Transfusión sanguínea profiláctica versus selectiva para la anemia falciforme del embarazo
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010378.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 22 diciembre 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Embarazo y parto
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
BO Okusanya and OT Oladapo independently assessed trials for inclusion and extracted data from the included study. BO Okusanya prepared the first draft of the review and has overall responsibility for maintaining the review. OT Oladapo revised and contributed to the final draft of the review.
Sources of support
Internal sources
-
UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research, World Health Organization, Geneva, Switzerland.
External sources
-
No sources of support supplied
Declarations of interest
Babasola O Okusanya: none known.
Olufemi T Oladapo: none known.
Acknowledgements
The contact author acknowledges the assistance of Reviews for Africa Programme (RAP) Nigeria run by the Nigerian Branch of the South African Cochrane Centre towards the completion of the first version of this review (Okusanya 2013b). RAP‐Nigeria was funded by a grant from the Department for International Development (DFID) through the Research Programme Consortium at the Liverpool School of Tropical Medicine.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
The World Health Organization and BO Okusanya retain copyright and all other rights in their respective contributions to the manuscript of this review as submitted for publication.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Dec 22 | Prophylactic versus selective blood transfusion for sickle cell disease in pregnancy | Review | Babasola O Okusanya, Olufemi T Oladapo | |
| 2013 Dec 03 | Prophylactic versus selective blood transfusion for sickle cell disease in pregnancy | Review | Babasola O Okusanya, Olufemi T Oladapo | |
| 2013 Feb 28 | Prophylactic versus selective blood transfusion for sickle cell disease in pregnancy | Protocol | Babasola O Okusanya, Olufemi T Oladapo | |
Differences between protocol and review
The frequency of sickle cell crises (vaso‐occlusive, sequestration or haemolytic) listed as a secondary outcome in the protocol was amended to the number of women experiencing the individual types of sickle cell crisis in the review. This is because the included trial (Koshy 1988) separately reported the number of women experiencing pain, sequestration and haemolytic crises and it was impossible to derive the number of women experiencing 'any sickle cell crisis' from the reported data. In future updates when sufficient data become available, both the frequency of sickle cell crises and the number of women experiencing each of them will be reported in the review.
The last published version of this review (Okusanya 2013b) included two studies (Koshy 1987; Koshy 1988). However, after careful scrutiny, it appears that these two studies are in fact reporting on a single trial (Koshy 1988) and so the current update now only includes one trial (Koshy 1988).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Female; Humans; Pregnancy;
PICO

Study flow diagram.
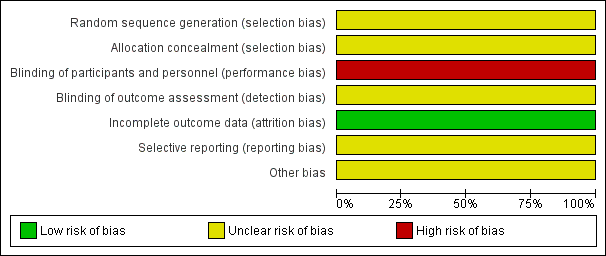
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
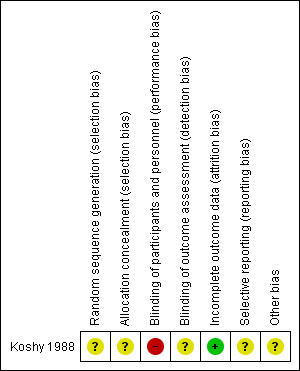
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Prophylactic versus selective blood transfusion, Outcome 1 Maternal death.

Comparison 1 Prophylactic versus selective blood transfusion, Outcome 2 Severe maternal morbidity (pulmonary embolism).
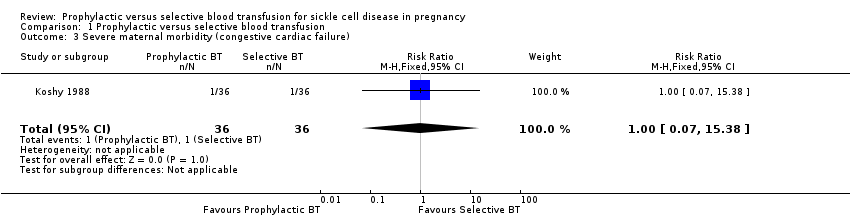
Comparison 1 Prophylactic versus selective blood transfusion, Outcome 3 Severe maternal morbidity (congestive cardiac failure).

Comparison 1 Prophylactic versus selective blood transfusion, Outcome 4 Severe maternal morbidity (acute chest syndrome).
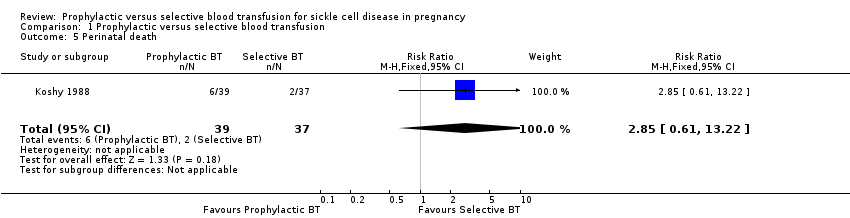
Comparison 1 Prophylactic versus selective blood transfusion, Outcome 5 Perinatal death.

Comparison 1 Prophylactic versus selective blood transfusion, Outcome 6 Sickle cell crisis (pain crisis).

Comparison 1 Prophylactic versus selective blood transfusion, Outcome 7 Sickle cell crises (acute splenic sequestration).

Comparison 1 Prophylactic versus selective blood transfusion, Outcome 8 Sickle cell crisis (haemolysis).

Comparison 1 Prophylactic versus selective blood transfusion, Outcome 9 Blood transfusion reaction.
| Prophylactic versus selective blood transfusion for sickle cell disease in pregnancy | ||||||
| Patient or population: patients with sickle cell disease in pregnancy Control: selective blood transfusion | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prophylactic versus selective blood transfusion | |||||
| Maternal death | See comment | See comment | Not estimable | 72 | ⊕⊝⊝⊝ | No woman in this study died. |
| Severe maternal morbidity (pulmonary embolism) | See comment | See comment | Not estimable | 72 | ⊕⊝⊝⊝ | No woman in this study experienced pulmonary embolism. |
| Severe maternal morbidity (congestive cardiac failure) | 28 per 1000 | 28 per 1000 | RR 1.00 | 72 | ⊕⊝⊝⊝ | |
| Perinatal death | 54 per 1000 | 154 per 1000 | RR 2.85 | 76 | ⊕⊝⊝⊝ | |
| Sickle cell crisis (pain crisis) | 500 per 1000 | 140 per 1000 | RR 0.28 | 72 | ⊕⊕⊝⊝ | |
| Sickle cell crises (acute splenic sequestration) | 28 per 1000 | 9 per 1000 | RR 0.33 | 72 | ⊕⊕⊝⊝ | |
| Blood transfusion reaction | 83 per 1000 | 167 per 1000 | RR 2.00 | 72 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The only study was at uncertain risk of bias due to unclear methods of random sequence generation and allocation concealment, and lack of blinding. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal death Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Severe maternal morbidity (pulmonary embolism) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Severe maternal morbidity (congestive cardiac failure) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.38] |
| 4 Severe maternal morbidity (acute chest syndrome) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.75] |
| 5 Perinatal death Show forest plot | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.61, 13.22] |
| 6 Sickle cell crisis (pain crisis) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.12, 0.67] |
| 7 Sickle cell crises (acute splenic sequestration) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.92] |
| 8 Sickle cell crisis (haemolysis) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.06] |
| 9 Blood transfusion reaction Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.54, 7.39] |

