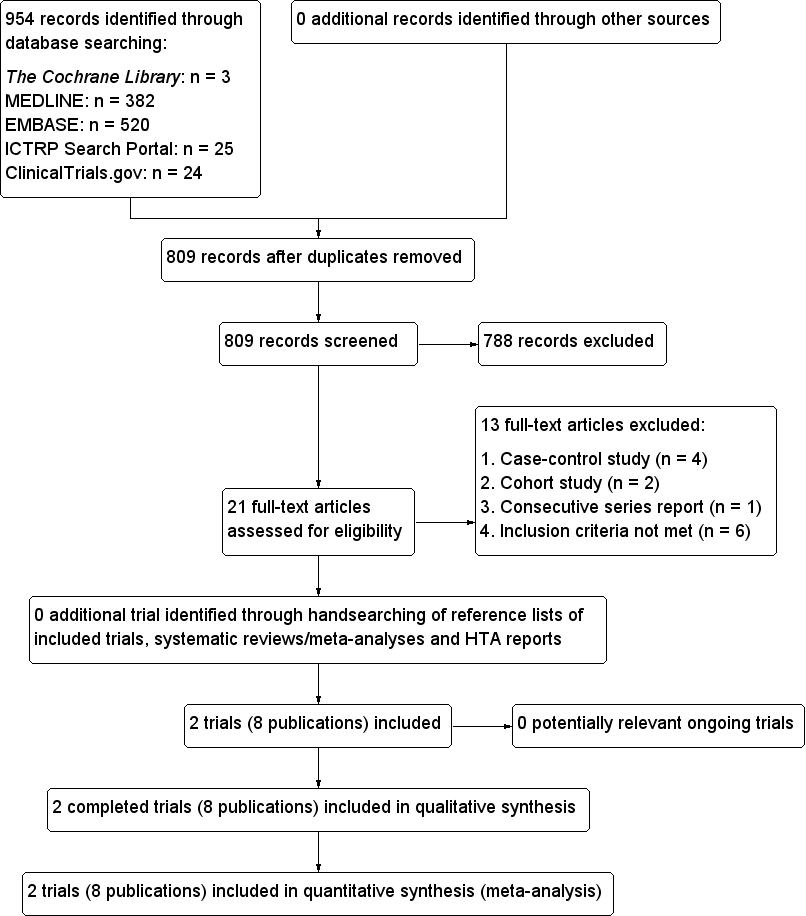
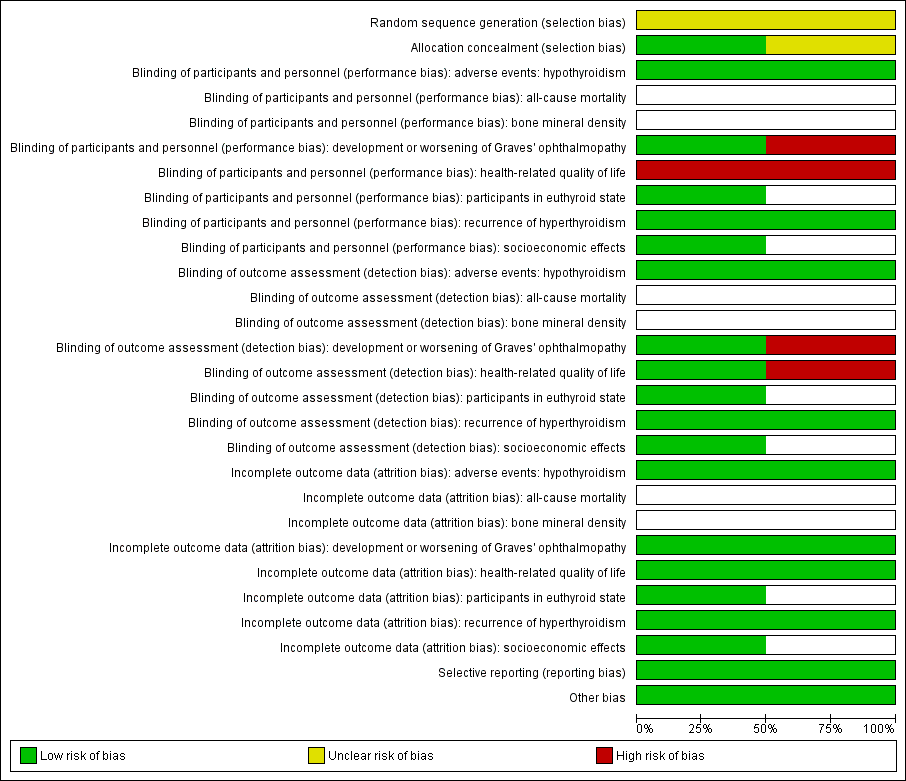
| Radioiodine therapy compared with antithyroid medications for Graves' disease |
| Patient: participants with Graves' disease Settings: outpatients Intervention: radioiodine Comparison: methimazole |
| Health‐related quality of life
[measured by the validated questionnaire SF‐36] Follow‐up: 4 years and 14 to 21 years | See comment | See comment | See comment | 425 (2) | See comment | 2 trials assessed this outcome but no quantitative data for comparisons between intervention groups were provided; trial authors in 1 trial reported that there were no differences in the results of the SF‐36 scores between the 2 treatment groups |
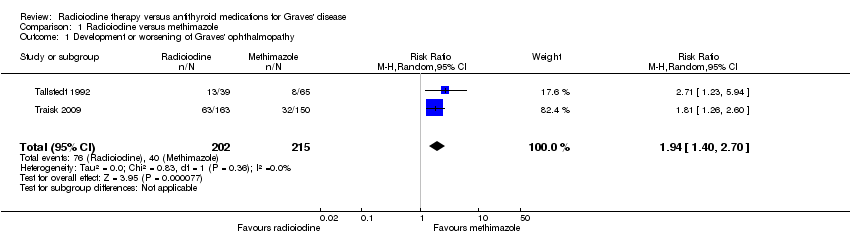
| Development and worsening of Graves' ophthalmopathy
[examination by ophthalmologists]
Follow‐up: 2 years and 4 years | 186 per 1000 | 361 of 1000 (260 to 502) | RR 1.94 (1.40 to 2.70) | 417 (2) | ⊕⊕⊝⊝
lowa | ‐ |
| Individuals in euthyroid state
[measured by serum thyroid hormone levels within the normal range] Follow‐up: at least 4 years | See comment | See comment | See comment | 112 (1) | See comment | No participant who underwent radioiodine treatment achieved an euthyroid state compared to 4/68 participants not becoming euthyroid treated by methimazole (Tallstedt 1992); however, in this trial thyroxine therapy was not introduced early in both treatment arms to avoid hypothyroidism |
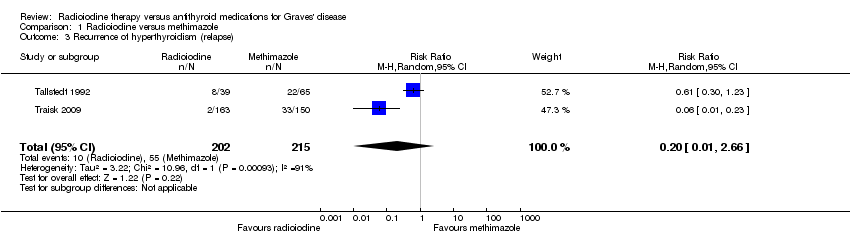
| Recurrence of hyperthyroidism (relapse)
[measured by serum thyroid hormone levels above the normal range after withdrawal of methimazole or end of radioiodine treatment] Follow‐up: at least 4 years | 256 per 1000 | 51 of 1000 (3 to 680) | RR 0.20 (0.01 to 2.66) | 417 (2) | ⊕⊝⊝⊝
very lowb | ‐ |
| Adverse events other than development or worsening of Graves' disease (a) Hypothyroidism [measured by TSH and/or thyroid hormones] (b) Drug reactions Follow‐up: (a) at least 2 years (b) at least 4 years | See comment | See comment | See comment | (a) 104 (1) (b) 215 (2) | a) ⊕⊝⊝⊝
very lowc b) ⊕⊝⊝⊝
very lowd | (a) 39 of 41 participants developed hypothyroidism after radioiodine treatment for Graves' disease, compared with 0 of 65 participants receiving methimazole (Tallstedt 1992); however, in this trial thyroxine was not introduced early in both treatment groups in order to avoid hypothyroidism (b) 23 of 215 participants (11%) reported adverse effects likely related to methimazole treatment |
| All‐cause mortality Follow‐up: at least 4 years and 14 to 21 years | See comment | See comment | See comment | 425 (2) | See comment | No quantitative data for all‐cause mortality were reported |
| Socioeconomic effects
[costs per patient, based on the official hospital reimbursement system in Sweden] Follow‐up: 2 years | See comment | See comment | See comment | 112 (1) | See comment | Costs for patients without relapse and methimazole treatment were USD 1126/USD 1164 (young/older methimazole group) and for radioiodine treatment USD 1862 Costs for patients with relapse and methimazole treatment were USD 2284/1972 (young/older methimazole group) and for radioiodine treatment USD 2760 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: confidence interval; RR: risk ratio; SF‐36: 36‐item Short Form Health Status Survey; TSH: thyroid‐stimulating hormone |
| GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate. |