Tratamiento con yodo radioactivo versus fármacos antitiroideos para la enfermedad de Graves
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010094.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 18 febrero 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Trastornos metabólicos y endocrinos
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
All review authors read and approved the final review draft.
Chao Ma (CM): protocol draft, search strategy development, data analysis, data interpretation and future review update.
Jiawei Xie (JWX): trial selection and data extraction.
Hui Wang (HW): search strategy development, trial selection, data extraction and data interpretation.
Jinsong Li (JL): trial selection, data analysis and data interpretation.
Suyun Chen (SYC): acquisition of trial copies, trial selection, data extraction, data analysis and future review update.
Sources of support
Internal sources
-
Dr. Ma was supported by National Natural Science Fund (no. 81271612), China.
External sources
-
No sources of support supplied
Declarations of interest
Chao Ma: none known.
Jiawei Xie: none known.
Hui Wang: none known.
Jinsong Li: none known.
Suyun Chen: none known.
Acknowledgements
The authors wish to acknowledge Dr. Al Driedge from the Department of Nuclear Medicine/Oncology, University of Western Ontario, London, Canada for his invaluable comments, editing and formatting. We also thank Dr. Ove Torring and Dr. Danyun Chen for providing the required data for the meta‐analysis. The search strategies were designed by Maria‐Inti Metzendorf (Trials Search Co‐ordinator of the CMED Group).
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Feb 18 | Radioiodine therapy versus antithyroid medications for Graves' disease | Review | Chao Ma, Jiawei Xie, Hui Wang, Jinsong Li, Suyun Chen | |
| 2012 Sep 12 | Radioiodine therapy versus anti‐thyroid medications for Graves' disease | Protocol | Chao Ma, Jiawei Xie, Hui Wang, Jinsong Li, Suyun Chen | |
Differences between protocol and review
In response to the requirements of the CMED Group, we also evaluated the outcome measure development or worsening of Graves' opthalmopathy as a primary outcome.
We also planned to evaluate the effects of steroids on preventing the development and progression of Graves' opthalmopathy. However, a systematic review reported that prednisolone prophylaxis was highly effective in preventing the progression of Graves' opthalmopathy in patients with pre‐existing Graves' opthalmopathy treated by radioiodine. Therefore, we did not assess the effects of steroids on Graves' opthalmopathy in this review. Moreover, these data were not reported in the two included trials.
We did not carry out the planned subgroup analyses 'dose of radioiodine', 'age' and 'gender' because of an insufficient number of trials, no information or both.
Due to the time lag between publication of the protocol and the final review draft the whole review was completely restructured by the editorial office of the CMED Group and the newest standards were implemented.
Notes
We have based parts of the background, the methods section, appendices, additional tables and figures 1 to 3 of this review on a standard template established by the CMED Group.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO
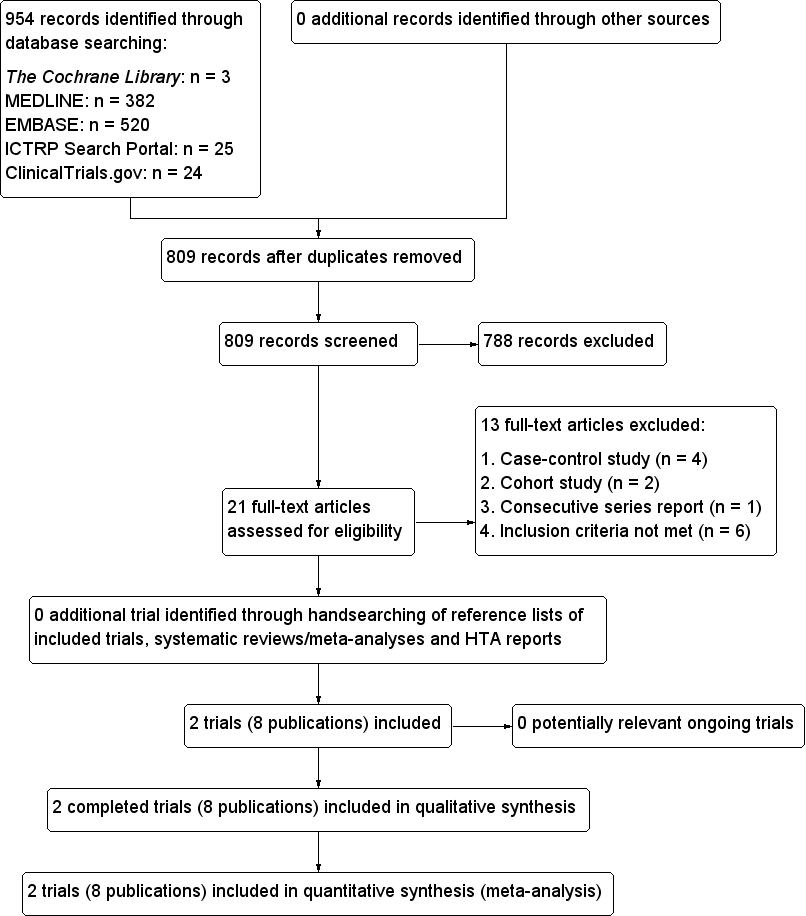
Study flow diagram.
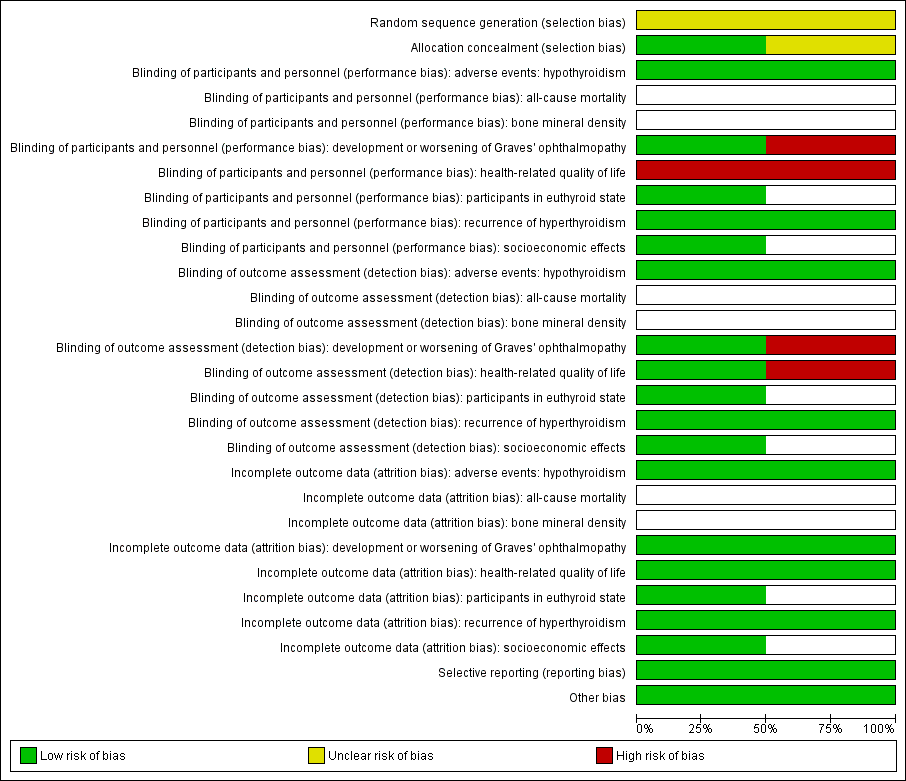
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial (blank cells indicate that the trial did not measure that particular outcome).
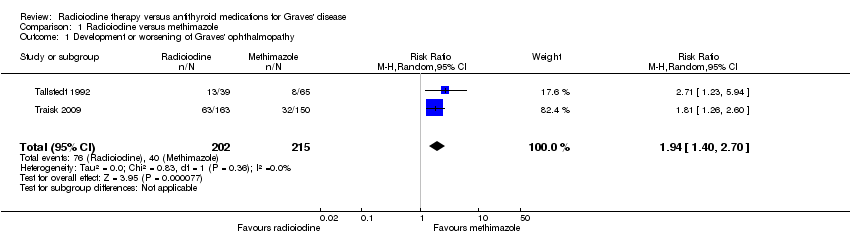
Comparison 1 Radioiodine versus methimazole, Outcome 1 Development or worsening of Graves' ophthalmopathy.

Comparison 1 Radioiodine versus methimazole, Outcome 2 Adverse events: hypothyroidism.
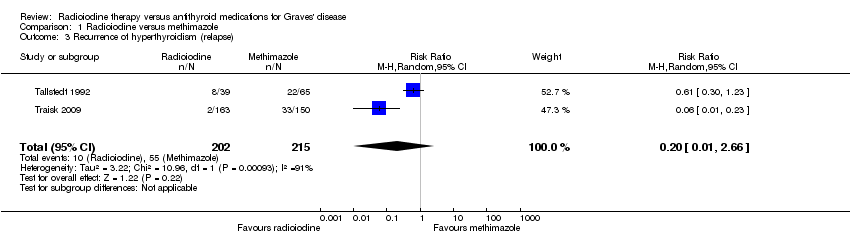
Comparison 1 Radioiodine versus methimazole, Outcome 3 Recurrence of hyperthyroidism (relapse).

Comparison 1 Radioiodine versus methimazole, Outcome 4 Participants in euthyroid state.
| Radioiodine therapy compared with antithyroid medications for Graves' disease | ||||||
| Patient: participants with Graves' disease Settings: outpatients Intervention: radioiodine Comparison: methimazole | ||||||
| Outcomes | Assumed risk | Corresponding risk | Relative effect | No of participants | Quality of the evidence | Comments |
| Methimazole | Radioiodine | |||||
| Health‐related quality of life Follow‐up: 4 years and 14 to 21 years | See comment | See comment | See comment | 425 (2) | See comment | 2 trials assessed this outcome but no quantitative data for comparisons between intervention groups were provided; trial authors in 1 trial reported that there were no differences in the results of the SF‐36 scores between the 2 treatment groups |
| Development and worsening of Graves' ophthalmopathy | 186 per 1000 | 361 of 1000 (260 to 502) | RR 1.94 (1.40 to 2.70) | 417 (2) | ⊕⊕⊝⊝ | ‐ |
| Individuals in euthyroid state Follow‐up: at least 4 years | See comment | See comment | See comment | 112 (1) | See comment | No participant who underwent radioiodine treatment achieved an euthyroid state compared to 4/68 participants not becoming euthyroid treated by methimazole (Tallstedt 1992); however, in this trial thyroxine therapy was not introduced early in both treatment arms to avoid hypothyroidism |
| Recurrence of hyperthyroidism (relapse) Follow‐up: at least 4 years | 256 per 1000 | 51 of 1000 (3 to 680) | RR 0.20 (0.01 to 2.66) | 417 (2) | ⊕⊝⊝⊝ | ‐ |
| Adverse events other than development or worsening of Graves' disease (a) Hypothyroidism [measured by TSH and/or thyroid hormones] (b) Drug reactions Follow‐up: (a) at least 2 years (b) at least 4 years | See comment | See comment | See comment | (a) 104 (1) (b) 215 (2) | a) ⊕⊝⊝⊝ b) ⊕⊝⊝⊝ | (a) 39 of 41 participants developed hypothyroidism after radioiodine treatment for Graves' disease, compared with 0 of 65 participants receiving methimazole (Tallstedt 1992); however, in this trial thyroxine was not introduced early in both treatment groups in order to avoid hypothyroidism (b) 23 of 215 participants (11%) reported adverse effects likely related to methimazole treatment |
| All‐cause mortality Follow‐up: at least 4 years and 14 to 21 years | See comment | See comment | See comment | 425 (2) | See comment | No quantitative data for all‐cause mortality were reported |
| Socioeconomic effects Follow‐up: 2 years | See comment | See comment | See comment | 112 (1) | See comment | Costs for patients without relapse and methimazole treatment were USD 1126/USD 1164 (young/older methimazole group) and for radioiodine treatment USD 1862 Costs for patients with relapse and methimazole treatment were USD 2284/1972 (young/older methimazole group) and for radioiodine treatment USD 2760 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Assumed risk was derived from the event rates in the comparator groups. | ||||||
| Intervention(s) and | Sample sizea | Screened/eligible | Randomised | ITT | Analysed | Finishing trial | Randomised finishing trial | Follow‐up | |
| Traisk 2009 | Radioiodine | Primary endpoint was the difference in the proportion of participants with worsening or development of thyroid‐associated ophthalmopathy during a 4‐year follow‐up in the 2 groups (intention‐to‐treat analysis). A comparison (0.05, two‐tailed test) of the binomial proportions between 2 groups of 300 patients each would give more than 90% probability (power) to detect a true difference of 10% | 482/333 | ‐ | 163 | 163 | 163 | ‐ | 24 months (4 years) |
| Methimazole | ‐ | 150 | 150 | 150 | ‐ | ||||
| total: | 333 | 313 | 313 | 313 | 94 | ||||
| Tallstedt 1992e | Radioiodine | ‐ | 179 | 41 | ‐ | 39f | 39 | 95 | At least 24 months (at least 48 months; 3 years; 5 years; 14‐21 years)g |
| Methimazole | 71 | 65f | 64 | 90 | |||||
| total: | 112 | 104 | 103 | 92 | |||||
| Grand total | All radioiodine‐treated participants | 204 | |||||||
| All participants treated with methimazole | 221 | ||||||||
| All interventions | 425 | ||||||||
| "‐" denotes not reported ITT: intention‐to‐treat | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Development or worsening of Graves' ophthalmopathy Show forest plot | 2 | 417 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [1.40, 2.70] |
| 2 Adverse events: hypothyroidism Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Recurrence of hyperthyroidism (relapse) Show forest plot | 2 | 417 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 2.66] |
| 4 Participants in euthyroid state Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

