Objetivos de presión arterial para el tratamiento de los pacientes con hipertensión y enfermedad cardiovascular
Appendices
Appendix 1. Search strategies
Database: Ovid MEDLINE(R) <1946 to Present with Daily Update>
Search Date: 13 February 2018
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp cardiovascular diseases/
2 ((heart or myocardial) adj5 (attack$ or disease$ or infarc$)).tw,kf.
3 (coronary adj5 (disease$ or syndrome$)).tw,kf.
4 ((cardiovascular or peripheral or vascular) adj5 disease$).tw,kf.
5 atrial fibril$.tw,kf.
6 ((cardiac or heart) adj failure).tw,kf.
7 angina$.tw,kf.
8 exp ischemia/
9 (ischaemi$ or ischemi$).tw,kf.
10 exp stroke/
11 (CVA or poststroke or post‐stroke or stroke or strokes).tw,kf.
12 apoplexy.tw,kf.
13 cerebrovascul$.tw,kf.
14 cerebral vascular.tw,kf.
15 ((brain$ or cerebral$ or lacunar) adj2 (accident$ or infarct$)).tw,kf.
16 or/1‐15
17 ((goal? or intensive$ or strict$ or target$ or tight$) adj6 (antihypertensive? or anti‐hypertensive? or bp or control or dbp or diastolic or pressure? or sbp or systolic or treat$)).tw,kf.
18 hypertension/
19 hypertens$.tw,kf.
20 exp blood pressure/
21 (blood pressure or bloodpressure).tw,kf.
22 or/18‐21
23 randomized controlled trial.pt.
24 controlled clinical trial.pt.
25 randomized.ab.
26 placebo.ab.
27 clinical trials as topic/
28 randomly.ab.
29 trial.ti.
30 or/23‐29
31 animals/ not (humans/ and animals/)
32 30 not 31
33 16 and 17 and 22 and 32
34 remove duplicates from 33
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: Hypertension Group Specialised Register via Cochrane Register of Studies (CRS‐Web)
Search Date: 13 February 2018
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
#1 ((intensive* NEAR bp) OR (intensive* NEAR dbp) OR (intensive* NEAR pressure*) OR (intensive* NEAR sbp)) AND INSEGMENT
#2 ((strict* NEAR bp) OR (strict* NEAR dbp) OR (strict* NEAR pressure*) OR (strict* NEAR sbp)) AND INSEGMENT
#3 ((target* NEAR bp) OR (target* NEAR dbp) OR (target* NEAR pressure*) OR (target* NEAR sbp)) AND INSEGMENT
#4 ((tight* NEAR bp) OR (tight* NEAR dbp) OR (tight* NEAR pressure*) OR (tight* NEAR sbp)) AND INSEGMENT
#5 #1 OR #2 OR #3 OR #4 AND INSEGMENT
#6 ((cardiovascular NEAR disease*) OR (heart NEAR attack*) OR (heart NEAR disease*) OR (heart NEAR infarct*)) AND INSEGMENT
#7 ((peripheral NEAR disease*) OR (myocardial NEAR attack*) OR (myocardial NEAR disease*) OR (myocardial NEAR infarct*)) AND INSEGMENT
#8 ((coronary NEAR disease*) OR (coronary NEAR syndrome*) OR (vascular NEAR disease*) OR (atrial fibril*)) AND INSEGMENT
#9 ((cardiac failure) OR (heart failure) OR (angina*) OR (ischemi*)) AND INSEGMENT
#10 (stroke OR (strokes) OR (ischaemi*) OR (CVA)) AND INSEGMENT
#11 (apoplexy OR (cerebrovascul*) OR (cerebral vascular) OR (brain accident*)) AND INSEGMENT
#12 ((brain infarct*) OR (cerebral NEAR accident*) OR (lacunar NEAR accident*) OR (lacunar NEAR infarct*)) AND INSEGMENT
#13 #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 AND INSEGMENT
#14 RCT:DE AND INSEGMENT
#15 Review:ODE AND INSEGMENT
#16 #14 OR #15 AND INSEGMENT
#17 #5 AND #13 AND #16 AND INSEGMENT
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: Cochrane Central Register of Controlled Trials via Cochrane Register of Studies (CRS‐Web)
Search Date: 13 February 2018
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
#1 ((intensive* NEAR bp) OR (intensive* NEAR dbp) OR (intensive* NEAR pressure*) OR (intensive* NEAR sbp)) AND CENTRAL:TARGET
#2 ((strict* NEAR bp) OR (strict* NEAR dbp) OR (strict* NEAR pressure*) OR (strict* NEAR sbp)) AND CENTRAL:TARGET
#3 ((target* NEAR bp) OR (target* NEAR dbp) OR (target* NEAR pressure*) OR (target* NEAR sbp)) AND CENTRAL:TARGET
#4 ((tight* NEAR bp) OR (tight* NEAR dbp) OR (tight* NEAR pressure*) OR (tight* NEAR sbp)) AND CENTRAL:TARGET
#5 #1 OR #2 OR #3 OR #4 AND CENTRAL:TARGET
#6 ((cardiovascular NEAR disease*) OR (heart NEAR attack*) OR (heart NEAR disease*) OR (heart NEAR infarct*)) AND CENTRAL:TARGET
#7 ((peripheral NEAR disease*) OR (myocardial NEAR attack*) OR (myocardial NEAR disease*) OR (myocardial NEAR infarct*)) AND CENTRAL:TARGET
#8 ((coronary NEAR disease*) OR (coronary NEAR syndrome*) OR (vascular NEAR disease*) OR (atrial fibril*)) AND CENTRAL:TARGET
#9 ((cardiac failure) OR (heart failure) OR (angina*) OR (ischemi*)) AND CENTRAL:TARGET
#10 (stroke OR (strokes) OR (ischaemi*) OR (CVA)) AND CENTRAL:TARGET
#11 (apoplexy OR (cerebrovascul*) OR (cerebral vascular) OR (brain accident*)) AND CENTRAL:TARGET
#12 ((brain infarct*) OR (cerebral NEAR accident*) OR (lacunar NEAR accident*) OR (lacunar NEAR infarct*)) AND CENTRAL:TARGET
#13 #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 AND CENTRAL:TARGET
#14 #5 AND #13 AND CENTRAL:TARGET
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: Embase <1974 to 2018 February 12>
Search Date: 13 February 2018
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp cardiovascular disease/
2 ((heart or myocardial) adj5 (attack$ or disease$ or infarc$)).tw.
3 (coronary adj5 (disease$ or syndrome$)).tw.
4 ((cardiovascular or peripheral or vascular) adj5 disease$).tw.
5 atrial fibril$.tw.
6 ((cardiac or heart) adj failure).tw.
7 angina$.tw.
8 exp ischemia/
9 (ischaemi$ or ischemi$).tw.
10 exp stroke/
11 (CVA or poststroke or post‐stroke or stroke or strokes).tw.
12 apoplexy.tw.
13 cerebrovascul$.tw.
14 cerebral vascular.tw.
15 ((brain$ or cerebral$ or lacunar) adj2 (accident$ or infarct$)).tw.
16 or/1‐15
17 ((goal? or intensive$ or strict$ or target$ or tight$) adj6 (antihypertensive? or anti‐hypertensive? or bp or control or dbp or diastolic or pressure? or sbp or systolic or treat$)).tw.
18 exp hypertension/
19 (antihypertens$ or anti‐hypertens$).tw.
20 exp blood pressure/
21 (blood pressure or bloodpressure).mp.
22 or/18‐21
23 randomized controlled trial/
24 crossover procedure/
25 double‐blind procedure/
26 (randomi?ed or randomly).tw.
27 (crossover$ or cross‐over$).tw.
28 placebo.ab.
29 (doubl$ adj blind$).tw.
30 assign$.ab.
31 allocat$.ab.
32 or/23‐31
33 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
34 32 not 33
35 16 and 17 and 22 and 34
36 remove duplicates from 35
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: LILACS
Search date: 13 February 2018
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
((cardiovascular disease?) or (heart attack$) or (myocardial infarct$) or (heart disease$) or (myocardial disease$) or (coronary disease$) or (coronary syndrome$) or (cardiovascular disease$) or (peripheral disease$) or (vascular disease$) or (atrial fibril$) or (cardiac failure) or (heart failure) or (angina$) or (ischaemi$) or (ischemi$) or (stroke$) or (CVA) or (poststroke) or (post‐stroke) or (apoplexy) or (cerebrovascul$) or (cerebral vascular) or (brain$ accident$) or (brain infarct$) or (cerebral$ accident$) or (cerebral$ infarct$) or (lacunar accident$) or (lacunar infarct$)) and ((intensive$ bp) or (intensive$ dbp) or (intensive$ blood pressure?) or (intensive$ sbp) or (strict$ bp) or (strict$ dbp) or (strict$ blood pressure?) or (strict$ sbp) or (target$ bp) or (target$ dbp) or (target$ blood pressure?) or (target$ sbp) or (tight$ bp) or (tight$ dbp) or (tight$ blood pressure?) or (tight$ sbp)) and ((hypertension) or (hypertens$) or (blood pressure) or (bloodpressure)) and (((PT:”randomised controlled trial”) or (PT:”controlled clinical trial”) or (AB:”randomi?ed”) or (AB:”placebo”) or (clinical trials) or (AB:”randomly”) or (TI:”trial”)) and not ((animals) and not (humans and animals)))
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: ClinicalTrials.gov
Search Date: 13 February 2018
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Condition or disease: (hypertension) AND (angina OR cardiovascular OR myocardial infarction OR peripheral vascular OR stroke)
Other terms: (intensive OR strict OR target OR tight) AND (blood pressure) AND (randomized)
Study type: Interventional Studies
Outcome Measure: blood pressure
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: WHO International Clinical Trials Registry Platform
Search Date: 13 February 2018
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
#1 intensive AND blood pressure AND randomized
#2 strict AND blood pressure AND randomized
#3 target* AND blood pressure AND randomized
#4 tight AND blood pressure AND randomized
#5 #1 OR #2 OR #3 OR #4 OR #5
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: TRIP Database
Search date: 6 April 2018
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
(blood) AND (pressure) AND (targets) AND (intensive) AND (standard)
Appendix 2. Reviews and guidelines checked
ACC‐AHA 2017; Arguedas 2009; Arguedas 2013; Bangalore 2011; Bangalore 2013; Bangalore 2017; BPLTTC 2013; BPLTTC 2014; CHEP 2018; Drozda 2011; ESH‐ESC 2013; Ettehad 2016; Feldstein 2014; Lv 2012; Lv 2013; McBrien 2012; NICE 2016; Rosendorff 2009; Rosendorff 2015; Roy 2010; SBU 2007; Verdecchia 2016; Xie 2016.

Results of the search.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Lower versus standard, outcome: 1.1 Total mortality.

Forest plot of comparison: 1 Lower versus standard, outcome: 1.2 Serious adverse events.

Forest plot of comparison: 1 Lower versus standard, outcome: 1.3 Cardiovascular events.
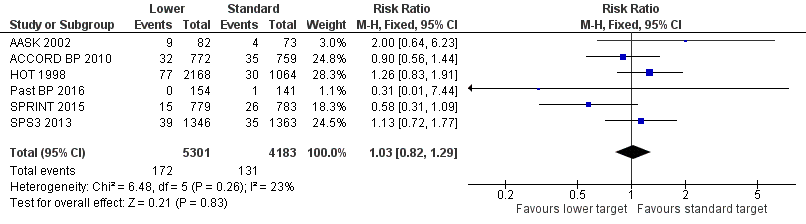
Forest plot of comparison: 1 Lower versus standard, outcome: 1.4 Cardiovascular mortality.

Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 1 Total mortality.

Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 2 Serious adverse events.
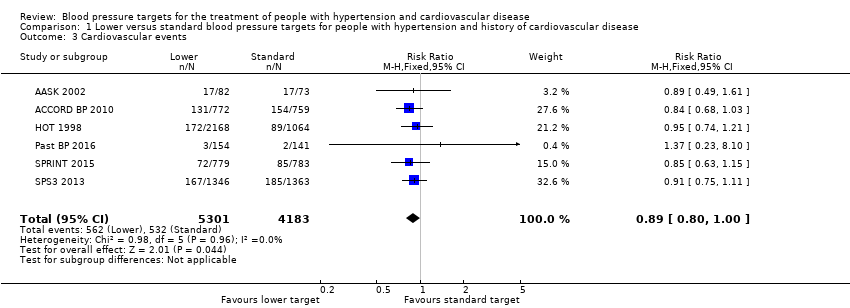
Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 3 Cardiovascular events.

Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 4 Cardiovascular mortality.

Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 5 Withdrawals due to adverse effects.

Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 6 Blood pressure target achieved at 1 year.

Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 7 Systolic blood pressure change from baseline at end of 1 year.

Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 8 Diastolic blood pressure change from baseline at end of 1 year.
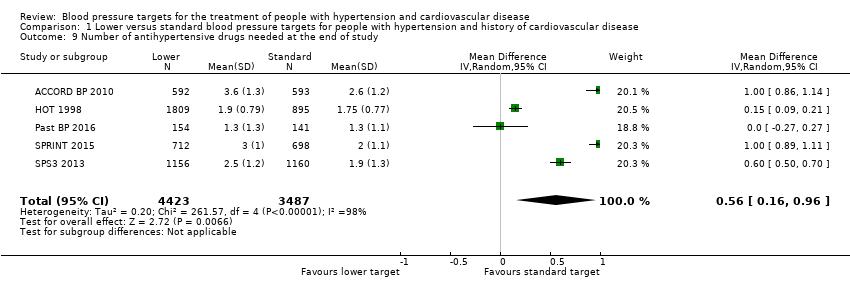
Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 9 Number of antihypertensive drugs needed at the end of study.
| Lower blood pressure targets compared with standard blood pressure targets for mortality and morbidity | ||||||
| Patient or population: cardiovascular disease with high blood pressure | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with standard blood pressure target | Risk with lower blood pressure target | |||||
| Total mortality | Study population | RR 1.06 | 9484 | ⊕⊕⊕⊝ | ||
| 68 per 1000 | 72 per 1000 | |||||
| Serious adverse events | Study population | RR 1.01 | 9484 | ⊕⊕⊝⊝ | ||
| 252 per 1000 | 255 per 1000 | |||||
| Total cardiovascular events | Study population | RR 0.89 | 9484 | ⊕⊕⊝⊝ | ||
| 127 per 1000 | 113 per 1000 | |||||
| Cardiovascular mortality | Study population | RR 1.03 (0.82 to 1.29) | 9484 (6 RCTs) | ⊕⊕⊕⊝ | ||
| 31 per 1000 | 32 per 1000 (25 to 40) | |||||
| Withdrawals due to adverse effects | Study population | RR 8.16 | 690 | ⊕⊝⊝⊝ | ||
| 7 per 1000 | 60 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aDowngraded one level owing to serious imprecision (95% CI is wider than the minimal important difference). bDowngraded one level owing to incomplete available data. cDowngraded one level owing to high risk of bias. dDowngraded two levels because only two of the smaller studies reported this outcome. | ||||||
| Mean (SD) unless otherwise stated | ||||||
| Number of participants | 155 | 1531 | 3232 | 295 | 1562 | 2709 |
| Sex (% male) | 68% | 63% | 53% | 64% | 76% | 62% |
| Age in years | 57 (9) | 62 (8) | 62 (‐) | 71 (9) | 70 (9) | 63 (11) |
| Ethnic group (% Caucasian) | 0% | 62% | 92% | 98% | 71% | 53% |
| Diabetes | 0% | 100% | 12% | 10% | 0% | 36% |
| Current smoker | 31% | 13% | 16% | 13% | 14% | 20% |
| Systolic blood pressure | 149 (28) | 138 (16) | 174 (15) | 143 (14) | 138 (16) | 146 (18) |
| Diastolic blood pressure | 93 (16) | 74 (11) | 106 (3) | 80 (10) | 74 (12) | 79 (11) |
| Ischemic heart disease (IHD) | 25% | 86% | 95% | 22% | ‐‐‐ | 11% |
| Stroke | 69% | 20% | 7% | 85% | 0% | 99% |
| Peripheral vascular disease | 23% | ‐‐‐ | ‐‐‐ | 7% | ‐‐‐ | ‐‐‐ |
| Thiazides | ‐‐‐ | 51% | ‐‐‐ | 35% | ‐‐‐ | 35% |
| ACEI/ARB | ‐‐‐ | 84% | ‐‐‐ | 65% | ‐‐‐ | 71% |
| Calcium channel blocker | ‐‐‐ | 26% | ‐‐‐ | 43% | ‐‐‐ | 28% |
| Beta blocker | ‐‐‐ | 57% | ‐‐‐ | 20% | ‐‐‐ | 27% |
| Other antihypertensive drugs | ‐‐‐ | 28% | ‐‐‐ | 11% | ‐‐‐ | 8% |
| Number of antihypertensive drugs | ‐‐‐ | 3.0 (1.4) | 1.0 (‐‐) | 1.1 (0.8) | 2.1 (1.0) | 1.7 (1.1) |
| (‐‐) no information is available. Ischemic heart disease, stroke, and peripheral vascular disease percentages are totally independent of each other because participants can have more than one cardiovascular event at the same time. A similar explanation can be offered with respect to percentages in the different classes of antihypertensive drugs. Abbreviations: ACEI: angiotensin‐converting enzyme inhibitor; ARB: angiotensin receptor blocker; IHD: ischemic heart disease; SD: standard deviation. | ||||||
| Outcome | Studies | Participants | Statistical Method | Effect Estimate |
| Total mortality | 2773 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.91,1.45] | |
| Cardiovascular mortality | 2773 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.69,1.39] | |
| Cardiovascular events | 2773 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.74,1.03] | |
| Serious adverse events | 2773 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.88,1.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total mortality Show forest plot | 6 | 9484 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.91, 1.23] |
| 2 Serious adverse events Show forest plot | 6 | 9484 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.94, 1.08] |
| 2.1 Total serious adverse events | 1 | 1562 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.91, 1.09] |
| 2.2 Subset of total serious adverse events | 5 | 7922 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.92, 1.11] |
| 3 Cardiovascular events Show forest plot | 6 | 9484 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.80, 1.00] |
| 4 Cardiovascular mortality Show forest plot | 6 | 9484 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.82, 1.29] |
| 5 Withdrawals due to adverse effects Show forest plot | 2 | 690 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.16 [2.06, 32.28] |
| 6 Blood pressure target achieved at 1 year Show forest plot | 6 | 8588 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.17, 1.24] |
| 7 Systolic blood pressure change from baseline at end of 1 year Show forest plot | 6 | 8546 | Mean Difference (IV, Random, 95% CI) | ‐8.90 [‐13.24, ‐4.56] |
| 8 Diastolic blood pressure change from baseline at end of 1 year Show forest plot | 6 | 8546 | Mean Difference (IV, Random, 95% CI) | ‐4.50 [‐6.35, ‐2.65] |
| 9 Number of antihypertensive drugs needed at the end of study Show forest plot | 5 | 7910 | Mean Difference (IV, Random, 95% CI) | 0.56 [0.16, 0.96] |

