Rosuvastatina para la reducción del nivel de lípidos
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | 4‐week washout period 12‐week before‐and‐after trial | |
| Participants | 90 men and women mean age 55 years with mixed dyslipidaemia LDL‐C > 160 mg/dl ( > 4.14 mmol/l TG > 200 mg/dl ( > 2.26 mmol/l) 30 participants randomized to rosuvastatin 40 mg/day 30 participants randomized to rosuvastatin 40 mg/day + fenofibrate 200 mg/day 30 participants randomized to rosuvastatin 40 mg/day + 2 g n‐3 fatty acids/day exclusion criteria: known coronary heart disease or atherosclerosis TG > 500 mg/dl ( > 5.645 mmol/l), renal disease, diabetes mellitus hypothyroidism, liver disease or dysfunction and medical conditions that would interfere with trial completion uncontrolled hypertension Rosuvastatin 40 mg/day baseline TC : 7.86 mmol/l (304 mg/dl) Rosuvastatin 40 mg/day baseline non‐HDL‐C: 6.54 mmol/l (253 mg/dl) | |
| Interventions | rosuvastatin 40 mg/day rosuvastatin 40 mg/day + fenofibrate 200 mg/day rosuvastatin 40 mg/day + 2 g n‐3 fatty acids/day | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, and non‐HDL‐C | |
| Notes | rosuvastatin 40 mg/day + fenofibrate 200 mg/day rosuvastatin 40 mg/day + 2 g n‐3 fatty acids/day groups were not included in the efficacy analysis SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the rosuvastatin 40 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the rosuvastatin 40 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the rosuvastatin 40 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all subjects were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | triglyceride data was not reported |
| Other bias | Low risk | trial was not funded by industry |
| Methods | 4‐week washout period 4‐week randomized, double‐blind, placebo‐controlled trial | |
| Participants | 65 men and women age 44‐80 years with chronic heart failure 18 randomized to placebo 21 randomized to rosuvastatin 10 mg/day 21 randomized to allopurinol 300 mg/day exclusion criteria: acute coronary syndromes during the last 6 months diabetes mellitus, cancer, RA, infections, pulmonary disease, thyroid disease liver dysfunction, severe hyperlipidaemia, renal dysfunction Placebo baseline TC : 5.61 mmol/l (217 mg/dl) Rosuvastatin 10 mg/day baseline TC : 5.77 mmol/l (223 mg/dl) | |
| Interventions | Placebo Rosuvastatin 10 mg/day Allopurinol 300 mg/day | |
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C and HDL‐C | |
| Notes | Allopurinol 300 mg/day group was not included in the efficacy analysis SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not reported |
| Blinding (performance bias and detection bias) | Low risk | double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | 5/65= 7.7% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | Triglycerides and WDAEs were not included in the analysis |
| Other bias | Low risk | There is no conflict of interest related with the present manuscript |
| Methods | 4‐week washout period 8‐week randomized, double‐blind study | |
| Participants | 509 men and non‐pregnant women age ≥ 18 years with type 2 diabetes glucose ≥ 7.0 mmol/l TG ≤ 6.0 mmol/l (531 mg/dl) 254 received rosuvastatin 255 received atorvastatin exclusion criteria: type 1 diabetes mellitus, glycated haemoglobin > 9.0 % history of cardiovascular disease or familial hypercholesterolaemia uncontrolled hypertension CK > 3 X ULN Rosuvastatin 10 mg/day baseline TC : 5.5 mmol/l (213 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 4.3 mmol/l (166 mg/dl) | |
| Interventions | Rosuvastatin 10 mg/day for 8 weeks Rosuvastatin 20 mg/day from 8‐16 weeks Atorvastatin 10 mg/day for 8 weeks Atorvastatin 20 mg/day from 8‐16 weeks | |
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | Rosuvastatin 20 mg/day from 8‐16 weeks Atorvastatin 10 mg/day for 8 weeks Atorvastatin 20 mg/day from 8‐16 weeks groups were not included in the analysis SD was imputed for triglycerides | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 14/254 were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | AstraZeneca funded the study. Data may support bias for rosuvastatin |
| Methods | 6‐week washout period 6‐week before‐and‐after trial | |
| Participants | 774 African‐American men and women age ≥ 18 years with type IIa or IIb hypercholesterolaemia LDL‐C ≥160 mg/dl and ≤300 mg/dl (≥4.14 mmol/l and 7.76 mmol/l) TG < 400 mg/dl (4.52 mmol/l) 391 randomized to rosuvastatin 383 randomized to atorvastatin exclusion criteria: homozygous familial hypercholesterolaemia or type I, III, or V hyperlipoproteinaemia active arterial disease, uncontrolled hypertension, poorly controlled diabetes mellitus, active liver disease or dysfunction serum CK > 3 X ULN Rosuvastatin 10 mg/day baseline TC : 7.00 mmol/l (271 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 5.67 mmol/l (219 mg/dl) Rosuvastatin 20 mg/day baseline TC : 7.01 mmol/l (271 mg/dl) Rosuvastatin 20 mg/day baseline non‐HDL‐C: 5.645 mmol/l (218 mg/dl) | |
| Interventions | Rosuvastatin 10 mg/day Rosuvastatin 20 mg/day Atorvastatin 10 mg/day Atorvastatin 20 mg/day | |
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | Atorvastatin 10 mg/day Atorvastatin 20 mg/day groups were not included in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day and Rosuvastatin 20 mg/day treatment arms were analyzed and since there was no placebo group to compare them to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day and Rosuvastatin 20 mg/day treatment arms were analyzed and since there was no placebo group to compare them to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day and Rosuvastatin 20 mg/day treatment arms were analyzed and since there was no placebo group to compare them to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 9/195 were not included in the efficacy analysis for rosuvastatin 10 mg/day 7/196 were not included in the efficacy analysis for rosuvastatin 20 mg/day 4.1 % participants receiving rosuvastatin were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were measured |
| Other bias | High risk | This research was supported by AstraZeneca. Data may support bias for rosuvastatin |
| Methods | 4‐week washout period 0 to 6‐week randomized double‐blind trial 6 to 12‐week open‐label trial | |
| Participants | 436 men and women ≥ 18 years with hypercholesterolaemia LDL‐C ≥ 3.36 mmol/l (130 mg/dl) and < 6.50 mmol/l (250 mg/dl) TG < 4.52 mmol/l (400 mg/dl) history of CHD or a CHD risk equivalent clinical evidence of atherosclerosis 10 year CHD risk of ≥10% 145 patients were randomized to rosuvastatin 5 mg/day for 0‐6 weeks 145 patients were randomized to rosuvastatin 10 mg/day for 0‐6 weeks 146 patients were randomized to atorvastatin 10 mg/day for 0 to 6 weeks 36 patients were titrated from 5 mg to 10 mg rosuvastatin for 6‐12 weeks 23 patients were titrated from 10 mg to 20 mg rosuvastatin for 6‐12 weeks exclusion criteria: none reported Rosuvastatin 5 mg/day baseline LDL‐C : 4.24 mmol/l (164 mg/dl) Rosuvastatin 5 mg/day baseline TG : 1.92 mmol/l (170 mg/dl) Rosuvastatin 10 mg/day baseline LDL‐C : 4.13 mmol/l (160 mg/dl) Rosuvastatin 10 mg/day baseline TG : 2.04 mmol/l (181 mg/dl) | |
| Interventions | rosuvastatin 5 mg/day for 0‐6 weeks rosuvastatin 10 mg/day for 0‐6 weeks atorvastatin 10 mg/day for 0‐6 weeks titrated from 5 mg to 10 mg rosuvastatin for 6‐12 weeks titrated from 10 mg to 20 mg rosuvastatin for 6‐12 weeks | |
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | atorvastatin 10 mg/day for 0 to 6 weeks titrated from 5 mg to 10 mg rosuvastatin for 6‐12 weeks titrated from 10 mg to 20 mg rosuvastatin for 6‐12 weeks groups were not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 5 mg/day for 0‐6 weeks and Rosuvastatin 10 mg/day for 0‐6 weeks treatment arms were analyzed and since there was no placebo group to compare them to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 5 mg/day for 0‐6 weeks and Rosuvastatin 10 mg/day for 0‐6 weeks treatment arms were analyzed and since there was no placebo group to compare them to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 5 mg/day for 0‐6 weeks and Rosuvastatin 10 mg/day for 0‐6 weeks treatment arms were analyzed and since there was no placebo group to compare them to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | rosuvastatin 5 mg/day group 9‐11/145 (6.2‐7.6)% patients were not included in the efficacy analysis rosuvastatin 10 mg/day group 6/145 (4.1%) patients were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | AstraZeneca funded the study. Data may support bias for rosuvastatin |
| Methods | no washout required because participants were not receiving any lipid‐altering agents for at least 6 months 8‐week randomized, double‐blind, placebo‐controlled trial | |
| Participants | 334 men and women with hypertriglyceridaemia mean age 52 years TG 200‐800 mg/dL (2.26‐9.03) mmol/l 111 randomized to placebo 111 randomized to rosuvastatin 10 mg/day 112 randomized to rosuvastatin 20 mg/day exclusion criteria: high LDL‐C, unstable cardiovascular condition or awaiting a myocardial revascularization congestive heart failure, uncontrolled diabetes mellitus cancer, uncontrolled hypothyroidism, familial hypercholesterolaemia, liver/muscle disease, pregnancy Placebo baseline TC : 5.53 mmol/l (214 mg/dl) Placebo baseline non‐HDL‐C : 4.71 mmol/l (182 mg/dl) Rosuvastatin 10 mg/day baseline TC : 5.61 mmol/l (216 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C : 4.64 mmol/l (179 mg/dl) Rosuvastatin 20 mg/day baseline TC : 5.57 mmol/l (215 mg/dl) Rosuvastatin 20 mg/day baseline non‐HDL‐C : 4.63 mmol/l (179 mg/dl) | |
| Interventions | Placebo Rosuvastatin 10 mg/day Rosuvastatin 20 mg/day | |
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C, non‐HDL‐C and Triglycerides | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not reported |
| Blinding (performance bias and detection bias) | Low risk | double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | WDAEs were not reported |
| Other bias | High risk | AstraZeneca funded the trial |
| Methods | no washout required because participants were not receiving any lipid‐altering agents for at least 2 months 8‐week open‐label, randomized study | |
| Participants | 877 men and women age > 20 years with hypercholesterolaemia 442 randomized to rosuvastatin 5 mg/day 435 randomized to atorvastatin 10 mg/day exclusion criteria: severe hypertension, type 1 diabetes mellitus, familial hypercholesterolaemia fasting TG > 400 mg/dL, MI or cerebrovascular disorder within 3 months prior to the start of the study serious cardiac insufficiency, revascularization during the study period active hepatic disease, renal dysfunction, pregnancy or possible pregnancy, hypothyroidism muscle disease, drug and alcohol abuse | |
| Interventions | rosuvastatin 5 mg/day atorvastatin 10 mg/day | |
| Outcomes | per cent change from baseline at 8 weeks of plasma LDL‐C, HDL‐C and triglycerides | |
| Notes | atorvastatin 10 mg/day data were not analyzed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 5 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 5 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 5 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 8/450 (1.8%) participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | TC and non‐HDL‐C data were not provided |
| Other bias | Low risk | Industry did not fund the trial |
| Methods | no washout required because participants were not receiving any lipid‐altering agents 12‐week randomized, double‐blind, placebo‐controlled trial | |
| Participants | 269 men and women age 18‐82 years with aortic stenosis 135 randomized to placebo 134 randomized to rosuvastatin 40 mg/day exclusion criteria: Patients with clinical indications for the use of statins as defined by Canadian guidelines such as coronary artery disease cerebrovascular disease, peripheral vascular disease, diabetes and Asian participants Placebo baseline LDL‐C : 3.12 mmol/l (121 mg/dl) Rosuvastatin 40 mg/day baseline LDL‐C : 3.18 mmol/l (123 mg/dl) | |
| Interventions | Placebo Rosuvastatin 40 mg/day | |
| Outcomes | per cent change from baseline at 12 weeks of blood LDL‐C and HDL‐C | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | centralized and generated by computer program a third party AstraZeneca Canada Inc |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not reported |
| Blinding (performance bias and detection bias) | Low risk | double‐blind "patients, site coordinators, investigators and statisticians were blinded" |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | TC, TGs and WDAEs were not reported |
| Other bias | High risk | this trial was partially funded by AstraZeneca |
| Methods | 6‐week washout period 18‐week randomized open‐label study | |
| Participants | 120 men and women with primary hyperlipidaemia TC > 240 mg/dl (6.2 mmol/l) TG < 350 mg/dl (4.0 mmol/l) 60 patients were randomized to rosuvastatin 60 patients were randomized to atorvastatin exclusion criteria: liver disease or dysfunction, renal dysfunction diabetes mellitus, raised TSH medical condition that could affect outcome of trial Rosuvastatin 10 mg/day baseline TC : 7.37 mmol/l (285 mg/dl) | |
| Interventions | Rosuvastatin 10 mg/day 0‐6 weeks Rosuvastatin 10 or 20 mg/day 6‐24 weeks Atorvastatin 20 mg/day for 0‐6 weeks Atorvastatin 20 or 40 mg/day for 6‐24 weeks | |
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Notes | Rosuvastatin 10 or 20 mg/day 6‐24 weeks Atorvastatin 20 mg/day for 0‐6 weeks Atorvastatin 20 or 40 mg/day for 6‐24 weeks groups were not included in the analysis SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day 0‐6 weeks treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day 0‐6 weeks treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day 0‐6 weeks treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Low risk | No company nor institution supported the trial financially |
| Methods | 6‐week washout 12‐week randomized open‐label trial | |
| Participants | 623 men and women mean age 60 years with and without metabolic syndrome LDL‐C ≥ 160 and < 250 mg/dl ( ≥ 4.14 and < 6.46 mmol/l) TG <400 mg/dl ( < 4.52 mmol/l) 194 metabolic syndrome patients received rosuvastatin 10 mg/day 382 non‐metabolic syndrome patients received rosuvastatin 10 mg/day 576 patients received rosuvastatin 10 mg/day exclusion criteria: none Metabolic syndrome group: Rosuvastatin 10 mg/day baseline LDL‐C : 4.84 mmol/l (187 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 5.94 mmol/l (230 mg/dl) Non‐metabolic syndrome group: Rosuvastatin 10 mg/day baseline LDL‐C : 4.81 mmol/l (186 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 5.61 mmol/l (217 mg/dl) Combined groups: Rosuvastatin 10 mg/day baseline LDL‐C : 4.82 mmol/l (186 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 5.72 mmol/l (221 mg/dl) | |
| Interventions | Rosuvastatin 10 mg/day | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | TC was calculated from HDL‐C and non‐HDL‐C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | There was only one group of participants analyzed therefore assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | There was only one group of participants analyzed therefore assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | There was only one group of participants analyzed therefore it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 47/623 (7.5%) were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | AstraZeneca funded the study. Data may support bias for rosuvastatin |
| Methods | 6‐week washout period 6‐week before‐and‐after trial evening doses | |
| Participants | 153 men and women with severe hypercholesterolaemia age ≥ 18 years LDL‐cholesterol 190‐400 mg/dl ( 4.9‐10.3 mmol/l ) TG < 400 mg/dl (4.5 mmol/l) all participants received 40 mg/day rosuvastatin exclusion criteria: liver disease, active arterial disease, cancer history, uncontrolled hypertension or hypothyroidism, homozygous familial hypercholesterolaemia or familial dysbetalipoproteinaemia, use of medications known to affect lipid measurements, present a safety concern or interfere with trial participation Rosuvastatin 40 mg/day baseline TC : 8.84 mmol/l (342 mg/dl) | |
| Interventions | Rosuvastatin 40 mg/day | |
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | There was only one group of participants analyzed therefore assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | There was only one group of participants analyzed therefore assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | There was only one group of participants analyzed therefore it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | AstraZeneca funded it. Data may support bias for rosuvastatin |
| Methods | 3‐week washout period 4‐week randomized single‐blind trial | |
| Participants | 29 men and women age 40‐60 years with type 2 diabetes mellitus BMI < 30, good glycaemic control, LDL‐C > 2.58 mmol/l ( > 100 mg/dl) 14 randomized to rosuvastatin 15 randomized to simvastatin exclusion criteria: history of cardiovascular, neoplastic or other systemic diseases Rosuvastatin 20 mg/day baseline TC : 5.01 mmol/l (194 mg/dl) Rosuvastatin 20 mg/day baseline non‐HDL‐C: 4.13 mmol/l (160 mg/dl) | |
| Interventions | Rosuvastatin 20 mg/day Simvastatin 20 mg/day | |
| Outcomes | per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and non‐HDL‐C | |
| Notes | Simvastatin 20 mg/day group was not included in the efficacy analysis SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 20 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 20 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 20 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | triglycerides were not included in the efficacy analysis because they were expressed as medians |
| Other bias | Low risk | study was not funded by pharmaceutical industry |
| Methods | 12‐week washout period 8‐week open study | |
| Participants | 187 men and women with dyslipidaemia Patients were classified according to their vascular risk factors based on the NCEP ATP III 2001 recommendations 98 were randomized to rosuvastatin 10 mg/day 89 were randomized to ezetimibe/simvastatin 10/20 mg/day exclusion criteria: not taking medications appropriately combining test drugs with other lipid‐lowering agents baseline or final data were missing Rosuvastatin 10 mg/day baseline TC : 6.42 mmol/l (248 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 5.76 mmol/l (223 mg/dl) | |
| Interventions | rosuvastatin 10 mg/day ezetimibe/simvastatin 10/20 mg/day | |
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | ezetimibe/simvastatin 10/20 mg/day group was not included in the efficacy analysis SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | Study was funded by AstaZeneca Mexico. Data may support bias for rosuvastatin |
| Methods | 6‐week washout period 52 week randomized double‐blind trial | |
| Participants | 477 men and women age ≥ 18 years with hypercholesterolaemia LDL‐C ≥160 and < 250 mg/dl (≥ 4.14 and < 6.465 mmol/l) TG ≤ 400 mg/dl ( ≤ 4.52 mmol/l) 123 patients received 5 mg rosuvastatin 116 patients received 10 mg rosuvastatin 118 patients received 20 mg pravastatin 120 patients received 20 mg simvastatin exclusion criteria: active liver disease or dysfunction, renal dysfunction, familial hypercholesterolaemia, pregnancy or lactation active arterial disease within 3 months of trial, cancer history, uncontrolled hypertension, hypothyroidism serum CK > 3X ULN Rosuvastatin 5 mg/day baseline TC : 7.15 mmol/l (276 mg/dl) Rosuvastatin 5 mg/day baseline non‐HDL‐C: 5.84 mmol/l (226 mg/dl) Rosuvastatin 10 mg/day baseline TC : 7.05 mmol/l (273 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 5.76 mmol/l (223 mg/dl) | |
| Interventions | Rosuvastatin 5 mg/day for 0‐12 weeks Rosuvastatin 5‐80 mg/day for 12‐52 weeks Rosuvastatin 10 mg/day for 0‐12 weeks Rosuvastatin 10‐80 mg/day for 12‐52 weeks Pravastatin 20 mg/day for 0‐12 weeks Pravastatin 20‐40 mg/day for 12‐52 weeks Simvastatin 20 mg/day for 0‐12 weeks Simvastatin 20‐80 mg/day for 12‐52 weeks | |
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | Rosuvastatin 5‐80 mg/day for 12‐52 weeks Rosuvastatin 10‐80 mg/day for 12‐52 weeks Pravastatin 20 mg/day for 0‐12 weeks Pravastatin 20‐40 mg/day for 12‐52 weeks Simvastatin 20 mg/day for 0‐12 weeks Simvastatin 20‐80 mg/day for 12‐52 weeks groups were not included in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 5 mg/day for 0‐12 weeks, 10 mg/day for 0‐12 weeks and 20 mg/day for 0‐12 weeks treatment arms were analyzed and since there was no placebo group to compare them to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 5 mg/day for 0‐12 weeks, 10 mg/day for 0‐12 weeks and 20 mg/day for 0‐12 weeks treatment arms were analyzed and since there was no placebo group to compare them to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 5 mg/day for 0‐12 weeks, 10 mg/day for 0‐12 weeks and 20 mg/day for 0‐12 weeks treatment arms were analyzed and since there was no placebo group to compare them to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 2/123 for rosuvastatin 5 mg/day were not included in the analysis 1/116 for rosuvastatin 10 mg/day were not included in the analysis 1.3% participants were not included in the analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | Trial was supported by AstraZeneca Pharmaceuticals. Data may support bias for rosuvastatin |
| Methods | 6‐week washout period for participants with ongoing statin treatment no washout required for participants naive to all lipid‐lowering treatment 8‐week randomized double‐blind trial | |
| Participants | 317 men and women ≥ 18 years with type IIa and IIb hypercholesterolaemia LDL‐C > 160 mg/dl ( > 4.14 mmol/l) in the presence of 2 other cardiovascular risk factors LDL‐C > 130 mg/dl (> 3.36 mmol/l) in the presence of more than 2 other cardiovascular risk factors 110 patients were randomized to rosuvastatin 5 mg/day 104 patients were randomized to atorvastatin 10 mg/day 103 patients were randomized to pravastatin 40 mg/day exclusion criteria: familial hypercholesterolaemia, TG > 400 mg/dl ( > 4.52 mmol/l) 10‐year CHD risk > 20 % statin hypersensitivity, concomitant drug use not authorized during the study active liver disease, CPK > 3 X ULN, renal dysfunction poorly controlled hypertension or hypothyroidism | |
| Interventions | rosuvastatin 5 mg/day atorvastatin 10 mg/day pravastatin 40 mg/day | |
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Notes | atorvastatin 10 mg/day pravastatin 40 mg/day groups were not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 5 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 5 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 5 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | non‐HDL‐C was not included in the efficacy analysis |
| Other bias | High risk | AstraZeneca funded the trial. Data may support bias for rosuvastatin |
| Methods | 6‐week washout period 4‐week randomized open study evening dosing | |
| Participants | 270 men and women age ≥ 18 years with combined hyperlipidaemia and low HDL‐C TC ≥200 mg/dl ( ≥ 5.17 mmol/l ) TG 200‐800 mg/dl (2.26‐9.03 mmol/l) HDL‐C <45 mg/dl (<1.16 mmol/l) 46 received rosuvastatin 72 received niacin 152 received rosuvastatin and niacin exclusion criteria: pregnancy or lactation, liver disease or dysfunction, active arterial disease within 3 months of trial uncontrolled hypertension or hypothyroidism CK > 3 X ULN use of concomitant medications known to affect serum lipid levels or potential safety concerns Rosuvastatin 40 mg/day baseline LDL‐C : 3.78 mmol/l (146 mg/dl) | |
| Interventions | Rosuvastatin 40 mg/day for 12 weeks ER niacin 2 g/day for 12 weeks Rosuvastatin/ER niacin 40 mg/1g/day for 12 weeks Rosuvastatin/ER niacin 10 mg/2g/day for 12 weeks | |
| Outcomes | per cent change from baseline at 4,6,12 weeks of serum LDL‐C | |
| Notes | ER niacin 2 g/day for 12 weeks Rosuvastatin/ER niacin 40 mg/1g/day for 12 weeks Rosuvastatin/ER niacin 10 mg/2g/day for 12 weeks groups were not included in the analysis SD was imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 40 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 40 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 40 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | TC, HDL‐C, TG and non‐HDL‐C were not included in the efficacy analysis |
| Other bias | High risk | AstraZeneca funded the study. Data may support bias for rosuvastatin |
| Methods | 4‐week washout period 6‐week double‐blind randomized study | |
| Participants | 2959 men and women age 18‐81 years LDL‐C ≥ 145 mg/dl ( ≥3.7 mmol/l) and ≤ 250 mg/dl ( ≤ 6.5 mmol/l) TG ≤ 350 mg/dl ( ≤ 4.0 mmol/l) ALT, AST, CK ≤ 1.5 X ULN haemoglobin Alc < 9.0% in patients with diabetes 1481 received rosuvastatin 1478 received ezetimibe/simvastatin exclusion criteria: none Rosuvastatin 10 mg/day baseline TC : 6.7 mmol/l (259 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C : 5.33 mmol/l (206 mg/dl) Rosuvastatin 20 mg/day baseline TC : 6.7 mmol/l (259 mg/dl) Rosuvastatin 20 mg/day baseline non‐HDL‐C : 5.35 mmol/l (207 mg/dl) Rosuvastatin 40 mg/day baseline TC : 6.7 mmol/l (259 mg/dl) Rosuvastatin 40 mg/day baseline non‐HDL‐C : 5.35 mmol/l (207 mg/dl) | |
| Interventions | Rosuvastatin 10 mg/day Rosuvastatin 20 mg/day Rosuvastatin 40 mg/day EZ/simvastatin 10/20 mg/day EZ/simvastatin 10/40 mg/day EZ/simvastatin 10/80 mg/day | |
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and non‐HDL‐C | |
| Notes | EZ/simvastatin 10/20 mg/day EZ/simvastatin 10/40 mg/day EZ/simvastatin 10/80 mg/day groups were not included in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day, Rosuvastatin 20 mg/day, Rosuvastatin 40 mg/day treatment arms were analyzed and since there was no placebo group to compare them to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day, Rosuvastatin 20 mg/day, Rosuvastatin 40 mg/day treatment arms were analyzed and since there was no placebo group to compare them to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day, Rosuvastatin 20 mg/day, Rosuvastatin 40 mg/day treatment arms were analyzed and since there was no placebo group to compare them to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 17/492 were not included in the efficacy analysis for rosuvastatin 10 mg/day 17/495 were not included in the efficacy analysis for rosuvastatin 20 mg/day 19/494 were not included in the efficacy analysis for rosuvastatin 40 mg/day 3.6 % participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | triglycerides were not included in the efficacy analysis |
| Other bias | High risk | Merck/Schering‐Plough Pharmaceutical funded the study. Data may support bias against rosuvastatin |
| Methods | participants were not receiving any lipid‐altering agents within 6 months of study no washout period was required 12‐week before‐and‐after trial | |
| Participants | 20 women received metformin for 12 weeks 18 women received metformin and 10 mg/day rosuvastatin for 12 weeks LDL‐C > 160 mg/dl (> 4.14 mmol/l) exclusion criteria:liver and kidney diseases, patients with Cushing's syndrome hyperprolactinaemia, diabetes mellitus, thyroid disease, congenital adrenal hyperplasia, androgen‐secreting tumours insufficient LH syndrome and other endocrinopathies, using drugs that affect insulin sensitivity, participants taking oral contraceptives Rosuvastatin 10 mg/day baseline TC : 6.56 mmol/l (254 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 5.34 mmol/l (206 mg/dl) | |
| Interventions | metformin metformin and rosuvastatin 10 mg/day | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | metformin group was not analyzed SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the metformin and rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the metformin and rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the metformin and rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Low risk | study was supported by the Research Fund of the University of Istanbul, Turkey not industry‐funded |
| Methods | 375 LLT‐naive participants no washout period required 12‐week open‐label trial | |
| Participants | 375 men and women aged ≥ 18 years with hypercholesterolaemia 375 patients received rosuvastatin 10 mg/day for 12 weeks exclusion criteria: homozygous familial hypercholesterolaemia, secondary hypercholesterolaemia liver disease or dysfunction, CK > 3 X ULN, renal dysfunction and statin hypersensitivity | |
| Interventions | rosuvastatin 10 mg/day | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | There was only one group of participants analyzed therefore assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | There was only one group of participants analyzed therefore assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | There was only one group of participants analyzed therefore it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | source of funding not reported |
| Methods | 4‐week washout period 12‐week open trial | |
| Participants | 30 men and women mean age 48 years with primary dyslipidaemia TG >200 mg/dl ( > 5.17 mmol/l) TC > 200 mg/dl (> 5.17 mmol/l) LDL‐C > 130 mg/dl ( > 3.36 mmol/l) HDL‐C < 35 mg/dl ( < 0.905 mmol/l) for men HDL‐C < 45 mg/dl ( < 1.16 mmol/l) for women 30 patients received rosuvastatin 10 mg/day exclusion criteria: use of lipid‐lowering drugs or drug that affect lipid metabolism supplements, hepatic or renal dysfunction, sustained hypertension diabetes mellitus, BMI ≥ 30, smoking , hypothyroidism, infection, cancer and/or major surgery or illness Rosuvastatin 10 mg/day baseline TC : 6.65 mmol/l (257 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 5.41 mmol/l (209 mg/dl) | |
| Interventions | Rosuvastatin 10 mg/day for 12 weeks | |
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | there was only one group of participants analyzed therefore assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | There was only one group of participants analyzed therefore assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | There was only one group of participants analyzed therefore it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | source of funding not reported |
| Methods | participants were not receiving any lipid‐altering agents no washout period is required 20‐week randomized open‐label trial | |
| Participants | 31 men and women age 40‐65 years with hypercholesterolaemia TC > 200 mg/dl (> 5.17 mmol/l) LDL‐C > 130 mg/dl (> 3.36 mmol/l) 16 participants were randomized to rosuvastatin 10 mg/day for 0‐10 weeks 16 participants were randomized to rosuvastatin 10 mg/day for 10‐20 weeks 15 participants were randomized to rosuvastatin 10 mg/day and exercise for 0‐10 weeks 15 participants were randomized to rosuvastatin 10 mg/day and exercise for 10‐20 weeks exclusion criteria:MI or stroke, liver or kidney disease, physical disability acute illness, hypothyroidism, diabetes mellitus, renal dysfunction other drugs that interfere with the study drug Rosuvastatin 10 mg/day baseline TC : 6.58 mmol/l (254 mg/dl) | |
| Interventions | rosuvastatin 10 mg/day for 0‐10 weeks rosuvastatin 10 mg/day for 10‐20 weeks rosuvastatin 10 mg/day and exercise for 0‐10 weeks rosuvastatin 10 mg/day and exercise for 10‐20 weeks | |
| Outcomes | per cent change from baseline at 10 weeks of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Notes | rosuvastatin 10 mg/day for 10‐20 weeks rosuvastatin 10 mg/day and exercise for 10‐20 weeks time periods were not included in the efficacy analysis SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day 0‐10 weeks treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day 0‐10 weeks treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day 0‐10 weeks treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | AstraZeneca sponsored the trial. Data may support bias for rosuvastatin |
| Methods | 4‐week washout period 12‐week randomized double‐blind placebo‐controlled trial | |
| Participants | 401 men and women age ≥ 18 years with metabolic syndrome LDL‐C ≥ 3.36 mmol/l (130 mg/dl) TG ≥ 1.70 mmol/l (150 mg/dl) HDL‐C < 1.04 mmol/l (40 mg/dl) for men HDL‐C < 1.30 mmol/l (50 mg/dl) for women glucose ≥ 6.11 mmol/l (110 mg/dl) 10 year CHD risk score of > 10% 79 patients were randomized to placebo 165 patients were randomized to rosuvastatin 157 patients were randomized to atorvastatin exclusion criteria:patients with diabetes mellitus,, use of lipid‐lowering agents within the past 6 months TG ≥ 5.65 mmol/l (500 mg/dl), LDL‐C ≥ 6.48 mmol/l (250 mg/dl) documented history of CHD or other atherosclerotic disease familial hypercholesterolaemia, statin hypersensitivity, uncontrolled hypertension or hypothyroidism acute liver disease or dysfunction, serum CK > 3 x ULN and the use of concomitant medications Placebo baseline TC : 6.60 mmol/l (255 mg/dl) Placebo baseline non‐HDL‐C: 5.35 mmol/l (207 mg/dl) Rosuvastatin 10 mg/day baseline TC : 6.48 mmol/l (251 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 5.4 mmol/l (209 mg/dl) | |
| Interventions | Placebo from 0‐6 weeks Rosuvastatin 10 mg/day from 0‐6 weeks Atorvastatin 10 mg/day from 0‐6 weeks Rosuvastatin 20 mg/day from 6‐12 weeks Atorvastatin 20 mg/day from 6‐12 weeks | |
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C WDAEs | |
| Notes | Atorvastatin 10 mg/day from 0‐6 weeks Rosuvastatin 20 mg/day from 6‐12 weeks Atorvastatin 20 mg/day from 6‐12 weeks groups were not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not reported |
| Blinding (performance bias and detection bias) | Low risk | double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | 1/79 placebo group was not included in the efficacy analysis 1/165 rosuvastatin group was not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | The study was supported by AstraZeneca. Data may support bias for rosuvastatin |
| Methods | 6‐week washout period 18‐week randomized open‐label study | |
| Participants | 263 men and women ≥ 18 years with type 2 diabetes mellitus LDL‐C > 2.99 to ≤ 5.00 mmol/l ( >116 to ≤193 mg/dl) TG <4.52 mmol/l ( < 400 mg/dl) 131 patients were randomized to rosuvastatin 132 patients were randomized to atorvastatin exclusion criteria:statin hypersensitivity, active cardiovascular disease, uncontrolled hypertension pregnancy lactation, renal dysfunction, uncontrolled hypothyroidism, TSH > 1.5 X ULN homozygous familial hypercholesterolaemia or familial dysbetalipoproteinaemia, active liver disease or dysfunction serum CK > 3 X ULN Rosuvastatin 10 mg/day baseline TC : 6.34 mmol/l (245 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 5.08 mmol/l (196 mg/dl) | |
| Interventions | Rosuvastatin 10 mg/day for 0‐6 weeks Rosuvastatin 20 mg/day for 6‐12 weeks Rosuvastatin 40 mg/day for 12‐18 weeks Atorvastatin 20 mg/day for 0‐6 weeks Atorvastatin 40 mg/day for 6‐12 weeks Atorvastatin 80 mg/day for 12‐18 weeks | |
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | Rosuvastatin 20 mg/day for 6‐12 weeks Rosuvastatin 40 mg/day for 12‐18 weeks Atorvastatin 20 mg/day for 0‐6 weeks Atorvastatin 40 mg/day for 6‐12 weeks Atorvastatin 80 mg/day for 12‐18 weeks groups were not included in the analysis SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day 0‐6 weeks treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day 0‐6 weeks treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day 0‐6 weeks treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the analysis |
| Other bias | High risk | AstraZeneca financially supported and monitored the study. Data may support bias for rosuvastatin |
| Methods | 6 week washout period 12 week randomized double‐blind placebo‐controlled trial | |
| Participants | 519 men and women age ≥ 18 years with type IIa or IIb hypercholesterolemia LDL‐C ≥4.14 mmol/l (160 mg/dl) and <6.47 mmol/l (250 mg/dl) TG ≤4.52 mmol/l (400 mg/dl) 132 randomized to placebo 259 randomized to rosuvastatin 128 randomized to atorvastatin exclusion criteria:active arterial disease within 3 months of study, homozygous familial hypercholesterolemia uncontrolled hypertension, liver disease or dysfunction, glyciated hemoglobin >9% Placebo baseline TC : 7.06 mmol/l (273 mg/dl) Rosuvastatin 5 mg/day baseline TC : 7.18 mmol/l (278 mg/dl) Rosuvastatin 10 mg/day baseline TC : 7.03 mmol/l (272 mg/dl) | |
| Interventions | Placebo for 12 weeks Rosuvastatin 5 mg/day for 12 weeks Rosuvastatin 10 mg/day for 12 weeks Atorvastatin 10 mg/day for 12 weeks | |
| Outcomes | percent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and triglycerides WDAEs | |
| Notes | Placebo and rosuvastatin groups were analyzed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not reported |
| Blinding (performance bias and detection bias) | Low risk | double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | 1/129 was not included in the efficacy analysis for the rosuvastatin 5 mg/day group 1/130 was not included in the efficacy analysis for the rosuvastatin 10 mg/day group 1% of the rosuvastatin group was not in the efficacy analysis all placebo subjects were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | AstraZeneca funded the study Data may support bias for rosuvastatin |
| Methods | 6‐week washout period for LLT‐naive patients 12‐week randomized open‐label study | |
| Participants | 1482 men and women aged ≥ 18 years with primary hypercholesterolaemia LDL‐C > 3.5 mmol/l ( > 135 mg/dl) TG ≤ 4.52 mmol/l (≤ 400 mg/dl) a history of CHD, atherosclerosis 10‐year CHD risk score of > 20% diabetes mellitus 995 patients were randomized to rosuvastatin 10 mg/day 487 patients were randomized to atorvastatin 10 mg/day exclusion criteria: familial hypercholesterolaemia or dysbetalipoproteinaemia secondary hypercholesterolaemia of any cause, uncontrolled hypertension or diabetes mellitus active liver disease or dysfunction, statin hypersensitivity serum CK > 3 X ULN, renal dysfunction and unstable angina within 3 months of the study Rosuvastatin 10 mg/day baseline TC : 6.47 mmol/l (248 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 5.16 mmol/l (200 mg/dl) | |
| Interventions | Rosuvastatin 10 mg/day 516 naive participants Rosuvastatin 10 mg/day 434 switched participants Atorvastatin 10 mg/day 267 naive participants Atorvastatin 10 mg/day 204 switched participants | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | Rosuvastatin 10 mg/day 434 switched participants Atorvastatin 10 mg/day 267 naive participants Atorvastatin 10 mg/day 204 switched participants groups were not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day naive participants treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day naive participants treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day naive participants treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | This study was sponsored by AstraZeneca. Data may support bias for rosuvastatin |
| Methods | 4‐week washout period 15‐week open‐label cross‐over trial | |
| Participants | 45 men and women age 18‐80 years with hypercholesterolaemia LDL‐C > 3.5 mmol/l ( > 135 mg/dl) 41 participants received rosuvastatin 10 mg/day for 0‐6 weeks 41 participants received rosuvastatin 20 mg every other day for 0‐6 weeks 41 participants received rosuvastatin 20 mg every other day for 9‐15 weeks 41 participants received rosuvastatin 10 mg/day for 9‐15 weeks | |
| Interventions | rosuvastatin 10 mg/day for 0‐6 weeks rosuvastatin 20 mg every other day for 0‐6 weeks rosuvastatin 20 mg every other day for 9‐15 weeks rosuvastatin 10 mg/day for 9‐15 weeks | |
| Outcomes | per cent change from baseline at 6 weeks of serum LDL‐C | |
| Notes | rosuvastatin 20 mg every other day for 0‐6 weeks rosuvastatin 20 mg every other day for 9‐15 weeks rosuvastatin 10 mg/day for 9‐15 weeks groups were not included in the efficacy analysis SD was imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 4/45 (8.9%) participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | total cholesterol, HDL‐C , non‐HDL‐C and triglycerides were not included in the efficacy analysis |
| Other bias | Low risk | funded by The Physicians` Services Incorporated Foundatioin, Grant RO4‐42 |
| Methods | 6‐week washout period 6‐week randomized double‐blind placebo‐controlled trial | |
| Participants | 216 men and women age ≥ 18 years with type 2 diabetes, hypertriglyceridaemia and HbA1c < 10% TG ≥ 200 to < 800 mg/dl (≥2.26 to <9.03 mmol/l) TC ≥ 200 mg/dl (≥ 5.17 mmol/), glycated haemoglobin < 10% 53 randomized to placebo for 5 mg/day rosuvastatin 49 randomized to placebo for 10 mg/day rosuvastatin 60 randomized to rosuvastatin 5 mg/day 54 randomized to rosuvastatin 10 mg/day exclusion criteria:type 1 diabetes, history of diabetic ketoacidosis, use of lipid‐lowering drugs or supplements, pregnancy or lactation uncontrolled hypertension, acute ischaemic event within 3 months of trial entry, alcohol abuse, active liver disease or dysfunction serum CK > 3 X ULN Placebo for 5 mg/day Rosuvastatin baseline TC : 6.2 mmol/l (240 mg/dl) Placebo for 10 mg/day Rosuvastatin baseline TC : 6.3 mmol/l (244 mg/dl) 5 mg/day Rosuvastatin baseline TC : 6.5 mmol/l (251 mg/dl) 5 mg/day Rosuvastatin baseline LDL‐C: 3.9 mmol/l (151 mg/dl) 5 mg/day Rosuvastatin baseline HDL‐C: 1.1 mmol/l (42.5 mg/dl) 5 mg/day Rosuvastatin baseline TG: 3.5 mmol/l (310 mg/dl) 10 mg/day Rosuvastatin baseline TC : 6.4 mmol/l (247 mg/dl) 10 mg/day Rosuvastatin baseline LDL‐C: 3.9 mmol/l (151 mg/dl) 10 mg/day Rosuvastatin baseline HDL‐C:1.0 mmol/l (38.7 mg/dl) 10 mg/day Rosuvastatin baseline TG: 3.5 mmol/l (310 mg/dl) | |
| Interventions | Placebo for 5 mg/day Rosuvastatin for 6 weeks Placebo for 10 mg/day Rosuvastatin for 6 weeks Rosuvastatin 5 mg/day for 6 weeks Rosuvastatin 10 mg/day for 6 weeks | |
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and triglycerides WDAEs | |
| Notes | all interventions were analyzed for efficacy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not reported |
| Blinding (performance bias and detection bias) | Low risk | double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | 2/53 placebo for 5 mg/day rosuvastatin were not included in the efficacy analysis 1/54 rosuvastatin 10 mg/day was not included in the efficacy analysis 1.4% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | non HDL‐C was not included in the efficacy analysis |
| Other bias | High risk | This research was supported by AstraZeneca. Data may support bias for rosuvastatin |
| Methods | participants were not receiving any lipid‐altering agents no washout period required 3‐week open‐label trial | |
| Participants | 47 men and women mean age 60 years with acute myocardial infarction 26 participants received rosuvastatin 10 mg/day for 3 weeks 21 participants received standard therapy but no statins exclusion criteria: active liver disease, renal dysfunction myopathy / rhabdomyolysis followed by a persistent increase in CPK more than 3 times upper limit normal prior to taking statins and other lipid‐reducing agents, cancer, connective tissue diseases, clinical and lab sign of inflammation and hypothyroidism Rosuvastatin 10 mg/day baseline TC : 5.81 mmol/l (225 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 4.41 mmol/l (171 mg/dl) | |
| Interventions | rosuvastatin 10 mg/day standard therapy but no statins | |
| Outcomes | per cent change from baseline at 3 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | standard therapy but no statins group was not included in the efficacy analysis SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | source of funding not reported |
| Methods | 6‐week washout period 24‐week open‐label randomized trial | |
| Participants | 1036 men and women aged ≥18 years with hypercholesterolaemia and a history of CHD, clinical evidence of atherosclerosis or a 10‐year CHD risk score > 20% LDL‐C ≥ 160 to < 250 mg/dl ( 4.14 to <6.47 mmol/l) TG <400 mg/dl ( < 4.52 mmol/l) 522 participants were randomized to rosuvastatin 10 mg/day 514 participants were randomized to atorvastatin 10 mg/day exclusion criteria: history of statin‐induced myopathy, statin hypersensitivity clinical instability after a cardiovascular event, homozygous familial hypercholesterolaemia uncontrolled hypothyroidism, severe hepatic impairment, serum CK > 3 X ULN, renal dysfunction pregnancy, lactation | |
| Interventions | rosuvastatin 10 mg/day for 0‐6 weeks rosuvastatin 20 mg/day for 6‐12 weeks rosuvastatin 40 mg/day for 12‐24 weeks atorvastatin 10 mg/day for 0‐6 weeks atorvastatin 20 mg/day for 6‐12 weeks atorvastatin 40 mg/day for 12‐18 weeks atorvastatin 80 mg/day for 18‐24 weeks | |
| Outcomes | per cent change from baseline at 6weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | rosuvastatin 20 mg/day for 6‐12 weeks rosuvastatin 40 mg/day for 12‐24 weeks atorvastatin 10 mg/day for 0‐6 weeks atorvastatin 20 mg/day for 6‐12 weeks atorvastatin 40 mg/day for 12‐18 weeks atorvastatin 80 mg/day for 18‐24 weeks groups were not included in the efficacy analysis SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 24/522 participants were not included in the efficacy analysis 4.6% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | AstraZeneca funded the study. Data may support bias for rosuvastatin |
| Methods | no lipid‐lowering medication had been administered within 3 months of trial enrolment no washout period required 12 week open‐label clinical study | |
| Participants | 97 men and women age 18‐69 years with metabolic syndrome LDL‐C > 130 mg/dl (3.36 mmol/l) HDL‐C < 40 mg/dl (1.03 mmol/l) in males < 50 mg/dl (1.29 mmol/l) in females TG < 400 mg/dl ( 4.52 mmol/l) 97 patients received rosuvastatin 10 mg/day for 0‐6 weeks 85 patients received rosuvastatin 20 mg/day for 6‐12 weeks exclusion criteria:concomitant coronary artery disease uncontrolled hypertension, homozygous familial hypercholesterolaemia uncontrolled hypothyroidism, renal failure, history of MI, renal dysfunction history of severe arrhythmia, heart failure, history of syncope cancer history, statin hypersensitivity or myopathy CK > 3 X ULN, alcohol or drug abuse, pregnancy or lactation concomitant medications with warfarin, cyclosporin, gemfibrozil, antacids participation in another investigational drug study less than 4 weeks before enrolment in the study Rosuvastatin 10 mg/day baseline TC : 6.12 mmol/l (237 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 5.10 mmol/l (197 mg/dl) | |
| Interventions | rosuvastatin 10 mg/day for 0‐6 weeks rosuvastatin 20 mg/day for 6‐12 weeks | |
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | rosuvastatin 20mg/day for 6‐12 weeks group was not included in the efficacy analysis SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | High risk | 12/97 = 12.4% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | AstraZeneca funded the study. Data may support bias for rosuvastatin |
| Methods | participants were not receiving any lipid‐altering agents no washout period is required 12‐week randomized double‐blind placebo‐controlled trial | |
| Participants | 42 men and women with chronic heart failure 22 patients were randomized to rosuvastatin 40 mg/day 20 patients were randomized to placebo exclusion criteria:renal failure, liver dysfunction, type 1 diabetes mellitus valvular heart disease, uncontrolled hypertension, muscle disease previous treatment with statins or other lipid‐altering agents fibrates immunosuppressants Placebo baseline LDL‐C : 3.91 mmol/l (151 mg/dl) Rosuvastatin 40 mg/day baseline LDL‐C : 3.66 mmol/l (142 mg/dl) | |
| Interventions | Placebo Rosuvastatin 40 mg/day | |
| Outcomes | per cent change from baseline at 12 weeks of serum LDL‐C, HDL‐C and triglycerides | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | method of random sequence generation not reported |
| Allocation concealment (selection bias) | Low risk | allocation was done by a third party Pharmacy of Heart Center and Parkkrankenhaus, Leipzig |
| Blinding (performance bias and detection bias) | Low risk | double‐blind "all patients, investigators and lab staff were blinded" |
| Incomplete outcome data (attrition bias) | Low risk | 2/42 = 4.8% were not included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | total cholesterol and non‐HDL‐C were not included in the efficacy analysis WDAEs were not reported |
| Other bias | High risk | AstraZeneca funded the trial. Data may support bias for rosuvastatin |
| Methods | 6‐week washout period 6‐week open‐label randomized study | |
| Participants | 469 men and women at high risk for cardiovascular disease with hypercholesterolaemia age ≥ 18 years LDL‐C ≥160 mg/dl and < 250 mg/dl ( ≥ 4.14 mmol/l and < 6.465 mmol/l) TG < 400 mg/dl ( 4.516 mmol/l) exclusion criteria: statin‐induced myopathy or serious hypersensitivity reaction history unstable heart disease, myocardial revascularization, coronary artery bypass graft, TIA or stroke, severe congestive heart failure, cancer, uncontrolled hypothyroidism homozygous familial hypercholesterolaemia, current active liver disease or dysfunction use of prohibited medications, pregnancy or lactation change in HRT or use of contraceptives within 3 months of enrolment Rosuvastatin 40 mg/day baseline TC : 7.19 mmol/l (278 mg/dl) Rosuvastatin 40 mg/day baseline non‐HDL‐C: 5.9 mmol/l (228 mg/dl) | |
| Interventions | Rosuvastatin 40 mg/day Rosuvastatin/Ezetimibe 40/10 mg/day combination therapy | |
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | Rosuvastatin group was analyzed SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 40 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 40 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 40 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were measured |
| Other bias | Unclear risk | source of funding not reported |
| Methods | no washout required because participants were not receiving any lipid‐altering agents for at least 4 weeks 3 month before‐and‐after trial | |
| Participants | 40 men and women with hypercholesterolaemia and impaired fasting glucose LDL‐C > 130 mg/dl (3.36 mmol/l), fasting serum glucose of 100‐125 mg/dl (5.6‐6.9 mmol/l) exclusion criteria:cardiovascular disease, DM, TG >300 mg/dl (3.39 mmol/l), renal disease hypothyroidism, liver disease, uncontrolled hypertension and patients taking other medications that could affect glucose homeostasis 20 participants received Rosuvastatin 5 mg/day plus colesevelam 3.75 grams/day 20 participants received Rosuvastatin 5 mg/day Rosuvastatin 5 mg/day baseline TC : 6.88 mmol/l (266 mg/dl) Rosuvastatin 5 mg/day baseline non‐HDL‐C: 5.35 mmol/l (207 mg/dl) | |
| Interventions | Rosuvastatin 5 mg/day plus colesevelam 3.75 grams/day Rosuvastatin 5 mg/day | |
| Outcomes | per cent change from baseline at 3 months of serum TC, LDL‐C, HDL‐C, and non‐HDL‐C | |
| Notes | Rosuvastatin 5 mg/day plus colesevelam 3.75 g/day group was not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 5 mg/day for 3 months treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 5 mg/day for 3 months treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 5 mg/day for 3 months treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | blood triglycerides were not included in the efficacy analysis because it was a median per cent change |
| Other bias | Unclear risk | source of funding not reported |
| Methods | 6‐week washout period 12‐week randomized double‐blind trial | |
| Participants | 304 patients age 18‐75 years with hypercholesterolaemia LDL‐C ≥ 160 mg/dl to < 250 mg/dl ( ≥ 4.14 mmol/l to < 6.465 mmol/l) TG < 400 mg/dl ( < 4.52 mmol/l) 191 patients were randomized to rosuvastatin 99 patients were randomized to atorvastatin Rosuvastatin 10 mg/day baseline TC : 6.81 mmol/l (263 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 5.35 mmol/l (207 mg/dl) | |
| Interventions | Rosuvastatin 10 mg/day for 0‐12 weeks Rosuvastatin 20 mg/day for 12‐20 weeks Atorvastatin 10 mg/day for 0‐12 weeks | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | Rosuvastatin 20 mg/day for 12‐20 weeks Atorvastatin 10 mg/day for 0‐12 weeks groups were not included in the efficacy analysis SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | AstraZeneca funded the study. Data may support bias for rosuvastatin |
| Methods | participants are not on any lipid‐altering agents 12‐week open‐label placebo‐controlled trial evening dosing | |
| Participants | 48 men and women age ≥ 65 years with hypertension and dyslipidaemia LDL‐C ≥100 mg/dl ( ≥ 2.59 mmol/l) TG ≥150 mg/dl ( ≥ 1.69 mmol/l) HDL‐C <40 mg/dl (<1.03 mmol/l) in men HDL‐C <50 mg/dl (<1.29 mmol/l) in women 16 participants received rosuvastatin 10 mg/day 16 participants received metformin 1.7 g/day 16 participants received placebo 10 mg/day Placebo baseline TC : 6.25 mmol/l (242 mg/dl) Rosuvastatin 10 mg/day baseline TC : 5.90 mmol/l (228 mg/dl) | |
| Interventions | placebo rosuvastatin metformin | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Notes | metformin 1.7 g/day group was not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | method of random sequence generation not reported |
| Allocation concealment (selection bias) | High risk | no allocation concealment |
| Blinding (performance bias and detection bias) | High risk | open‐label |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | WDAEs were not included in the analysis |
| Other bias | Low risk | not funded by the pharmaceutical industry |
| Methods | 6‐week washout period 12‐week randomized open‐label trial | |
| Participants | 833 patients ≥ 18 years with hypercholesterolaemia and CHD or CHD risk score > 20% LDL‐C ≥130 to < 220 mg/dl ( ≥3.36 mmol/l to < 5.69 mmol/l) TG < 400 mg/dl ( 4.52 mmol/l) 214 participants randomized to rosuvastatin 10 mg/day for 0‐6 weeks 214 participants randomized to rosuvastatin 10 mg/day+ezetimibe 10 mg/day for 6‐12 weeks 214 participants randomized to rosuvastatin 20 mg/day for 0‐6 weeks 214 participants randomized to rosuvastatin 20 mg/day+ezetimibe 10 mg/day for 6‐12 weeks 202 participants randomized to simvastatin 40 mg/day for 0‐6 weeks 202 participants randomized to simvastatin 40 mg/day+ezetimibe 10 mg/day for 6‐12 weeks 203 participants randomized to simvastatin 80 mg/day for 0‐6 weeks 203 participants randomized to simvastatin 80 mg/day+ezetimibe 10 mg/day for 6‐12 weeks exclusion criteria: myocardial infarction, recent episode of angina, PTCA, CABG, TIA stroke and patients awaiting a planned myocardial revascularization statin or ezetimibe hypersensitivity, use of lipid‐lowering drugs and other prohibited concomitant medications | |
| Interventions | rosuvastatin 10 mg/day rosuvastatin 10 mg/day+ezetimibe 10 mg/day rosuvastatin 20 mg/day rosuvastatin 20 mg/day+ezetimibe 10 mg/day simvastatin 40 mg/day simvastatin 40 mg/day+ezetimibe 10 mg/day simvastatin 80 mg/day simvastatin 80 mg/day+ezetimibe 10 mg/day | |
| Outcomes | LDL‐C | |
| Notes | rosuvastatin 10 mg/day+ezetimibe 10 mg/day rosuvastatin 20 mg/day+ezetimibe 10 mg/day rosuvastatin 20 mg/day+ezetimibe 10 mg/day simvastatin 40 mg/day simvastatin 40 mg/day+ezetimibe 10 mg/day simvastatin 80 mg/day simvastatin 80 mg/day+ezetimibe 10 mg/day groups were not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 8/214 participants rosuvastatin 10 mg/day group 15/214 participants rosuvastatin 20 mg/day group 23/428 (5.4%) were not included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | only LDL‐C was included in the efficacy analysis |
| Other bias | High risk | AstraZeneca funded the trial. Data may support bias for rosuvastatin |
| Methods | participants were not receiving any lipid‐altering agents within 3 months of study no washout period was required 12‐week before‐and‐after trial | |
| Participants | 30 participants received 10 mg/day rosuvastatin for 12 weeks 30 participants received 40 mg/day simvastatin for 12 weeks 30 participants received control diet for 12 weeks exclusion criteria: hepatic dysfunction, endocrine disorders, recent major surgery or cancer statin intolerance and participation in other clinical trials Rosuvastatin 10 mg/day baseline TC : 6.11 mmol/l (236 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 5.35 mmol/l (207 mg/dl) | |
| Interventions | rosuvastatin 10 mg/day simvastatin 40 mg/day diet | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | simvastatin 40 mg/day and control diet groups were not analyzed SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | no source of funding was reported |
| Methods | 4‐week washout period 4‐week randomized single‐blind trial | |
| Participants | 67 patients 65‐70 years with hypercholesterolaemia LDL‐C ≥ 4.14 mmol/l and ≤ 6.50 mmol/l ( ≥ 160 mg/dl and ≤ 251 mg/dl TG ≤ 4.52 mmol/l ( ≤ 400 mmol/l) 33 patients received rosuvastatin 10 mg/day 34 patients received atorvastatin 20 mg/day Rosuvastatin 10 mg/day baseline TC : 5.5 mmol/l (213 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 4.26 mmol/l (165 mg/dl) | |
| Interventions | rosuvastatin 10 mg/day atorvastatin 20 mg/day | |
| Outcomes | per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | atorvastatin 20 mg/day group was not included in the efficacy analysis SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | source of funding was not reported |
| Methods | 6‐week washout period 18‐week weighted randomized double‐blind study | |
| Participants | 623 men and women age 45‐50 with heterozygous familial hypercholesterolaemia LDL‐C 220 to < 500 mg/dl (5.7 to < 12.9 mmol/l) TG ≤ 400 mg/dl ( ≤4.5 mmol/l) 436 were randomized to rosuvastatin 187 were randomized to atorvastatin exclusion criteria: hepatic dysfunction, active arterial disease within 3 months of trial uncontrolled hypertension, uncontrolled hypothyroidism, renal dysfunction, CK > 3 X ULN cyclic hormone therapy, use of medication that could affect serum lipid profiles or safety issues Rosuvastatin 20 mg/day baseline TC : 9.62 mmol/l (372 mg/dl) | |
| Interventions | Rosuvastatin 20 mg/day for 0‐6 weeks Rosuvastatin 40 mg/day for 6‐12 weeks Rosuvastatin 80 mg/day for 12‐18 weeks Atorvastatin 20 mg/day for 0‐6 weeks Atorvastatin 40 mg/day for 6‐12 weeks Atorvastatin 80 mg/day for 12‐18 weeks | |
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Notes | Rosuvastatin 40 mg/day for 6‐12 weeks Rosuvastatin 80 mg/day for 12‐18 weeks Atorvastatin 20 mg/day for 0‐6 weeks Atorvastatin 40 mg/day for 6‐12 weeks Atorvastatin 80 mg/day for 12‐18 weeks groups were not included in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 20 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 20 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 20 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 1/436 was not included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | non‐HDL‐C was not included in the efficacy analysis |
| Other bias | High risk | The research was supported by AstraZeneca. Data may support bias for rosuvastatin |
| Methods | 4‐week washout period 12‐week randomized open‐label trial | |
| Participants | 90 men and women aged 20‐79 years with hypercholesterolaemia LDL‐C > 130 mg/dl (> 3.36 mmol/l) TG < 400 mg/dl (< 4.52 mmol/l) 25 were randomized to rosuvastatin 10 mg/day 25 were randomized to atorvastatin 20 mg/day 26 were randomized to atorvastatin/ezetimibe 5 mg/5 mg/day exclusion criteria: familial hypercholesterolaemia, diabetes mellitus pregnancy, lactation, stroke or MI within 3 months of enrolment renal dysfunction, thyroid dysfunction, serum CK > 2.5 X ULN infection, cancer, history of adverse reaction to test drugs Rosuvastatin 10 mg/day baseline TC : 6.26 mmol/l (242 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 4.96 mmol/l (192 mg/dl) | |
| Interventions | rosuvastatin 10 mg/day for 8 weeks atorvastatin 20 mg/day for 8 weeks atorvastatin/ezetimibe 5 mg/5 mg/day for 8 weeks | |
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | atorvastatin 20 mg/day for 8 weeks atorvastatin/ezetimibe 5 mg/5 mg/day for 8 weeks groups were not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 2/27 participants in the rosuvastatin group were not included in the efficacy analysis due to protocol violation 7.4% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Low risk | pharmaceutical industry did not fund this study |
| Methods | 6‐week washout period 6‐week randomized double‐blind placebo‐controlled trial | |
| Participants | 156 men and women with hypertriglyceridaemia type IIb or IV aged 18 years or older were enrolled in the study TG ≥ 300 and < 800 mg/dl ( ≥3.39 and < 9.03mmol/l) glucose ≤ 180 mg/dl (≤ 9.99 mmol/l) or glycosylated haemoglobin ≤ 8% 26 were randomized to placebo 26 were randomized to rosuvastatin 5 mg/day 23 were randomized to rosuvastatin 10 mg/day 28 were randomized to rosuvastatin 20 mg/day 26 were randomized to rosuvastatin 40 mg/day 27 were randomized to rosuvastatin 80 mg/day exclusion criteria: pregnancy, lactation, heterozygous or homozygous familial hypercholesterolaemia type III hyperlipoproteinaemia, active arterial disease within 3 months of trial entry, uncontrolled hypertension cancer history, uncontrolled hypothyroidism, alcohol or drug abuse, active liver disease or dysfunction, elevated serum CK concomitant medications known to affect lipid profiles or present a safety concern Placebo baseline TC : 6.62 mmol/l (256 mg/dl) Placebo baseline non‐HDL‐C : 5.715 mmol/l (221 mg/dl) Rosuvastatin 5 mg/day baseline TC : 6.31 mmol/l (244 mg/dl) Rosuvastatin 5 mg/day baseline non‐HDL‐C : 5.38 mmol/l (208 mg/dl) Rosuvastatin 10 mg/day baseline TC : 6.67 mmol/l (258 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C : 5.69 mmol/l (220 mg/dl) Rosuvastatin 20 mg/day baseline TC : 6.49 mmol/l (251 mg/dl) Rosuvastatin 20 mg/day baseline non‐HDL‐C : 5.61 mmol/l (217 mg/dl) Rosuvastatin 40 mg/day baseline TC : 6.41 mmol/l (248 mg/dl) Rosuvastatin 40 mg/day baseline non‐HDL‐C : 5.53 mmol/l (214 mg/dl) Rosuvastatin 80 mg/day baseline TC : 7.03 mmol/l (272 mg/dl) Rosuvastatin 80 mg/day baseline non‐HDL‐C : 6.08 mmol/l (235 mg/dl) | |
| Interventions | Placebo Rosuvastatin 5 mg/day Rosuvastatin 10 mg/day Rosuvastatin 20 mg/day Rosuvastatin 40 mg/day Rosuvastatin 80 mg/day | |
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not reported |
| Blinding (performance bias and detection bias) | Low risk | double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | 3/156 were not included in the efficacy analysis 1.9 % participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were measured WDAEs were also reported |
| Other bias | High risk | The study was supported by AstraZeneca. Data may support bias for rosuvastatin |
| Methods | no washout period required no participant received lipid‐altering agents 4 week before‐and‐after trial | |
| Participants | 137 men and women with dyslipidaemia who suffered acute ischaemic stroke age 60‐80 years exclusion criteria: history of previous stroke or lipid‐lowering treatment Rosuvastatin 2.5 mg/day baseline TC : 5.87 mmol/l (227 mg/dl) Rosuvastatin 2.5 mg/day baseline non‐HDL‐C : 4.65 mmol/l (180 mg/dl) Rosuvastatin 2.5 mg/day baseline TG : 1.72 mmol/l (152 mg/dl) | |
| Interventions | rosuvastatin 2.5 mg/day for 4 weeks | |
| Outcomes | per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C, non‐HDL‐C and triglycerides | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | there was only one group of participants analyzed therefore assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | there was only one group of participants analyzed therefore assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | there was only one group of participants analyzed therefore it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Low risk | pharmaceutical industry did not funded this trial |
| Methods | participants were not receiving any lipid‐altering agents no washout period is required 12 week before‐and‐after trial | |
| Participants | 26 postmenopausal women age 55 years or older with dyslipidaemia LDL‐C ≥ 140 mg/dl (3.62 mmol/l) exclusion criteria: uncontrolled hypertension, diabetes mellitus, cerebrovascular disease, CAD, peripheral artery disease Rosuvastatin 2.5 mg/day baseline TC : 6.39 mmol/l (247 mg/dl) Rosuvastatin 2.5 mg/day baseline non‐HDL‐C: 4.68 mmol/l (181 mg/dl) | |
| Interventions | rosuvastatin 2.5 mg/day for 12 weeks | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | there was only one group of participants analyzed therefore assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | there was only one group of participants analyzed therefore assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | there was only one group of participants analyzed therefore it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Low risk | not industry funded |
| Methods | 6‐week washout period 6‐week randomized open‐label study | |
| Participants | 740 men and women at risk of CHD LDL‐C ≥100 mg/dl (≥ 2.6 mmol/l); or ≥2 risk factors, 10‐year CHD risk 10% to 20% LDL‐C≥130 mg/dl (≥ 3.4 mmol/l); or 0 or 1 risk factor LDL‐C≥160 mg/dl (≥ 4.1mmol/l) For eligibility, LDL‐C ≤ 300 mg/dl (≤ 7.8 mmol/l TG < 500 mg/dl (5.6mmol/l) 371 randomized to rosuvastatin 369 randomized to atorvastatin exclusion criteria: homozygous familial hypercholesterolaemia, type I, III or V hyperlipoproteinaemia active arterial disease within 3 months of entry into trial, uncontrolled hypertension, poorly controlled diabetes mellitus active liver disease or liver dysfunction CK > 3XULN Rosuvastatin 10 mg/day baseline TC : 6.13 mmol/l (237 mg/dl) Rosuvastatin 20 mg/day baseline TC : 6.03 mmol/l (233 mg/dl) | |
| Interventions | Rosuvastatin 10 mg/day for 6 weeks Rosuvastatin 20 mg/day for 6 weeks Atorvastatin 10 mg/day for 6 weeks Atorvastatin 20 mg/day for 6 weeks | |
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Notes | Atorvastatin 10 mg/day for 6 weeks Atorvastatin 20 mg/day for 6 weeks groups were not included in the analysis SDs were imputed for LDL‐C and HDL‐C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 6/189 was not included in the efficacy analysis for the rosuvastatin 10 mg/day group 11/182 was not included in the efficacy analysis for the rosuvastatin 20 mg/day group 3% rosuvastatin participants was not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | AstraZeneca funded the study. Data may support bias for rosuvastatin |
| Methods | 1‐month washout period 8‐week before‐and‐after trial | |
| Participants | 348 patients with hypercholesterolaemia aged 20 years or older LDL‐C ≥ 140 mg/dL (≥3.62 mmol/l) with maximum IMT ≥ 1.1 mm measured at the carotid artery 173 patients randomized to rosuvastatin 5 mg/day 175 patients randomized to pravastatin 10 mg/day exclusion criteria: severe carotid artery stenosis (≥ 80%) or severe calcification, familial hypercholesterolaemia or secondary hypercholesterolaemia fasting TG ≥ 400 mg/dL ( ≥ 4.52 mmol/l), statin hypersensitivity, uncontrolled hypertension, type 1 diabetes mellitus and uncontrolled type 2 diabetes mellitus MI or stroke within 3 months, severe congestive heart failure, hepatic dysfunction or renal dysfunction, cyclosporine treatment, cancer, hypothyroidism, muscle disease drug and alcohol abuse, pregnancy or potential for pregnancy Rosuvastatin 5 mg/day baseline LDL‐C : 4.24 mmol/l (164 mg/dl) Rosuvastatin 5 mg/day baseline non‐HDL‐C : 4.99 mmol/l (193 mg/dl) Rosuvastatin 5 mg/day baseline TG : 1.69 mmol/l (150 mg/dl) | |
| Interventions | Rosuvastatin 5 mg/day for 8 weeks Pravastatin 10 mg/day for 8 weeks | |
| Outcomes | per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C, non‐HDL‐C and triglycerides | |
| Notes | Pravastatin 10 mg/day for 8 weeks group was not analyzed TC was calculated from HDL‐C and non‐HDL‐C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 5 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 5 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 5 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | High risk | 24/173 (13.9%) patients were not included in the LDL‐C efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Low risk | pharmaceutical industry did not fund this trial |
| Methods | 4‐week placebo run‐in period 4‐week before‐and‐after trial | |
| Participants | 345 men and women with hypercholesterolaemia aged 18‐70 years TC ≥ 5.72 mmol/l (221 mg/dl) LDL‐C ≥ 3.64 mmol/l (141 mg/dl) TG < 4.5 mmol/l (399 mg/dl) exclusion criteria: pregnancy, lactation, liver and kidney dysfunction, myopathy MI major surgery or angioplasty within the last 6 months congestive heart failure or unstable angina, systolic blood pressure ≥180 mmHg and/or diastolic blood pressure ≥ 110 mmHg HMG‐CoA reductase inhibitor hypersensitivity Rosuvastatin 5 mg/day baseline TC : 6.74 mmol/l (261 mg/dl) Rosuvastatin 5 mg/day baseline non‐HDL‐C: 5.35 mmol/l (207 mg/dl) Rosuvastatin 10 mg/day baseline TC : 6.61 mmol/l (256 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 5.27 mmol/l (204 mg/dl) | |
| Interventions | 115 patients randomized to rosuvastatin 5 mg/day 115 patients randomized to rosuvastatin 10 mg/day 115 patients randomized to atorvastatin 10 mg/day | |
| Outcomes | per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C, non‐HDL‐C and triglycerides | |
| Notes | atorvastatin 10 mg/day group was not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 5 mg/day and 10 mg/day treatment arms were analyzed and since there was no placebo group to compare them to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 5 mg/day and 10 mg/day treatment arms were analyzed and since there was no placebo group to compare them to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 5 mg/day and 10 mg/day treatment arms were analyzed and since there was no placebo group to compare them to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 1/115 participants in the rosuvastatin group was not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | source of funding was not reported |
| Methods | 6‐week washout period 12‐week randomized double‐blind trial | |
| Participants | 1445 men and women ≥18 years with mixed dyslipidaemia TG ≥ 150 mg/dl ( ≥ 1.69 mmol/l) HDL‐C < 40 mg/dl ( < 1.03 mmol/l) for men HDL‐C < 50 mg/dl ( < 1.29 mmol/l) for women LDL‐C ≥ 130 mg/dl ( ≥ 3.36 mmol/l) 260 participants were randomized to fenofibrate 135 mg/day 265 participants were randomized to rosuvastatin 10 mg/day 261 participants were randomized to fenofibrate/rosuvastatin 135 mg/10 mg/day 266 participants were randomized to rosuvastatin 20 mg/day 262 participants were randomized to fenofibrate/rosuvastatin 135 mg/20 mg/day 131 participants were randomized to rosuvastatin 40 mg/day exclusion criteria: hypersensitivity to test drugs Asian ancestry, type 1 diabetes mellitus, uncontrolled type 2 diabetes mellitus GI disease, hepatic disease, renal disease, unstable cardiovascular disease, myopathy solid organ transplant, HIV positive, drug or alcohol abuse, mental instability changes in HRT, treatment with excluded medications, abnormal TSH study unsuitability Rosuvastatin 10 mg/day baseline TC : 6.68 mmol/l (258 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 5.66 mmol/l (219 mg/dl) Rosuvastatin 20 mg/day baseline TC : 6.72 mmol/l (260 mg/dl) Rosuvastatin 20 mg/day baseline non‐HDL‐C: 5.71 mmol/l (221 mg/dl) Rosuvastatin 40 mg/day baseline TC : 6.67 mmol/l (258 mg/dl) Rosuvastatin 40 mg/day baseline non‐HDL‐C: 5.66 mmol/l (219 mg/dl) | |
| Interventions | fenofibrate 135 mg/day rosuvastatin 10 mg/day fenofibrate/rosuvastatin 135 mg/10 mg/day rosuvastatin 20 mg/day fenofibrate/rosuvastatin 135 mg/20 mg/day rosuvastatin 40 mg/day | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | fenofibrate 135 mg/day fenofibrate/rosuvastatin 135 mg/10 mg/day fenofibrate/rosuvastatin 135 mg/20 mg/day groups were not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 22/165 participants from the rosuvastatin 10 mg/day group were not included in the efficacy analysis 28/266 participants from the rosuvastatin 20 mg/day group were not included in the efficacy analysis 11/131 participants from the rosuvastatin 40 mg/day group were not included in the efficacy analysis 61/662 = 9.2% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | Financial support was provided by Abbott. Abbott make fenofibrate. Data may be bias against rosuvastatin |
| Methods | no one had treatments with any lipid‐altering drugs, washout not required 3‐month randomized open trial | |
| Participants | 36 men and women mean age 60 years with hypercholesterolaemia and type 2 diabetes mellitus LDL‐C ≥ 100 mg/dl ( ≥ 2.59 mmol/l) 18 were randomized to rosuvastatin 2.5 mg/day 18 were randomized to ezetimibe 10 mg/day exclusion criteria: hepatic or renal dysfunction nutritional derangement Rosuvastatin 2.5 mg/day baseline TC : 5.82 mmol/l (225 mg/dl) Rosuvastatin 2.5 mg/day baseline non‐HDL‐C : 4.19 mmol/l ( 162 mg/dl) | |
| Interventions | Rosuvastatin 2.5 mg/day | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | there was only one group of participants analyzed therefore assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | there was only one group of participants analyzed therefore assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | there was only one group of participants analyzed therefore it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Low risk | source of funding not reported |
| Methods | no washout required because participants were not receiving any lipid‐altering agents within 8 weeks of randomization 8‐week randomized single‐blind placebo‐controlled trial | |
| Participants | 53 men and women with mild to moderate hypertension mean age 60 years SBP < 170 mmHg DBP < 105 mm Hg exclusion criteria: renal disease, hepatic disease, any thyroid disease, uncontrolled diabetes uncontrolled severe hypertension, stroke, acute coronary syndrome and unstable angina Placebo baseline TC : 5.14 mmol/l (199 mg/dl) Placebo baseline non‐HDL‐C : 3.856 mmol/l ( 149 mg/dl) Rosuvastatin 20 mg/day baseline TC : 5.64 mmol/l (218 mg/dl) Rosuvastatin 20 mg/day baseline non‐HDL‐C : 4.345 mmol/l ( 168 mg/dl) | |
| Interventions | 26 participants received placebo for 8 weeks 27 participants received Rosuvastatin 20 mg/day for 8 weeks | |
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not reported "medication was provided in envelopes" |
| Blinding (performance bias and detection bias) | High risk | single‐blinded, patients were blinded |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis WDAE data were provided |
| Other bias | Low risk | pharmaceutical companies did not fund the trial |
| Methods | no washout required because participants were not receiving any lipid‐altering agents within 8 weeks of randomization 8‐week randomized single‐blind placebo‐controlled trial | |
| Participants | 162 men and women with hypercholesterolaemia age 54‐56 years LDL‐C ≥ 130 mg/dl ( 3.36 mmol/l) exclusion criteria: liver disease, renal failure, hypothyroidism, myopathy, uncontrolled diabetes, severe hypertension, stroke acute coronary events, coronary revascularization within 3 months of trial, alcohol abuse Placebo baseline TC : 6.41 mmol/l (248 mg/dl) Placebo baseline non‐HDL‐C : 5.017 mmol/l ( 194 mg/dl) Rosuvastatin 20 mg/day baseline TC : 6.36 mmol/l (246 mg/dl) Rosuvastatin 20 mg/day baseline non‐HDL‐C : 4.991 mmol/l ( 193 mg/dl) | |
| Interventions | 54 participants received placebo 54 participants received rosuvastatin 10 mg/day 54 participants received pravastatin 40 mg/day | |
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | the pravastatin group was not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not reported |
| Blinding (performance bias and detection bias) | High risk | single‐blind |
| Incomplete outcome data (attrition bias) | Low risk | 1/54 of the placebo participants were not included in the efficacy analysis 2/54 of the rosuvastatin participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | all lipid parameters were included in the efficacy analysis WDAEs were not reported |
| Other bias | Low risk | pharmaceutical companies did not fund the trial |
| Methods | 6‐week washout period 12‐week open study | |
| Participants | 40 men and women with primary dyslipidaemia type IIa age ≥18 years LDL‐C > 160 mg/dl ( > 4.13 mmol/l) TG ≤ 350 mg/dl (≤ 3.95 mmol/l) 40 patients received rosuvastatin 10 mg/day exclusion criteria:renal impairment, diabetes mellitus, raised thyroid stimulating hormone levels liver disease, child‐bearing potential, antihypertensive therapy modified 12 or fewer weeks before enrolment Rosuvastatin 10 mg/day baseline TC : 7.95 mmol/l (307 mg/dl) | |
| Interventions | rosuvastatin 10 mg/day | |
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | there was only one group of participants analyzed therefore assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | there was only one group of participants analyzed therefore assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | there was only one group of participants analyzed therefore it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | non‐HDL‐C was not included in the efficacy analysis |
| Other bias | Unclear risk | source of funding not provided |
| Methods | 6‐week washout period 12‐week open‐label trial | |
| Participants | 75 men and women mean age 52 years with hyperlipidaemia LDL‐C >160 mg/dl (> 4.14 mmol/l) TG < 400 mg/dl ( < 4.52 mmol/l) 75 received rosuvastatin 10 mg/day exclusion criteria: renal dysfunction, liver disease or dysfunction elevated TSH, diabetes mellitus, childbearing potential lipid‐lowering therapy agents within 8 weeks of trial Rosuvastatin 10 mg/day baseline TC : 7.71 mmol/l (298 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 6.23 mmol/l (241 mg/dl) | |
| Interventions | Rosuvastatin 10 mg/day | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | there was only one group of participants analyzed therefore assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | there was only one group of participants analyzed therefore assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | there was only one group of participants analyzed therefore it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Low risk | pharmaceutical companies did not fund this trial |
| Methods | 6‐week washout period 12‐week randomized open‐label trial | |
| Participants | 130 men and women mean age 52 years with primary hyperlipidaemia 45 participants randomized to rosuvastatin 10 mg/day 45 participants randomized to rosuvastatin 20 mg/day 40 participants randomized to control dietary treatment only not a placebo exclusion criteria: renal dysfunction, liver disease or dysfunction elevated TSH, diabetes mellitus, child‐bearing potential lipid‐lowering therapy agents within 8 weeks of trial Rosuvastatin 10 mg/day baseline TC : 7.99 mmol/l (309 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 6.34 mmol/l (245 mg/dl) Rosuvastatin 20 mg/day baseline TC : 8.07 mmol/l (312 mg/dl) Rosuvastatin 20 mg/day baseline non‐HDL‐C: 6.44 mmol/l (249 mg/dl) | |
| Interventions | rosuvastatin 10 mg/day rosuvastatin 20 mg/day control dietary treatment only not a placebo | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | control dietary treatment only not a placebo group was not included in the efficacy analysis SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | source of funding not reported |
| Methods | 6‐week washout period 12‐week randomized open‐label trial | |
| Participants | 80 men and women mean age 52 years non‐diabetic with primary hyperlipidaemia 40 participants randomized to rosuvastatin 10 mg/day 40 participants randomized to control non‐statin group dietary treatment only not a placebo exclusion criteria: renal dysfunction, liver disease or dysfunction elevated TSH, diabetes mellitus, childbearing potential lipid‐lowering therapy agents within 8 weeks of trial Rosuvastatin 10 mg/day baseline TC : 7.9 mmol/l (305 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 6.3 mmol/l (244 mg/dl) | |
| Interventions | Rosuvastatin 10 mg/day control non‐statin group dietary treatment only not a placebo | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | control non‐statin group dietary treatment only not a placebo group was not included in the efficacy analysis SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | source of funding not reported |
| Methods | 6‐week washout period 12‐week randomized open‐label trial | |
| Participants | 120 men and women mean age 50 years with primary dyslipidaemia 60 participants randomized to rosuvastatin 20 mg/day 60 participants randomized to control non‐statin group dietary treatment only not a placebo exclusion criteria: renal dysfunction, liver disease or dysfunction elevated TSH, diabetes mellitus, childbearing potential lipid‐lowering therapy agents within 8 weeks of trial Rosuvastatin 20 mg/day baseline TC : 8.59 mmol/l (332 mg/dl) Rosuvastatin 20 mg/day baseline non‐HDL‐C: 7.01 mmol/l (271 mg/dl) | |
| Interventions | Rosuvastatin 20 mg/day control non‐statin group dietary treatment only not a placebo | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and non‐HDL‐C | |
| Notes | control non‐statin group dietary treatment only not a placebo was not included in the efficacy analysis SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 20 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 20 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 20 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | source of funding not reported |
| Methods | 6‐week washout period 12‐week randomized open‐label trial | |
| Participants | 150 men and women mean age 51 years with primary dyslipidaemia 50 participants randomized to rosuvastatin 10 mg/day 50 participants randomized to rosuvastatin 20 mg/day 50 participants randomized to control non‐statin group dietary treatment only not a placebo exclusion criteria: renal dysfunction, liver disease or dysfunction elevated TSH, diabetes mellitus, childbearing potential lipid‐lowering therapy agents within 8 weeks of trial Rosuvastatin 10 mg/day baseline TC : 7.86 mmol/l (304 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 6.23 mmol/l (241 mg/dl) Rosuvastatin 20 mg/day baseline TC : 7.94 mmol/l (307 mg/dl) Rosuvastatin 20 mg/day baseline non‐HDL‐C: 6.39 mmol/l (247 mg/dl) | |
| Interventions | rosuvastatin 10 mg/day rosuvastatin 20 mg/day control dietary treatment only not a placebo | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | control dietary treatment only not a placebo group was not included in the efficacy analysis SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day and Rosuvastatin 20 mg/day treatment arms were analyzed and since there was no placebo group to compare them to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day and Rosuvastatin 20 mg/day treatment arms were analyzed and since there was no placebo group to compare them to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day and Rosuvastatin 20 mg/day treatment arms were analyzed and since there was no placebo group to compare them to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | source of funding not reported |
| Methods | 6‐week washout 12‐week randomized open study | |
| Participants | 39 men and women with combined dyslipidaemia aged 51‐54 years old TC > 200 mg/dl (> 5.17 mmol/l) TG > 200 mg/dl (> 2.26 mmol/l) BMI <33.0 20 participants randomized to rosuvastatin 19 participants randomized to gemfibrozil exclusion criteria: none reported Rosuvastatin 40 mg/day baseline TC : 6.26 mmol/l (242 mg/dl) Rosuvastatin 40 mg/day baseline non‐HDL‐C: 5.22 mmol/l (202 mg/dl) | |
| Interventions | Rosuvastatin 40 mg/day Gemfibrozil 1.2 g/day | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | Gemfibrozil 1.2 g/day group was not analyzed SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 40 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 40 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 40 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | AstraZeneca funded the trial. Data may support bias for rosuvastatin |
| Methods | participants were not receiving any lipid‐altering agents within 3 months of trial no washout required 12‐week before‐and‐after trial | |
| Participants | 40 men and women with impaired fasting glucose, hypertension and mixed dyslipidaemia LDL‐C >160 mg/dl (4.14 mmol/l) TG >150 mg/dl (1.69 mmol/l) 20 participants were randomized to manidipine and rosuvastatin 10 mg/day 20 participants were randomized to olmesartan and rosuvastatin 10 mg/day exclusion criteria: diabetes mellitus, cardiovascular disease, renal disease hypothyroidism, liver dysfunction and females not taking sufficient contraceptive measures Rosuvastatin 10 mg/day baseline TC : 6.745 mmol/l (261 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 5.315 mmol/l (206 mg/dl) | |
| Interventions | manidipine and rosuvastatin 10 mg/day for 12 weeks olmesartan and rosuvastatin 10 mg/day for 12 weeks both sets of data were combined | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and non HDL‐C | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arms were analyzed and since there was no placebo group to compare them to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arms were analyzed and since there was no placebo group to compare them to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day treatment arms were analyzed and since there was no placebo group to compare them to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | triglyceride data were not included in the efficacy analysis |
| Other bias | Low risk | no financial support for the study not industry funded |
| Methods | 6‐week washout period 6‐week randomized, double‐blind, placebo‐controlled trial | |
| Participants | 46 men and women with hypercholesterolaemia LDL‐C > 160 mg/dl ( > 4.14 mmol/l) TG <350 mg/dl (< 3.95 mmol/l) 25 were randomized to placebo 25 were randomized to rosuvastatin exclusion criteria:renal dysfunction, hepatic dysfunction, thyroid disease, chronic or acute inflammation, cancer history, uncompensated heart failure, uncontrolled hypertension uncontrolled diabetes mellitus, acute coronary syndrome within 1 month of trial entry were also excluded Placebo baseline TC : 6.63 mmol/l (256 mg/dl) Rosuvastatin 10 mg/day baseline TC : 6.72 mmol/l (260 mg/dl) | |
| Interventions | Placebo Rosuvastatin 10 mg/day | |
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not reported |
| Blinding (performance bias and detection bias) | Low risk | double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | 2/25 placebo group was not included in the efficacy analysis 2/25 rosuvastatin group was not included in the efficacy analysis 8% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | AstraZeneca partially funded the study. Data may support bias for rosuvastatin |
| Methods | 4‐week washout period 4‐week open‐label trial | |
| Participants | 146 men and women mean age 56 years with hyperlipidaemia TC ≥ 5.2 mmol/l ( ≥ 201 mg/dl) TG ≥ 2.3 mmol/l ( ≥ 204 mg/dl) LDL‐C ≥3.4 mmol/l ( ≥ 131 mg/dl) 146 participants received rosuvastatin 10 mg/day exclusion criteria:renal dysfunction, hepatic dysfunction drugs known to affect lipid profiles or rosuvastatin interaction Rosuvastatin 10 mg/day baseline TC : 7.84 mmol/l (303 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 6.3 mmol/l (244 mg/dl) | |
| Interventions | Rosuvastatin 10 mg/day | |
| Outcomes | per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | SD was imputed for non‐HDL‐C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | there was only one group of participants analyzed therefore assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | there was only one group of participants analyzed therefore assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | there was only one group of participants analyzed therefore it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 9/146 = 6.2% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | source of funding not provided |
| Methods | no washout period required no participant received lipid‐altering agents for at least 4 weeks 12 week before‐and‐after trial | |
| Participants | 825 patients with acute coronary syndrome age 18 to 75 years LDL‐C > 70 mg/dl (1.81 mmol/l) TG< 500 mg/dl (5.645 mmol/l) 277 patients received rosuvastatin 20 mg/day 270 patients received rosuvastatin 40 mg/day 278 patients received atorvastatin 80 mg/day exclusion criteria: HRT within 3 months, Q‐wave MI, pulmonary oedema, moderate or severe congestive heart failure severe mitral regurgitation, acute ventricular septal defect, heart disease, stroke, sepsis, coronary artery bypass graft within 3 months HMG CoA reductase inhibitor hypersensitivity, pregnancy, uncontrolled diabetes mellitus, hypertension, hypothyroidism, systolic hypotension hepatic dysfunction, severe anaemia, serum creatinine >2 mg/dL and serum creatine kinase > 3 times ULN Rosuvastatin 20 mg/day baseline TC : 5.19 mmol/l (201 mg/dl) Rosuvastatin 20 mg/day baseline non‐HDL‐C : 4.17 mmol/l (161 mg/dl) Rosuvastatin 20 mg/day baseline TG : 2.04 mmol/l (181 mg/dl) Rosuvastatin 40 mg/day baseline TC : 5.22 mmol/l (202 mg/dl) Rosuvastatin 40 mg/day baseline non‐HDL‐C : 4.21 mmol/l (163 mg/dl) Rosuvastatin 40 mg/day baseline TG : 2.06 mmol/l (182 mg/dl) | |
| Interventions | rosuvastatin 20 mg/day for 12 weeks rosuvastatin 40 mg/day for 12 weeks atorvastatin 80 mg/day for 12 weeks | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, non‐HDL‐C and triglycerides | |
| Notes | atorvastatin group was not analyzed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 20 mg/day and the Rosuvastatin 40 mg/day treatment arms were analyzed and since there was no placebo group to compare them to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 20 mg/day and the Rosuvastatin 40 mg/day treatment arms were analyzed and since there was no placebo group to compare them to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 20 mg/day and the Rosuvastatin 40 mg/day treatment arms were analyzed and since there was no placebo group to compare them to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 31/277 rosuvastatin 20 mg/day were not included in the efficacy analysis 19/270 rosuvastatin 40 mg/day were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | AstraZeneca funded the trial. Data may support bias for rosuvastatin |
| Methods | 8‐week washout period 18‐week open‐label study | |
| Participants | 37 men and non‐pregnant women age ≥ 18 years with heterozygous familial hypercholesterolaemia LDL‐C ≥ 220 and < 500 mg/dl (≥5.69 and < 12.93 mmol/l) TG ≤ 400 mg/dl ( ≤ 4.52 mmol/l) 37 patients received rosuvastatin exclusion criteria: statin sensitivity, serious or unstable medical or psychological conditions that could compromise the patient's safety or successful trial participation history of homozygous FH, use of medications that affect lipid profile or safety concern, drug or alcohol abuse CK > 3 X ULN, renal dysfunction, liver disease or dysfunction Rosuvastatin 10 mg/day baseline TC : 9.91 mmol/l (383 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 8.62 mmol/l (333 mg/dl) | |
| Interventions | Rosuvastatin 10 mg/day for 0‐6 weeks Rosuvastatin 20 mg/day for 6‐12 weeks Rosuvastatin 40 mg/day for 12‐18 weeks | |
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | Rosuvastatin 20 mg/day for 6‐12 weeks Rosuvastatin 40 mg/day for 12‐18 weeks groups were not analyzed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | source of funding not reported |
| Methods | participants were not receiving any lipid‐altering agents within 4 weeks of trial no washout required 12 week before‐and‐after trial | |
| Participants | 60 men and women mean age of 55 years with mixed lipidaemia LDL‐C >160 mg/dl (4.14 mmol/l) TG >200 mg/dl (2.26 mmol/l) 22 participants were randomized to rosuvastatin 40 mg/day for 12 weeks 21 participants were randomized to rosuvastatin 10 mg/day plus 200 mg fenofibrate for 12 weeks 17 participants were randomized to rosuvastatin 10 mg/day plus 2 g omega‐3 fatty acids for 12 weeks exclusion criteria: coronary heart disease, atherosclerotic disease, TG > 500 mg/dl renal disease, diabetes mellitus, hypothyroidism, liver disease, uncontrolled hypertension Rosuvastatin 40 mg/day baseline TC : 8.22 mmol/l (318 mg/dl) Rosuvastatin 40 mg/day baseline non‐HDL‐C: 6.83 mmol/l (264 mg/dl) | |
| Interventions | rosuvastatin 40 mg/day rosuvastatin 10 mg/day plus 200 mg fenofibrate rosuvastatin 10 mg/day plus 2 g omega‐3 fatty acids | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and non HDL‐C | |
| Notes | rosuvastatin 10 mg/day plus 200 mg fenofibrate rosuvastatin 10 mg/day plus 2 g omega‐3 fatty acids groups were not analyzed SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 40 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 40 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 40 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | triglyceride data were not included in the efficacy analysis |
| Other bias | Low risk | no financial support for the study not industry funded |
| Methods | 4‐week washout period 0‐18 week open‐label forced dose titration trial then a 18‐24 week randomized double‐blind cross‐over trial | |
| Participants | 44 patients ≥ 10 years with homozygous familial hypercholesterolaemia LDLC ≥ 500 mg/dl (12.9 mmol/l) TG < 600 mg/dl (6.8 mmol/l) 41 patients received rosuvastatin exclusion criteria:active liver disease or dysfunction, serum CK > 3 X ULN, renal dysfunction, uncontrolled hypertension Rosuvastatin 20 mg/day baseline TC : 15.0 mmol/l (580 mg/dl) | |
| Interventions | Rosuvastatin 20 mg/day for 0‐6 weeks Rosuvastatin 40 mg/day for 6‐12 weeks Rosuvastatin 80 mg/day for 12‐18 weeks Crossover rosuvastatin 80 mg/day and atorvastatin 80 mg/day 18‐24 and 24‐30 weeks | |
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Notes | Rosuvastatin 40 mg/day for 6‐12 weeks Rosuvastatin 80 mg/day for 12‐18 weeks Cros‐sover rosuvastatin 80 mg/day and atorvastatin 80 mg/day 18‐24 and 24‐30 weeks groups were not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 20 mg/day for 0‐6 weeks treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 20 mg/day for 0‐6 weeks treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 20 mg/day for 0‐6 weeks treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 3/41 participants were not included in the efficacy analysis 7.3% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | AstraZeneca funded the study. Data may support bias for rosuvastatin |
| Methods | participants were not receiving any lipid‐altering agents no washout period required 12‐week before‐and‐after trial | |
| Participants | 10 participants had type 2 diabetes and hypercholesterolaemia mean age was 68 years 10 participants had hypercholesterolaemia mean age was 65 years exclusion criteria: infection, smoking habits and competitive sporting activities Rosuvastatin 10 mg/day baseline TC : 6.615 mmol/l (256 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 5.26 mmol/l (203 mg/dl) | |
| Interventions | rosuvastatin 10 mg/day for 12 weeks | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | there was only one group of participants analyzed therefore assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | there was only one group of participants analyzed therefore assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | there was only one group of participants analyzed therefore it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | source of funding was not reported |
| Methods | 6‐week washout period 16‐week randomized open‐label trial | |
| Participants | 3161 patients ≥ 18 years history of CHD or atherosclerosis type 2 diabetes mellitus CHD risk > 20% over 10 years LDL‐C ≥ 2.99 mmol/l ( ≥ 115 mg/dl) TG <4.52 mmol/l ( < 400 mg/dl) 538 participants were randomized to rosuvastatin 10 mg/day for 0‐8 weeks 529 participants were randomized to atorvastatin 10 mg/day for 0‐8 weeks 925 participants were randomized to atorvastatin 20 mg/day for 0‐8 weeks 543 participants were randomized to simvastatin 20 mg/day for 0‐8 weeks 521 participants were randomized to pravastatin 40 mg/day for 0‐8 weeks 521 participants were randomized to rosuvastatin 10 mg/day for 8‐16 weeks 276 participants switched from atorvastatin 10 mg/day to rosuvastatin 10 mg/day for 8‐16 weeks 240 participants were randomized to atorvastatin 10 mg/day for 8‐16 weeks 293 participants switched from atorvastatin 20 mg/day to rosuvastatin 10 mg/day for 8‐16 weeks 305 participants switched from atorvastatin 20 mg/day to rosuvastatin 20 mg/day for 8‐16 weeks 299 participants were randomized to atorvastatin 20 mg/day for 8‐16 weeks 277 participants switched from simvastatin 20 mg/day to rosuvastatin 10 mg/day for 8‐16 weeks 250 participants were randomized to simvastatin 20 mg/day for 8‐16 weeks 253 participants switched from pravastatin 40 mg/day to rosuvastatin 10 mg/day for 8‐16 weeks 253 participants were randomized to pravastatin 40 mg/day for 8‐16 weeks exclusion criteria: pregnancy, lactation homozygous familial hypercholesterolaemia, type III hyperlipoproteinaemia active arterial disease, uncontrolled hypertension, active liver disease or hepatic dysfunction serum CK > 3 X ULN and renal dysfunction Rosuvastatin 10 mg/day baseline LDL‐C : 4.26 mmol/l (165 mg/dl) | |
| Interventions | rosuvastatin 10 mg/day for 0‐8 weeks atorvastatin 10 mg/day for 0‐8 weeks atorvastatin 20 mg/day for 0‐8 weeks simvastatin 20 mg/day for 0‐8 weeks pravastatin 40 mg/day for 0‐8 weeks rosuvastatin 10 mg/day for 8‐16 weeks switched from atorvastatin 10 mg/day to rosuvastatin 10 mg/day for 8‐16 weeks atorvastatin 10 mg/day for 8‐16 weeks switched from atorvastatin 20 mg/day to rosuvastatin 20 mg/day for 8‐16 weeks atorvastatin 20 mg/day for 8‐16 weeks switched from simvastatin 20 mg/day to rosuvastatin 10 mg/day for 8‐16 weeks simvastatin 20 mg/day for 8‐16 weeks switched from pravastatin 40 mg/day to rosuvastatin 10 mg/day for 8‐16 weeks pravastatin 40 mg/day for 8‐16 weeks | |
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Notes | atorvastatin 10 mg/day for 0‐8 weeks atorvastatin 20 mg/day for 0‐8 weeks simvastatin 20 mg/day for 0‐8 weeks pravastatin 40 mg/day for 0‐8 weeks rosuvastatin 10 mg/day for 8‐16 weeks switched from atorvastatin 10 mg/day to rosuvastatin 10 mg/day for 8‐16 weeks atorvastatin 10 mg/day for 8‐16 weeks switched from atorvastatin 20 mg/day to rosuvastatin 20 mg/day for 8‐16 weeks atorvastatin 20 mg/day for 8‐16 weeks switched from simvastatin 20 mg/day to rosuvastatin 10 mg/day for 8‐16 weeks simvastatin 20 mg/day for 8‐16 weeks switched from pravastatin 40 mg/day to rosuvastatin 10 mg/day for 8‐16 weeks pravastatin 40 mg/day for 8‐16 weeks groups were not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day for 0‐8 weeks treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day for 0‐8 weeks treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day for 0‐8 weeks treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 105/3161 (3.3%) participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | non‐HDL‐C was not included in the efficacy analysis |
| Other bias | High risk | Trial was supported by AstraZeneca. Data may support bias for rosuvastatin |
| Methods | 6‐week washout period 8‐week open randomized study | |
| Participants | 1993 men and women with hypercholesterolaemia age ≥ 18 years LDL‐C ≥ 130 to < 250 mg/dl ( ≥3.36 to < 6.46 mmol/l) TG <400 mg/dl ( <4.52 mmol/l) 392 received rosuvastatin 798 received atorvastatin 803 received simvastatin exclusion criteria: pregnancy or lactation, homozygous familial hypercholesterolaemia known hyperlipoproteinaemia types I, III, IV or V, unstable arterial disease within 3 months of trial uncontrolled hypertension, fasting serum glucose of >180 mg/dl (> 10.0 mmol/l ) during dietary lead‐in active liver disease or dysfunction Rosuvastatin 20 mg/day baseline TC : 6.48 mmol/l (251 mg/dl) Rosuvastatin 20 mg/day baseline non‐HDL‐C: 5.26 mmol/l (203 mg/dl) | |
| Interventions | Rosuvastatin 20 mg/day Atorvastatin 10 mg/day Atorvastatin 20 mg/day Simvastatin 20 mg/day Simvastatin 40 mg/day | |
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | Atorvastatin 10 mg/day Atorvastatin 20 mg/day Simvastatin 20 mg/day Simvastatin 40 mg/day groups were not included in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 20 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 20 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 20 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 9/392 were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | source of funding not reported |
| Methods | 6‐week washout period 20‐week before‐and‐after trial | |
| Participants | 55 men and women age 55‐66 years with primary hyperlipidaemia TC > 240 mg/dl ( >6.2 mmol/l) TG < 350 mg/dl (4.0 mmol/l) 55 patients received rosuvastatin exclusion criteria: liver disease or dysfunction, renal dysfunction, diabetes mellitus, raised FSH levels, medical conditions that might preclude successful completion of trial participants receiving drugs that could affect lab parameters tested were also excluded Rosuvastatin 10 mg/day baseline TC : 7.63 mmol/l (295 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 6.26 mmol/l (242 mg/dl) | |
| Interventions | Rosuvastatin 10 mg/day for 0‐6 weeks Rosuvastatin 10 mg/day for 6‐20 weeks | |
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | Rosuvastatin 10 mg/day for 6‐20 weeks group was not analyzed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day for 0‐6 weeks treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day for 0‐6 weeks treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day for 0‐6 weeks treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the analysis |
| Other bias | Unclear risk | source of funding not reported |
| Methods | 1‐month or more washout period 3‐month before‐and‐after trial | |
| Participants | 128 men and women with hypercholesterolaemia and type 2 diabetes aged 20‐80 years LDL‐C ≥ 100 mg /dl (2.59 mmol/l) 44 participants received atorvastatin 42 participants received rosuvastatin 42 participants received pravastatin exclusion criteria: history of stoke or ischaemic heart disease during the previous 6 months hepatic dysfunction, renal dysfunction, females that were pregnant or possibly pregnant Rosuvastatin 5 mg/day baseline TC : 6.25 mmol/l (242 mg/dl) Rosuvastatin 5 mg/day baseline non‐HDL‐C: 4.67 mmol/l (181 mg/dl) | |
| Interventions | Atorvastatin 10 mg/day for 3 months Rosuvastatin 5 mg/day for 3 months Pravastatin 10 mg/day for 3 months | |
| Outcomes | per cent change from baseline at 3 months of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | Atorvastatin 10 mg/day for 3 months and Pravastatin 10 mg/day for 3 months groups were not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 5 mg/day for 3 months treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 5 mg/day for 3 months treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 5 mg/day for 3 months treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | High risk | 5/42 (11.9%) of the rosuvastatin group were not included in the efficacy analysis due to dropout or incomplete evaluation |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the analysis |
| Other bias | Low risk | no pharmaceutical company funded the study |
| Methods | 4‐week washout period 12‐week randomized open‐label trial | |
| Participants | 153 men and women with primary hypercholesterolaemia TG ≤ 500 mg/dl ( ≤ 565 mmol/l) 55 participants were randomized to simvastatin 40 mg/day 45 participants were randomized to rosuvastatin 10 mg/day 53 participants were randomized to simvastatin/ezetimibe 10/10 mg/day exclusion criteria:CVD, carotid artery disease, peripheral artery disease abdominal aortic aneurysm, diabetes mellitus, renal disease hypothyroidism, liver disease, cancer, inflammatory or infectious diseases, uncontrolled hypertension Rosuvastatin 10 mg/day baseline TC : 7.09 mmol/l (274 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 5.51 mmol/l (213 mg/dl) | |
| Interventions | rosuvastatin 10 mg/day simvastatin 40 mg/day simvastatin/ezetimibe 10/10 mg/day | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and non‐HDL‐C | |
| Notes | simvastatin 40 mg/day simvastatin/ezetimibe 10/10 mg/day groups were not included in the efficacy analysis SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | triglycerides were not included in the efficacy analysis |
| Other bias | Low risk | authors state no conflict of interest |
| Methods | 6‐week washout period 6‐week randomized double‐blind placebo‐controlled trial | |
| Participants | 206 men aged 18‐70 and postmenopausal women with hypercholesterolaemia aged 50‐70 years LDL‐C > 4.14 mmol/l (>160 mg/dl) and < 6.21 mmol/l (< 240 mg/dl) TG < 3.39 mmol/l (< 300 mg/dl) BMI ≤ 30 29 received placebo 137 received rosuvastatin 23 received atorvastatin exclusion criteria:active arterial disease, cancer history, uncontrolled hypertension, diabetes mellitus uncontrolled hypothyroidism, homozygous familial hypercholesterolaemia, active liver disease or dysfunction CK > 3 X ULN Placebo baseline TC : 7.00 mmol/l (271 mg/dl) Rosuvastatin 1 mg/day baseline TC : 6.90 mmol/l (267 mg/dl) Rosuvastatin 2.5 mg/day baseline TC : 6.80 mmol/l (236 mg/dl) Rosuvastatin 5 mg/day baseline TC : 7.00 mmol/l (271 mg/dl) Rosuvastatin 10 mg/day baseline TC : 6.90 mmol/l (267 mg/dl) Rosuvastatin 20 mg/day baseline TC : 6.80 mmol/l (263 mg/dl) Rosuvastatin 40 mg/day baseline TC : 6.70 mmol/l (259 mg/dl) Rosuvastatin 80 mg/day baseline TC : 6.80 mmol/l (236 mg/dl) | |
| Interventions | Placebo Rosuvastatin 1 mg/day Rosuvastatin 2.5 mg/day Rosuvastatin 5 mg/day Rosuvastatin 10 mg/day Rosuvastatin 20 mg/day Rosuvastatin 40 mg/day Rosuvastatin 80 mg/day Atorvastatin 10 mg/day Atorvastatin 80 mg/day | |
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and triglycerides WDAEs reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not reported |
| Blinding (performance bias and detection bias) | Low risk | double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | 17/206 were not included in the efficacy analysis 8.25 % were not analyzed |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | AstraZeneca funded the study. Data may support bias for rosuvastatin |
| Methods | 6‐week washout period 52‐week randomized double‐blind trial | |
| Participants | 412 men and women aged ≥ 18 years with hypercholesterolaemia LDL‐C between 160 and < 250 mg/dl (4.14 and <6.5 mmol/l) TG ≤ 400 mg/dl ( ≤ 4.5 mmol/l) EPAT score ≤28 138 participants were randomized to rosuvastatin 5 mg/day 134 participants were randomized to rosuvastatin 10 mg/day 140 participants were randomized to atorvastatin 10 mg/day Conventional exclusion criteria for lipid‐modifying drugs under development were applied Rosuvastatin 5 mg/day baseline TC : 7.07 mmol/l (273 mg/dl) Rosuvastatin 10 mg/day baseline TC : 7.00 mmol/l (271 mg/dl) | |
| Interventions | Rosuvastatin 5 mg/day for 0‐12 weeks Rosuvastatin 5‐80 titration mg/day 12‐52 weeks Rosuvastatin 10 mg/day for 0‐12 weeks Rosuvastatin 10‐80 titration mg/day 12‐52 weeks Atorvastatin 10 mg/day for 0‐12 weeks Atorvastatin 10‐80 titration mg/day 12‐52 weeks | |
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Notes | Rosuvastatin 5‐80 titration mg/day 12‐52 weeks Rosuvastatin 10‐80 titration mg/day 12‐52 weeks Atorvastatin 10 mg/day for 0‐12 weeks Atorvastatin 10‐80 titration mg/day 12‐52 weeks groups were not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 5 mg/day for 0‐12 weeks and Rosuvastatin 10 mg/day for 0‐12 weeks treatment arms were analyzed and since there was no placebo group to compare them to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 5 mg/day for 0‐12 weeks and Rosuvastatin 10 mg/day for 0‐12 weeks treatment arms were analyzed and since there was no placebo group to compare them to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 5 mg/day for 0‐12 weeks and Rosuvastatin 10 mg/day for 0‐12 weeks treatment arms were analyzed and since there was no placebo group to compare them to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 3/138 participants from the rosuvastatin 5 mg/day group were not included in the efficacy analysis 2/134 participants from the rosuvastatin 10 mg/day group were not included in the efficacy analysis 1.8% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | AstraZeneca funded the trial. Data may support bias for rosuvastatin |
| Methods | 6‐week washout period 12‐week double‐blind randomized study evening doses | |
| Participants | 502 patients with hypercholesterolaemia age ≥ 18 years LDL‐C ≥ 160 mg/dl (4.14 mmol/l) and < 250 mg/dl (6.50 mmol/l) TG ≤ 400 mg/dl (4.52 mmol/l) 235 received rosuvastatin 137 received pravastatin 130 received simvastatin exclusion criteria:active arterial disease within 3 months trial entry, familial hypercholesterolaemia uncontrolled hypertension, liver disease, alcohol or drug abuse, use of cyclic hormonal therapy Rosuvastatin 5 mg/day baseline TC : 7.1 mmol/l (276 mg/dl) Rosuvastatin 10 mg/day baseline TC : 7.0 mmol/l (271 mg/dl) | |
| Interventions | Rosuvastatin 5 mg/day Rosuvastatin 10 mg/day Pravastatin 20 mg/day Simvastatin 20 mg/day | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Notes | rosuvastatin groups were analyzed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 5 mg/day and Rosuvastatin10 mg/day treatment arms were analyzed and since there was no placebo group to compare them to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 5 mg/day and Rosuvastatin10 mg/day treatment arms were analyzed and since there was no placebo group to compare them to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 5 mg/day and Rosuvastatin10 mg/day treatment arms were analyzed and since there was no placebo group to compare them to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 5 mg/day rosuvastatin: 1/120 were not included in the efficacy analysis 10 mg/day rosuvastatin: 4/115 were not included in the efficacy analysis 2.1 % participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | AstraZeneca funded it. Data may support bias for rosuvastatin |
| Methods | 6‐week washout period 6‐week randomized open‐label trial evening dosing | |
| Participants | 351 men and women ≥ 18 years with nondiabetic metabolic syndrome and hypercholesterolaemia LDL‐C ≥ 130 mg/dl to < 220 mg/dl ( ≥ 3.36 mmol/l to < 5.69 mmol/l) TG ≥ 150 mg/dl (1.70 mmol/l) HDL‐C < 40 mg/dl (< 1.03 mmol/l) in men HDL‐C < 50 mg/dl (< 1.29 mmol/l) in women well‐controlled hypertension glucose 110 mg/dl (6.11 mmol/l) to 125 mg/dl (6.94 mmol/l) 172 participants were randomized to rosuvastatin 178 participants were randomized to atorvastatin exclusion criteria: pregnancy, cancer, diabetes mellitus, active arterial disease Rosuvastatin 10 mg/day baseline TC : 6.14 mmol/l (237 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 5.12 mmol/l (198 mg/dl) | |
| Interventions | Rosuvastatin 10 mg/day for 6 weeks Atorvastatin 10 mg/day for 6 weeks | |
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | Atorvastatin 10 mg/day for 6 weeks group was not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 2/170 (1.2%) participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | AstraZenca Korea funded the trial. Data might support bias for the drug |
| Methods | no washout required because participants were not receiving any lipid‐altering agents 4‐week before‐and‐after trial | |
| Participants | 11 men and women with chronic hepatitis C mean age 51 years exclusion criteria: patients with CHC with other co‐morbid states, renal impairment, hepatic dysfunction, low TC <80 mg/dl familial hypercholesterolaemia, combined hyperlipidaemia, secondary dyslipidaemias participants receiving thiazide diuretics, retinoids, corticosteroids known hypersensitivity and/or myopathy to previous lipid‐lowering therapy Rosuvastatin 20 mg/day baseline TC : 4.52 mmol/l (175 mg/dl) Rosuvastatin 20 mg/day baseline non‐HDL‐C: 3.14 mmol/l (121 mg/dl) | |
| Interventions | Rosuvastatin 20 mg/day for 4 weeks Rosuvastatin 40 mg/day for 4‐8 weeks a posttreatment period of 8‐16 weeks | |
| Outcomes | per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | Rosuvastatin 40 mg/day for 4‐8 weeks period and the posttreatment period of 8‐16 weeks were not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | there was only one group of participants analyzed therefore assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | there was only one group of participants analyzed therefore assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | there was only one group of participants analyzed therefore it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | Schering Plough Research Institute supported the study in part. Data might support bias against the drug |
| Methods | no participant was receiving lipid‐altering drugs no washout period required 4‐week randomized open‐label trial | |
| Participants | 71 men and women mean age 57 years with primary hypercholesterolaemia LDL‐C > 160 mg/dl ( > 4.14 mmol/l) 35 patients were randomized to rosuvastatin 10mg/day 36 patients were randomized to diet exclusion criteria: secondary hyperlipidaemia, diabetes mellitus renal, liver dysfunction, thyroid disease, alcohol consumption > 40 g/day active arterial disease Rosuvastatin 10 mg/day baseline TC : 6.67 mmol/l (258 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 5.33 mmol/l (206 mg/dl) | |
| Interventions | Rosuvastatin 10 mg/day Diet | |
| Outcomes | per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | diet group was not included in the efficacy analysis SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | source of funding not reported |
| Methods | 8‐week washout period 4‐week randomized open‐label trial | |
| Participants | 48 men and women age 50‐60 years with primary hypercholesterolaemia LDL‐C > 160 mg/dl ( > 4.14 mmol/l) 32 patients were randomized to rosuvastatin 10 mg/day 16 patients were randomized to no pharmacological treatment exclusion criteria: secondary hyperlipidaemia, diabetes mellitus renal, liver dysfunction, thyroid disease, alcohol consumption > 40 g/day active arterial disease Rosuvastatin 10 mg/day baseline LDL‐C : 5.35 mmol/l (207 mg/dl) | |
| Interventions | rosuvastatin 10 mg/day no pharmacological treatment | |
| Outcomes | per cent change from baseline at 4 weeks of serum LDL‐C | |
| Notes | no pharmacological treatment group was not included in the efficacy analysis SD was imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | TC, HDL‐C, non‐HDL‐C and triglycerides were not included in the efficacy analysis |
| Other bias | Low risk | pharmaceutical industry did not fund this study |
| Methods | 6‐week washout period 12‐week randomized, double‐blind placebo‐controlled trial | |
| Participants | 177 pubertal children ages 10‐17 years with familial hypercholesterolaemia LDL‐C >160 mg/dl ( > 4.14 mmol/l) 46 randomized to placebo 42 randomized to Rosuvastatin 5 mg/day 44 randomized to Rosuvastatin 10 mg/day 44 randomized to Rosuvastatin 20 mg/day Placebo baseline TC : 7.58 mmol/l (293 mg/dl) Rosuvastatin 5 mg/day baseline TC : 7.76 mmol/l (300 mg/dl) Rosuvastatin 10 mg/day baseline TC : 7.68 mmol/l (297 mg/dl) Rosuvastatin 20 mg/day baseline TC : 7.81 mmol/l (302 mg/dl) | |
| Interventions | placebo Rosuvastatin 5 mg/day Rosuvastatin 10 mg/day Rosuvastatin 20 mg/day | |
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C , HDL‐C and non‐HDL‐C | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not reported |
| Blinding (performance bias and detection bias) | Low risk | double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | 1/177 participants were not included in th efficacy analysis |
| Selective reporting (reporting bias) | High risk | triglycerides were not included because they were expressed as medians WDAEs were not reported |
| Other bias | High risk | AstraZeneca funded the trial. Data may support bias for rosuvastatin |
| Methods | 4‐week washout period 12‐week randomized open‐label trial | |
| Participants | 30 men and women age 47‐74 years with type 2 diabetes mellitus low HDL‐C HDL‐C <1.0 mmol/l ( < 39 mg/dl) for men HDL‐C <1.2 mmol/l ( < 46 mg/dl) for women all participants had hypertension, BMI > 25 17 participants were randomized to rosuvastatin 10 mg/day 13 participants were randomized to fenofibrate 200 mg/day exclusion criteria: persistent atrial fibrillation, participants with acute coronary syndrome within the previous 3 months, type 1 diabetes mellitus and decompensated diabetes (glycated haemoglobin level HbA1C > 10.5 %) Rosuvastatin 10 mg/day baseline TC : 5.59 mmol/l (216 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 4.64 mmol/l (179 mg/dl) | |
| Interventions | rosuvastatin 10 mg/day fenofibrate 200 mg/day | |
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | fenofibrate 200 mg/day group was not included in the efficacy analysis SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | source of funding not reported |
| Methods | patients received no lipid‐altering agents during the past 6 months, no washout was required 12‐week randomized trial | |
| Participants | 30 men and women age 50‐60 years with unstable angina or non STEMI 16 patients received rosuvastatin 10 mg/day 14 patients received rosuvastatin 20 mg/day exclusion criteria: acute of chronic inflammatory diseases, cancer renal dysfunction, liver disease, on immunosuppressants and antibiotics acute ST elevation MI, diabetes mellitus, active arterial disease within 1 month of trial Rosuvastatin 10 mg/day baseline TC : 5.66 mmol/l (219 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 4.67 mmol/l (181 mg/dl) Rosuvastatin 20 mg/day baseline TC : 6.07 mmol/l (235 mg/dl) Rosuvastatin 20 mg/day baseline non‐HDL‐C: 5.16 mmol/l (200 mg/dl) | |
| Interventions | Rosuvastatin 10 mg/day Rosuvastatin 20 mg/day | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day and Rosuvastatin 20 mg/day treatment arms were analyzed and since there was no placebo group to compare them to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day and Rosuvastatin 20 mg/day treatment arms were analyzed and since there was no placebo group to compare them to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day and Rosuvastatin 20 mg/day treatment arms were analyzed and since there was no placebo group to compare them to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | source of funding not reported |
| Methods | 6‐week washout period 6‐week open randomized study | |
| Participants | 996 men and women of high risk with hypercholesterolaemia age ≥ 18 years LDL‐C ≥ 3.4 and < 5.7 mmol/l ( 130 and 220 mg/dl) TG < 4.5 mmol/l (400 mg/dl) 504 received 10 mg rosuvastatin 492 received 20 mg atorvastatin exclusion criteria: history of statin‐induced myopathy or hypersensitivity, unstable cardiovascular system cancer history, homozygous familial hypercholesterolaemia, current active liver disease, uncontrolled hypothyroidism history of alcohol and drug abuse, pregnancy or lactation, changes in HRT within 3 months of enrolment Rosuvastatin 10 mg/day baseline TC : 6.49 mmol/l (251 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C : 5.19 mmol/l (201 mg/dl) | |
| Interventions | Rosuvastatin 10 mg/day Atorvastatin 20 mg/day | |
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | rosuvastatin group was analyzed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 11/504 were not analyzed |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were measured |
| Other bias | High risk | AstraZeneca sponsored the trial. Data may support bias for rosuvastatin |
| Methods | 6‐week washout period 6‐week open‐label randomized trial | |
| Participants | 461 men and women aged 40‐80 years with cardiovascular disease and a low HDL‐C HDL‐C < 1.0 mmol/l (40 mg/dl) TG ≤4.5 mmol/l (400 mg/dl) 230 participants were randomized to rosuvastatin 231 participants were randomized to atorvastatin exclusion criteria: use of lipid‐altering drugs or supplements after enrolment, statin hypersensitivity pregnancy lactation, active arterial disease, uncontrolled hypertension, glycated haemoglobin > 8%, cancer history, uncontrolled hypothyroidism homozygous familial hypercholesterolaemia or type III hyperlipoproteinaemia, alcohol or drug abuse, active liver disease serum CK > 3 X ULN, received an investigational drug within 4 weeks of enrolment serious or unstable medical or psychological conditions that could affect the trial or safety concerns Rosuvastatin 10 mg/day baseline TC : 5.8 mmol/l (224 mg/dl) | |
| Interventions | Rosuvastatin 10 mg/day for 0‐6 weeks Rosuvastatin 20 mg/day for 6‐12 weeks Rosuvastatin 40 mg/day for 12‐18 weeks Atorvastatin 20 mg/day for 0‐6 weeks Atorvastatin 40 mg/day for 6‐12 weeks Atorvastatin 80 mg/day for 12‐18 weeks | |
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | Rosuvastatin 20 mg/day for 6‐12 weeks Rosuvastatin 40 mg/day for 12‐18 weeks Atorvastatin 20 mg/day for 0‐6 weeks Atorvastatin 40 mg/day for 6‐12 weeks Atorvastatin 80 mg/day for 12‐18 weeks outcomes were not analyzed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day for 0‐6 weeks treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day for 0‐6 weeks treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day for 0‐6 weeks treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | The study was supported by AstraZeneca. Data may support bias for rosuvastatin |
| Methods | 6‐week washout period 6‐week randomized double‐blind placebo‐controlled trial evening dosing | |
| Participants | 108 men and women ≥ 18 years LDL‐C >4.14 mmol/l to < 6.21 mmol/l ( > 160 mg/dl to < 240 mg/dl) TG < 3.39 mmol/l ( < 300 mg/dl) BMI ≤ 30 12 participants were randomized to placebo 12 participants were randomized to rosuvastatin 1 mg/day 12 participants were randomized to rosuvastatin 2.5 mg/day 12 participants were randomized to rosuvastatin 5 mg/day 12 participants were randomized to rosuvastatin 10 mg/day 12 participants were randomized to rosuvastatin 20 mg/day 12 participants were randomized to rosuvastatin 40 mg/day 12 participants were randomized to atorvastatin 10 mg/day 12 participants were randomized to atorvastatin 80 mg/day exclusion criteria: statin hypersensitivity, active arterial disease cancer, uncontrolled hypertension, diabetes mellitus, uncontrolled hypothyroidism homozygous familial hypercholesterolaemia or type III hyperlipoproteinaemia use of some concomitant medications, alcohol or drug abuse active liver disease or dysfunction, renal dysfunction participation in another study less than 3 months before enrolment serum CK ? 3 X ULN, serious of unstable medical or psychological conditions that would affect safety or successful participation in the study HRT, participants receiving digoxin and/or coumarin anti‐coagulants, immunosuppressants | |
| Interventions | placebo rosuvastatin 1 mg/day rosuvastatin 2.5 mg/day rosuvastatin 5 mg/day rosuvastatin 10 mg/day rosuvastatin 20 mg/day rosuvastatin 40 mg/day atorvastatin 10 mg/day atorvastatin 80 mg/day | |
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Notes | atorvastatin 10 mg/day atorvastatin 80 mg/day groups were not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not reported |
| Blinding (performance bias and detection bias) | Low risk | double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | non‐HDL‐C and WDAEs were not reported |
| Other bias | High risk | AstraZeneca funded the trial. Data may support bias for rosuvastatin |
| Methods | 6‐week washout period 6 ‐week randomized open‐label trial | |
| Participants | 258 men and women ≥ 18 years with metabolic syndrome LDL‐C ≥ 130 mg/dl (3.36 mmol/l) < 220 mg/dl (5.69 mmol/l) 132 were randomized to rosuvastatin 126 were randomized to atorvastatin exclusion criteria: none reported | |
| Interventions | rosuvastatin 10 mg/day for 0‐6 weeks atorvastatin 10 mg/day for 0‐6 weeks | |
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Notes | atorvastatin 10 mg/day for 0‐6 weeks group was not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 5/132 participants from the rosuvastatin group were not included in the efficacy analysis 3.8 % participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | non‐HDL‐C was not included in the efficacy analysis |
| Other bias | High risk | AstraZeneca provided the data. Data may support bias for rosuvastatin |
| Methods | 6‐week washout period 12‐week randomized double‐blind trial | |
| Participants | 499 patients with mixed dyslipidaemia and type 2 diabetes mellitus LDL‐C ≥130 mg/dl ( ≥3.36 mmol/l) HDL‐C < 40/50 mg/dl in men/women ( < 1.03/1.29 mmol/l ) in men/women TG ≥150 mg/dl ( ≥ 1.69 mmol/l) 123 participants were randomized to fenofibric acid 135 mg/day 68 participants were randomized to rosuvastatin 5 mg/day 73 participants were randomized to rosuvastatin fenofibric acid 5 mg/135 mg/day 53 participants were randomized to rosuvastatin 10 mg/day 52 participants were randomized to rosuvastatin fenofibric acid 10 mg/135 mg/day 53 participants were randomized to rosuvastatin 20 mg/day 52 participants were randomized to rosuvastatin fenofibric acid 20 mg/135 mg/day 25 participants were randomized to rosuvastatin 40 mg/day exclusion criteria: none reported Rosuvastatin 5 mg/day baseline LDL‐C : 3.82 mmol/l (148 mg/dl) Rosuvastatin 5 mg/day baseline non‐HDL‐C: 5.63 mmol/l (218 mg/dl) Rosuvastatin 10 mg/day baseline LDL‐C : 3.79 mmol/l (147 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 5.62 mmol/l (217 mg/dl) Rosuvastatin 20 mg/day baseline LDL‐C : 3.93 mmol/l (152 mg/dl) Rosuvastatin 20 mg/day baseline non‐HDL‐C: 5.75 mmol/l (222 mg/dl) | |
| Interventions | rosuvastatin 5 mg/day rosuvastatin 10 mg/day rosuvastatin 20 mg/day rosuvastatin 40 mg/day fenofibric acid 135 mg/day rosuvastatin fenofibric acid 5 mg/135 mg/day rosuvastatin fenofibric acid 10 mg/135 mg/day rosuvastatin fenofibric acid 20 mg/135 mg/day | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, non‐HDL‐C and triglycerides | |
| Notes | fenofibric acid 135 mg/day rosuvastatin fenofibric acid 5 mg/135 mg/day rosuvastatin fenofibric acid 10 mg/135 mg/day rosuvastatin fenofibric acid 20 mg/135 mg/day rosuvastatin 40 mg/day groups were not included in the efficacy analysis TC was calculated from HDL‐C and non‐HDL‐C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 5 mg/day, the Rosuvastatin 10 mg/day, the Rosuvastatin 20 mg/day and the Rosuvastatin 40 mg/day treatment arms were analyzed and since there was no placebo group to compare them to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 5 mg/day, the Rosuvastatin 10 mg/day, the Rosuvastatin 20 mg/day and the Rosuvastatin 40 mg/day treatment arms were analyzed and since there was no placebo group to compare them to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 5 mg/day, the Rosuvastatin 10 mg/day, the Rosuvastatin 20 mg/day and the Rosuvastatin 40 mg/day treatment arms were analyzed and since there was no placebo group to compare them to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 5 mg rosuvastatin group 2/68 = 2.9% participants were not included in the efficacy analysis 10 mg rosuvastatin group 7/53 = 13.2% participants were not included in the efficacy analysis 4/53 = 7.5% participants were not included in the efficacy analysis all rosuvastatin groups 13/174 = 7.5 % of all the participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | Abbott and AstraZeneca sponsored the studies. Data may support bias for rosuvastatin |
| Methods | 6‐week washout period 6‐week randomized, double‐blind, placebo‐controlled trial evening dosing | |
| Participants | 112 men aged 18‐70 years and postmenopausal women aged 50‐70 years with hypercholesterolaemia LDL‐C > 160 and < 240 mg/dl ( > 4.14 and < 6.21 mmol/l) TG < 300 mg/dl ( < 3.39 mmol/l) 15 participants were randomized to placebo 16 participants were randomized to rosuvastatin 1 mg/day 18 participants were randomized to rosuvastatin 2.5 mg/day 15 participants were randomized to rosuvastatin 5 mg/day 15 participants were randomized to rosuvastatin 10 mg/day 19 participants were randomized to rosuvastatin 20 mg/day 14 participants were randomized to rosuvastatin 40 mg/day exclusion criteria: use of lipid‐lowering agents within 4 weeks of enrolment active arterial disease, BMI > 30, active liver disease or dysfunction, renal dysfunction serum CK > 3 X ULN Placebo baseline TC : 6.96 mmol/l (269 mg/dl) Rosuvastatin 1 mg/day baseline TC : 6.80 mmol/l (263 mg/dl) Rosuvastatin 2.5 mg/day baseline TC : 6.94 mmol/l (268 mg/dl) Rosuvastatin 5 mg/day baseline TC : 6.89 mmol/l (266 mg/dl) Rosuvastatin 10 mg/day baseline TC : 6.68 mmol/l (258 mg/dl) Rosuvastatin 20 mg/day baseline TC : 7.02 mmol/l (271 mg/dl) Rosuvastatin 40 mg/day baseline TC : 6.94 mmol/l (268 mg/dl) | |
| Interventions | placebo rosuvastatin 1 mg/day rosuvastatin 2.5 mg/day rosuvastatin 5 mg/day rosuvastatin 10 mg/day rosuvastatin 20 mg/day rosuvastatin 40 mg/day | |
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and triglycerides WDAEs were reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not reported (tablets encapsulated in dark‐yellowish red, opaque capsule shells) |
| Blinding (performance bias and detection bias) | Low risk | double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | 3/15 participants for the placebo group were not included in the efficacy analysis 1/16 participants for the rosuvastatin 1 mg/day group were not included in the efficacy analysis 1/18 participants for the rosuvastatin 2.5 mg/day group were not included in the efficacy analysis 3/15 participants for the rosuvastatin 5 mg/day group were not included in the efficacy analysis 1/15 participants for the rosuvastatin 10 mg/day group were not included in the efficacy analysis 1/19 participants for the rosuvastatin 20 mg/day group were not included in the efficacy analysis 1/14 participants for the rosuvastatin 40 mg/day group were not included in the efficacy analysis 9.8% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | non‐HDL‐C was not reported for the efficacy analysis |
| Other bias | Low risk | AstraZeneca sponsored the trial. Data may support bias for rosuvastatin |
| Methods | 6‐9 week washout period 8‐week randomized double‐blind placebo‐controlled trial | |
| Participants | 154 patients age 20‐75 years with hypertriglyceridaemia TG ≥ 200 to < 800 mg/dl ( ≥ 2.26 to < 9.0 mmol/l) 35 patients were randomized to placebo 32 patients were randomized to rosuvastatin 5 mg/day 34 patients were randomized to rosuvastatin 10 mg/day 26 patients were randomized to rosuvastatin 20 mg/day 27 patients were randomized to bezafibrate 200 mg bid exclusion criteria: pancreatitis, use of glitazones within 3 months of trial entry pregnancy, lactation, active arterial disease, uncontrolled diabetes serum glucose ≥140 mg/dl or glycated haemoglobin ≥8%, serum CK > 3 X ULN active liver disease or hepatic dysfunction, familial hypercholesterolaemia Placebo baseline TC : 7.00 mmol/l (246 mg/dl) Placebo baseline non‐HDL‐C: 5.24 mmol/l (203 mg/dl) Rosuvastatin 5 mg/day baseline TC : 6.00 mmol/l (232 mg/dl) Rosuvastatin 5 mg/day baseline non‐HDL‐C: 4.92 mmol/l (190 mg/dl) Rosuvastatin 10 mg/day baseline TC : 6.00 mmol/l (232 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 4.97 mmol/l (192 mg/dl) Rosuvastatin 20 mg/day baseline TC : 6.06 mmol/l (234 mg/dl) Rosuvastatin 20 mg/day baseline non‐HDL‐C: 4.49 mmol/l (192 mg/dl) | |
| Interventions | Placebo rosuvastatin 5 mg/day rosuvastatin 10 mg/day rosuvastatin 20 mg/day bezafibrate 200 mg bid | |
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C WDAEs | |
| Notes | bezafibrate 200 mg bid group was not included the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not reported |
| Blinding (performance bias and detection bias) | Low risk | double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis WDAEs were also reported |
| Other bias | High risk | This study was supported by AstraZeneca. Data may support bias for rosuvastatin |
| Methods | 6‐week washout period 6‐week randomized, double‐blind trial | |
| Participants | 374 men and women age ≥ 18 years with hypercholesterolaemia without active arterial disease within 3 months of trial entry or uncontrolled hypertension LDL‐C ≥ 160 mg/dl ( ≥ 4.14 mmol/l) and < 250 mg/dl (< 6.47 mmol/l) TG ≤ 400 mg/dl ( ≤ 4.52 mmol/l) EPAT score of ≤28 38 participants were randomized to rosuvastatin 5 mg/day 45 participants were randomized to rosuvastatin 10 mg/day 39 participants were randomized to rosuvastatin 20 mg/day 45 participants were randomized to rosuvastatin 40 mg/day 42 participants were randomized to rosuvastatin 80 mg/day 165 participants randomized to atorvastatin exclusion criteria:pregnancy, lactation, familial hypercholesterolaemia type III hyperlipoproteinaemia Rosuvastatin 5 mg/day baseline TC : 7.27 mmol/l (281 mg/dl) Rosuvastatin 5 mg/day baseline non‐HDL‐C : 5.90mmol/l (228 mg/dl) Rosuvastatin 10 mg/day baseline TC : 7.14 mmol/l (276 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C : 5.82 mmol/l (225 mg/dl) Rosuvastatin 20 mg/day baseline TC : 6.98 mmol/l (270 mg/dl) Rosuvastatin 20 mg/day baseline non‐HDL‐C : 5.72 mmol/l (221 mg/dl) Rosuvastatin 40 mg/day baseline TC : 7.14 mmol/l (276 mg/dl) Rosuvastatin 40 mg/day baseline non‐HDL‐C : 5.77 mmol/l (223 mg/dl) Rosuvastatin 80 mg/day baseline TC : 7.40 mmol/l (286 mg/dl) Rosuvastatin 80 mg/day baseline non‐HDL‐C : 6.025 mmol/l (233 mg/dl) | |
| Interventions | Rosuvastatin 5 mg/day Rosuvastatin 10 mg/day Rosuvastatin 20 mg/day Rosuvastatin 40 mg/day Rosuvastatin 80 mg/day Atorvastatin 10 mg/day Atorvastatin 20 mg/day Atorvastatin 40 mg/day Atorvastatin 80 mg/day | |
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | Atorvastatin 10 mg/day Atorvastatin 20 mg/day Atorvastatin 40 mg/day Atorvastatin 80 mg/day groups were not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 5 mg/day, the Rosuvastatin 10 mg/day, the Rosuvastatin 20 mg/day, the Rosuvastatin 40 mg/day, and the rosuvastatin 80 mg/day treatment arms were analyzed and since there was no placebo group to compare them to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 5 mg/day, the Rosuvastatin 10 mg/day, the Rosuvastatin 20 mg/day, the Rosuvastatin 40 mg/day, and the rosuvastatin 80 mg/day treatment arms were analyzed and since there was no placebo group to compare them to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 5 mg/day, the Rosuvastatin 10 mg/day, the Rosuvastatin 20 mg/day, the Rosuvastatin 40 mg/day, and the Rosuvastatin 80 mg/day treatment arms were analyzed and since there was no placebo group to compare them to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 1/39 participants in the rosuvastatin 20 mg/day and 1/45 participants in the rosuvastatin 40 mg/day were not included in the efficacy analysis 1.2% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | The research was supported by AstaZeneca. Data may support bias for rosuvastatin |
| Methods | 6‐week washout period 24‐week randomized, double‐blind trial evening dosing | |
| Participants | 383 men and women age ≥ 18 years with type 2 diabetes mellitus or atherosclerosis LDL‐C ≥ 160 mg/dl ( ≥ 4.14 mmol/l) and < 250 mg/dl (< 6.47 mmol/l) TG ≤ 400 mg/dl ( ≤ 4.52 mmol/l) 127 participants received 5/20/80 mg/day rosuvastatin 128 participants received 10/40/80 mg/day rosuvastatin 128 participants received 10/40/80 mg/day atorvastatin exclusion criteria: pregnant, receiving concomitant medications that affect lipid profile or safety concern active arterial disease, familial hypercholesterolaemia, uncontrolled hypertension and hypothyroidism cancer history, acute liver disease or dysfunction, serum CK > 3 X ULN, renal disease uncontrolled diabetes mellitus Rosuvastatin 5 mg/day baseline TC : 7.09 mmol/l (274 mg/dl) Rosuvastatin 5 mg/day baseline non‐HDL‐C: 5.87 mmol/l (227 mg/dl) Rosuvastatin 10 mg/day baseline TC : 7.03 mmol/l (272 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 5.82 mmol/l (225 mg/dl) | |
| Interventions | Rosuvastatin 5 mg/day for 0‐12 weeks Rosuvastatin 20 mg/day for 12‐18 weeks Rosuvastatin 80 mg/day for 18‐24 weeks Rosuvastatin 10 mg/day for 0‐12 weeks Rosuvastatin 40 mg/day for 12‐18 weeks Rosuvastatin 80 mg/day for 18‐24 weeks Atorvastatin 10 mg/day for 0‐12 weeks Atorvastatin 40 mg/day for 12‐18 weeks Atorvastatin 80 mg/day for 18‐24 weeks | |
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | Rosuvastatin 20 mg/day for 12‐18 weeks Rosuvastatin 80 mg/day for 18‐24 weeks Rosuvastatin 40 mg/day for 12‐18 weeks Rosuvastatin 80 mg/day for 18‐24 weeks Atorvastatin 10 mg/day for 0‐12 weeks Atorvastatin 40 mg/day for 12‐18 weeks Atorvastatin 80 mg/day for 18‐24 weeks groups were not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 5 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 5 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 5 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | AstraZeneca supported the study. Data may support bias for rosuvastatin |
| Methods | 1‐month washout period 12‐week open‐label trial | |
| Participants | 70 men and women age 57 years with CAD BMI =25‐29 40 men and women received aspirin and statins 30 men received rosuvastatin 10 mg/day for 12 weeks exclusion criteria: congestive heart failure, diabetes mellitus, hypothyroidism liver or kidney dysfunction, acute coronary syndrome inflammatory disease and surgical intervention during the last 3 months exclusion criteria in the rosuvastatin group: TG > 4.5 mmol/l (> 399 mg/dl) liver dysfunction, serum CK > 2 X ULN Rosuvastatin 10 mg/day baseline TC : 6.52 mmol/l (252 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 5.37 mmol/l (208 mg/dl) | |
| Interventions | aspirin and statins rosuvastatin 10 mg/day for 12 weeks | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | aspirin and statins group was not included in the efficacy analysis SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | source of funding not reported |
| Methods | 6‐week washout 12‐week randomized double‐blind placebo‐controlled trial | |
| Participants | 135 postmenopausal women age 55‐60 years with hypercholesterolaemia receiving HRT LDL‐C ≥ 130 mg/dl ( ≥ 3.4 mmol/l) and < 250 mg/dl (< 6.5 mmol/l) TG ≤ 400 mg/dl ( ≤ 4.5 mmol/l) 46 patients were randomized to placebo 45 patients were randomized to rosuvastatin 5 mg/day 44 patients were randomized to rosuvastatin 10 mg/day exclusion criteria: statin hypersensitivity, active arterial disease within 3 months of trial entry cancer history excluded skin cancer, uncontrolled hypertension or hypothyroidism glucose > 180 mg/dl ( > 9.99 mmol/l), glucosylated haemoglobin > 9%, familial hypercholesterolaemia use of concomitant medications that affect trial, alcohol or drug abuse active liver disease or dysfunction, serum CK > 3 X ULN serious or unstable medical or psychological condition that could affect safety or trial participation Placebo baseline TC : 6.57 mmol/l (254 mg/dl) Rosuvastatin 5 mg/day baseline TC : 6.72 mmol/l (260 mg/dl) Rosuvastatin 10 mg/day baseline TC : 6.57 mmol/l (254 mg/dl) | |
| Interventions | Placebo Rosuvastatin 5 mg/day Rosuvastatin 10 mg/day | |
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not reported (randomization scheme was predetermined) |
| Blinding (performance bias and detection bias) | Low risk | double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | research was reported by AstraZeneca. Data may support bias for rosuvastatin |
| Methods | 6‐week washout period 6‐week randomized double‐blind trial | |
| Participants | 55 men and women with hypercholesterolaemia LDL‐C ≥ 4.00 mmol/l ( ≥ 155 mg/dl) TG< 4.52 mmol/l ( < 400 mg/dl) exclusion criteria: none reported 30 participants were randomized to rosuvastatin 5 mg/day 25 participants were randomized to atorvastatin 10 mg/day | |
| Interventions | rosuvastatin 5 mg/day atorvastatin 10 mg/day | |
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and non‐HDL‐C | |
| Notes | atorvastatin 10 mg/day group was not included in the efficacy analysis SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 5 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 5 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 5 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | serum triglycerides were not included in the efficacy analysis |
| Other bias | High risk | This study was supported by AstraZeneca. Data may support bias for rosuvastatin |
| Methods | no washout required because participants were not receiving any lipid‐altering agents within the last 6 months of the trial 12‐week before‐and‐after trial | |
| Participants | 135 men and women with metabolic syndrome age 30‐70 years LDL‐C ≥ 130 mg/dl ( ≥ 3.36 mmol/l), TG ≥ 150 mg/dl (1.69 mmol/l), HDL‐C < 40 mg/dl (1.03 mmol/l) for men, HDL‐C < 50 mg/dl (1.29 mmol/l) for women BP ≥ 130/85 mmHg, 10‐year CHD risk score of >10% 68 patients received Atorvastatin/Ezetimibe (10/10 mg/day) 67 patients received Rosuvastatin 5 mg/day exclusion criteria: TG ≥ 500 mg/dl (5.65 mmol/l), LDL‐C ≥ 250 mg/dl (6.48 mmol/l) documented history of CHD or other atherosclerotic disease, familial hypercholesterolaemia, statin hypersensitivity uncontrolled hypertension, hypothyroidism, acute liver disease of hepatic dysfunction, CK > 3X ULN and the use of prohibited concomitant medications Rosuvastatin 5 mg/day baseline TC : 6.047 mmol/l (234 mg/dl) Rosuvastatin 5 mg/day baseline non‐HDL‐C: 4.792 mmol/l (185 mg/dl) | |
| Interventions | Atorvastatin/Ezetimibe (10/10 mg/day) Rosuvastatin 5 mg/day | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | The atorvastatin/ezetimibe (10/10 mg/day) group was not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 5 mg/day for 12 weeks treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 5 mg/day for 12 weeks treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 5 mg/day for 12 weeks treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | source of funding not reported |
| Methods | 6‐week washout period 6‐week open‐label randomized trial | |
| Participants | 1632 men and women 18 years or older at high risk for CHD LDL‐C ≥ 130 mg/dl to < 250 mg/dl ( ≥ 3.36 mmol/l to < 6.465 mmol/l) TG < 400 mg/dl ( < 4.52 mmol/l) 542 participants were randomized to rosuvastatin 544 participants were randomized to atorvastatin 546 participants were randomized to simvastatin exclusion criteria: active arterial disease, uncontrolled hypertension glucose ≥180 mg/dl, glucosylated haemoglobin ≥ 9% active liver disease or dysfunction, serum CK > 3 X ULN Rosuvastatin 10 mg/day baseline TC : 6.57 mmol/l (254 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C : 5.35 mmol/l (207 mg/dl) | |
| Interventions | Rosuvastatin 10 mg/day Atorvastatin 10 mg/day Simvastatin 20 mg/day | |
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | Atorvastatin 10 mg/day Simvastatin 20 mg/day were not analyzed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to, it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 6/542 were not included in the efficacy analysis 1.1% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | source of funding not reported |
| Methods | 6‐week washout period 6‐week randomized open study | |
| Participants | 696 patients age ≥ 18 years with hypercholesterolaemia with a 10‐year risk ≥10% for coronary heart disease or its equivalent LDL‐C (130‐300 mg/dl) ( 3.36‐7.76 mmol/l) TG < 400 mg/dl (< 4.516 mmol/l) 357 were randomized to rosuvastatin 339 were randomized to atorvastatin exclusion criteria: homozygous familial hypercholesterolaemia, known type I, III, or V hyperlipoproteinaemia active arterial disease, uncontrolled hypertension, poorly controlled diabetes active liver disease or dysfunction, serum CK > 3 X ULN Rosuvastatin 10 mg/day baseline TC : 6.465 mmol/l (250 mg/dl) Rosuvastatin 20 mg/day baseline TC : 6.26 mmol/l (242 mg/dl) | |
| Interventions | Rosuvastatin 10 mg/day Rosuvastatin 20 mg/day Atorvastatin 10 mg/day Atorvastatin 20 mg/day | |
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Notes | Atorvastatin 10 mg/day Atorvastatin 20 mg/day groups were not analyzed SDs were imputed for LDL‐C and HDL‐C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day and the Rosuvastatin 20 mg/day treatment arms were analyzed and since there was no placebo group to compare them to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day and the Rosuvastatin 20 mg/day treatment arms were analyzed and since there was no placebo group to compare them to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day and the Rosuvastatin 20 mg/day treatment arms were analyzed and since there was no placebo group to compare them to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 10/184 Rosuvastatin 10 mg/day were not included in the efficacy analysis 6/173 Rosuvastatin 20 mg/day were not included in the efficacy analysis 4.5% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | AstaZeneca supported the study. Data may support bias for rosuvastatin |
| Methods | 4‐5 week washout period 6‐week open‐label randomized trial | |
| Participants | 626 patients aged 18 years or older with dyslipidaemia LDL‐C 175‐350 mg/dl ( 4.52‐9.04 mmol/l) TG <400 mg/dl (4.52 mmol/l) 308 participants were randomized to rosuvastatin 318 participants were randomized to simvastatin exclusion criteria:active arterial disease within 3 months of study entry renal dysfunction,uncontrolled hypertension, uncontrolled diabetes mellitus active liver disease or hepatic dysfunction serum CK > 3 X ULN Rosuvastatin 40 mg/day baseline TC : 8.0 mmol/l (309 mg/dl) | |
| Interventions | Rosuvastatin 40 mg/day Simvastatin 80 mg/day | |
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Notes | Simvastatin 80 mg/day group was not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 40 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 40 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 40 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | The study was funded by AstraZeneca. Data may support bias for rosuvastatin |
| Methods | 6‐week washout period 96‐week open‐label trial | |
| Participants | 1382 men and women ≥ 18 years with severe hypercholesterolaemia LDL‐C 190‐260 mg/dl ( 4.91‐6.72 mmol/l) TG <400 mg/dl ( < 4.52 mmol/l) 1382 patients received rosuvastatin 40 mg/day exclusion criteria: homozygous familial hypercholesterolaemia, type III hyperlipoproteinaemia hepatic dysfunction,, active arterial disease serum CK > 3 X ULN, renal dysfunction, poorly controlled diabetes mellitus uncontrolled hypothyroidism Rosuvastatin 40 mg/day baseline TC : 7.81 mmol/l (302 mg/dl) Rosuvastatin 40 mg/day baseline non‐HDL‐C : 6.54 mmol/l (253 mg/dl) | |
| Interventions | Rosuvastatin 40 mg/day for 0‐12 weeks Rosuvastatin 40 mg/day for 12‐48 weeks Rosuvastatin 40 mg/day for 48‐96 weeks Rosuvastatin 40‐20‐40 titrated dosing mg/day any time | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | Rosuvastatin 40 mg/day for 12‐48 weeks Rosuvastatin 40 mg/day for 48‐96 weeks Rosuvastatin 40‐20‐40 titrated dosing mg/day any time groups were not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 40 mg/day for 0‐12 weeks treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 40 mg/day for 0‐12 weeks treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 40 mg/day for 0‐12 weeks treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 152/1382( 11%) were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | The study was sponsored by AstraZeneca. Data may support bias for rosuvastatin |
| Methods | 6‐week washout period | |
| Participants | 2431 men and women from the USA mean age 58 (21‐86) 641 patients received atorvastatin 655 patients received simvastatin 492 patients received pravastatin 643 patients received rosuvastatin exclusion criteria: women who are likely to become pregnant , statin sensitivity, serious or unstable medical conditions, familial hypercholesterolaemia, lipid‐altering drug use and drug and alcohol abuse Rosuvastatin 10 mg/day baseline TC : 7.11 mmol/l (275 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C : 5.79 mmol/l (224 mg/dl) Rosuvastatin 20 mg/day baseline TC : 7.09 mmol/l (274 mg/dl) Rosuvastatin 20 mg/day baseline non‐HDL‐C : 5.77 mmol/l (223 mg/dl) Rosuvastatin 40 mg/day baseline TC : 7.24 mmol/l (280 mg/dl) Rosuvastatin 40 mg/day baseline non‐HDL‐C : 5.95 mmol/l (230 mg/dl) | |
| Interventions | Atorvastatin 10 mg/day Atorvastatin 20 mg/day Atorvastatin 40 mg/day Atorvastatin 80 mg/day Rosuvastatin 10 mg/day Rosuvastatin 20 mg/day Rosuvastatin 40 mg/day Simvastatin 10 mg/day Simvastatin 20 mg/day Simvastatin 40 mg/day Simvastatin 80 mg/day Pravastatin 10 mg/day Pravastatin 20 mg/day Pravastatin 40 mg/day | |
| Outcomes | per cent change from baseline at 6 weeks of plasma TC, LDL‐C, HDL‐C, non‐HDL‐C and triglycerides | |
| Notes | rosuvastatin groups were analyzed SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day, the Rosuvastatin 20 mg/day, the Rosuvastatin 40 mg/day, treatment arms were analyzed and since there was no placebo group to compare them to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day, the Rosuvastatin 20 mg/day, the Rosuvastatin 40 mg/day, treatment arms were analyzed and since there was no placebo group to compare them to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day, the Rosuvastatin 20 mg/day, the Rosuvastatin 40 mg/day, treatment arms were analyzed and since there was no placebo group to compare them to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 10 mg/day rosuvastatin: 2/158 were not included in the efficacy analysis 20 mg/day rosuvastatin: 4/164 were not included in the efficacy analysis 40 mg/day rosuvastatin: 1/158 were not included in the efficacy analysis 1.5% participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | High risk | AstraZeneca funded it. Data may support bias for rosuvastatin |
| Methods | no washout required because participants were not receiving any lipid‐altering agents 12‐week before‐and‐after trial | |
| Participants | 109 men and women age 72 years with cerebrovascular disease exclusion criteria: none Rosuvastatin 20 mg/day baseline TC : 5.47 mmol/l (212 mg/dl) Rosuvastatin 20 mg/day baseline non‐HDL‐C: 4.2 mmol/l (162 mg/dl) | |
| Interventions | Rosuvastatin 20 mg/day | |
| Outcomes | per cent change from baseline at 12 weeks of blood TC, LDL‐C, HDL‐C, non‐HDL‐C and triglycerides | |
| Notes | SD was imputed for non‐HDL‐C data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | there was only one group of participants analyzed therefore assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | there was only one group of participants analyzed therefore assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | there was only one group of participants analyzed therefore it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | source of funding was not reported |
| Methods | no patient was receiving lipid‐altering medications no washout period was required 12‐week randomized open‐label trial | |
| Participants | 40 men and women age 55‐65 with type 2 diabetes mellitus and hyperlipidaemia TC > 220 mg/dl ( > 5.69 mmol/l) TG > 150 mg/dl ( > 1.69 mmol/l) 20 patients were randomized to rosuvastatin 2.5 mg/day 20 patients were randomized to colestimide 3.0 g/day exclusion criteria: liver dysfunction, renal dysfunction, infections autoimmune disease Rosuvastatin 2.5 mg/day baseline TC : 6.60 mmol/l (255 mg/dl) Rosuvastatin 2.5 mg/day baseline non‐HDL‐C: 5.22 mmol/l (202 mg/dl) | |
| Interventions | rosuvastatin 2.5 mg/day colestimide 3.0 g/day | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | colestimide 3.0 g/day group was not included in the efficacy analysis SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 2.5 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 2.5 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 2.5 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 1/20 (5%) in the rosuvastatin group was not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | source of funding was not reported |
| Methods | participants were not receiving any lipid‐altering agents no washout period is required 12 week | |
| Participants | exclusion criteria: none Rosuvastatin 2.5 mg/day baseline LDL‐C : 4.22 mmol/l (163 mg/dl) | |
| Interventions | rosuvastatin 2.5 mg/day for 0‐12 weeks rosuvastatin 5 mg/day for 12‐24 weeks atorvastatin 10 mg/day pitavastatin 2 mg/day | |
| Outcomes | per cent change from baseline at 8 weeks of serum LDL‐C, HDL‐C and triglycerides | |
| Notes | rosuvastatin 5 mg/day for 12‐24 weeks atorvastatin 10 mg/day pitavastatin 2 mg/day groups were not included in the efficacy analysis SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 2.5 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 2.5 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 2.5 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | total cholesterol and non‐HDL‐C were not included in the efficacy analysis |
| Other bias | Unclear risk | source of funding was not reported |
| Methods | participants were not receiving any lipid‐altering agents no washout period is required 12‐week open‐label trial | |
| Participants | exclusion criteria: serum triglycerides > 400 mg/dl Rosuvastatin 2.5 mg/day baseline TC : 6.53 mmol/l (253 mg/dl) Rosuvastatin 2.5 mg/day baseline non‐HDL‐C: 5.15 mmol/l (199 mg/dl) | |
| Interventions | rosuvastatin 2.5 mg/day atorvastatin 10 mg/day | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | atorvastatin 10 mg/day group was not included in the efficacy analysis SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 2.5 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 2.5 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 2.5 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | source of funding was not reported |
| Methods | participants were not receiving any lipid‐altering agents within 1 month of study no washout period was required 12‐week before‐and‐after trial | |
| Participants | 90 patients with mild to moderate chronic kidney disease with dyslipidaemia age 26‐81 years TC ≥ 5.18 mmol / l, LDL ‐ C ≥ 3.37 mmol / l, TG ≥ 1.7 mmol/l 30 participants received rosuvastatin 5 mg/day for 12 weeks 30 participants received rosuvastatin 10 mg/day for 12 weeks 30 participants received atorvastatin 10 mg/day for 12 weeks exclusion criteria: statin allergies, familial hypercholesterolaemia, thyroid dysfunction, acute and chronic Rosuvastatin 5 mg/day baseline TC : 6.18 mmol/l (239 mg/dl) Rosuvastatin 5 mg/day baseline non‐HDL‐C: 5.33 mmol/l (206 mg/dl) Rosuvastatin 10 mg/day baseline TC : 6.21 mmol/l (240 mg/dl) Rosuvastatin 10 mg/day baseline non‐HDL‐C: 5.34 mmol/l (206 mg/dl) | |
| Interventions | rosuvastatin 5 mg/day rosuvastatin 10 mg/day atorvastatin 10 mg/day | |
| Outcomes | per cent change from baseline at 4 and 12 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | atorvastatin 10 mg/day group was not analyzed SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 5 mg/day and the Rosuvastatin 10 mg/day treatment arms were analyzed and since there was no placebo group to compare them to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 5 mg/day and the Rosuvastatin 10 mg/day treatment arms were analyzed and since there was no placebo group to compare them to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 5 mg/day and the Rosuvastatin 10 mg/day treatment arms were analyzed and since there was no placebo group to compare them to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | source of funding not reported |
| Methods | 6‐week washout period 0‐8 week before‐and‐after trial 8‐16 week dose escalation study 16‐24 week posttreatment washout phase | |
| Participants | 280 men and women ≥18 years of age with stage 3 CKD and mixed dyslipidaemia TG ≥ 150 mg/dl (3.88 mmol/l), LDL‐C ≥ 130 mg/dl (3.36 mmol/l), HDL‐C < 40 mg/dl (1.03 mmol/l) for men and < 50 mg/dl (1.29 mmol/l) for women 140 patients received 45 mg/day fenofibric acid and 5‐10 mg/day rosuvastatin 140 patients received 5‐10 mg/day rosuvastatin exclusion criteria:hypersensitivity to fenofibrate or fenofibric acid or statins BP >140/90 mm Hg, unstable cardiovascular disease within 3‐6 months of trial, type 1 diabetes, uncontrolled type 2 diabetes, history of diabetic ketoacidosis, malignancy except non‐melanoma skin cancer within 2 years, neurologic or blood disorders, GI/hepatic dysfunction, myopathy, renal dysfunction and patients of Asian ancestry Rosuvastatin 5 mg/day baseline TC : 6.55 mmol/l (253 mg/dl) Rosuvastatin 5 mg/day baseline non‐HDL‐C: 5.53 mmol/l (214 mg/dl) | |
| Interventions | Fenofibric acid 45 mg/day and Rosuvastatin 5 mg/day for 0‐8 weeks Fenofibric acid 45 mg/day and Rosuvastatin 10 mg/day for 8‐16 weeks Fenofibric acid and Rosuvastatin posttreatment washout phase for 16‐24 weeks Rosuvastatin 5 mg/day for 0‐8 weeks Rosuvastatin 10 mg/day for 8‐16 weeks Rosuvastatin posttreatment washout phase for 16‐24 weeks | |
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C, and non‐HDL‐C | |
| Notes | Fenofibric acid 45 mg/day and Rosuvastatin 5 mg/day for 0‐8 weeks Fenofibric acid 45 mg/day and Rosuvastatin 10 mg/day for 8‐16 weeks Fenofibric acid and Rosuvastatin posttreatment washout phase for 16‐24 weeks Rosuvastatin 10 mg/day for 8‐16 weeks Rosuvastatin posttreatment washout phase for 16‐24 weeks groups were not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 5 mg/day for 8 weeks treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 5 mg/day for 8 weeks treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 5 mg/day for 8 weeks treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 2/140 (1.4%) participants were not included in the efficacy analysis for TC , HDL‐C and non‐HDL‐C 7/140 (5%) participants were not included in the efficacy analysis for LDL‐C |
| Selective reporting (reporting bias) | High risk | blood triglycerides were not included in the efficacy analysis because it was a median per cent change |
| Other bias | High risk | AbbVie and AstraZeneca sponsored the study. Data may be biased for Rosuvastaitn |
| Methods | participants were not receiving any lipid‐altering agents no washout period is required 8‐week randomized open‐label trial | |
| Participants | 80 men and women > 20 years of age with hypercholesterolaemia coronary heart disease or risk equivalents LDL‐C ≥100 mg/dl ( ≥2.59 mmol/l), ≥2 risk factors and LDL‐C ≥130 mg/dl (≥3.36 mmol/l) <2 risk factors and LDL‐C ≥160 mg/dl ( ≥4.14 mmol/l) 38 patients randomized to rosuvastatin 10mg/day 38 patients randomized to rosuvastatin 10 mg EOD exclusion criteria: taking drugs known to affect lipid metabolism or interact with rosuvastatin active liver disease, liver dysfunction, serum CK > 3 X ULN renal dysfunction, pregnancy, lactation Rosuvastatin 40 mg/day baseline TC : 6.63 mmol/l (256 mg/dl) Rosuvastatin 40 mg/day baseline non‐HDL‐C: 5.27 mmol/l (204 mg/dl) | |
| Interventions | rosuvastatin 10 mg/day rosuvastatin 10 mg EOD | |
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | rosuvastatin 10 mg EOD group was not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 10 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 2/40 = 5% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | source of funding was not reported |
| Methods | ≥ 4 week washout period 8‐week randomized double‐blind trial | |
| Participants | 68 men and women age 28‐72 years with primary hypercholesterolaemia TC ≥220 mg/dl ( 5.68 mmol/l) TG ≤400 mg/dl ( 4.50 mmol/l) 20 patients were randomized to rosuvastatin 1 mg/day 19 patients were randomized to rosuvastatin 2 mg/day 21 patients were randomized to rosuvastatin 4 mg/day exclusion criteria: none reported Rosuvastatin 1 mg/day baseline TC : 7.61 mmol/l (294 mg/dl) Rosuvastatin 2 mg/day baseline TC : 7.85 mmol/l (304 mg/dl) Rosuvastatin 4 mg/day baseline TC : 7.37 mmol/l (285 mg/dl) | |
| Interventions | rosuvastatin 1 mg/day rosuvastatin 2 mg/day rosuvastatin 4 mg/day | |
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 1 mg/day, the Rosuvastatin 2 mg/day, the Rosuvastatin 4 mg/day, treatment arms were analyzed and since there was no placebo group to compare them to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 1 mg/day, the Rosuvastatin 2 mg/day, the Rosuvastatin 4 mg/day, treatment arms were analyzed and since there was no placebo group to compare them to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 1 mg/day, the Rosuvastatin 2 mg/day, the Rosuvastatin 4 mg/day, treatment arms were analyzed and since there was no placebo group to compare them to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | High risk | 8/68 = 11.8% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | source of funding not reported |
| Methods | no lipid‐lowering medication had been administered no washout period required 24‐week randomized open‐label cross‐over trial | |
| Participants | 90 men and women with type 2 diabetes mellitus and hyperlipidaemia LDL‐C ≥140 mg/dl (≥ 3.62 mmol/l) TG < 300 mg/dl (<3.39 mmol/l) glycated haemoglobin <8.5% serum creatinine < 2.0 mg/d urinary albumin excretion < 300 mg/Cr 21 patients were randomized to rosuvastatin 2.5 mg/day for 0‐12 weeks then pitavastatin 2 mg/day for 12‐24 weeks 21 patients were randomized to pitavastatin 2 mg/day for 0‐12 weeks then rosuvastatin 2.5 mg/day for 12‐24 weeks 22 patients were randomized to rosuvastatin 2.5 mg/day for 0‐12 weeks then rosuvastatin 2.5 mg/day for 12‐24 weeks 22 patients were randomized to pitavastatin 2 mg/day for 0‐12 weeks then pitavastatin 2 mg/day for 12‐24 weeks exclusion criteria: history of stroke,or other cardiovascular event ROS‐PIT group for 0‐12 weeks Rosuvastatin 2.5 mg/day baseline LDL‐C : 4.98 mmol/l (193 mg/dl) ROS‐ROS group for 0‐12 weeks Rosuvastatin 2.5 mg/day baseline LDL‐C : 4.87 mmol/l (188 mg/dl) Combined groups for 0‐12 weeks Rosuvastatin 2.5 mg/day baseline LDL‐C : 4.92 mmol/l (190 mg/dl) | |
| Interventions | rosuvastatin 2.5 mg/day for 0‐12 weeks pitavastatin 2 mg/day for 12‐24 weeks pitavastatin 2 mg/day for 0‐12 weeks rosuvastatin 2.5 mg/day for 12‐24 weeks | |
| Outcomes | per cent change from baseline at 12 weeks of serum LDL‐C, HDL‐C and triglycerides | |
| Notes | pitavastatin 2 mg/day for 12‐24 weeks pitavastatin 2 mg/day for 0‐12 weeks rosuvastatin 2.5 mg/day for 12‐24 weeks groups and time periods were not included in the efficacy analysis SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | the Rosuvastatin 2.5 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | the Rosuvastatin 2.5 mg/day treatment arm was analyzed and since there was no placebo group to compare it to assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | the Rosuvastatin 2.5 mg/day treatment arm was analyzed and since there was no placebo group to compare it to it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | 4/90 = 4.4% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | total cholesterol and non‐HDL‐C were not included in the efficacy analysis |
| Other bias | Low risk | authors declare they have no conflict of interest |
| Methods | 6‐week dietary washout baseline stabilization period 12‐week before‐and‐after trial | |
| Participants | 23 men and women with diabetes and hypercholesterolaemia and 33 non diabetic men and women with hypercholesterolaemia for a total of 56 age 36‐78 years all participants received 2.5 mg/day rosuvastatin for 12 weeks exclusion criteria: renal dysfunction, hepatic dysfunction hyperthyroidism, pregnancy, receiving lipid‐altering agents within 8 weeks of study diabetic participants receiving pioglitazone Rosuvastatin 2.5 mg/day baseline TC : 6.75 mmol/l (261 mg/dl) Rosuvastatin 2.5 mg/day baseline non‐HDL‐C: 5.24 mmol/l (203 mg/dl) | |
| Interventions | rosuvastatin 2.5 mg/day | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and non‐HDL‐C | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | there was only one group of participants analyzed therefore assessment of adequate sequence generation is not applicable |
| Allocation concealment (selection bias) | High risk | there was only one group of participants analyzed therefore assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | High risk | there was only one group of participants analyzed therefore it is an unblinded trial |
| Incomplete outcome data (attrition bias) | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | all lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | source of funding not reported |
ALT: Alanine transaminase
AST: aspartate aminotransferase
BMI: body mass index
CABG: coronary artery bypass graft
CAD: coronary artery disease
CHD: coronary heart disease
CK: creatine kinase
CKD: chronic kidney disease
CPK: creatine phosphokinase
DBP: diastolic blood pressure
GI: gastrointestinal
HDL‐C: high‐density lipoprotein cholesterol
HRT: hormone replacement therapy
IMT: intima‐media thickness
LDL‐C: low‐density lipoprotein cholesterol
LLT: lipid‐lowering therapy
MI: myocardial infarction
PTCA: percutaneous transluminal coronary angioplasty
SBP: systolic blood pressure
SD: standard deviation
STEMI: ST segment elevation myocardial infarction
TC: total cholesterol
TG: triglycerides
TIA: transient ischaemic attack
TSH: thyroid stimulating hormone
ULN: upper limit of normal
WDAEs: withdrawals due to adverse effects
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Exact number of participants receiving rosuvastatin not reported | |
| Participants received confounding drug ezetimibe added to the rosuvastatin | |
| Specific dose not reported, range of doses given | |
| Confounding factor: anti retroviral drugs for the treatment of HIV inappropriate outcomes median per cent change from baseline | |
| Confounding factor: haemodialysis | |
| Confounding factor: anti retroviral drugs for the treatment of HIV | |
| Confounding factor: anti retroviral drugs for the treatment of HIV | |
| Confounding factor: anti retroviral drugs for the treatment of HIV | |
| Titrated dose sequential data | |
| 447/1002 (44.6%) participants were not included in the efficacy analysis high incomplete outcome bias | |
| Confounding factor: anti retroviral drugs for the treatment of HIV | |
| Data from statin‐naive patients and switched patients were combined. No baseline dietary washout stabilization period for at least 3 weeks was performed for the switched patients | |
| Some participants were receiving confounding drugs | |
| length of washout period not recorded | |
| No dose of rosuvastatin reported | |
| Confounding factor: anti retroviral drugs for the treatment of HIV | |
| Not available via interlibrary loan | |
| length of washout period not recorded | |
| length of washout period not recorded | |
| Confounding factor: participants are receiving protease inhibitors for the treatment of HIV | |
| LDL‐C values are incorrect from the Friedewald formula all values are suspect | |
| LDL‐C values are incorrect from the Friedewald formula all values are suspect | |
| Pooled data across all statin doses | |
| Inappropriate outcomes, median per cent change | |
| Article is not available via interlibrary loan | |
| 4/15 (26.6%) participants were not included in the efficacy analysis high incomplete outcome bias | |
| Rosuvastatin fenofibrate combination | |
| Median per cent change was reported | |
| Confounding factor: participants received protease inhibitors for the treatment of HIV | |
| Confounding factor: participants received protease inhibitors for the treatment of HIV | |
| 167/723 (23%) participants were not included in the efficacy analysis; high incomplete outcome bias |
LDL‐C: low‐density lipoprotein cholesterol
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total cholesterol Show forest plot | 3 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐21.83 [‐25.59, ‐18.08] |
| Analysis 1.1  Comparison 1 1.0 mg vs control, Outcome 1 Total cholesterol. | ||||
| 2 LDL‐cholesterol Show forest plot | 3 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐31.17 [‐35.32, ‐27.02] |
| Analysis 1.2  Comparison 1 1.0 mg vs control, Outcome 2 LDL‐cholesterol. | ||||
| 3 HDL‐cholesterol Show forest plot | 3 | 93 | Mean Difference (IV, Fixed, 95% CI) | 8.16 [2.93, 13.38] |
| Analysis 1.3  Comparison 1 1.0 mg vs control, Outcome 3 HDL‐cholesterol. | ||||
| 4 non‐HDL‐cholesterol Show forest plot | 2 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐30.13 [‐38.06, ‐22.20] |
| Analysis 1.4 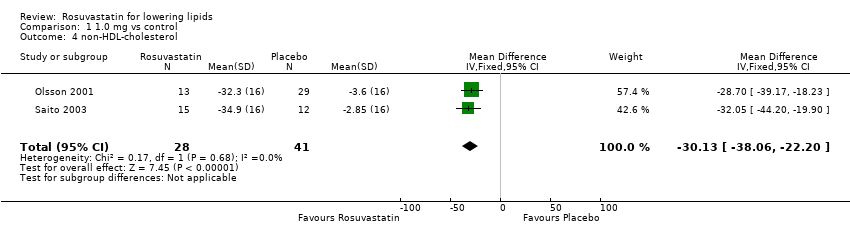 Comparison 1 1.0 mg vs control, Outcome 4 non‐HDL‐cholesterol. | ||||
| 5 Triglycerides Show forest plot | 3 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐20.77 [‐32.73, ‐8.80] |
| Analysis 1.5  Comparison 1 1.0 mg vs control, Outcome 5 Triglycerides. | ||||
| 6 WDAE Show forest plot | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.6  Comparison 1 1.0 mg vs control, Outcome 6 WDAE. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total cholesterol Show forest plot | 3 | 95 | Mean Difference (IV, Fixed, 95% CI) | ‐27.44 [‐31.17, ‐23.70] |
| Analysis 2.1  Comparison 2 2.5 mg vs control, Outcome 1 Total cholesterol. | ||||
| 2 LDL‐cholesterol Show forest plot | 3 | 95 | Mean Difference (IV, Fixed, 95% CI) | ‐38.27 [‐42.79, ‐33.75] |
| Analysis 2.2  Comparison 2 2.5 mg vs control, Outcome 2 LDL‐cholesterol. | ||||
| 3 HDL‐cholesterol Show forest plot | 3 | 95 | Mean Difference (IV, Fixed, 95% CI) | 6.02 [0.88, 11.16] |
| Analysis 2.3  Comparison 2 2.5 mg vs control, Outcome 3 HDL‐cholesterol. | ||||
| 4 non‐HDL‐cholesterol Show forest plot | 2 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐36.47 [‐44.30, ‐28.63] |
| Analysis 2.4  Comparison 2 2.5 mg vs control, Outcome 4 non‐HDL‐cholesterol. | ||||
| 5 Triglycerides Show forest plot | 3 | 95 | Mean Difference (IV, Fixed, 95% CI) | ‐13.11 [‐24.97, ‐1.25] |
| Analysis 2.5  Comparison 2 2.5 mg vs control, Outcome 5 Triglycerides. | ||||
| 6 Total cholesterol Show forest plot | 6 | 286 | % change from baseline (Fixed, 95% CI) | ‐26.52 [‐27.90, ‐25.13] |
| Analysis 2.6  Comparison 2 2.5 mg vs control, Outcome 6 Total cholesterol. | ||||
| 7 LDL‐cholesterol Show forest plot | 8 | 355 | % change from baseline (Fixed, 95% CI) | ‐39.21 [‐40.76, ‐37.65] |
| Analysis 2.7 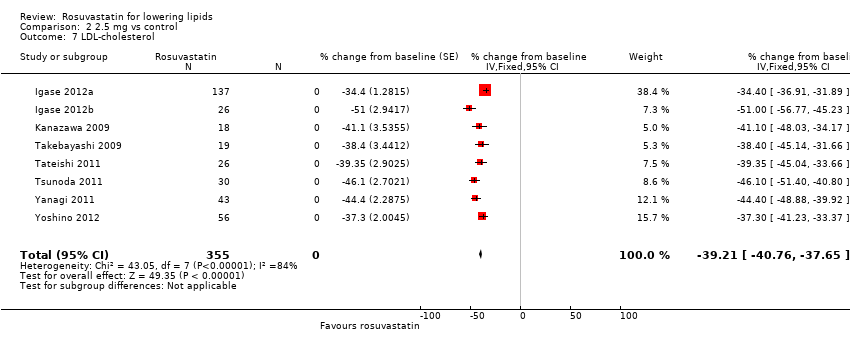 Comparison 2 2.5 mg vs control, Outcome 7 LDL‐cholesterol. | ||||
| 8 HDL‐cholesterol Show forest plot | 8 | 355 | % change from baseline (Fixed, 95% CI) | 4.20 [2.54, 5.85] |
| Analysis 2.8  Comparison 2 2.5 mg vs control, Outcome 8 HDL‐cholesterol. | ||||
| 9 non‐HDL‐cholesterol Show forest plot | 6 | 286 | Mean Difference (Fixed, 95% CI) | ‐35.27 [‐37.13, ‐33.41] |
| Analysis 2.9  Comparison 2 2.5 mg vs control, Outcome 9 non‐HDL‐cholesterol. | ||||
| 10 Triglycerides Show forest plot | 8 | 355 | % change from baseline (Fixed, 95% CI) | ‐13.70 [‐16.97, ‐10.43] |
| Analysis 2.10  Comparison 2 2.5 mg vs control, Outcome 10 Triglycerides. | ||||
| 11 WDAE Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [0.11, 57.83] |
| Analysis 2.11 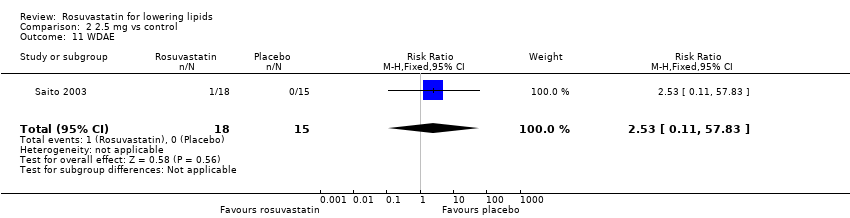 Comparison 2 2.5 mg vs control, Outcome 11 WDAE. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total cholesterol Show forest plot | 9 | 762 | Mean Difference (IV, Fixed, 95% CI) | ‐29.13 [‐30.56, ‐27.70] |
| Analysis 3.1  Comparison 3 5.0 mg vs control, Outcome 1 Total cholesterol. | ||||
| 2 LDL‐cholesterol Show forest plot | 9 | 762 | Mean Difference (IV, Fixed, 95% CI) | ‐39.12 [‐41.11, ‐37.12] |
| Analysis 3.2  Comparison 3 5.0 mg vs control, Outcome 2 LDL‐cholesterol. | ||||
| 3 HDL‐cholesterol Show forest plot | 9 | 762 | Mean Difference (IV, Fixed, 95% CI) | 8.64 [6.93, 10.35] |
| Analysis 3.3 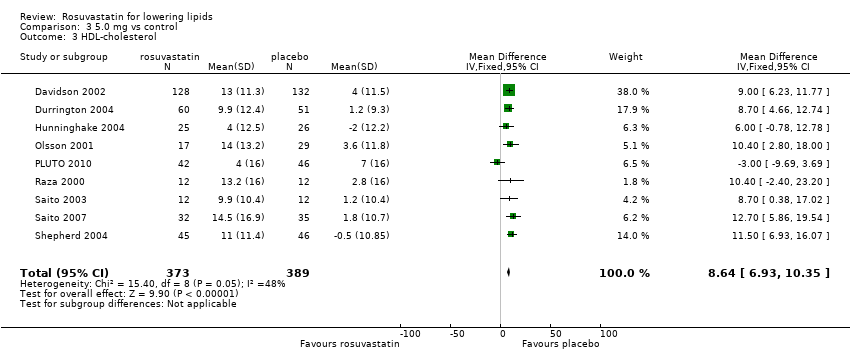 Comparison 3 5.0 mg vs control, Outcome 3 HDL‐cholesterol. | ||||
| 4 non‐HDL‐cholesterol Show forest plot | 8 | 738 | Mean Difference (IV, Fixed, 95% CI) | ‐36.79 [‐38.85, ‐34.72] |
| Analysis 3.4  Comparison 3 5.0 mg vs control, Outcome 4 non‐HDL‐cholesterol. | ||||
| 5 Triglycerides Show forest plot | 8 | 674 | Mean Difference (IV, Fixed, 95% CI) | ‐23.08 [‐26.97, ‐19.19] |
| Analysis 3.5  Comparison 3 5.0 mg vs control, Outcome 5 Triglycerides. | ||||
| 6 Total cholesterol Show forest plot | 15 | 1411 | % change from baseline (Fixed, 95% CI) | ‐29.11 [‐29.69, ‐28.53] |
| Analysis 3.6  Comparison 3 5.0 mg vs control, Outcome 6 Total cholesterol. | ||||
| 7 LDL‐cholesterol Show forest plot | 16 | 1840 | % change from baseline (Fixed, 95% CI) | ‐41.57 [‐42.22, ‐40.92] |
| Analysis 3.7  Comparison 3 5.0 mg vs control, Outcome 7 LDL‐cholesterol. | ||||
| 8 HDL‐cholesterol Show forest plot | 16 | 1845 | % change from baseline (Fixed, 95% CI) | 6.69 [6.04, 7.34] |
| Analysis 3.8  Comparison 3 5.0 mg vs control, Outcome 8 HDL‐cholesterol. | ||||
| 9 non‐HDL‐cholesterol Show forest plot | 14 | 1307 | % change from baseline (Fixed, 95% CI) | ‐37.77 [‐38.53, ‐37.01] |
| Analysis 3.9 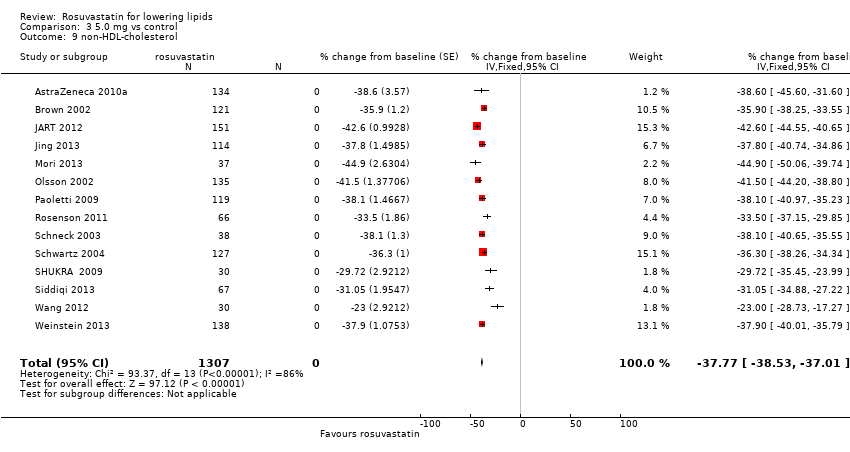 Comparison 3 5.0 mg vs control, Outcome 9 non‐HDL‐cholesterol. | ||||
| 10 Triglycerides Show forest plot | 14 | 1678 | % change from baseline (Fixed, 95% CI) | ‐16.96 [‐18.33, ‐15.60] |
| Analysis 3.10  Comparison 3 5.0 mg vs control, Outcome 10 Triglycerides. | ||||
| 11 WDAE Show forest plot | 5 | 561 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.70, 2.72] |
| Analysis 3.11 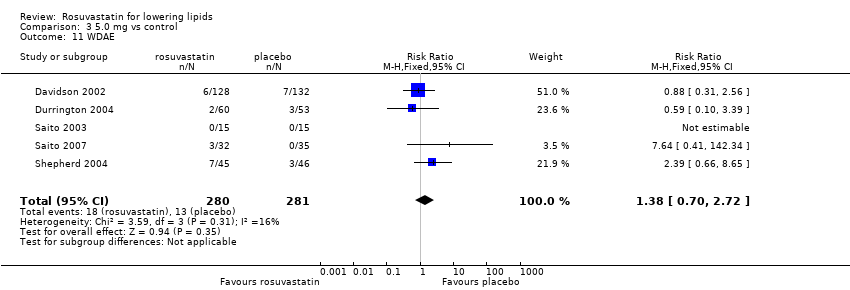 Comparison 3 5.0 mg vs control, Outcome 11 WDAE. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total cholesterol Show forest plot | 15 | 1442 | Mean Difference (IV, Fixed, 95% CI) | ‐31.34 [‐32.45, ‐30.23] |
| Analysis 4.1  Comparison 4 10 mg vs control, Outcome 1 Total cholesterol. | ||||
| 2 LDL‐cholesterol Show forest plot | 15 | 1442 | Mean Difference (IV, Fixed, 95% CI) | ‐42.80 [‐44.26, ‐41.35] |
| Analysis 4.2 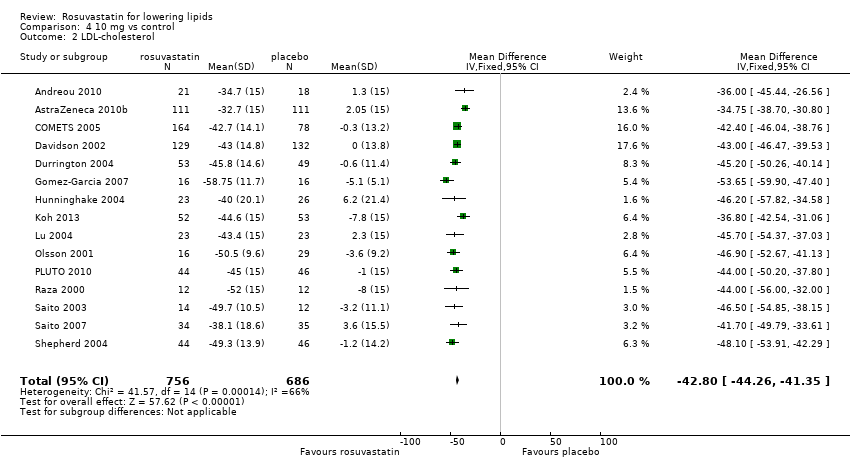 Comparison 4 10 mg vs control, Outcome 2 LDL‐cholesterol. | ||||
| 3 HDL‐cholesterol Show forest plot | 15 | 1442 | Mean Difference (IV, Fixed, 95% CI) | 10.46 [9.40, 11.52] |
| Analysis 4.3  Comparison 4 10 mg vs control, Outcome 3 HDL‐cholesterol. | ||||
| 4 non‐HDL‐cholesterol Show forest plot | 14 | 1418 | Mean Difference (IV, Fixed, 95% CI) | ‐39.28 [‐40.82, ‐37.74] |
| Analysis 4.4  Comparison 4 10 mg vs control, Outcome 4 non‐HDL‐cholesterol. | ||||
| 5 Triglycerides Show forest plot | 13 | 1313 | Mean Difference (IV, Fixed, 95% CI) | ‐19.97 [‐22.81, ‐17.12] |
| Analysis 4.5  Comparison 4 10 mg vs control, Outcome 5 Triglycerides. | ||||
| 6 Total cholesterol Show forest plot | 55 | 8100 | % change from baseline (Fixed, 95% CI) | ‐32.89 [‐33.14, ‐32.64] |
| Analysis 4.6  Comparison 4 10 mg vs control, Outcome 6 Total cholesterol. | ||||
| 7 LDL‐cholesterol Show forest plot | 59 | 8413 | % change from baseline (Fixed, 95% CI) | ‐45.77 [‐46.09, ‐45.46] |
| Analysis 4.7  Comparison 4 10 mg vs control, Outcome 7 LDL‐cholesterol. | ||||
| 8 HDL‐cholesterol Show forest plot | 55 | 8085 | % change from baseline (Fixed, 95% CI) | 6.25 [5.93, 6.58] |
| Analysis 4.8 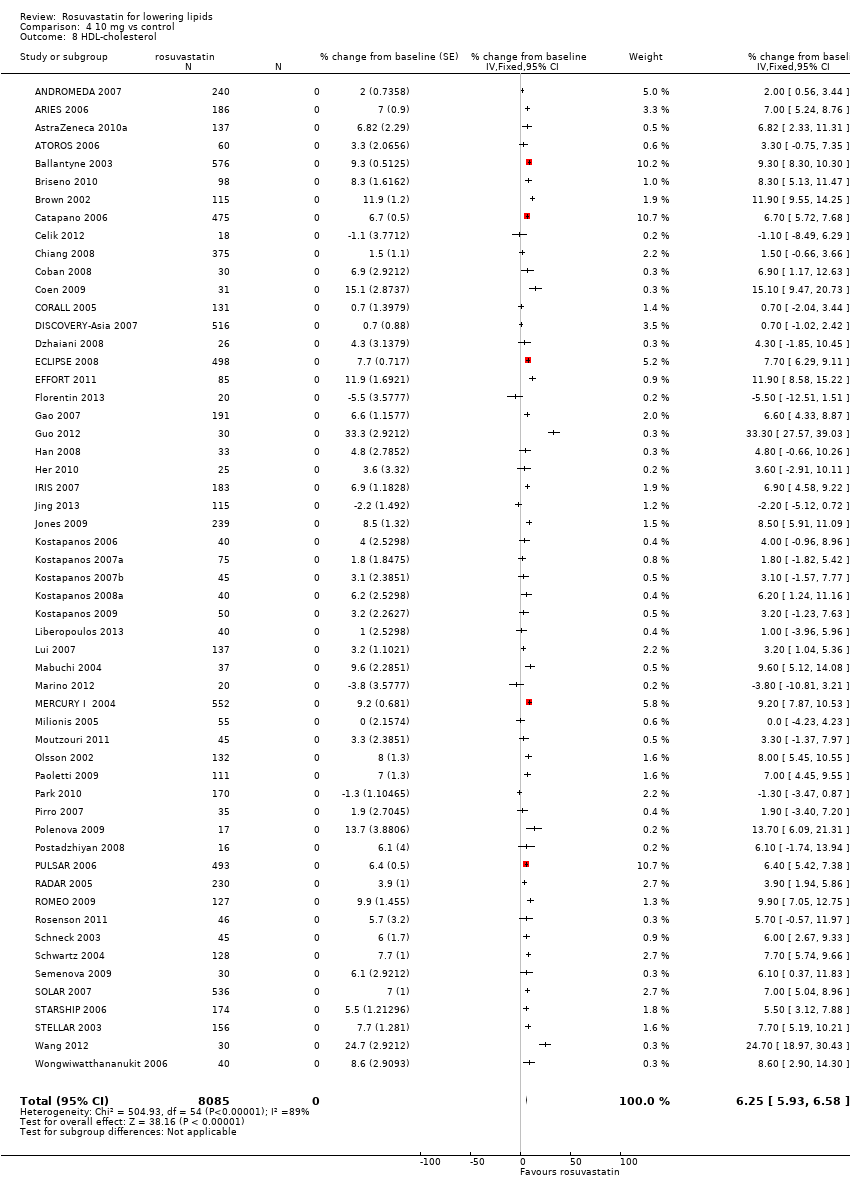 Comparison 4 10 mg vs control, Outcome 8 HDL‐cholesterol. | ||||
| 9 non‐HDL‐cholesterol Show forest plot | 53 | 7405 | % change from baseline (Fixed, 95% CI) | ‐42.06 [‐42.39, ‐41.72] |
| Analysis 4.9  Comparison 4 10 mg vs control, Outcome 9 non‐HDL‐cholesterol. | ||||
| 10 Triglycerides Show forest plot | 51 | 7524 | % change from baseline (Fixed, 95% CI) | ‐19.72 [‐20.38, ‐19.07] |
| Analysis 4.10  Comparison 4 10 mg vs control, Outcome 10 Triglycerides. | ||||
| 11 WDAE Show forest plot | 6 | 724 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.29, 1.39] |
| Analysis 4.11  Comparison 4 10 mg vs control, Outcome 11 WDAE. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total cholesterol Show forest plot | 8 | 576 | Mean Difference (IV, Fixed, 95% CI) | ‐33.58 [‐35.41, ‐31.75] |
| Analysis 5.1 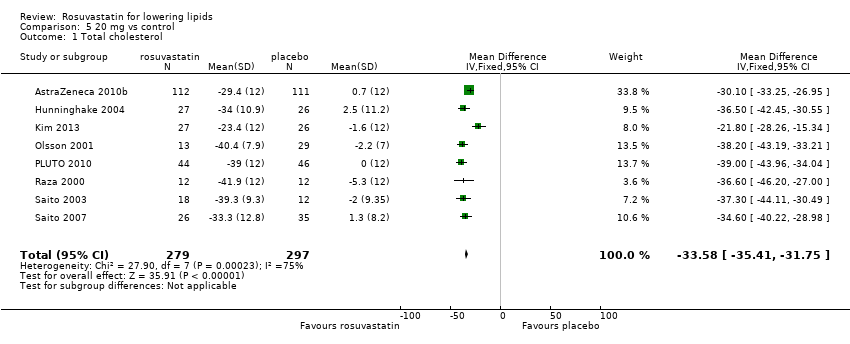 Comparison 5 20 mg vs control, Outcome 1 Total cholesterol. | ||||
| 2 LDL‐cholesterol Show forest plot | 8 | 576 | Mean Difference (IV, Fixed, 95% CI) | ‐45.83 [‐48.22, ‐43.44] |
| Analysis 5.2  Comparison 5 20 mg vs control, Outcome 2 LDL‐cholesterol. | ||||
| 3 HDL‐cholesterol Show forest plot | 8 | 576 | Mean Difference (IV, Fixed, 95% CI) | 6.82 [4.42, 9.21] |
| Analysis 5.3  Comparison 5 20 mg vs control, Outcome 3 HDL‐cholesterol. | ||||
| 4 non‐HDL‐cholesterol Show forest plot | 7 | 552 | Mean Difference (IV, Fixed, 95% CI) | ‐40.67 [‐43.16, ‐38.19] |
| Analysis 5.4  Comparison 5 20 mg vs control, Outcome 4 non‐HDL‐cholesterol. | ||||
| 5 Triglycerides Show forest plot | 7 | 486 | Mean Difference (IV, Fixed, 95% CI) | ‐22.61 [‐27.94, ‐17.28] |
| Analysis 5.5  Comparison 5 20 mg vs control, Outcome 5 Triglycerides. | ||||
| 6 Total cholesterol Show forest plot | 19 | 2915 | % change from baseline (Fixed, 95% CI) | ‐36.30 [‐36.70, ‐35.90] |
| Analysis 5.6  Comparison 5 20 mg vs control, Outcome 6 Total cholesterol. | ||||
| 7 LDL‐cholesterol Show forest plot | 20 | 3099 | % change from baseline (Fixed, 95% CI) | ‐50.07 [‐50.55, ‐49.58] |
| Analysis 5.7  Comparison 5 20 mg vs control, Outcome 7 LDL‐cholesterol. | ||||
| 8 HDL‐cholesterol Show forest plot | 19 | 2896 | % change from baseline (Fixed, 95% CI) | 8.03 [7.51, 8.55] |
| Analysis 5.8  Comparison 5 20 mg vs control, Outcome 8 HDL‐cholesterol. | ||||
| 9 non‐HDL‐cholesterol Show forest plot | 18 | 2461 | % change from baseline (Fixed, 95% CI) | ‐45.77 [‐46.31, ‐45.24] |
| Analysis 5.9  Comparison 5 20 mg vs control, Outcome 9 non‐HDL‐cholesterol. | ||||
| 10 Triglycerides Show forest plot | 16 | 2367 | % change from baseline (Fixed, 95% CI) | ‐21.65 [‐22.80, ‐20.50] |
| Analysis 5.10  Comparison 5 20 mg vs control, Outcome 10 Triglycerides. | ||||
| 11 WDAE Show forest plot | 5 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.25, 4.48] |
| Analysis 5.11  Comparison 5 20 mg vs control, Outcome 11 WDAE. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total cholesterol Show forest plot | 4 | 163 | Mean Difference (IV, Fixed, 95% CI) | ‐42.54 [‐45.22, ‐39.86] |
| Analysis 6.1  Comparison 6 40 mg vs control, Outcome 1 Total cholesterol. | ||||
| 2 LDL‐cholesterol Show forest plot | 6 | 472 | Mean Difference (IV, Fixed, 95% CI) | ‐55.85 [‐58.31, ‐53.40] |
| Analysis 6.2  Comparison 6 40 mg vs control, Outcome 2 LDL‐cholesterol. | ||||
| 3 HDL‐cholesterol Show forest plot | 6 | 472 | Mean Difference (IV, Fixed, 95% CI) | 6.85 [4.29, 9.40] |
| Analysis 6.3  Comparison 6 40 mg vs control, Outcome 3 HDL‐cholesterol. | ||||
| 4 non‐HDL‐cholesterol Show forest plot | 3 | 139 | Mean Difference (IV, Fixed, 95% CI) | ‐53.75 [‐58.57, ‐48.94] |
| Analysis 6.4  Comparison 6 40 mg vs control, Outcome 4 non‐HDL‐cholesterol. | ||||
| 5 Triglycerides Show forest plot | 5 | 203 | Mean Difference (IV, Fixed, 95% CI) | ‐31.76 [‐39.40, ‐24.12] |
| Analysis 6.5  Comparison 6 40 mg vs control, Outcome 5 Triglycerides. | ||||
| 6 Total cholesterol Show forest plot | 11 | 3017 | % change from baseline (Fixed, 95% CI) | ‐40.42 [‐40.83, ‐40.02] |
| Analysis 6.6  Comparison 6 40 mg vs control, Outcome 6 Total cholesterol. | ||||
| 7 LDL‐cholesterol Show forest plot | 11 | 3010 | % change from baseline (Fixed, 95% CI) | ‐54.84 [‐55.35, ‐54.33] |
| Analysis 6.7  Comparison 6 40 mg vs control, Outcome 7 LDL‐cholesterol. | ||||
| 8 HDL‐cholesterol Show forest plot | 11 | 3005 | % change from baseline (Fixed, 95% CI) | 9.90 [9.34, 10.46] |
| Analysis 6.8  Comparison 6 40 mg vs control, Outcome 8 HDL‐cholesterol. | ||||
| 9 non‐HDL‐cholesterol Show forest plot | 11 | 3005 | % change from baseline (Fixed, 95% CI) | ‐50.69 [‐51.22, ‐50.16] |
| Analysis 6.9 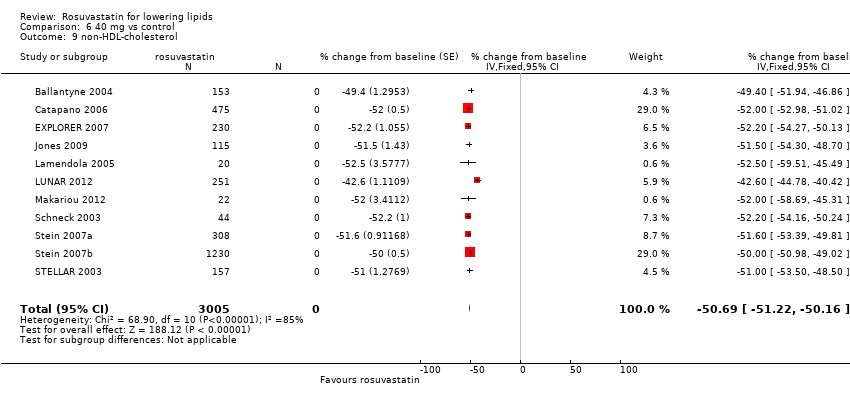 Comparison 6 40 mg vs control, Outcome 9 non‐HDL‐cholesterol. | ||||
| 10 Triglycerides Show forest plot | 9 | 2520 | % change from baseline (Fixed, 95% CI) | ‐26.53 [‐27.76, ‐25.29] |
| Analysis 6.10  Comparison 6 40 mg vs control, Outcome 10 Triglycerides. | ||||
| 11 WDAE Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 6.11  Comparison 6 40 mg vs control, Outcome 11 WDAE. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total cholesterol Show forest plot | 2 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐44.5 [‐47.84, ‐41.16] |
| Analysis 7.1  Comparison 7 80 mg vs control, Outcome 1 Total cholesterol. | ||||
| 2 LDL‐cholesterol Show forest plot | 2 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐59.47 [‐64.15, ‐54.79] |
| Analysis 7.2  Comparison 7 80 mg vs control, Outcome 2 LDL‐cholesterol. | ||||
| 3 HDL‐cholesterol Show forest plot | 2 | 113 | Mean Difference (IV, Fixed, 95% CI) | 10.68 [5.92, 15.44] |
| Analysis 7.3  Comparison 7 80 mg vs control, Outcome 3 HDL‐cholesterol. | ||||
| 4 non‐HDL‐cholesterol Show forest plot | 2 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐55.50 [‐60.70, ‐50.29] |
| Analysis 7.4 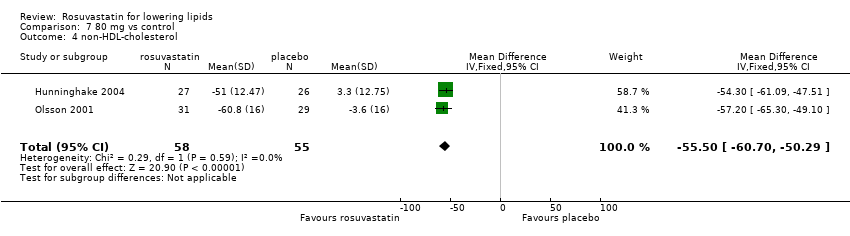 Comparison 7 80 mg vs control, Outcome 4 non‐HDL‐cholesterol. | ||||
| 5 Triglycerides Show forest plot | 2 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐34.49 [‐43.89, ‐25.10] |
| Analysis 7.5  Comparison 7 80 mg vs control, Outcome 5 Triglycerides. | ||||
| 6 Total cholesterol Show forest plot | 1 | 42 | % change from baseline (Fixed, 95% CI) | ‐43.00 [‐47.16, ‐42.84] |
| Analysis 7.6  Comparison 7 80 mg vs control, Outcome 6 Total cholesterol. | ||||
| 7 LDL‐cholesterol Show forest plot | 1 | 42 | % change from baseline (Fixed, 95% CI) | ‐61.9 [‐64.64, ‐59.16] |
| Analysis 7.7  Comparison 7 80 mg vs control, Outcome 7 LDL‐cholesterol. | ||||
| 8 HDL‐cholesterol Show forest plot | 1 | 42 | % change from baseline (Fixed, 95% CI) | 9.6 [6.27, 12.93] |
| Analysis 7.8 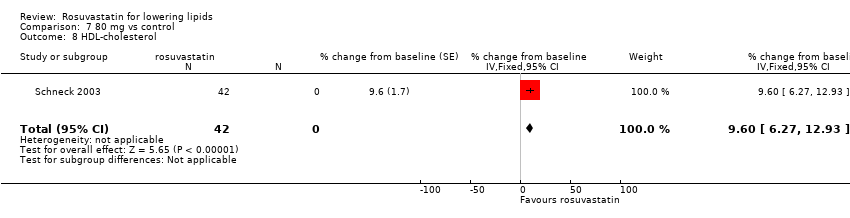 Comparison 7 80 mg vs control, Outcome 8 HDL‐cholesterol. | ||||
| 9 non‐HDL‐cholesterol Show forest plot | 1 | 42 | % change from baseline (Fixed, 95% CI) | ‐57.0 [‐59.55, ‐54.45] |
| Analysis 7.9  Comparison 7 80 mg vs control, Outcome 9 non‐HDL‐cholesterol. | ||||
| 10 Triglycerides Show forest plot | 1 | 42 | % change from baseline (Fixed, 95% CI) | ‐19.7 [‐28.32, ‐11.08] |
| Analysis 7.10 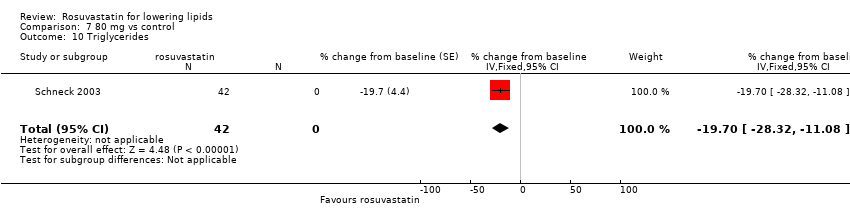 Comparison 7 80 mg vs control, Outcome 10 Triglycerides. | ||||
| 11 WDAE Show forest plot | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.05, 4.99] |
| Analysis 7.11  Comparison 7 80 mg vs control, Outcome 11 WDAE. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 WDAEs Show forest plot | 10 | 1330 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.48, 1.47] |
| Analysis 8.1  Comparison 8 all doses vs control, Outcome 1 WDAEs. | ||||

Values represent the results of each trial for each dose comparison.
The standard error bars cannot be seen because they all lie within the points.

Values represent the results of each trial for each dose comparison.
The standard error bars cannot be seen because they all lie within the points.
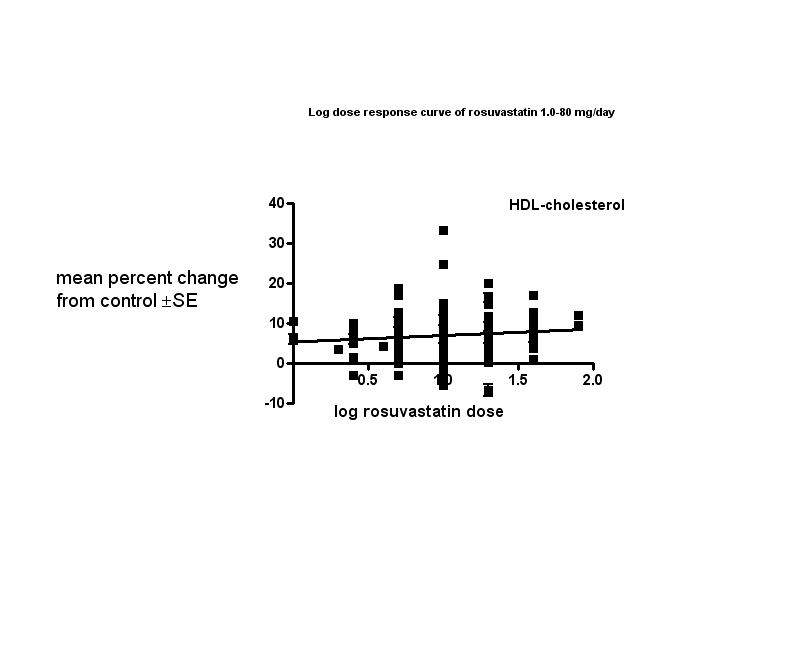
Values represent the results of each trial for each dose comparison.
The standard error bars cannot be seen because they all lie within the points.

Values represent the results of each trial for each dose comparison.
The standard error bars cannot be seen because they all lie within the points.

Values represent the results of each trial for each dose comparison.
The standard error bars cannot be seen because they all lie within the points.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 1.0 mg vs control, Outcome 1 Total cholesterol.

Comparison 1 1.0 mg vs control, Outcome 2 LDL‐cholesterol.

Comparison 1 1.0 mg vs control, Outcome 3 HDL‐cholesterol.

Comparison 1 1.0 mg vs control, Outcome 4 non‐HDL‐cholesterol.

Comparison 1 1.0 mg vs control, Outcome 5 Triglycerides.
Comparison 1 1.0 mg vs control, Outcome 6 WDAE.
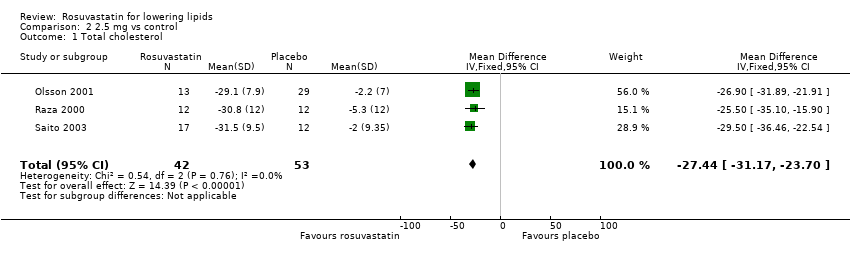
Comparison 2 2.5 mg vs control, Outcome 1 Total cholesterol.

Comparison 2 2.5 mg vs control, Outcome 2 LDL‐cholesterol.

Comparison 2 2.5 mg vs control, Outcome 3 HDL‐cholesterol.

Comparison 2 2.5 mg vs control, Outcome 4 non‐HDL‐cholesterol.
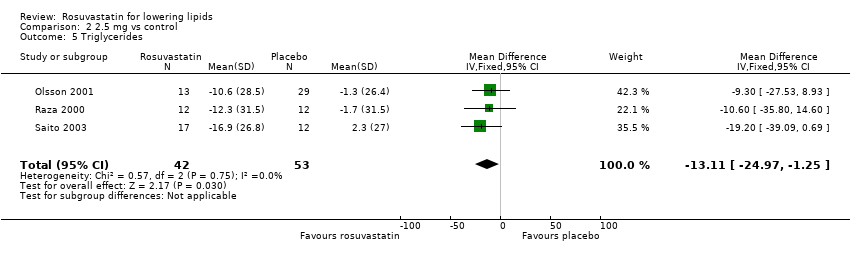
Comparison 2 2.5 mg vs control, Outcome 5 Triglycerides.

Comparison 2 2.5 mg vs control, Outcome 6 Total cholesterol.

Comparison 2 2.5 mg vs control, Outcome 7 LDL‐cholesterol.

Comparison 2 2.5 mg vs control, Outcome 8 HDL‐cholesterol.

Comparison 2 2.5 mg vs control, Outcome 9 non‐HDL‐cholesterol.

Comparison 2 2.5 mg vs control, Outcome 10 Triglycerides.
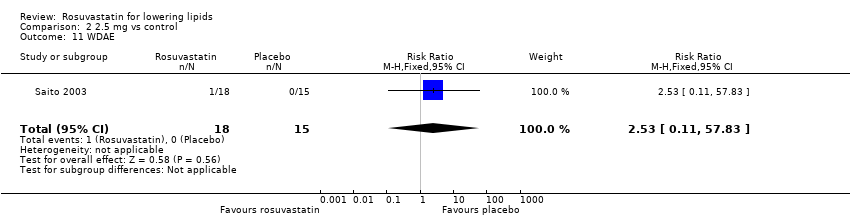
Comparison 2 2.5 mg vs control, Outcome 11 WDAE.

Comparison 3 5.0 mg vs control, Outcome 1 Total cholesterol.

Comparison 3 5.0 mg vs control, Outcome 2 LDL‐cholesterol.

Comparison 3 5.0 mg vs control, Outcome 3 HDL‐cholesterol.

Comparison 3 5.0 mg vs control, Outcome 4 non‐HDL‐cholesterol.

Comparison 3 5.0 mg vs control, Outcome 5 Triglycerides.

Comparison 3 5.0 mg vs control, Outcome 6 Total cholesterol.

Comparison 3 5.0 mg vs control, Outcome 7 LDL‐cholesterol.
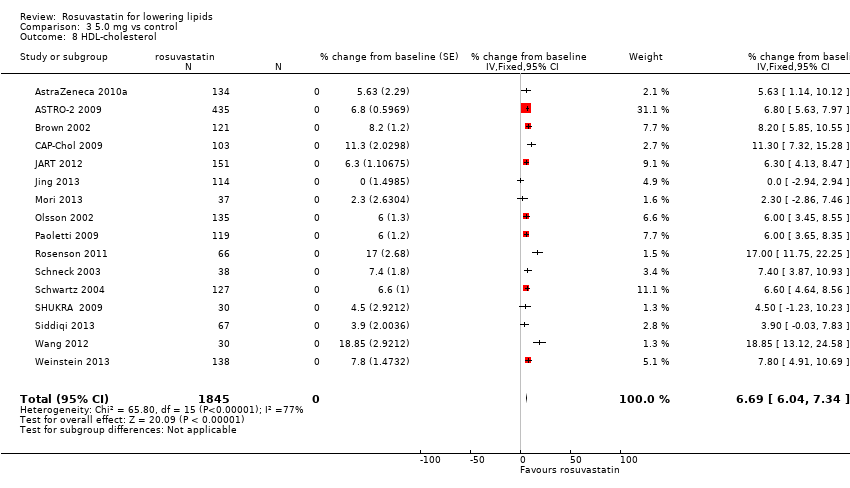
Comparison 3 5.0 mg vs control, Outcome 8 HDL‐cholesterol.

Comparison 3 5.0 mg vs control, Outcome 9 non‐HDL‐cholesterol.

Comparison 3 5.0 mg vs control, Outcome 10 Triglycerides.

Comparison 3 5.0 mg vs control, Outcome 11 WDAE.

Comparison 4 10 mg vs control, Outcome 1 Total cholesterol.

Comparison 4 10 mg vs control, Outcome 2 LDL‐cholesterol.

Comparison 4 10 mg vs control, Outcome 3 HDL‐cholesterol.

Comparison 4 10 mg vs control, Outcome 4 non‐HDL‐cholesterol.

Comparison 4 10 mg vs control, Outcome 5 Triglycerides.

Comparison 4 10 mg vs control, Outcome 6 Total cholesterol.

Comparison 4 10 mg vs control, Outcome 7 LDL‐cholesterol.

Comparison 4 10 mg vs control, Outcome 8 HDL‐cholesterol.

Comparison 4 10 mg vs control, Outcome 9 non‐HDL‐cholesterol.

Comparison 4 10 mg vs control, Outcome 10 Triglycerides.

Comparison 4 10 mg vs control, Outcome 11 WDAE.

Comparison 5 20 mg vs control, Outcome 1 Total cholesterol.

Comparison 5 20 mg vs control, Outcome 2 LDL‐cholesterol.

Comparison 5 20 mg vs control, Outcome 3 HDL‐cholesterol.
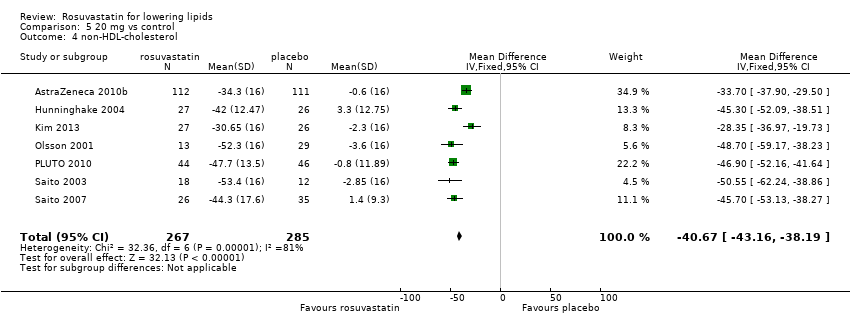
Comparison 5 20 mg vs control, Outcome 4 non‐HDL‐cholesterol.
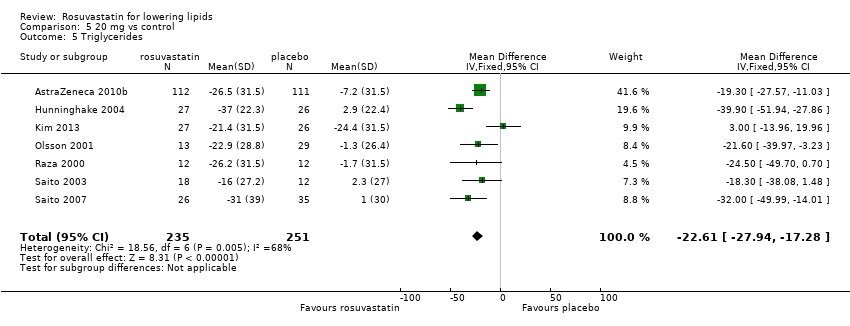
Comparison 5 20 mg vs control, Outcome 5 Triglycerides.
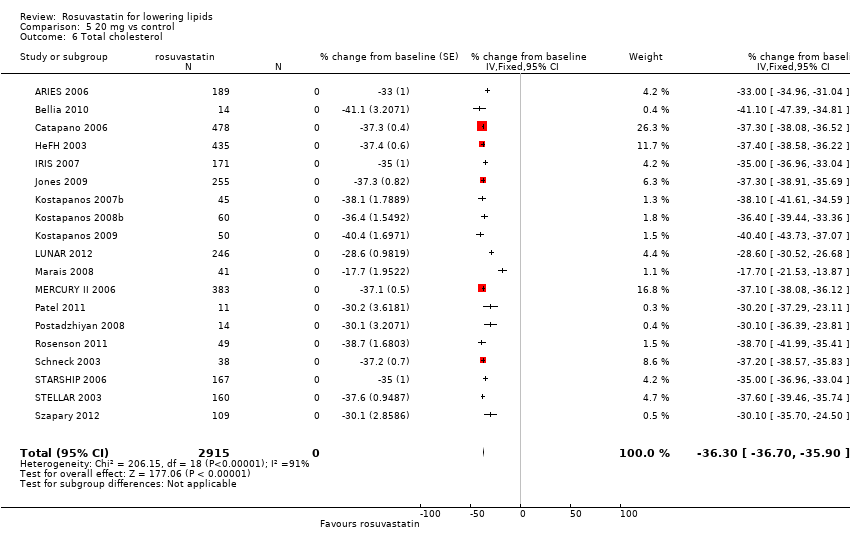
Comparison 5 20 mg vs control, Outcome 6 Total cholesterol.

Comparison 5 20 mg vs control, Outcome 7 LDL‐cholesterol.

Comparison 5 20 mg vs control, Outcome 8 HDL‐cholesterol.

Comparison 5 20 mg vs control, Outcome 9 non‐HDL‐cholesterol.
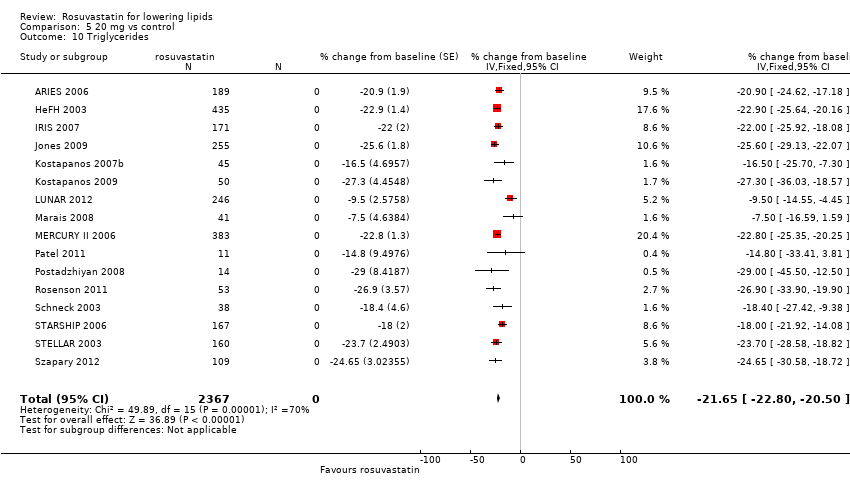
Comparison 5 20 mg vs control, Outcome 10 Triglycerides.
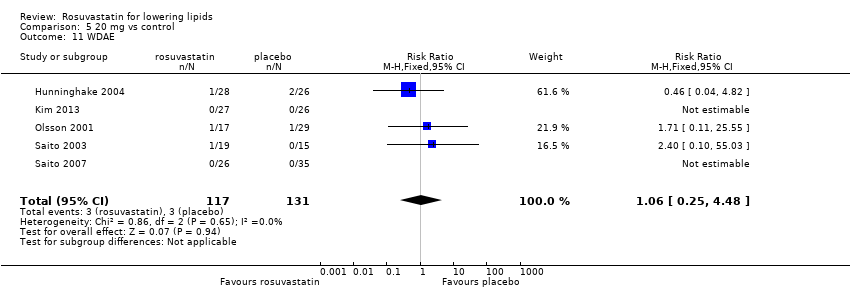
Comparison 5 20 mg vs control, Outcome 11 WDAE.
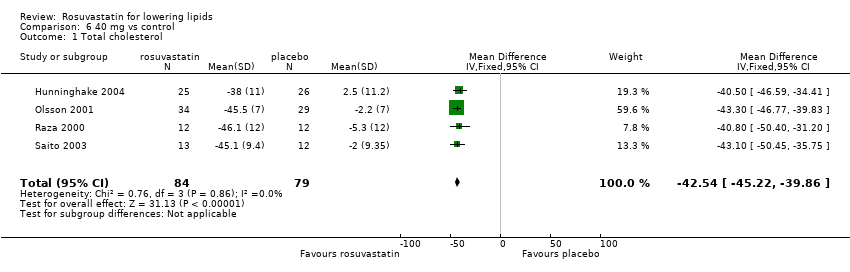
Comparison 6 40 mg vs control, Outcome 1 Total cholesterol.

Comparison 6 40 mg vs control, Outcome 2 LDL‐cholesterol.

Comparison 6 40 mg vs control, Outcome 3 HDL‐cholesterol.
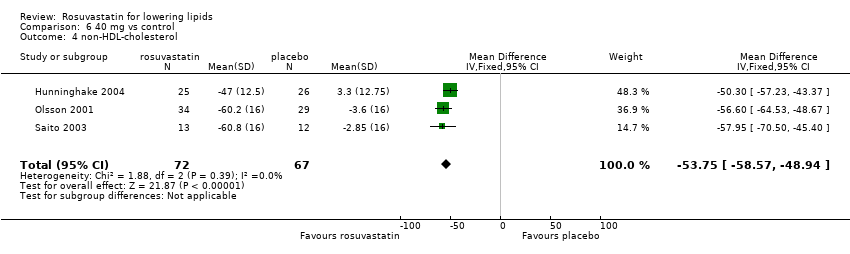
Comparison 6 40 mg vs control, Outcome 4 non‐HDL‐cholesterol.
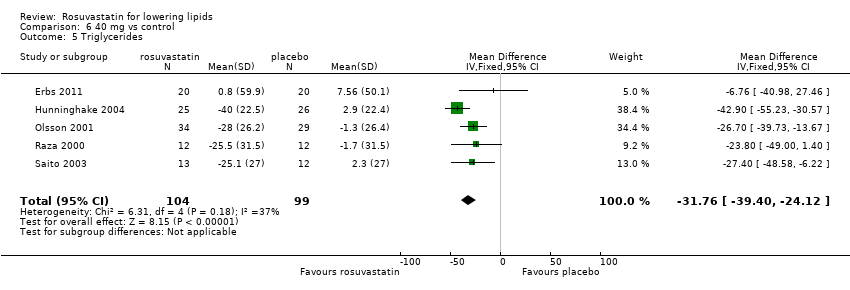
Comparison 6 40 mg vs control, Outcome 5 Triglycerides.
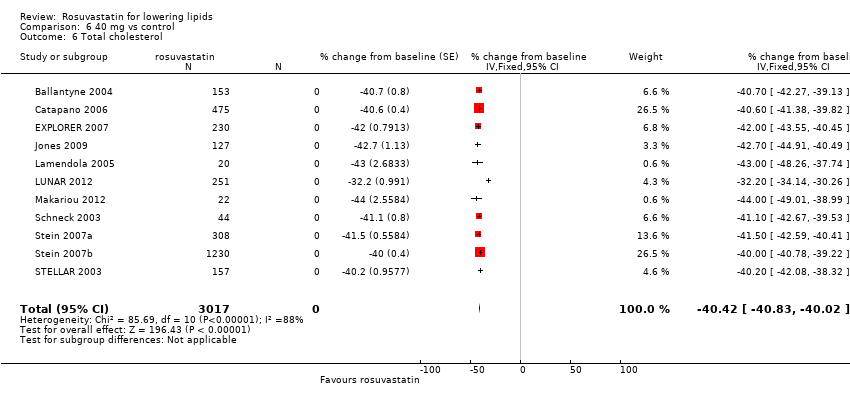
Comparison 6 40 mg vs control, Outcome 6 Total cholesterol.
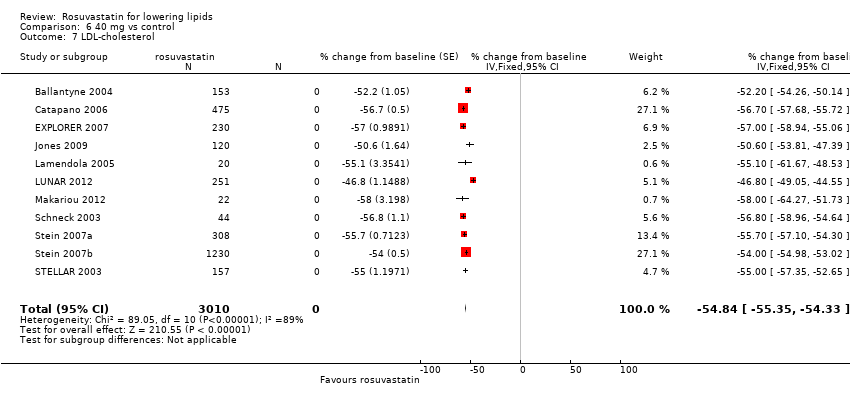
Comparison 6 40 mg vs control, Outcome 7 LDL‐cholesterol.
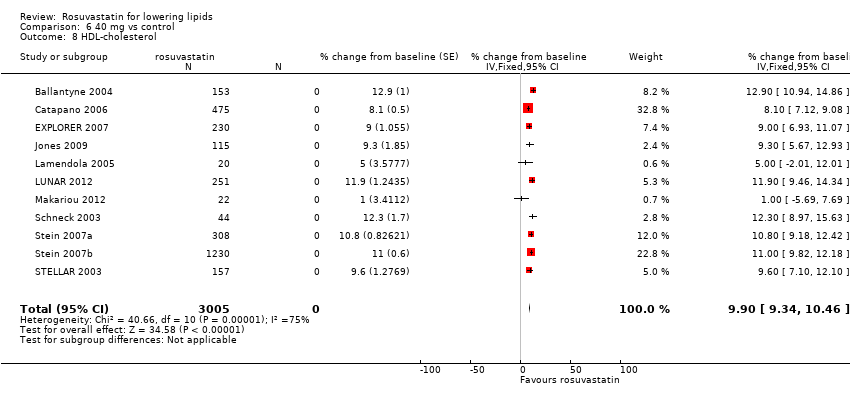
Comparison 6 40 mg vs control, Outcome 8 HDL‐cholesterol.

Comparison 6 40 mg vs control, Outcome 9 non‐HDL‐cholesterol.
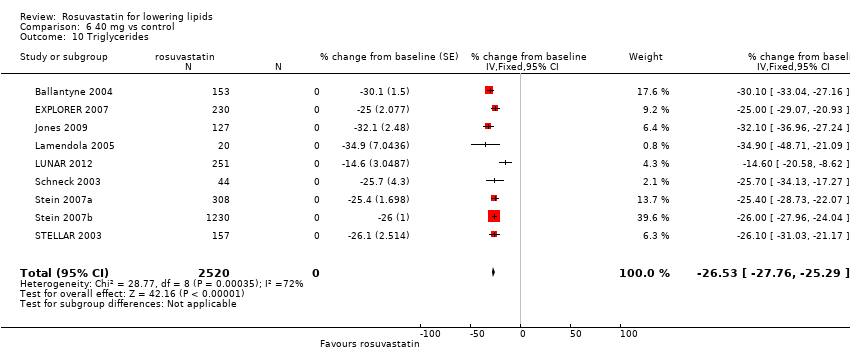
Comparison 6 40 mg vs control, Outcome 10 Triglycerides.

Comparison 6 40 mg vs control, Outcome 11 WDAE.

Comparison 7 80 mg vs control, Outcome 1 Total cholesterol.

Comparison 7 80 mg vs control, Outcome 2 LDL‐cholesterol.

Comparison 7 80 mg vs control, Outcome 3 HDL‐cholesterol.
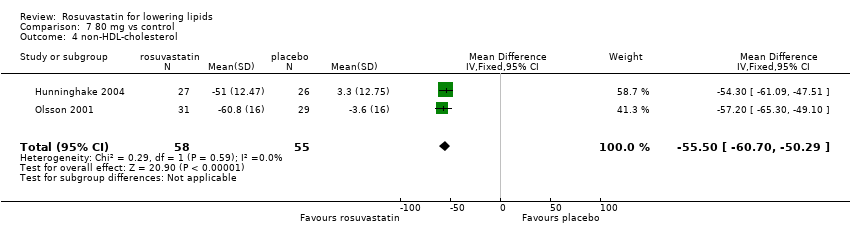
Comparison 7 80 mg vs control, Outcome 4 non‐HDL‐cholesterol.

Comparison 7 80 mg vs control, Outcome 5 Triglycerides.

Comparison 7 80 mg vs control, Outcome 6 Total cholesterol.

Comparison 7 80 mg vs control, Outcome 7 LDL‐cholesterol.

Comparison 7 80 mg vs control, Outcome 8 HDL‐cholesterol.

Comparison 7 80 mg vs control, Outcome 9 non‐HDL‐cholesterol.

Comparison 7 80 mg vs control, Outcome 10 Triglycerides.
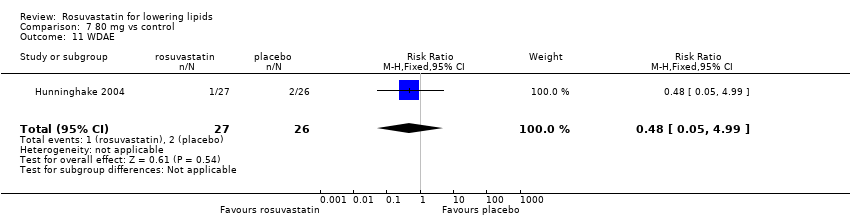
Comparison 7 80 mg vs control, Outcome 11 WDAE.

Comparison 8 all doses vs control, Outcome 1 WDAEs.
| LDL‐cholesterol lowering efficacy of rosuvastatin | |||||
| Patient or population: participantswith normal or abnormal lipid profiles Settings: clinics of hospitals Intervention: rosuvastatin Comparison: LDL‐Cholesterol per cent change from baseline for all trials Comparison: WDAEs rosuvastatin versus placebo | |||||
| Outcomes | Mean % reduction (95% CI)1 | No of Participants | Quality of the evidence | Comments | |
| LDL‐Cholesterol rosuvastatin 2.5 mg/day | ‐39.1 (‐40.6 to ‐37.6) | 450 (11) | low4 ⊕⊕⊝⊝ | Likely an overestimate of the effect; effect predicted from log dose response equation is ‐36.9% | |
| LDL‐Cholesterol rosuvastatin 5 mg/day | ‐41.3 (‐42.0 to ‐40.7) | 2602 (25) | ⊕⊕⊕⊕ | Effect predicted from log dose response equation is ‐41.4%. | |
| LDL‐Cholesterol rosuvastatin 10 mg/day | ‐45.6 (‐46.0 to ‐45.3) | 9855 (74) | ⊕⊕⊕⊕ | Effect predicted from log dose response equation is ‐45.8%. | |
| LDL‐Cholesterol rosuvastatin 20 mg/day | ‐49.9 (‐50.4 to ‐49.4) | 3675 (28) | ⊕⊕⊕⊕ | Effect predicted from log dose response equation is ‐50.2%. | |
| LDL‐Cholesterol rosuvastatin 40 mg/day | ‐54.9 (‐55.4 to ‐54.4) | 3512 (18) | ⊕⊕⊕⊕ | Effect predicted from log dose response equation is ‐54.6%. | |
| WDAE2 all doses | RR3 (0.84) (0.48 to 1.47) | 1330 | ⊕⊝⊝⊝ very low6 | Only 10 out of 18 placebo‐controlled trials reported withdrawals due to adverse effects. | |
| GRADE Working Group grades of evidence | |||||
| 1. CI: confidence interval. 2. WDAE: withdrawal due to adverse effects. 3. RR: risk ratio. 4. Small number of studies and participants with relatively wide confidence intervals and high risk of publication bias. 5. Narrow confidence intervals. 6. High risk of selective reporting bias and wide confidence interval. | |||||
| Rosuvastatin dose mg/day | 1 | 2.5 | 5 | 10 | 20 | 40 | 80 |
| Mean per cent change from control of total cholesterol | ‐22.1 | ‐26.6 | ‐29.1 | ‐32.8 | ‐36.2 | ‐40.5 | ‐44.8 |
| 95% CI1 | (‐24.9 to ‐19.3) | (‐27.9 to ‐25.3) | (‐29.6 to ‐28.6) | (‐33.1 to ‐32.6) | (‐36.6 to ‐35.8) | (‐40.9 to ‐40.1) | (‐46.6 to ‐43.1) |
| Mean per cent change from control of LDL‐C2 | ‐31.2 | ‐39.1 | ‐41.3 | ‐45.6 | ‐49.9 | ‐54.9 | ‐61.2 |
| 95% CI1 | (‐34.5 to ‐27.9) | (‐40.6 to ‐37.6) | (‐42.0 to ‐40.7) | (‐45.95 to ‐45.3) | (‐50.4 to ‐49.4) | (‐55.4 to ‐54.4) | (‐63.6 to ‐58.9) |
| Mean per cent change from control of non‐HDL‐C3 | ‐28.9 | ‐35.4 | ‐37.6 | ‐41.9 | ‐45.5 | ‐50.8 | ‐56.7 |
| 95% CI1 | (‐34.1 to ‐23.7) | (‐37.2 to ‐33.5) | (‐38.4 to ‐36.9) | (‐42.3 to ‐41.6) | (‐46.1 to ‐45.0) | (‐51.3 to ‐50.2 ) | (‐59.0 to ‐54.4) |
| Mean per cent change from control of triglycerides | ‐14.4 | ‐13.4 | ‐17.7 | ‐19.7 | ‐21.7 | ‐26.7 | ‐26.6 |
| 95% CI1 | (‐22.1 to ‐6.8) | (‐16.5 to ‐10.2) | (‐19.0 to ‐16.4) | (‐20.4 to ‐19.1) | (‐22.8 to ‐20.6) | (‐27.9 to ‐25.4) | (‐32.9 to ‐20.4) |
| 1. CI: confidence interval 2. LDL‐C: low‐density lipoprotein cholesterol 3. non‐HDL‐C: non high‐density lipoprotein cholesterol | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total cholesterol Show forest plot | 3 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐21.83 [‐25.59, ‐18.08] |
| 2 LDL‐cholesterol Show forest plot | 3 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐31.17 [‐35.32, ‐27.02] |
| 3 HDL‐cholesterol Show forest plot | 3 | 93 | Mean Difference (IV, Fixed, 95% CI) | 8.16 [2.93, 13.38] |
| 4 non‐HDL‐cholesterol Show forest plot | 2 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐30.13 [‐38.06, ‐22.20] |
| 5 Triglycerides Show forest plot | 3 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐20.77 [‐32.73, ‐8.80] |
| 6 WDAE Show forest plot | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total cholesterol Show forest plot | 3 | 95 | Mean Difference (IV, Fixed, 95% CI) | ‐27.44 [‐31.17, ‐23.70] |
| 2 LDL‐cholesterol Show forest plot | 3 | 95 | Mean Difference (IV, Fixed, 95% CI) | ‐38.27 [‐42.79, ‐33.75] |
| 3 HDL‐cholesterol Show forest plot | 3 | 95 | Mean Difference (IV, Fixed, 95% CI) | 6.02 [0.88, 11.16] |
| 4 non‐HDL‐cholesterol Show forest plot | 2 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐36.47 [‐44.30, ‐28.63] |
| 5 Triglycerides Show forest plot | 3 | 95 | Mean Difference (IV, Fixed, 95% CI) | ‐13.11 [‐24.97, ‐1.25] |
| 6 Total cholesterol Show forest plot | 6 | 286 | % change from baseline (Fixed, 95% CI) | ‐26.52 [‐27.90, ‐25.13] |
| 7 LDL‐cholesterol Show forest plot | 8 | 355 | % change from baseline (Fixed, 95% CI) | ‐39.21 [‐40.76, ‐37.65] |
| 8 HDL‐cholesterol Show forest plot | 8 | 355 | % change from baseline (Fixed, 95% CI) | 4.20 [2.54, 5.85] |
| 9 non‐HDL‐cholesterol Show forest plot | 6 | 286 | Mean Difference (Fixed, 95% CI) | ‐35.27 [‐37.13, ‐33.41] |
| 10 Triglycerides Show forest plot | 8 | 355 | % change from baseline (Fixed, 95% CI) | ‐13.70 [‐16.97, ‐10.43] |
| 11 WDAE Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [0.11, 57.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total cholesterol Show forest plot | 9 | 762 | Mean Difference (IV, Fixed, 95% CI) | ‐29.13 [‐30.56, ‐27.70] |
| 2 LDL‐cholesterol Show forest plot | 9 | 762 | Mean Difference (IV, Fixed, 95% CI) | ‐39.12 [‐41.11, ‐37.12] |
| 3 HDL‐cholesterol Show forest plot | 9 | 762 | Mean Difference (IV, Fixed, 95% CI) | 8.64 [6.93, 10.35] |
| 4 non‐HDL‐cholesterol Show forest plot | 8 | 738 | Mean Difference (IV, Fixed, 95% CI) | ‐36.79 [‐38.85, ‐34.72] |
| 5 Triglycerides Show forest plot | 8 | 674 | Mean Difference (IV, Fixed, 95% CI) | ‐23.08 [‐26.97, ‐19.19] |
| 6 Total cholesterol Show forest plot | 15 | 1411 | % change from baseline (Fixed, 95% CI) | ‐29.11 [‐29.69, ‐28.53] |
| 7 LDL‐cholesterol Show forest plot | 16 | 1840 | % change from baseline (Fixed, 95% CI) | ‐41.57 [‐42.22, ‐40.92] |
| 8 HDL‐cholesterol Show forest plot | 16 | 1845 | % change from baseline (Fixed, 95% CI) | 6.69 [6.04, 7.34] |
| 9 non‐HDL‐cholesterol Show forest plot | 14 | 1307 | % change from baseline (Fixed, 95% CI) | ‐37.77 [‐38.53, ‐37.01] |
| 10 Triglycerides Show forest plot | 14 | 1678 | % change from baseline (Fixed, 95% CI) | ‐16.96 [‐18.33, ‐15.60] |
| 11 WDAE Show forest plot | 5 | 561 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.70, 2.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total cholesterol Show forest plot | 15 | 1442 | Mean Difference (IV, Fixed, 95% CI) | ‐31.34 [‐32.45, ‐30.23] |
| 2 LDL‐cholesterol Show forest plot | 15 | 1442 | Mean Difference (IV, Fixed, 95% CI) | ‐42.80 [‐44.26, ‐41.35] |
| 3 HDL‐cholesterol Show forest plot | 15 | 1442 | Mean Difference (IV, Fixed, 95% CI) | 10.46 [9.40, 11.52] |
| 4 non‐HDL‐cholesterol Show forest plot | 14 | 1418 | Mean Difference (IV, Fixed, 95% CI) | ‐39.28 [‐40.82, ‐37.74] |
| 5 Triglycerides Show forest plot | 13 | 1313 | Mean Difference (IV, Fixed, 95% CI) | ‐19.97 [‐22.81, ‐17.12] |
| 6 Total cholesterol Show forest plot | 55 | 8100 | % change from baseline (Fixed, 95% CI) | ‐32.89 [‐33.14, ‐32.64] |
| 7 LDL‐cholesterol Show forest plot | 59 | 8413 | % change from baseline (Fixed, 95% CI) | ‐45.77 [‐46.09, ‐45.46] |
| 8 HDL‐cholesterol Show forest plot | 55 | 8085 | % change from baseline (Fixed, 95% CI) | 6.25 [5.93, 6.58] |
| 9 non‐HDL‐cholesterol Show forest plot | 53 | 7405 | % change from baseline (Fixed, 95% CI) | ‐42.06 [‐42.39, ‐41.72] |
| 10 Triglycerides Show forest plot | 51 | 7524 | % change from baseline (Fixed, 95% CI) | ‐19.72 [‐20.38, ‐19.07] |
| 11 WDAE Show forest plot | 6 | 724 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.29, 1.39] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total cholesterol Show forest plot | 8 | 576 | Mean Difference (IV, Fixed, 95% CI) | ‐33.58 [‐35.41, ‐31.75] |
| 2 LDL‐cholesterol Show forest plot | 8 | 576 | Mean Difference (IV, Fixed, 95% CI) | ‐45.83 [‐48.22, ‐43.44] |
| 3 HDL‐cholesterol Show forest plot | 8 | 576 | Mean Difference (IV, Fixed, 95% CI) | 6.82 [4.42, 9.21] |
| 4 non‐HDL‐cholesterol Show forest plot | 7 | 552 | Mean Difference (IV, Fixed, 95% CI) | ‐40.67 [‐43.16, ‐38.19] |
| 5 Triglycerides Show forest plot | 7 | 486 | Mean Difference (IV, Fixed, 95% CI) | ‐22.61 [‐27.94, ‐17.28] |
| 6 Total cholesterol Show forest plot | 19 | 2915 | % change from baseline (Fixed, 95% CI) | ‐36.30 [‐36.70, ‐35.90] |
| 7 LDL‐cholesterol Show forest plot | 20 | 3099 | % change from baseline (Fixed, 95% CI) | ‐50.07 [‐50.55, ‐49.58] |
| 8 HDL‐cholesterol Show forest plot | 19 | 2896 | % change from baseline (Fixed, 95% CI) | 8.03 [7.51, 8.55] |
| 9 non‐HDL‐cholesterol Show forest plot | 18 | 2461 | % change from baseline (Fixed, 95% CI) | ‐45.77 [‐46.31, ‐45.24] |
| 10 Triglycerides Show forest plot | 16 | 2367 | % change from baseline (Fixed, 95% CI) | ‐21.65 [‐22.80, ‐20.50] |
| 11 WDAE Show forest plot | 5 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.25, 4.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total cholesterol Show forest plot | 4 | 163 | Mean Difference (IV, Fixed, 95% CI) | ‐42.54 [‐45.22, ‐39.86] |
| 2 LDL‐cholesterol Show forest plot | 6 | 472 | Mean Difference (IV, Fixed, 95% CI) | ‐55.85 [‐58.31, ‐53.40] |
| 3 HDL‐cholesterol Show forest plot | 6 | 472 | Mean Difference (IV, Fixed, 95% CI) | 6.85 [4.29, 9.40] |
| 4 non‐HDL‐cholesterol Show forest plot | 3 | 139 | Mean Difference (IV, Fixed, 95% CI) | ‐53.75 [‐58.57, ‐48.94] |
| 5 Triglycerides Show forest plot | 5 | 203 | Mean Difference (IV, Fixed, 95% CI) | ‐31.76 [‐39.40, ‐24.12] |
| 6 Total cholesterol Show forest plot | 11 | 3017 | % change from baseline (Fixed, 95% CI) | ‐40.42 [‐40.83, ‐40.02] |
| 7 LDL‐cholesterol Show forest plot | 11 | 3010 | % change from baseline (Fixed, 95% CI) | ‐54.84 [‐55.35, ‐54.33] |
| 8 HDL‐cholesterol Show forest plot | 11 | 3005 | % change from baseline (Fixed, 95% CI) | 9.90 [9.34, 10.46] |
| 9 non‐HDL‐cholesterol Show forest plot | 11 | 3005 | % change from baseline (Fixed, 95% CI) | ‐50.69 [‐51.22, ‐50.16] |
| 10 Triglycerides Show forest plot | 9 | 2520 | % change from baseline (Fixed, 95% CI) | ‐26.53 [‐27.76, ‐25.29] |
| 11 WDAE Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total cholesterol Show forest plot | 2 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐44.5 [‐47.84, ‐41.16] |
| 2 LDL‐cholesterol Show forest plot | 2 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐59.47 [‐64.15, ‐54.79] |
| 3 HDL‐cholesterol Show forest plot | 2 | 113 | Mean Difference (IV, Fixed, 95% CI) | 10.68 [5.92, 15.44] |
| 4 non‐HDL‐cholesterol Show forest plot | 2 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐55.50 [‐60.70, ‐50.29] |
| 5 Triglycerides Show forest plot | 2 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐34.49 [‐43.89, ‐25.10] |
| 6 Total cholesterol Show forest plot | 1 | 42 | % change from baseline (Fixed, 95% CI) | ‐43.00 [‐47.16, ‐42.84] |
| 7 LDL‐cholesterol Show forest plot | 1 | 42 | % change from baseline (Fixed, 95% CI) | ‐61.9 [‐64.64, ‐59.16] |
| 8 HDL‐cholesterol Show forest plot | 1 | 42 | % change from baseline (Fixed, 95% CI) | 9.6 [6.27, 12.93] |
| 9 non‐HDL‐cholesterol Show forest plot | 1 | 42 | % change from baseline (Fixed, 95% CI) | ‐57.0 [‐59.55, ‐54.45] |
| 10 Triglycerides Show forest plot | 1 | 42 | % change from baseline (Fixed, 95% CI) | ‐19.7 [‐28.32, ‐11.08] |
| 11 WDAE Show forest plot | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.05, 4.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 WDAEs Show forest plot | 10 | 1330 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.48, 1.47] |



