 0 to 10. 0 = no impairment and 10 = extremely impaired) | 45.0 | 4.4 | 26 | 43.8 | 3.6 | 24 | ─ |
| Secondary outcome | ─ | ||||||
| Beck Depression Inventory (BDI) | 7.5 | 7.0 | 26 | 11.3 | 10.9 | 24 | ─ |
| von Zersen Mood Scale (ZMS) | 10.7 | 7.5 | 26 | 18.8 | 14.6 | 24 | ─ |
| Zinc in serum (microgram/dL) | 81.53 | 19.61 | 26 | 72.01 | 10.22 | 24 | ─ |
| Type of Intervention | Event (success) Improved | No event (fail) | Total |
| Experimental intervention (Zinc) | SE= 13 | FE= 13 | NE= 26 |
| Control intervention | SC= 6 | FC= 18 | NC= 24 |
| RR = 2; OR = 3; RD = 0.25 | |||
| RR = risk ratio: risk of event in experimental group/risk of event in control group. OR = odds ratio: odds of event in experimental group/ odds of event in control group. RD = risk difference: risk of event in experimental group – risk of event in control group. | |||
| Outcome | Gp A | Gp B | Time when measured | ||||||
| Mean | Std Dev* | N=17 | Mean | Std Dev* | N=20 | ||||
| Taste discrimination | 11.7 (before)/ 17.5 (after) | 4 (before)/ | ─ | 11.9 (before)/ 14.7(after) | 5 (before)/ | ─ | Before and after treatment | ||
| Quality of life | Not estimable (changes per group only given for each of the 5 individual questions of the questionnaire, but no combined score/analysis stated) Only information given: ‘both treatments resulted in an increased quality of life, however, no statistically significant difference could be found’ | Before and after treatment | |||||||
| Depressive symptoms | 11 (before)/ 6 (after)* | 5 (before) / 4 (after)* | ─ | 10,5 (before)/ 10 (after)* | 7 (before)/ 7 (after)* | ─ | Before and after treatment | ||
| “The psychological well‐being of the intervention groups increased for 94,1% of all patients in the intervention group, but only for 60% of patients in the control group. This difference was statistically significant” | |||||||||
| Subjective well‐being | 16 (before)/ 12 (after)* | 10 (before)/ 7 (after)* | ─ | 20 (before)/ 18 (after)* | 9 (before)/ 14 (after)* | ─ | Before and after treatment | ||
| “58.8% of all patients in the intervention group felt better, whereas only 45% of all patients in the control group felt better. This difference was not statistically significant” | |||||||||
| *Only given in graph ‐> estimated from graph | |||||||||
| Outcome | Gp A – Zinc picolinate events | Gp A total | Gp B – Placebo Events | Gp B total | Time when measured |
| Adverse events | 6 | 37 | 0 | 36 | 3 months |
| Outcome | Gp A ‐ 17 mg Zinc events | Gp A total | Gp B – 34 mg zinc Events | Gp B total | Gp C – 68 mg zinc events | Gp C total | Gp D ‐ Placebo events | Gp D total | Time when measured |
| Side effects | 5 | 27 | 6 | 25 | 7 | 28 | 3 | 27 | 12 weeks |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Taste acuity improvement ‐ Patient reported outcome Show forest plot | 2 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.00, 2.10] |
| 2 Taste acuity improvement ‐ Objective outcome ‐ Continuous data Show forest plot | 3 | 366 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.44 [0.23, 0.65] |
| 3 Taste acuity improvement for different taste sensations Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Salt | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Sweet | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Sour | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Bitter | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Cross‐over study Show forest plot | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 3.00 [0.66, 5.34] |
| 5 Taste acuity improvement ‐ Objective outcome ‐ Dichotomous Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Idiopathic and zinc deficient taste disorders | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [1.13, 2.56] |
| 5.2 Taste disorder secondary to chronic renal failure | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 25.0 [1.65, 379.57] |
| 6 Adverse events Show forest plot | 3 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.21 [1.12, 34.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Taste discrimination Show forest plot | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 2.80 [‐1.18, 6.78] |

