Transfusión sanguínea profiláctica versus selectiva para la anemia falciforme del embarazo
Resumen
Antecedentes
Las embarazadas con anemia falciforme (HbSS, HbSC y HbSβThal) pueden necesitar transfusión sanguínea para prevenir la anemia grave o tratar las complicaciones médicas potenciales. La transfusión sanguínea preventiva antes de que ocurran las complicaciones que comienzan desde las primeras semanas de embarazo o la transfusión sanguínea solamente por indicaciones médicas u obstétricas se han utilizado como políticas de tratamiento. Actualmente no hay consenso sobre una política de transfusión sanguínea que garantice efectos clínicos beneficiosos óptimos con riesgos mínimos en dichas pacientes y sus fetos. Ésta es una actualización de una revisión Cochrane publicada en el 2013.
Objetivos
Evaluar los efectos beneficiosos y perjudiciales de una política de transfusión sanguínea profiláctica versus selectiva en embarazadas con anemia falciforme.
Métodos de búsqueda
Se hicieron búsquedas en el registro de ensayos del Grupo Cochrane de Embarazo y Parto (Cochrane Pregnancy and Childbirth Group) (30 de mayo 2016) y en las listas de referencias de los estudios recuperados. No se aplicó ninguna restricción de idioma o de fecha.
Criterios de selección
Ensayos controlados que evaluaron los efectos de la transfusión sanguínea profiláctica versus selectiva (urgencia) en embarazadas con anemia falciforme. Se consideraba la inclusión de ensayos cuasialeatorizados y los que usaran un diseño aleatorizado grupal, pero no se identificó ninguno.
Obtención y análisis de los datos
Dos autores de la revisión de forma independiente evaluaron los ensayos para la inclusión y el riesgo de sesgo, extrajeron los datos y verificaron su exactitud. Dos autores de la revisión evaluaron la calidad de la evidencia mediante los criterios GRADE de forma independiente.
Resultados principales
De seis informes relevantes identificados mediante la estrategia de búsqueda, un ensayo que incluyeron a 72 pacientes con anemia de células falciformes (HbSS) cumplieron con los criterios de inclusión. El ensayo presentó un riesgo de sesgo incierto. En general hubo pocos eventos en la mayoría de los resultados informados y generalmente los resultados no fueron precisos. El ensayo incluido no informó que ocurriera mortalidad materna en las pacientes que recibieron transfusión sanguínea profiláctica o selectiva. Evidencia de calidad muy baja no indicó diferencias claras en la mortalidad materna, la mortalidad perinatal (riesgo relativo [RR] 2,85; intervalo de confianza [IC] del 95%: 0,61 a 13,22; evidencia de calidad muy baja) ni marcadores de morbilidad materna grave (embolia pulmonar [sin eventos]; insuficiencia cardíaca congestiva [RR 1,00, IC del 95%: 0,07 a 15,38; evidencia de muy baja calidad); síndrome torácico agudo[RR 0,67; IC del 95%: 0,12 a 3,75]) entre los grupos de tratamiento (transfusión sanguínea profiláctica versus transfusión sanguínea selectiva). La evidencia de baja calidad indicó que la transfusión sanguínea profiláctica redujo el riesgo de crisis de dolor en comparación con la transfusión sanguínea selectiva (RR 0,28; IC del 95%: 0,12 a 0,67; un ensayo, 72 mujeres; evidencia de baja calidad), y no hubo diferencias en la aparición de secuestro esplénico agudo (RR 0.33, IC del 95%: 0,01 a 7,92; evidencia de calidad baja), crisis hemolíticas (RR 0,33, IC del 95%: 0,04 a 3,06) ni en la reacción retardada a la transfusión de sangre (RR 2,00, IC del 95%: 0,54 a 7,39; evidencia de calidad muy baja) entre los grupos de comparación.
En el ensayo no se informaron otros resultados maternos pertinentes preespecificados para esta revisión, como la duración acumulada de la estancia hospitalaria, la hemorragia posparto y la sobrecarga de hierro, ni los resultados del lactante, el ingreso en la unidad de cuidados intensivos neonatales (UCIN) ni la enfermedad hemolítica del recién nacido.
Conclusiones de los autores
La evidencia de un ensayo pequeños de calidad muy baja indica que la transfusión sanguínea profiláctica a las embarazadas con anemia de células falciformes (HbSS) no proporciona efectos clínicos beneficiosos claros en comparación con la transfusión selectiva. Actualmente no hay evidencia de ensayos aleatorizados o cuasialeatorizados para proporcionar asesoramiento confiable sobre la política de transfusión sanguínea óptima en las pacientes con otras variantes de anemia falciforme (es decir, HbSC y HbSβThal). Los datos disponibles y la calidad de la evidencia sobre este tema no son suficientes para abogar por un cambio en la práctica clínica y la política existente.
PICOs
Resumen en términos sencillos
Políticas de transfusión sanguínea para la anemia falciforme en el embarazo
¿Cuál es el problema?
La anemia falciforme es un trastorno hereditario de la hemoglobina, la proteína en los eritrocitos que transporta el oxígeno. En esta afección, una hemoglobina S anormal de la madre se combina con la hemoglobina anormal del padre. La hemoglobina S heredada de ambos padres (genotipo HbSS), descrita como anemia de células falciformes, es la forma más frecuente.
¿Por qué es esto importante?
Cuando la tensión de oxígeno es baja, la hemoglobina S cristaliza y hace que los eritrocitos se deformen en forma de "hoz". La deformación en "hoz" de los eritrocitos reduce la capacidad de los eritrocitos de pasar a través de los vasos sanguíneos muy pequeños, lo que causa bloqueo vascular y destrucción temprana de los eritrocitos. La rotura de los eritrocitos y la acumulación masiva de eritrocitos dañados en el hígado y el vaso causan la anemia. La enfermedad aguda incluye crisis dolorosas, embolia pulmonar, síndrome torácico agudo e insuficiencia cardíaca congestiva. Por lo tanto, las embarazadas con anemia falciforme requieren un tratamiento cuidadoso.
Según la política institucional, a la embarazada con HbSS con relativamente pocos o ningún síntoma se le pueden administrar transfusiones sanguíneas a intervalos para mejorar la capacidad de transportar el oxígeno de la sangre al aumentar la concentración sanguínea de hemoglobina y disminuir los niveles de hemoglobina S;o solamente cuando esté indicado por el desarrollo de complicaciones médicas o del embarazo. Transfundir sangre a intervalos frecuentes conlleva riesgo de infecciones transmitidas por la sangre y niveles excesivos de hierro.
Esta revisión tuvo como objetivo determinar si transfundir sangre a intervalos antes de que ocurran complicaciones graves en comparación con transfundir sangre solamente cuando está médicamente indicado logra un cambio en la salud de la madre y su feto.
¿Qué evidencia se encontró?
Se buscó evidencia el 30 de mayo de 2016 y se identificó un ensayo controlado con riesgo de sesgo poco claro que asignaron al azar a 72 mujeres con anemia de células falciformes (hemoglobina SS) antes de las 28 semanas de gestación a una de las dos políticas de transfusión sanguínea. El ensayo no indicó diferencias en la enfermedad grave ni la muerte de la madre o el feto. No hubo diferencias en el riesgo de reacción retardada a la transfusión sanguínea. Los ensayos indicaron que transfundir sangre a intervalos frecuentes reduce el riesgo de crisis de dolor, con un grado grande de incertidumbre acerca del tamaño del efecto, en comparación con transfundir sangre solamente cuando está médicamente indicado. La transfusión sanguínea se administró a una proporción de cuatro a una para la transfusión sanguínea profiláctica versus selectiva, respectivamente. En general, la calidad de la evidencia es muy baja para resultados que son importantes para las mujeres.
¿Qué significa esto?
La evidencia disponible sobre este tema no son suficientes para abogar por un cambio en la política y la práctica clínicas. Se deben realizar más investigaciones.
Authors' conclusions
Summary of findings
| Prophylactic versus selective blood transfusion for sickle cell disease in pregnancy | ||||||
| Patient or population: patients with sickle cell disease in pregnancy Control: selective blood transfusion | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prophylactic versus selective blood transfusion | |||||
| Maternal death | See comment | See comment | Not estimable | 72 | ⊕⊝⊝⊝ | No woman in this study died. |
| Severe maternal morbidity (pulmonary embolism) | See comment | See comment | Not estimable | 72 | ⊕⊝⊝⊝ | No woman in this study experienced pulmonary embolism. |
| Severe maternal morbidity (congestive cardiac failure) | 28 per 1000 | 28 per 1000 | RR 1.00 | 72 | ⊕⊝⊝⊝ | |
| Perinatal death | 54 per 1000 | 154 per 1000 | RR 2.85 | 76 | ⊕⊝⊝⊝ | |
| Sickle cell crisis (pain crisis) | 500 per 1000 | 140 per 1000 | RR 0.28 | 72 | ⊕⊕⊝⊝ | |
| Sickle cell crises (acute splenic sequestration) | 28 per 1000 | 9 per 1000 | RR 0.33 | 72 | ⊕⊕⊝⊝ | |
| Blood transfusion reaction | 83 per 1000 | 167 per 1000 | RR 2.00 | 72 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The only study was at uncertain risk of bias due to unclear methods of random sequence generation and allocation concealment, and lack of blinding. | ||||||
Background
Sickle cell disease (SCD) is an inherited disorder of haemoglobin S synthesis. The genetic defects of haemoglobin (Hb) are the most common genetic disorders worldwide and homozygous sickle cell anaemia (HbSS) is the most frequent (Hoffbrand 2011).
Description of the condition
The inherited disorders of haemoglobin S are either in the homozygous form (HbSS) or in combination with another Hb variant such as haemoglobin C (HbSC), haemoglobin D (HbSD) or β‐thalassaemia (HbSβo‐thalassaemia and HbSβ+‐thalassaemia). The homozygous form, HbSS (sickle cell anaemia), is the most common followed by HbSC and the HbSβ‐thalassaemias (Hoffbrand 2011).
SCD is a relatively prevalent condition in Africa, the Mediterranean and the Caribbean; about 10% of the Jamaican population is estimated to carry HbS. However, the disease prevalence now has a more global outlook as a result of immigration (RCOG 2011). Population estimates in the USA indicated that 72,000 to 98,000 people have SCD (Hassel 2010). Similarly, the UK has the largest population of people with SCD in Europe, a population that increased from approximately 5000 in 1995 (Howard 1995) to about 12,000 to 15,000 in 2011 (RCOG 2011).
Pregnancy in women with SCD carries significant risks of maternal and perinatal morbidity and mortality, particularly in resource‐poor settings where facilities are lacking to adequately manage associated complications (Afolabi 2009; Odum 2002). Complications that may arise include various forms of crises resulting from haemolysis(destruction of red blood cells), vascular occlusion and sequestration (massive pooling) of damaged red blood cells in the liver and spleen, all having the potential to cause profound anaemia (Hoffbrand 2011). Another recognised complication, and an important cause of death in women with SCD, particularly in women with sickle cell anaemia (HbSS) is acute chest syndrome (ACS), which is characterised by cough, chest pain, dyspnoea (laboured breathing), fever, increasing anaemia and fluid infiltrate on chest X‐ray, all resulting from the sickling of red cells in the lungs. ACS is the most common cause of death in patients with SCD after puberty (Hoffbrand 2011), and it occurs in 7% to 20% of pregnant women with SCD (RCOG 2011). While all these complications are not specific to pregnancy in women with SCD, they are more frequent and are exacerbated during pregnancy and are all major causes of severe ill health and death among women with the condition. Although the complications could arise in all forms of SCD, they are more frequent and severe among those with sickle cell anaemia (HbSS) (Nomura 2009; Odum 2002; RCOG 2011).
Pregnancy‐specific complications include increased risk of spontaneous abortion (Serjeant 2004), urinary tract infection (Howard 1995), pre‐eclampsia and thromboembolism (RCOG 2011). Pregnant women with SCD have an increased risk of preterm birth, repeated antepartum hospitalisations, placenta abruption, induction of labour, caesarean section and postpartum sepsis (ACOG 2007; Asnani 2011; Barfield 2010). As a result of chronic anaemia, women with SCD are more likely to require blood transfusions (Grossetti 2009) and subsequent alloimmunisation of red cells which also increases the risk of haemolytic disease of the newborn. Fetal complications include intrauterine growth restriction, prematurity and its sequelae, low birthweight and death (ACOG 2007; Barfield 2010; Howard 1995).
Painful crisis secondary to vascular occlusion is the most frequent manifestation of SCD, which is often precipitated by conditions such as infection, stress, acidosis, dehydration and hypoxia (low oxygenation state) (Hoffbrand 2011). Furthermore, visceral sequestration crises as a result of pooling of blood within the reticuloendothelial system (liver and spleen), as well as haemolytic and aplastic crises, are all associated with worsening anaemia (Hoffbrand 2011) that often have to be corrected to avert severe morbidity and mortality.
In spite of these complications, successful pregnancy outcomes have been reported in up to 57% of women with HbSS and 85% of women with HbSC (Asnani 2011; Serjeant 2004). These outcomes have been achieved with interventions to improve the complications occurring in these women. Measures to improve pregnancy outcomes have included the use of analgesia, intravenous fluid replacement, antibiotics and packed red cell transfusion for treatment of vaso‐occlusive complications (ACOG 2007; RCOG 2011). However, there is no compelling evidence from randomised trials that regimens comprising the various combination of these interventions actually improve pregnancy outcomes (Marti‐Carvajal 2009).
Description of the intervention
Pregnancy in women with SCD requires management by a multi‐disciplinary team of haematologists, obstetricians, anaesthetists and physicians to reduce the risk of likely complications (Boga 2016). Although longitudinal follow‐up studies of women with HbSC have reported a relatively benign course of pregnancy (Serjeant 2005), complications occurred in 96.6% of pregnant women with HbSS (Odum 2002). While pregnant women with HbSβ+‐thalassaemia share similarly mild clinical behaviours with those with HbSC, the clinical course of pregnancy in HbSβo‐thalassaemia is quite similar to women with sickle cell anaemia (Hoffbrand 2011).
Blood transfusion for women with SCD during pregnancy could either be selective or prophylactic. Selective blood transfusion is performed when conditions such as anaemia, pain crises, or ACS necessitates blood transfusion. Other indications for selective blood transfusion are malaria infection and sepsis as these may lead to haemolysis of red cells. On the other hand, prophylactic blood transfusion is performed with the aim of optimising the oxygen‐carrying capacity of the blood and reducing complications related to sickled red cells and anaemia in an asymptomatic pregnant woman with SCD. Prophylactic blood transfusion is often started early in pregnancy and performed at intervals to reduce the chances of transfusion on an emergency basis. Whenever blood is transfused, it is aimed at reducing the proportion of HbS in the circulation to less than 40% and also to achieve an Hb concentration of 10 g/dL (ACOG 2007; Grossetti 2009).
Prophylactic blood transfusion can either be simple "top‐up" (transfusion without prior withdrawal of blood from the recipient) or exchange blood transfusion (Howard 1995). Prophylactic exchange blood transfusion was first proposed by Ricks in 1965 (ACOG 2007). He recommended exchange blood transfusion four to six weeks before the delivery date to optimise the woman's Hb level towards the end of pregnancy when complications are most frequent (ACOG 2007). Prophylactic transfusion reduces the risk of sickling by reducing maternal erythropoiesis and thereby, increasing the partial O2 pressure (Grossetti 2009). It has the advantage of avoiding the risk of alloimmunisation from transfusion of inadequately phenotyped red blood cells and the transfusion‐related reaction or overload that could occur with emergency transfusion (Grossetti 2009).
How the intervention might work
Depending on the institutional policy, prophylactic blood transfusion could be started during the first, second or beginning of the third trimester of pregnancy (Grossetti 2009; Howard 1995; Ngo 2010). When it commences during the third trimester of pregnancy, it preferably begins at 28 weeks (Gilli 2007) and repeated every two to four weeks until delivery. This intervention carries the risks of iron overload (from the woman's inability to adequately excrete iron released from sickled and dead red blood cells), blood‐related infections and alloimmunisation (due to exposure to multiple sources of allogeneic blood) with significant cost implications, especially in resource‐poor settings. Compliance to schedules of transfusion and the need for repeated hospitalisation are other issues of concern for affected women as well as health services (Makani 2007). On the other hand, selective blood transfusion, which has a comparatively lower risk of transfusion‐related morbidities, may become indicated and performed at a time when it is already too late to improve maternal, or fetal outcomes.
Why it is important to do this review
Pregnant women with SCD are at increased risk of severe complications as a result of chronic anaemia, sickling of red cells and their consequences. Repeated blood transfusions to optimise the Hb level is an intervention to avert some of the complications encountered despite the potential morbidity that such practice constitutes. In spite of its use, the benefit of prophylactic blood transfusion to improve the outcome of pregnancy is uncertain. For instance, 11.6% of women who had prophylactic transfusion still needed emergency blood transfusions for severe anaemia during the same pregnancy (Ngo 2010). Therefore, there is lack of consensus among clinicians, hospitals and even countries regarding the optimal transfusion regimen, which makes evaluation of their impact on pregnancy outcomes difficult. This is partly because the usefulness of the practice had been largely derived from observational studies that have inherent limitations for policy formulation on the subject (Cunningham 1983; Grossetti 2009; Howard 1995; Ngo 2010). One systematic review, mostly of observational studies, reported a reduction of vaso‐occlusive crises, pulmonary complications, preterm birth, perinatal mortality and maternal mortality in women who received prophylactic blood transfusion (Malinowski 2015). However, the inherent limitations of observational studies reduce the confidence in the certainty of these findings. The continuing controversy about the approaches to care requires a more rigorous evaluation of the evidence in order to carefully balance effectiveness with safety. This is an update of the Cochrane review published in 2013 (Okusanya 2013b)). It evaluated the use of blood transfusion in pregnant women with SCD based on rigorous studies derived from up‐to‐date searches.
Objectives
To assess the benefits and harms of a policy of prophylactic versus selective blood transfusion in pregnant women with sickle cell disease (SCD).
Methods
Criteria for considering studies for this review
Types of studies
All published randomised controlled trials evaluating the effects of prophylactic versus selective blood transfusion in pregnant women with SCD. We planned to include, but did not find any eligible quasi‐randomised trials or trials using a cluster‐randomised design. Reports presented only as abstract were eligible for inclusion. However the one report presented only as abstract was excluded because there was no evidence that the trial was finally published many years after the conference presentation.
Types of participants
Pregnant women with SCD (genotype HbSS, HbSC and HbSβ‐thalassaemias). Studies of pregnant women with sickle cell trait (HbAS) were not eligible for inclusion.
Types of interventions
Prophylactic blood transfusion to optimise Hb concentration to a specified level versus selective (emergency) blood transfusion when indicated by specific complication or a critically low level of Hb concentration. Studies were eligible for inclusion regardless of whether whole blood or packed red cells were transfused.
Types of outcome measures
Primary outcomes
-
Maternal death
-
Severe maternal morbidity (e.g. organ failure, pulmonary embolism, fat embolism, stroke, intensive care unit admission; or as defined by trial authors)
-
Perinatal death
Secondary outcomes
Mother
-
Sickle cell crisis (due to vaso‐occlusion, sequestration or haemolysis)
-
Total units of blood transfused
-
Blood transfusion reaction
-
Iron overload in the woman (as assessed by trial authors)
-
Postpartum haemorrhage (greater than 500 mL blood loss or haemodynamic compromise following any degree of blood loss; or as defined by trial authors)
-
Cumulative duration of hospital stay
Infant
-
Admission to neonatal intensive care
-
Haemolytic disease of the newborn
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting their Information Specialist (30 May 2016)
The Register is a database containing over 22,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate the Pregnancy and Childbirth Group’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth Group in The Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, the Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by their Information Specialist and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
monthly searches of CINAHL (EBSCO);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth Group review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included studies; Excluded studies).
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For this update, no new studies were identified.
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group. These methods were also used for assessing the studies identified in the previous version of this review (Okusanya 2013b).
Selection of studies
Two review authors (BO Okusanya and OT Oladapo) independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved disagreements through discussion.
Data extraction and management
We designed a form as specified by the Cochrane Pregnancy and Childbirth Group to extract data. For eligible studies, both review authors independently extracted the data using the agreed form. We resolved discrepancies through discussion. We entered data into Review Manager software (RevMan 2014) and checked them for accuracy.
When information regarding any of the above was unclear, we made efforts to contact authors of the original reports to provide further details.
There was no masking of authors or journals.
Assessment of risk of bias in included studies
Both review authors independently assessed risk of bias for the included study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion.
(1) Random sequence generation (checking for possible selection bias)
We described for the included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for the included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for the included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered the study was at low risk of bias if it was blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for the included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for the included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for the included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for the included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether the included study was at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
We assessed the quality of the evidence using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes for the main comparisons.
-
Maternal death
-
Severe maternal morbidity (pulmonary embolism)
-
Severe maternal morbidity (congestive cardiac failure)
-
Perinatal death
-
Sickle cell crisis (pain crisis)
-
Sickle cell crisis (acute splenic sequestration)
-
Blood transfusion reaction
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create a ’Summary of findings’ table. A summary of the intervention effect and a measure of quality for each of the above outcomes were produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
No continuous data were analysed in this review. In future updates, if appropriate, we will use the mean difference if outcomes are measured in the same way between trials. We will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
The search strategy did not find any eligible cluster‐randomised trials. However, in future updates, if we identify any cluster‐randomised trials we will include them in the analyses along with individually‐randomised trials. We will adjust their standard errors using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Dealing with missing data
For the included study, we noted levels of attrition. In future updates, if more eligible studies are included, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We did not assess statistical heterogeneity as only one study was included. However, in future updates when more studies are included, statistical heterogeneity would be assessed using the Tau², I² and Chi² statistics. We would regard heterogeneity as substantial if an I² was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identify substantial heterogeneity (above 30%), we plan to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We did not conduct a meta‐analysis as only one study was included. In future updates, we will use a fixed‐effect meta‐analysis to combine data where it is reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If we find clinical heterogeneity sufficient to expect that the underlying treatment effects differs between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials. If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We did not investigate heterogeneity as only one study was included. If we identify substantial heterogeneity in future updates, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
We plan to carry out the following subgroup analyses for the primary outcomes.
-
Number of fetuses (singleton versus multiple).
-
Type/clinical severity of haemoglobinopathy (homozygous (HbSS) versus heterozygous (HbSC and or HbSβ+‐thal).
-
Type of transfused blood (whole blood versus packed red cells).
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We did not conduct a sensitivity analysis because only one study was included. In future updates, we plan to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this makes any difference to the overall result.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
See (Figure 1).

Study flow diagram.
The search of the Cochrane Pregnancy and Childbirth Group's Trials Register retrieved six trial reports relating to three studies. We included one study (Koshy 1988) involving 72 women. Two studies have been excluded (Cerqueira 1999; Koshy 1991).
The last published version of this review (Okusanya 2013b) included two studies (Koshy 1987; Koshy 1988). However, after careful scrutiny, it appears that these two studies are in fact reporting on a single trial (Koshy 1988) and so the current update now only includes one trial (Koshy 1988).
Included studies
We included one trial (Koshy 1988), involving 72 women, with outcome data on effectiveness variables available for all participating women. The trial was very small and was conducted in USA in the late 1980s. It was a multicentre study conducted in secondary and university hospitals.
Participants
The included trial (Koshy 1988) recruited pregnant women with sickle cell anaemia (genotype HbSS) before 28 weeks of gestation. The diagnosis of sickle cell anaemia in the study was confirmed with haemoglobin (Hb) electrophoresis on cellulose acetate with citrate agar and solubility testing, and quantitative chromatography. Exclusion criteria included pregnant women with other types of sickle cell disease (SCD) (HbSC and HbSβThal); religious belief against blood transfusion (i.e. Jehovah's witness); pregnancy greater than 28 weeks; those presenting with several other medical complications; or women who had several red blood cell antibodies.
Interventions
Prophylactic blood transfusion in asymptomatic pregnant women with sickle cell anaemia was compared with selective blood transfusion when indicated by medical or obstetric complications. Prophylactic blood transfusion was commenced prior to 28 weeks of gestation and continued at intervals until delivery. The goal of prophylactic transfusion was to maintain HbS at less than 35% and Hb concentration at 10 g/dL to 11 g/dL. Prophylactic blood transfusion took place in an outpatient clinic setting with simple transfusion or partial exchange transfusion. Women were transfused with two units of packed washed frozen red cells weekly, immediately following trial entry, for three weeks or until goals of prophylactic transfusion were reached.
Selective (emergency) blood transfusion was carried out when indicated by a medical or obstetric complication. The indication for blood transfusion was mainly haematological ‐ Hb concentration less than 6 g/dL (or haematocrit (HCT) less than 18%) and a reticulocyte count less than 3%.
Outcomes
The included study did not pre‐specify maternal death as an outcome variable but it reported it. The study reported some markers of severe maternal morbidities including pulmonary embolism, congestive heart failure, and acute chest syndrome. For the baby, perinatal death was reported as an outcome.
Regarding the secondary outcomes for this review, the included study reported pain (vaso‐occlusive) crisis, haemolytic and acute sequestration crises, total units of blood transfused and blood transfusion reaction. Other relevant maternal outcomes pre‐specified for this review such as cumulative duration of hospital stay, postpartum haemorrhage and iron overload, and infant outcomes were not reported by the trial.
For more information about included studies, see Characteristics of included studies.
Excluded studies
Two studies (Cerqueira 1999; Koshy 1991) were excluded. Koshy 1991 was an observational study that compared the findings of two studies on pregnant women with SCD. Cerqueira 1999 was a conference abstract report of the preliminary findings of a trial of more than two decades without any evidence that the trial was ever completed.
For more information, see Characteristics of excluded studies.
Risk of bias in included studies
Overall, the risk of bias for the included study was uncertain as the trial reports contained little methodological description. The details of the trial are given in the Characteristics of included studies table. See Figure 2 and Figure 3 for a summary of the risk of bias of included studies.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
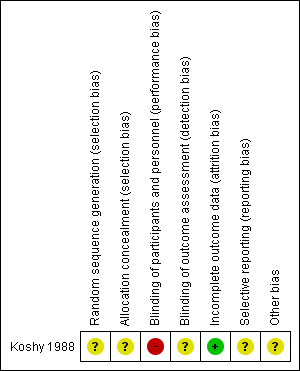
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The risk of selection bias was unclear for the trial as its methods of generating and concealing allocation sequence were not described. It only reported that participants were "randomly assigned" into two treatment groups.
Blinding
With regard to blinding of participants and key study personnel, we considered the trial to be at high risk of performance bias even though the nature of the interventions made it impracticable to blind participants or study personnel. The risk of detection bias was, however, assessed as unclear as the study gave no description on blinding of outcome assessors.
Incomplete outcome data
The included study was considered to be at low risk of bias as outcome data were available for all participating women in both treatment groups.
Selective reporting
The risk of reporting bias was considered unclear for the study. The comparison of outcome measure in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting although there was a reference to occurrence of maternal death in the 'Discussion' section.
Other potential sources of bias
We considered the study to be at unclear risk of other potential sources of bias. It is unclear what level of bias the knowledge of preliminary results from the same trial by the lead investigator in Koshy 1987 had on the implementation and reporting of the trial procedures.
Effects of interventions
Prophlactic versus selective blood transfusion
Overall, there were few events in the included trial and the results were generally imprecise.
Primary outcomes
Maternal death
The included trial (Koshy 1988; 72 women) reported no maternal mortality in women who received either prophylactic or selective blood transfusion.
Severe maternal morbidity (Outcomes 1.2 to 1.4)
The included trial (Koshy 1988; 72 women) reported the occurrence of markers of severe acute maternal morbidity including pulmonary embolism, acute chest syndrome and congestive cardiac failure. The trial suggested no difference in the risk of pulmonary embolism (no events) between the prophylactic blood transfusion and the selective blood transfusion group (Koshy 1988; 72 women). The trial also indicated no difference in the risk of congestive cardiac failure (risk ratio (RR) 1.00, 95% confidence interval (CI) 0.07 to 15.38; very low‐quality evidence, Analysis 1.3) and acute chest syndrome (RR 0.67, 95% CI 0.12 to 3.75, Analysis 1.4) between the two comparison groups.
Perinatal death
The trial indicated no difference in the risk of perinatal death between women with sickle cell anaemia (HbSS) who received prophylactic blood transfusion compared with those who received transfusion when indicated by medical or obstetric complications (RR 2.85, 95% CI 0.61 to 13.22, 76 infants; very low‐quality evidence, Analysis 1.5).
Secondary outcomes
Sickle cell crises
The trial (Koshy 1988, 72 women) reported pain, sequestration and haemolytic crises as outcomes.
The trial (72 women) indicated that prophylactic blood transfusion reduced the risk of pain crises compared with selective blood transfusion in pregnant women with HbSS (RR 0.28, 95% CI 0.12 to 0.67; low‐quality evidence, Analysis 1.6). However, the few events and small sample size widen the uncertainty around the treatment effect estimate. The trial (Koshy 1988, 72 women) suggested no difference in the occurrence of sequestration (RR 0.33, 95% CI 0.01 to 7.92; low‐quality evidence, Analysis 1.7) and haemolytic crises (RR 0.33, 95% CI 0.04 to 3.06, Analysis 1.8).
Total units of blood transfused
The trial (Koshy 1988, 72 women) reported the amount of blood transfused. The trial reported the total units of blood transfused per treatment group as well as the average units of blood received per participant in each group. The units of blood transfused were 432 units (with mean of 12 units) and 108 units (with mean of 3 units) to women with HbSS in the prophylactic and selective transfusion groups, respectively. The lack of information on either standard deviation, standard error or CIs of the means precluded the inclusion of the data in the analysis table.
Blood transfusion reaction
The trial (Koshy 1988, 72 women) indicated no difference in the risk of delayed blood transfusion reaction between women with HbSS who received prophylactic compared with those who received blood transfusion only when indicated (RR 2.00, 95% CI 0.54 to 7.39; very low‐quality evidence,Analysis 1.9).
Discussion
Sickle cell disease (SCD) poses a threat to the well‐being of a pregnant woman and her unborn child. Preventing the primary underlying pathophysiological process ‐ sickling of red blood cells and reduction in oxygen‐carrying capacity of the blood ‐ is expected to improve the outcome for both mother and baby. In view of the pregnancy demand on the persistent state of anaemia, there is no doubt that a significant proportion of pregnant women with SCD may require blood transfusion during the course of their pregnancies. However, whether this intervention should be preventive or therapeutic was the question for this review. In spite of its importance, it is surprising to note that very few rigorous studies have been conducted on the subject.
Summary of main results
This review shows that there is weak evidence indicating that prophylactic blood transfusion to achieve set levels of haemoglobin (Hb) concentration and sickle haemoglobin (HbS) confers no clear advantage in clinical outcomes beyond a reduction in the risk of pain crisis compared to emergency blood transfusion indicated by either medical or obstetric complications in women with sickle cell anaemia (HbSS). This evidence was derived from a small study (involving 72 women), at unclear risk of bias and wide confidence intervals. Other clinical end‐points such as maternal mortality and severe morbidity and perinatal death may not be significantly different between the two transfusion regimens for women with HbSS as indicated by the included study. The benefits in terms of pain crisis reduction in women with HbSS was achieved at the expense of blood transfusion at a ratio of four to one for prophylactic versus selective blood transfusion groups, respectively. This expected difference in the frequency of blood transfusion between the comparison groups also translates to a significant difference in healthcare resource use given the required clinic visits and hospital admissions for both policies.There is no information from randomised or quasi‐randomised trials to assess the benefits or risks of a policy of prophylactic versus selective blood transfusion in women with other variants of SCD, i.e. HbSC and HbSβThal.
Overall completeness and applicability of evidence
In general, there is a paucity of randomised studies required to generate rigorous evidence regarding the primary question for this review. The available evidence pertains to the most severe form of SCD ‐ sickle cell anaemia (HbSS) ‐ and precludes other variants that are also important. There is a general agreement that these other variants do not carry the same risk of pregnancy‐associated adverse outcomes as HbSS as their baseline level of anaemia is somewhat better. In fact, in the trial included in this review, the trialists primarily excluded women with HbSC and HbSβThal with the justification that complications are less frequent and repeated blood transfusion was not necessary. While this assumption might be true in settings where women with such variants already know their Hb genotype status, it could prove dangerous in settings where many women are first diagnosed of SCD when presenting with related complications in pregnancy. As demonstrated among such women excluded in the included study for this review, serious complications similar to those found in women with HbSS sometimes occur with considerable frequency and frequent blood transfusion might also be necessary to save lives (Koshy 1988). As the underlying pathophysiological process of SCD variants is the same, it is reasonable to assume that the findings of this review may be applicable across the board to other sickle cell haemoglobinopathies. However, in view of the relatively fewer frequency of crises in HbSC and HbSβThal, it is possible that the benefit of prophylactic blood transfusion in terms of reduction of pain crisis may become less apparent due to reduced frequency of events.
Repeated allogeneic blood transfusion has many challenges including allo‐immunisation of red blood cells, blood transfusion reactions and increased risks of blood‐borne infections and iron overload in women with SCD. It is uncertain whether the same level of refinement in blood typing, grouping, cross‐matching and red cell processing techniques, albeit in late 1980s, as performed in the included trial can be achieved in resource‐constrained settings. Where such standards cannot be met, prophylactic transfusion of an average of 11 to 12 units of packed red cells might increase transfusion‐related complications for prophylactic transfusion in excess of the findings of this review.
As a result of the growing concern about blood‐borne infections, particularly HIV and hepatitis virus, selective blood transfusion is now generally favoured in most clinical practice and it is unlikely that the only benefit in favour of prophylactic transfusion would be enough to influence such practice.
Quality of the evidence
The review found only one trial (involving 72 women). Overall, the study was at unclear risk for many of the 'Risk of bias' domains. Particularly, there was no description of random sequence generation and allocation concealment. The nature of the intervention also would not allow blinding of the intervention for the participants and personnel and as such puts it at high risk of detection bias particularly for a somewhat subjective outcome such as pain crises, the only outcome with a difference between the comparison groups. The small number of the participants and the methodological limitations of the included study does not permit confident conclusion on the safety and effectiveness of prophylactic blood transfusion for pregnant women with sickle cell anaemia (HbSS).
We also assessed the evidence using the GRADE approach and found the evidence to be very low quality for the outcomes maternal death, severe maternal morbidity, perinatal death and blood transfusion and low quality for sickle cell crises (pain; acute splenic sequestration) and blood transfusion reaction. Evidence was downgraded due to limitations in study design and imprecision relating to a small‐sample size from a single trial with few events.
Potential biases in the review process
We minimised potential biases by the use of a comprehensive search strategy and restriction of the study design to randomised and quasi‐randomised trials. As the search strategy found trials as far back as the late 1980s, it is unlikely that we missed out any important study. It could be that the authors' conclusions in the small trials found (included and excluded), the changing trend towards fewer blood transfusion and the challenges of setting up this type of trial have discouraged further work in more recent times. As a crucial step to limit bias as much as possible, we limited the studies considered for inclusion to those with some form of random component in participants' recruitment given the impracticability of blinding of the intervention, which already exposed them to significant risk of detection bias.
Agreements and disagreements with other studies or reviews
In a Cochrane review (Marti‐Carvajal 2009) evaluating intervention regimens (including prophylactic blood transfusion) to treat sickle cell crises, the review authors found no randomised trials to examine the safety and effectiveness of different regimens that have been used. The lack of prophylactic blood transfusion as an important component of any regimen to treat sickle cell crises supports the benefit regarding pain crisis reduction as demonstrated by the current review.
One systematic review (Malinowski 2015) including 11 cohort studies and one randomised trial concluded that prophylactic transfusion reduced maternal mortality, vaso‐occlusive pain episodes, pulmonary complications, pulmonary embolism, preterm birth, perinatal mortality, and neonatal deaths. The majority (82%; 9/11 cohort studies) of the studies included in Malinowski 2015 were assessed to be at high risk of bias and therefore the certainty of effect estimate is limited. The inherent methodological limitations of non‐randomised studies that informed the conclusion of the review (Malinowski 2015) may have over‐estimated the effects size of the intervention, and therefore its conclusions need to be interpreted with caution.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Prophylactic versus selective blood transfusion, Outcome 1 Maternal death.

Comparison 1 Prophylactic versus selective blood transfusion, Outcome 2 Severe maternal morbidity (pulmonary embolism).

Comparison 1 Prophylactic versus selective blood transfusion, Outcome 3 Severe maternal morbidity (congestive cardiac failure).

Comparison 1 Prophylactic versus selective blood transfusion, Outcome 4 Severe maternal morbidity (acute chest syndrome).
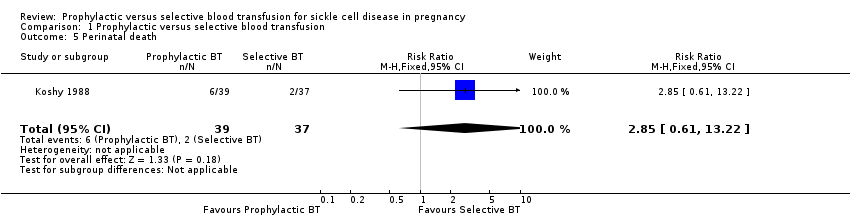
Comparison 1 Prophylactic versus selective blood transfusion, Outcome 5 Perinatal death.

Comparison 1 Prophylactic versus selective blood transfusion, Outcome 6 Sickle cell crisis (pain crisis).

Comparison 1 Prophylactic versus selective blood transfusion, Outcome 7 Sickle cell crises (acute splenic sequestration).

Comparison 1 Prophylactic versus selective blood transfusion, Outcome 8 Sickle cell crisis (haemolysis).

Comparison 1 Prophylactic versus selective blood transfusion, Outcome 9 Blood transfusion reaction.
| Prophylactic versus selective blood transfusion for sickle cell disease in pregnancy | ||||||
| Patient or population: patients with sickle cell disease in pregnancy Control: selective blood transfusion | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prophylactic versus selective blood transfusion | |||||
| Maternal death | See comment | See comment | Not estimable | 72 | ⊕⊝⊝⊝ | No woman in this study died. |
| Severe maternal morbidity (pulmonary embolism) | See comment | See comment | Not estimable | 72 | ⊕⊝⊝⊝ | No woman in this study experienced pulmonary embolism. |
| Severe maternal morbidity (congestive cardiac failure) | 28 per 1000 | 28 per 1000 | RR 1.00 | 72 | ⊕⊝⊝⊝ | |
| Perinatal death | 54 per 1000 | 154 per 1000 | RR 2.85 | 76 | ⊕⊝⊝⊝ | |
| Sickle cell crisis (pain crisis) | 500 per 1000 | 140 per 1000 | RR 0.28 | 72 | ⊕⊕⊝⊝ | |
| Sickle cell crises (acute splenic sequestration) | 28 per 1000 | 9 per 1000 | RR 0.33 | 72 | ⊕⊕⊝⊝ | |
| Blood transfusion reaction | 83 per 1000 | 167 per 1000 | RR 2.00 | 72 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The only study was at uncertain risk of bias due to unclear methods of random sequence generation and allocation concealment, and lack of blinding. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal death Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Severe maternal morbidity (pulmonary embolism) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Severe maternal morbidity (congestive cardiac failure) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.38] |
| 4 Severe maternal morbidity (acute chest syndrome) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.75] |
| 5 Perinatal death Show forest plot | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.61, 13.22] |
| 6 Sickle cell crisis (pain crisis) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.12, 0.67] |
| 7 Sickle cell crises (acute splenic sequestration) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.92] |
| 8 Sickle cell crisis (haemolysis) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.06] |
| 9 Blood transfusion reaction Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.54, 7.39] |

