Objetivos de presión arterial para el tratamiento de los pacientes con hipertensión y enfermedad cardiovascular
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010315.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 20 julio 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Hipertensión
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
LC Saiz is the lead author. He coordinated the review, entered the text of the review into RevMan, conducted external correspondence, appraised inclusion criteria and quality, and extracted and analyzed study data.
J Gorricho led the protocol, appraised inclusion criteria and quality of studies, extracted study data, and drafted the final review.
J Garjón appraised inclusion criteria and quality of studies, extracted study data, and drafted the final review.
MC Celaya appraised inclusion criteria and quality of studies and drafted the final review.
J Erviti appraised inclusion criteria and quality of studies and drafted the final review.
L Leache appraised inclusion criteria and quality of studies, extracted study data, and drafted the final review.
All review authors participated in writing of the Discussion and Conclusions.
Sources of support
Internal sources
-
Navarre Health Service and Health Department of the Government of Navarre, Spain.
Working time of authors (employees of the Government of Navarre).
Facilities.
External sources
-
European Social Fund Operational Programme 2007‐2013, Other.
50% of the full research project, as salary from September 2012 to December 2015 for the Pharmacotherapy Research Coordinator in the Navarre Health Service (LCS).
-
University of British Columbia, Vancouver, Canada.
Bibliographic searches. Methodological support.
Declarations of interest
LC Saiz: none known.
J Gorricho: none known.
J Garjón: none known.
MC Celaya: none known.
J Erviti: none known.
L Leache: none known.
Acknowledgements
We are grateful to:
-
James M Wright and the Cochrane Hypertension Group, for their encouragement, support, and assistance;
-
Lourdes Muruzábal, María del Mar Malón, Rodolfo Montoya, and Antonio López, for their relevant contributions as authors to a previous version of this systematic review;
-
the Biomedical Information Center of Navarre, which provided most of the published documents reviewed in this report (Stephen Adams, Vancouver, Canada, provided assistance with some references that were especially difficult to find);
-
Miguel Ángel Imízcoz, cardiologist, who provided specific advice related to ascertainment of cardiovascular events from individual patient data;
-
Agustín Ciapponi and Demian Glujovsky, of the Institute of Clinical Effectiveness and Health Policy (IECS), Buenos Aires, Argentina, who provided access to Early Review Organizing Software;
-
Annalisa Perna, Giuseppe Remuzzi, and Piero Ruggenenti, of Istituto di Ricerche Farmacologiche Mario Negri, Bergamo, Italy, who provided individual patient data for REIN‐2 2005;
-
Kate Fletcher, of the University of Birmingham, Birmingham, UK, and Jonathan Mant, of the University of Cambridge, Cambridge, UK, who provided individual patient data for Past BP 2016; and
-
Larrye Loss and Julie Ye, from AstraZeneca, Wilmington (DE), USA, who provided access to protocol, forms, Clinical Study Report, and individual patient data for the HOT 1998 study. This manuscript was not prepared in collaboration with AstraZeneca staff and does not necessarily reflect the opinions or views of the company. According to the Data Transfer Agreement, AstraZeneca was entitled to make comments to the final report but approval was given with no remarks;
Data from SPS3 2013 were supplied by the National Institute of Neurological Disorders and Stroke (NINDS). This manuscript does not necessarily reflect the opinions or views of the SPS3 study, the NINDS Central Repositories, or the NINDS.
AASK 2002 and MDRD 1994 were conducted by the AASK/MDRD Investigators and supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Data from the AASK 2002 and MDRD 1994 studies reported here were supplied by the NIDDK Central Repositories. This manuscript was not prepared in collaboration with investigators in the AASK 2002 and MDRD 1994 studies and does not necessarily reflect the opinions or views of the AASK/MDRD studies, the NIDDK Central Repositories, or the NIDDK.
This manuscript was prepared with ACCORD and SPRINT_POP Research Materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordination Center and does not necessarily reflect the opinions or views of ACCORD, SPRINT_POP, or the National Heart, Lung and Blood Institute (NHLBI).
Version history
| Published | Title | Stage | Authors | Version |
| 2022 Nov 18 | Blood pressure targets for the treatment of people with hypertension and cardiovascular disease | Review | Luis Carlos Saiz, Javier Gorricho, Javier Garjón, Mª Concepción Celaya, Juan Erviti, Leire Leache | |
| 2020 Sep 09 | Blood pressure targets for the treatment of people with hypertension and cardiovascular disease | Review | Luis Carlos Saiz, Javier Gorricho, Javier Garjón, Mª Concepción Celaya, Juan Erviti, Leire Leache | |
| 2018 Jul 20 | Blood pressure targets for the treatment of people with hypertension and cardiovascular disease | Review | Luis Carlos Saiz, Javier Gorricho, Javier Garjón, Mª Concepción Celaya, Juan Erviti, Leire Leache | |
| 2017 Oct 11 | Blood pressure targets for the treatment of people with hypertension and cardiovascular disease | Review | Luis Carlos Saiz, Javier Gorricho, Javier Garjón, Mª Concepción Celaya, Lourdes Muruzábal, Mª del Mar Malón, Rodolfo Montoya, Antonio López | |
| 2013 Jan 31 | Blood pressure targets for the treatment of patients with hypertension and cardiovascular disease | Protocol | Javier Gorricho, Javier Garjón, Mª Concepción Celaya, Lourdes Muruzábal, Rodolfo Montoya, Antonio López Andrés, Mª del Mar Malón, Luis Carlos Saiz | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Antihypertensive Agents [adverse effects, *therapeutic use];
- Blood Pressure [*drug effects, physiology];
- Cardiovascular Diseases [*drug therapy, mortality];
- Diastole;
- Hypertension [complications, *drug therapy, mortality];
- Patient Dropouts [statistics & numerical data];
- Randomized Controlled Trials as Topic;
- Reference Values;
- Systole;
Medical Subject Headings Check Words
Humans;
PICO

Results of the search.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Lower versus standard, outcome: 1.1 Total mortality.

Forest plot of comparison: 1 Lower versus standard, outcome: 1.2 Serious adverse events.

Forest plot of comparison: 1 Lower versus standard, outcome: 1.3 Cardiovascular events.
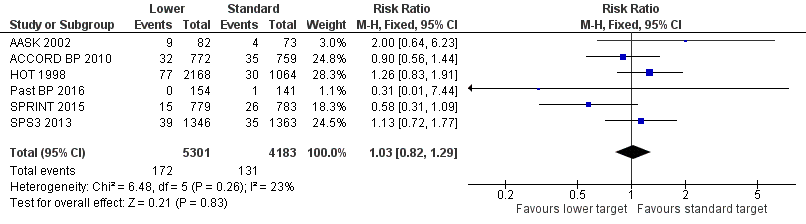
Forest plot of comparison: 1 Lower versus standard, outcome: 1.4 Cardiovascular mortality.

Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 1 Total mortality.

Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 2 Serious adverse events.
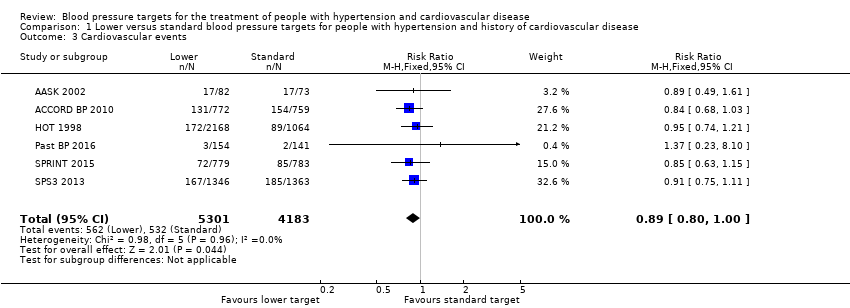
Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 3 Cardiovascular events.

Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 4 Cardiovascular mortality.

Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 5 Withdrawals due to adverse effects.

Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 6 Blood pressure target achieved at 1 year.

Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 7 Systolic blood pressure change from baseline at end of 1 year.

Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 8 Diastolic blood pressure change from baseline at end of 1 year.
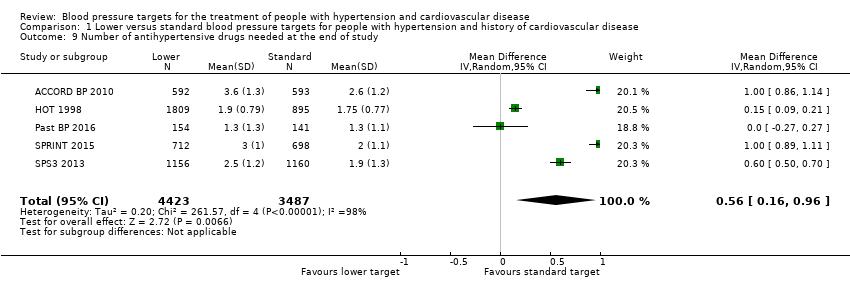
Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 9 Number of antihypertensive drugs needed at the end of study.
| Lower blood pressure targets compared with standard blood pressure targets for mortality and morbidity | ||||||
| Patient or population: cardiovascular disease with high blood pressure | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with standard blood pressure target | Risk with lower blood pressure target | |||||
| Total mortality | Study population | RR 1.06 | 9484 | ⊕⊕⊕⊝ | ||
| 68 per 1000 | 72 per 1000 | |||||
| Serious adverse events | Study population | RR 1.01 | 9484 | ⊕⊕⊝⊝ | ||
| 252 per 1000 | 255 per 1000 | |||||
| Total cardiovascular events | Study population | RR 0.89 | 9484 | ⊕⊕⊝⊝ | ||
| 127 per 1000 | 113 per 1000 | |||||
| Cardiovascular mortality | Study population | RR 1.03 (0.82 to 1.29) | 9484 (6 RCTs) | ⊕⊕⊕⊝ | ||
| 31 per 1000 | 32 per 1000 (25 to 40) | |||||
| Withdrawals due to adverse effects | Study population | RR 8.16 | 690 | ⊕⊝⊝⊝ | ||
| 7 per 1000 | 60 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aDowngraded one level owing to serious imprecision (95% CI is wider than the minimal important difference). bDowngraded one level owing to incomplete available data. cDowngraded one level owing to high risk of bias. dDowngraded two levels because only two of the smaller studies reported this outcome. | ||||||
| Mean (SD) unless otherwise stated | ||||||
| Number of participants | 155 | 1531 | 3232 | 295 | 1562 | 2709 |
| Sex (% male) | 68% | 63% | 53% | 64% | 76% | 62% |
| Age in years | 57 (9) | 62 (8) | 62 (‐) | 71 (9) | 70 (9) | 63 (11) |
| Ethnic group (% Caucasian) | 0% | 62% | 92% | 98% | 71% | 53% |
| Diabetes | 0% | 100% | 12% | 10% | 0% | 36% |
| Current smoker | 31% | 13% | 16% | 13% | 14% | 20% |
| Systolic blood pressure | 149 (28) | 138 (16) | 174 (15) | 143 (14) | 138 (16) | 146 (18) |
| Diastolic blood pressure | 93 (16) | 74 (11) | 106 (3) | 80 (10) | 74 (12) | 79 (11) |
| Ischemic heart disease (IHD) | 25% | 86% | 95% | 22% | ‐‐‐ | 11% |
| Stroke | 69% | 20% | 7% | 85% | 0% | 99% |
| Peripheral vascular disease | 23% | ‐‐‐ | ‐‐‐ | 7% | ‐‐‐ | ‐‐‐ |
| Thiazides | ‐‐‐ | 51% | ‐‐‐ | 35% | ‐‐‐ | 35% |
| ACEI/ARB | ‐‐‐ | 84% | ‐‐‐ | 65% | ‐‐‐ | 71% |
| Calcium channel blocker | ‐‐‐ | 26% | ‐‐‐ | 43% | ‐‐‐ | 28% |
| Beta blocker | ‐‐‐ | 57% | ‐‐‐ | 20% | ‐‐‐ | 27% |
| Other antihypertensive drugs | ‐‐‐ | 28% | ‐‐‐ | 11% | ‐‐‐ | 8% |
| Number of antihypertensive drugs | ‐‐‐ | 3.0 (1.4) | 1.0 (‐‐) | 1.1 (0.8) | 2.1 (1.0) | 1.7 (1.1) |
| (‐‐) no information is available. Ischemic heart disease, stroke, and peripheral vascular disease percentages are totally independent of each other because participants can have more than one cardiovascular event at the same time. A similar explanation can be offered with respect to percentages in the different classes of antihypertensive drugs. Abbreviations: ACEI: angiotensin‐converting enzyme inhibitor; ARB: angiotensin receptor blocker; IHD: ischemic heart disease; SD: standard deviation. | ||||||
| Outcome | Studies | Participants | Statistical Method | Effect Estimate |
| Total mortality | 2773 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.91,1.45] | |
| Cardiovascular mortality | 2773 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.69,1.39] | |
| Cardiovascular events | 2773 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.74,1.03] | |
| Serious adverse events | 2773 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.88,1.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total mortality Show forest plot | 6 | 9484 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.91, 1.23] |
| 2 Serious adverse events Show forest plot | 6 | 9484 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.94, 1.08] |
| 2.1 Total serious adverse events | 1 | 1562 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.91, 1.09] |
| 2.2 Subset of total serious adverse events | 5 | 7922 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.92, 1.11] |
| 3 Cardiovascular events Show forest plot | 6 | 9484 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.80, 1.00] |
| 4 Cardiovascular mortality Show forest plot | 6 | 9484 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.82, 1.29] |
| 5 Withdrawals due to adverse effects Show forest plot | 2 | 690 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.16 [2.06, 32.28] |
| 6 Blood pressure target achieved at 1 year Show forest plot | 6 | 8588 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.17, 1.24] |
| 7 Systolic blood pressure change from baseline at end of 1 year Show forest plot | 6 | 8546 | Mean Difference (IV, Random, 95% CI) | ‐8.90 [‐13.24, ‐4.56] |
| 8 Diastolic blood pressure change from baseline at end of 1 year Show forest plot | 6 | 8546 | Mean Difference (IV, Random, 95% CI) | ‐4.50 [‐6.35, ‐2.65] |
| 9 Number of antihypertensive drugs needed at the end of study Show forest plot | 5 | 7910 | Mean Difference (IV, Random, 95% CI) | 0.56 [0.16, 0.96] |

