Tratamiento con yodo radioactivo versus fármacos antitiroideos para la enfermedad de Graves
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Parallel randomised controlled clinical trial Superiority design | |
| Participants | Inclusion criteria: participants between 20 and 55 years with hyperthyroidism caused by Graves' disease and without a history of previous thyroid disease Exclusion criteria: participants who wanted treatment options other than those offered by randomisation Diagnostic criteria: diagnosis of Graves' disease: presence of symptoms and signs of hyperthyroidism, non‐nodular thyroid gland, and elevated total and free serum T4, and/or total T3 levels; if the serum T4 level was between 150 and 180 nmol/L or T3 was between 2.5 and 3.5 nmol/L, the diagnosis was supported by a TRH test. Furthermore, all participants should have a thyroid uptake of 131I that was not suppressed, a diffuse pattern of isotope uptake on the radionuclide scan and a goitre size that should enable a single dose of 131I to render the participant euthyroid or hypothyroid | |
| Interventions | Number of study centres: unclear Treatment before study: unclear | |
| Outcomes | Composite outcome measures reported: no | |
| Study details | Run‐in period: no Study terminated before regular end: no | |
| Publication details | Language of publication: English Funding: non‐commercial funding Publication status: peer review journal | |
| Stated aim for study | Quote from publication: "First, is there a difference in the frequency of the development or aggravation of Graves' ophthalmopathy among young patients treated with an antithyroid drug as compared with subtotal thyroidectomy and among older patients treated with an antithyroid drug, subtotal thyroidectomy, or iodine‐131? And second, can factors that predict the development of Graves' ophthalmopathy be identified?" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "Each patient was assigned a treatment group consecutively using two lists, one for each age group, on which the treatment group occurred in random order but balanced to equalize the size of the treatment groups" Quote from publication: "The patients who were between 20 and 34 years old were randomly assigned to treatment with antithyroid drug plus thyroxine (young medical group) or subtotal thyroidectomy (young surgical group); the patients who were between 35 and 55 years old were randomly assigned to antithyroid drug plus thyroxine (old medical group), subtotal thyroidectomy (old surgical group) or to iodine‐131 (iodine‐131 group)" Comment: no details on how randomisation was performed |
| Allocation concealment (selection bias) | Low risk | Quote from the publication (Laurberg 2008): "Randomization was performed by assigning each patient a treatment group consecutively using two lists, one for each age group. On the list, each treatment group occurred in a random order but was balanced to equalize the size of the treatment groups. The lists were unavailable to the clinicians throughout the study, and randomization was performed over the phone." Comment: probably adequate concealment of allocation |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from the publication (Laurberg 2008): "Randomization was performed by assigning each patient a treatment group consecutively using two lists, one for each age group. On the list, each treatment group occurred in a random order but was balanced to equalize the size of the treatment groups. The lists were unavailable to the clinicians throughout the study, and randomization was performed over the phone." Comment: investigator‐assessed outcome measurement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from the publication (Laurberg 2008): "Randomization was performed by assigning each patient a treatment group consecutively using two lists, one for each age group. On the list, each treatment group occurred in a random order but was balanced to equalize the size of the treatment groups. The lists were unavailable to the clinicians throughout the study, and randomization was performed over the phone." Comment: investigator‐assessed outcome measurement |
| Blinding of participants and personnel (performance bias) | High risk | Comment: self reported outcome measurement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from the publication (Laurberg 2008): "Randomization was performed by assigning each patient a treatment group consecutively using two lists, one for each age group. On the list, each treatment group occurred in a random order but was balanced to equalize the size of the treatment groups. The lists were unavailable to the clinicians throughout the study, and randomization was performed over the phone." Comment: investigator‐assessed outcome measurement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from the publication (Laurberg 2008): "Randomization was performed by assigning each patient a treatment group consecutively using two lists, one for each age group. On the list, each treatment group occurred in a random order but was balanced to equalize the size of the treatment groups. The lists were unavailable to the clinicians throughout the study, and randomization was performed over the phone." Comment: investigator‐assessed outcome measurement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from the publication (Laurberg 2008): "Randomization was performed by assigning each patient a treatment group consecutively using two lists, one for each age group. On the list, each treatment group occurred in a random order but was balanced to equalize the size of the treatment groups. The lists were unavailable to the clinicians throughout the study, and randomization was performed over the phone." Comment: investigator‐assessed outcome measurement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from publication: "Outcomes were assessed by physicians blinded to participants' treatment assignments" Comment: investigator‐assessed outcome measurement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from publication: "Outcomes were assessed by physicians blinded to participants' treatment assignments" Comment: investigator‐assessed outcome measurement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from publication: "quality of life was assessed by physicians blinded to participants' treatment assignments" Comment: investigator‐assessed outcome measurement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from publication: "quality of life was assessed by physicians blinded to participants' treatment assignments" Comment: investigator‐assessed outcome measurement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from publication: "recurrence of hyperthyroidism was assessed by physicians blinded to participants' treatment assignments" Comment: investigator‐assessed outcome measurement Comment: low risk |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from publication: "quality of life was assessed by physicians blinded to participants' treatment assignments" Comment: investigator‐assessed outcome measurement |
| Incomplete outcome data (attrition bias) | Low risk | Quote from publication: "outcomes data including health‐related quality of life are available for all included patients, two patients rejected the assigned treatment" Comment: no intention‐to‐treat analysis for most outcome measures |
| Incomplete outcome data (attrition bias) | Low risk | Quote from publication: "outcomes data including health‐related quality of life are available for all included patients, two patients rejected the assigned treatment" Comment: no intention‐to‐treat analysis for most outcome measures |
| Incomplete outcome data (attrition bias) | Low risk | Quote from publication: "outcomes data including health‐related quality of life are available for all included patients, two patients rejected the assigned treatment" Comment: no intention‐to‐treat analysis for most outcome measures |
| Incomplete outcome data (attrition bias) | Low risk | Quote from publication: "outcomes data including health‐related quality of life are available for all included patients, two patients rejected the assigned treatment" Comment: no intention‐to‐treat analysis for most outcome measures |
| Incomplete outcome data (attrition bias) | Low risk | Quote from publication: "outcomes data including health‐related quality of life are available for all included patients, two patients rejected the assigned treatment" Comment: no intention‐to‐treat analysis for most outcome measures |
| Incomplete outcome data (attrition bias) | Low risk | Quote from publication: "outcomes data including health‐related quality of life are available for all included patients, two patients rejected the assigned treatment" Comment: no intention‐to‐treat analysis for most outcome measures |
| Selective reporting (reporting bias) | Low risk | Comment: identified outcomes adequately reported as compared with the description in methods |
| Other bias | Low risk | Comment: no source of other bias noted |
| Methods | Parallel randomised controlled clinical trial Superiority design | |
| Participants | Inclusion criteria: participants aged 35 to 69 years; symptomatic Graves' hyperthyroidism; confirmation of the diagnosis by serum TSH (≤ 0.1 mIU/L) and T3 and/or free T4 (elevated), thyroid uptake of iodine‐131, and radionuclide scans compatible with Graves' disease, i.e. an even distribution of the radionuclide Exclusion criteria: participants with a previous history of treatment with antithyroid drugs, iodine‐131 or thyroid surgery were excluded as well as participants with severe TAO requiring treatment with corticosteroids at the time of inclusion Diagnostic criteria: diagnosis of Graves' disease was made according to the hyperthyroidism and diffuse goitre. For the set criteria (worsening or development and improvement of TAO), 2 of the following 4 decisive factors were required (compared with baseline data): 1) change in exophthalmometry readings of 2 mm or more; 2) improvement or deterioration of the participant's eye movements between the 4 scoring levels (no impairment, clearly impaired, diplopia in the primary position, fixation of the globe); 3) changes of visual acuity caused by optic neuropathy; and 4) changes in 2 of the 3 TAO activity measures (chemosis, eyelid oedema and conjunctival redness). The participants who did not meet the criteria for improvement or worsening or development of TAO were referred to as having no change of TAO | |
| Interventions | Number of study centres: 4 Treatment before study: beta‐blockers | |
| Outcomes | Composite outcome measures reported: no | |
| Study details | Run‐in period: no Study terminated before regular end: no | |
| Publication details | Language of publication: English Funding: non‐commercial funding (Medical Council for Research) Publication status: peer review journal | |
| Stated aim for study | Quote from publication: "... to compare radioiodine treatment and medical therapy for long‐term worsening or development of TAO. Smoking and hypothyroidism as confounding risk factors were controlled for, and L‐thyroxine supplementation was given early, i.e. at 2 wk after the initiation of treatment for hyperthyroidism in both arms" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "Patients were randomised to radioiodine and medical treatment within each center (stratified randomization). Randomization was made in blocks over time and was performed by the Oncological Centre at the Karolinska University Hospital in Stockholm" Comment: no details of the randomisation procedure provided |
| Allocation concealment (selection bias) | Unclear risk | Quote from publication: "Patients were randomised to radioiodine and medical treatment within each center (stratified randomization). Randomization was made in blocks over time and was performed by the Oncological Centre at the Karolinska University Hospital in Stockholm" Comment: probably concealed allocation (by the oncological centre) |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from publication: "The study was designed as an open, randomized, prospective multicenter trial" Comment: investigator‐assessed outcome measurement |
| Blinding of participants and personnel (performance bias) | High risk | Quote from publication: "The study was designed as an open, randomized, prospective multicenter trial" Comment: investigator‐assessed outcome measurement |
| Blinding of participants and personnel (performance bias) | High risk | Quote from publication: "The study was designed as an open, randomized, prospective multicenter trial" Comment: self reported outcome measurement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from publication: "The study was designed as an open, randomized, prospective multicenter trial" Comment: investigator‐assessed outcome measurement; outcome probably not influenced by non‐blinding conditions |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: no details provided; outcome probably not influenced by non‐blinding conditions |
| Blinding of outcome assessment (detection bias) | High risk | Comment: no details provided; investigator‐assessed outcome measurement |
| Blinding of outcome assessment (detection bias) | High risk | Comment: self reported outcome measurement |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: no details provided; outcome probably not influenced by non‐blinding conditions |
| Incomplete outcome data (attrition bias) | Low risk | Comment: intention‐to‐treat analysis; few drop‐outs |
| Incomplete outcome data (attrition bias) | Low risk | Comment: intention‐to‐treat analysis; few drop‐outs |
| Incomplete outcome data (attrition bias) | Low risk | Comment: intention‐to‐treat analysis; few drop‐outs |
| Incomplete outcome data (attrition bias) | Low risk | Comment: intention‐to‐treat analysis; few drop‐outs |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes adequately reported as compared with the description in methods |
| Other bias | Low risk | Comment: no source of other bias noted |
Note: where the judgement is 'Unclear' and the description is blank, the trial did not report that particular outcome
131I: radioiodine; TAO: thyroid‐associated ophthalmopathy; T4: thyroxine; T3: tri‐iodothyronine; TRH: thyrotropin‐releasing hormone
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| RCT but included participants with recurrence of hyperthyroidism after methimazole treatment for 18 months before the study | |
| RCT but with 1‐year follow‐up only | |
| Consecutive series trial | |
| Case‐control trial | |
| RCT but with 1‐year follow‐up only | |
| Case‐control trial | |
| RCT but included participants with Graves' disease, multinodular toxic goitre and uninodular goitre | |
| Some of the participants were randomised; the others were not randomised; with 1‐year follow‐up | |
| Cohort trial | |
| Case‐control trial | |
| Case‐control trial | |
| Cohort trial | |
| RCT but with 1‐year follow‐up only |
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Development or worsening of Graves' ophthalmopathy Show forest plot | 2 | 417 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [1.40, 2.70] |
| Analysis 1.1  Comparison 1 Radioiodine versus methimazole, Outcome 1 Development or worsening of Graves' ophthalmopathy. | ||||
| 2 Adverse events: hypothyroidism Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Radioiodine versus methimazole, Outcome 2 Adverse events: hypothyroidism. | ||||
| 3 Recurrence of hyperthyroidism (relapse) Show forest plot | 2 | 417 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 2.66] |
| Analysis 1.3  Comparison 1 Radioiodine versus methimazole, Outcome 3 Recurrence of hyperthyroidism (relapse). | ||||
| 4 Participants in euthyroid state Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4 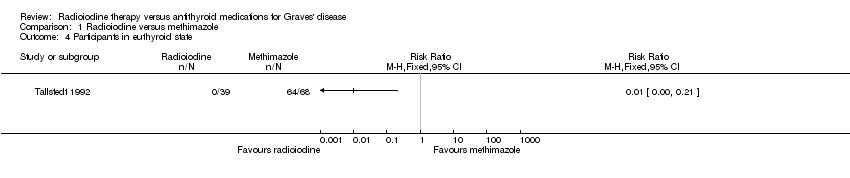 Comparison 1 Radioiodine versus methimazole, Outcome 4 Participants in euthyroid state. | ||||
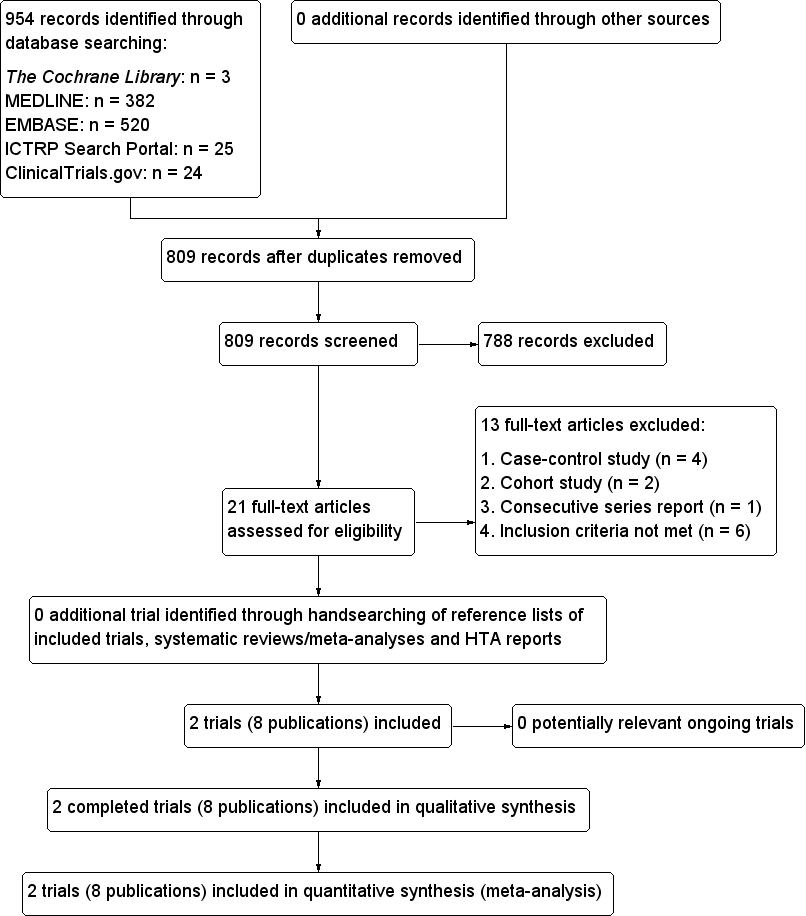
Study flow diagram.
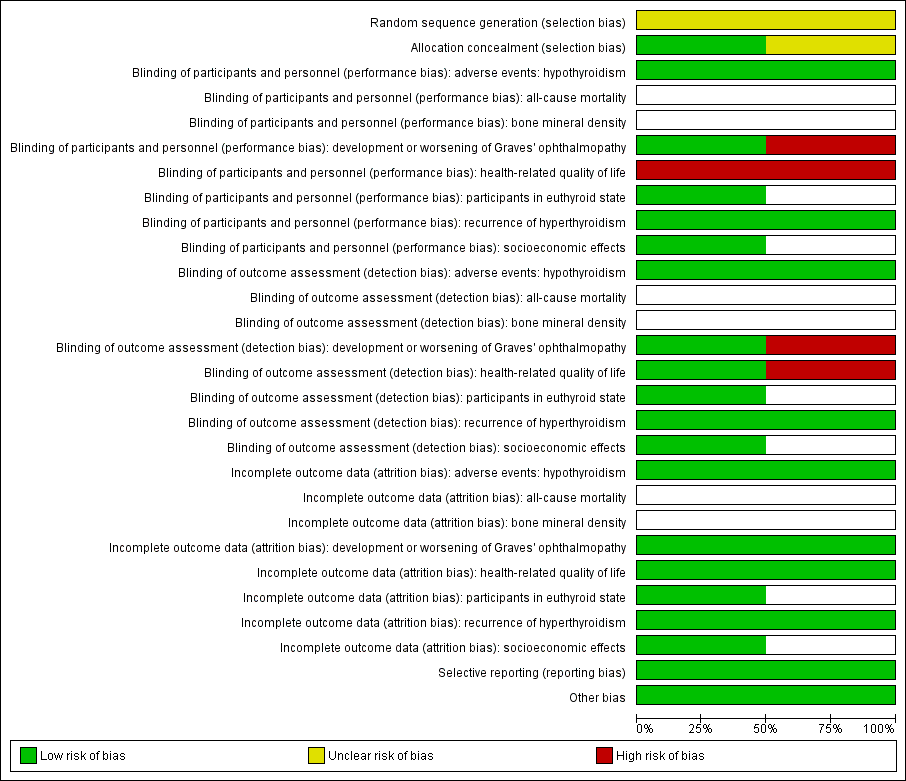
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial (blank cells indicate that the trial did not measure that particular outcome).
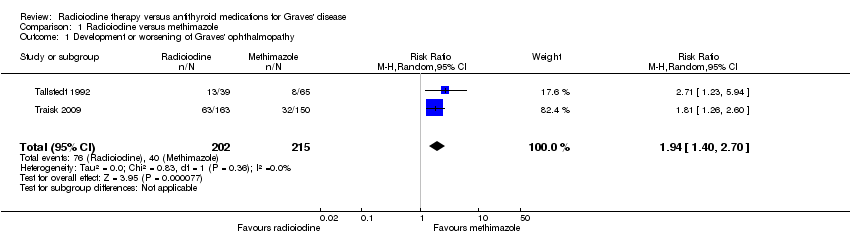
Comparison 1 Radioiodine versus methimazole, Outcome 1 Development or worsening of Graves' ophthalmopathy.

Comparison 1 Radioiodine versus methimazole, Outcome 2 Adverse events: hypothyroidism.
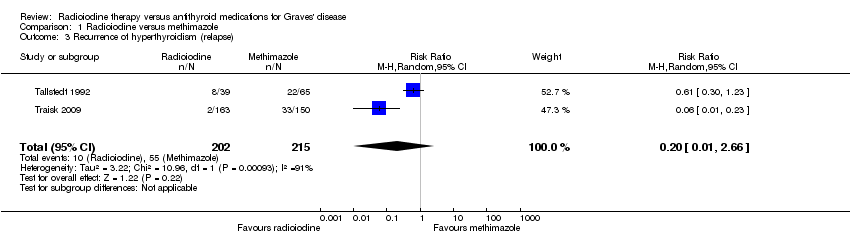
Comparison 1 Radioiodine versus methimazole, Outcome 3 Recurrence of hyperthyroidism (relapse).

Comparison 1 Radioiodine versus methimazole, Outcome 4 Participants in euthyroid state.
| Radioiodine therapy compared with antithyroid medications for Graves' disease | ||||||
| Patient: participants with Graves' disease Settings: outpatients Intervention: radioiodine Comparison: methimazole | ||||||
| Outcomes | Assumed risk | Corresponding risk | Relative effect | No of participants | Quality of the evidence | Comments |
| Methimazole | Radioiodine | |||||
| Health‐related quality of life Follow‐up: 4 years and 14 to 21 years | See comment | See comment | See comment | 425 (2) | See comment | 2 trials assessed this outcome but no quantitative data for comparisons between intervention groups were provided; trial authors in 1 trial reported that there were no differences in the results of the SF‐36 scores between the 2 treatment groups |
| Development and worsening of Graves' ophthalmopathy | 186 per 1000 | 361 of 1000 (260 to 502) | RR 1.94 (1.40 to 2.70) | 417 (2) | ⊕⊕⊝⊝ | ‐ |
| Individuals in euthyroid state Follow‐up: at least 4 years | See comment | See comment | See comment | 112 (1) | See comment | No participant who underwent radioiodine treatment achieved an euthyroid state compared to 4/68 participants not becoming euthyroid treated by methimazole (Tallstedt 1992); however, in this trial thyroxine therapy was not introduced early in both treatment arms to avoid hypothyroidism |
| Recurrence of hyperthyroidism (relapse) Follow‐up: at least 4 years | 256 per 1000 | 51 of 1000 (3 to 680) | RR 0.20 (0.01 to 2.66) | 417 (2) | ⊕⊝⊝⊝ | ‐ |
| Adverse events other than development or worsening of Graves' disease (a) Hypothyroidism [measured by TSH and/or thyroid hormones] (b) Drug reactions Follow‐up: (a) at least 2 years (b) at least 4 years | See comment | See comment | See comment | (a) 104 (1) (b) 215 (2) | a) ⊕⊝⊝⊝ b) ⊕⊝⊝⊝ | (a) 39 of 41 participants developed hypothyroidism after radioiodine treatment for Graves' disease, compared with 0 of 65 participants receiving methimazole (Tallstedt 1992); however, in this trial thyroxine was not introduced early in both treatment groups in order to avoid hypothyroidism (b) 23 of 215 participants (11%) reported adverse effects likely related to methimazole treatment |
| All‐cause mortality Follow‐up: at least 4 years and 14 to 21 years | See comment | See comment | See comment | 425 (2) | See comment | No quantitative data for all‐cause mortality were reported |
| Socioeconomic effects Follow‐up: 2 years | See comment | See comment | See comment | 112 (1) | See comment | Costs for patients without relapse and methimazole treatment were USD 1126/USD 1164 (young/older methimazole group) and for radioiodine treatment USD 1862 Costs for patients with relapse and methimazole treatment were USD 2284/1972 (young/older methimazole group) and for radioiodine treatment USD 2760 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Assumed risk was derived from the event rates in the comparator groups. | ||||||
| Intervention(s) and | Sample sizea | Screened/eligible | Randomised | ITT | Analysed | Finishing trial | Randomised finishing trial | Follow‐up | |
| Traisk 2009 | Radioiodine | Primary endpoint was the difference in the proportion of participants with worsening or development of thyroid‐associated ophthalmopathy during a 4‐year follow‐up in the 2 groups (intention‐to‐treat analysis). A comparison (0.05, two‐tailed test) of the binomial proportions between 2 groups of 300 patients each would give more than 90% probability (power) to detect a true difference of 10% | 482/333 | ‐ | 163 | 163 | 163 | ‐ | 24 months (4 years) |
| Methimazole | ‐ | 150 | 150 | 150 | ‐ | ||||
| total: | 333 | 313 | 313 | 313 | 94 | ||||
| Tallstedt 1992e | Radioiodine | ‐ | 179 | 41 | ‐ | 39f | 39 | 95 | At least 24 months (at least 48 months; 3 years; 5 years; 14‐21 years)g |
| Methimazole | 71 | 65f | 64 | 90 | |||||
| total: | 112 | 104 | 103 | 92 | |||||
| Grand total | All radioiodine‐treated participants | 204 | |||||||
| All participants treated with methimazole | 221 | ||||||||
| All interventions | 425 | ||||||||
| "‐" denotes not reported ITT: intention‐to‐treat | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Development or worsening of Graves' ophthalmopathy Show forest plot | 2 | 417 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [1.40, 2.70] |
| 2 Adverse events: hypothyroidism Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Recurrence of hyperthyroidism (relapse) Show forest plot | 2 | 417 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 2.66] |
| 4 Participants in euthyroid state Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

