Tratamientos farmacológicos para la prevención de la epilepsia después del traumatismo craneoencefálico
Información
- DOI:
- https://doi.org/10.1002/14651858.CD009900.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 10 agosto 2015see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Epilepsia
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
KT initiated the topic.
KT, BP and LA all contributed to drafting of the protocol.
Tracey Daley along with the Cochrane Epilepsy Group Trials Search Co‐ordinator devised the search strategy and carried out the literature searches.
KT, BP and LA reviewed and approved protocol.
KT, HA and BP all contributed to the data extraction, and drafted, reviewed and approved the text.
Sources of support
Internal sources
-
Research Office, Department of Medicine, Dalhousie University, Canada.
-
Research Methods Unit, CDHA, Canada.
External sources
-
Nova Scotia Health Research Foundation's (NSHRF) Systematic Review grant, Canada.
Funding was provided for research assistant.
Declarations of interest
Leslie Anne Campbell and Bernhard Pohlmann‐Eden were supported by a Nova Scotia Health Research Foundation Knowledge Programs Grant.
Bernhard Pohlmann‐Eden is a member of the Advisory Board of UCB Pharma. He has also acted as a consultant for UCB Pharma Canada. Berhard has received speaker's honoraria from UCB Pharma and Desitin Pharam Germany on several occasions.
Kara Thompson is a Biostatistician for the Department of Medicine, therefore, additional funding received from Nova Scotia Health Research Foundation was to provide a research assistant to help with work on project.
Hannah Abel provided research assistance in the review.
Acknowledgements
The review authors would like to thank Tracey Daley, Research Assistant for her valued assistance in writing the protocol. We would also like to thank the Nova Scotia Health Research Foundation (NSHRF) for their funding support though the Systematic Review Grant.
Version history
| Published | Title | Stage | Authors | Version |
| 2015 Aug 10 | Pharmacological treatments for preventing epilepsy following traumatic head injury | Review | Kara Thompson, Bernhard Pohlmann‐Eden, Leslie A Campbell, Hannah Abel | |
| 2012 Jun 13 | Pharmacological treatments for preventing epilepsy following traumatic head injury | Protocol | Kara Thompson, Bernhard Pohlmann‐Eden, Leslie Anne Campbell | |
Differences between protocol and review
We found too few studies; therefore, we produced no funnel plots or orbit tables. Other sources of bias were also considered in addition to the bias domains listed in protocol. We performed no subgroup analysis by trauma severity as only one study reported including participants of moderate to severe trauma, all the other studies included severe only. The subgroup analyses for age were broken down by proportion of participants aged over 17 years and under 17 years, such that studies that exclusively included adults or exclusively children were separated out. Data were not provided in a manner that we were able to separate by mean age under six years, age between six and 17 years and age over 17 years as planned in protocol. We performed no subgroup analysis for the early seizure outcome due to insufficient data. However, we added a sensitivity analysis for age of population for early seizure. Subgroup analysis was only performed by type of AED and treatment duration for the late seizure outcome due to insufficient data for other analyses. We added a sensitivity analysis for age of population, and type of control group (placebo versus usual care) comparison for late seizure. Insufficient data were available to perform a subgroup analysis by type of AED for all‐cause mortality; however, we performed a sensitivity analysis. Time‐to‐event data were not provided in the papers; therefore, we did not use hazard ratios to summarize time‐to‐event outcomes. Time from first seizure to second seizure was not recorded or analyzed as these data were not provided in the papers. No trial reported comparing a pharmacologic agent other than an AED with a placebo on adverse events; therefore, we completed no analysis. We performed no imputation for missing data, as this was not relevant based on data collected. We performed no sensitivity analysis for analysis based on imputation.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Anticonvulsants [*therapeutic use];
- Carbamazepine [therapeutic use];
- Cause of Death;
- Craniocerebral Trauma [*complications, mortality];
- Epilepsy [etiology, mortality, *prevention & control];
- Levetiracetam;
- Magnesium Sulfate [therapeutic use];
- Neuroprotective Agents [*therapeutic use];
- Phenytoin [therapeutic use];
- Piracetam [analogs & derivatives, therapeutic use];
- Randomized Controlled Trials as Topic;
- Valproic Acid [therapeutic use];
Medical Subject Headings Check Words
Adult; Child; Humans;
PICO
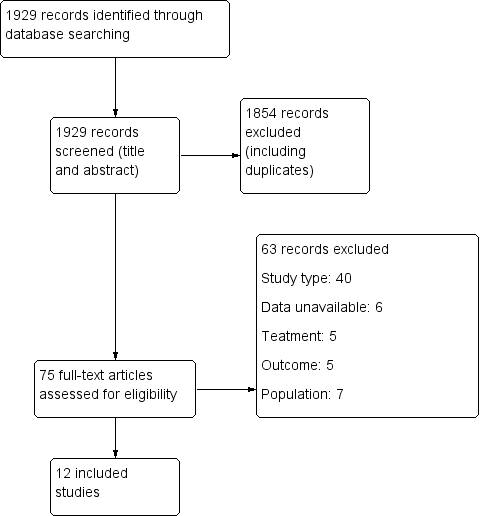
Study flow diagram.

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
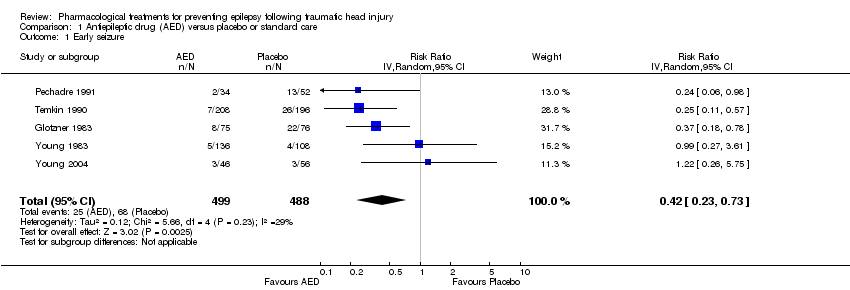
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 1 Early seizure.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 2 Late seizure.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 3 All‐cause mortality.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 4 Any serious event.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 5 Skin rash.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 6 Sensitivity analysis ‐ early seizure: age of population.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 7 Sensitivity analysis ‐ early seizure: study quality.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 8 Subgroup: late seizure: type of AED.
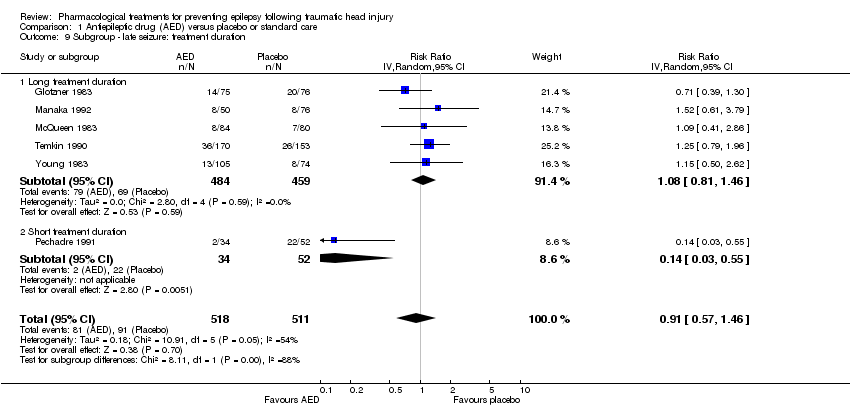
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 9 Subgroup ‐ late seizure: treatment duration.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 10 Sensitivity analysis ‐ late seizure: age of population.
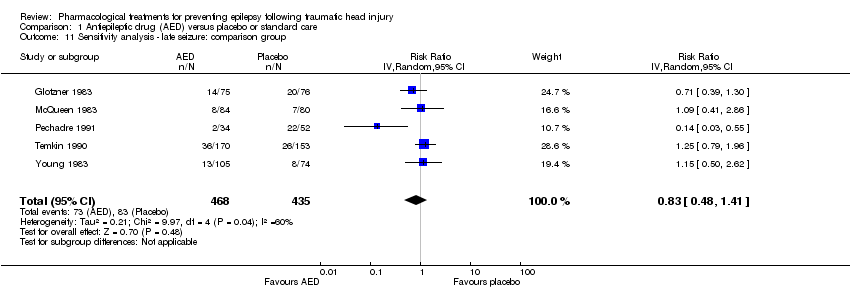
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 11 Sensitivity analysis ‐ late seizure: comparison group.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 12 Sensitivity analysis ‐ late seizure: study quality.
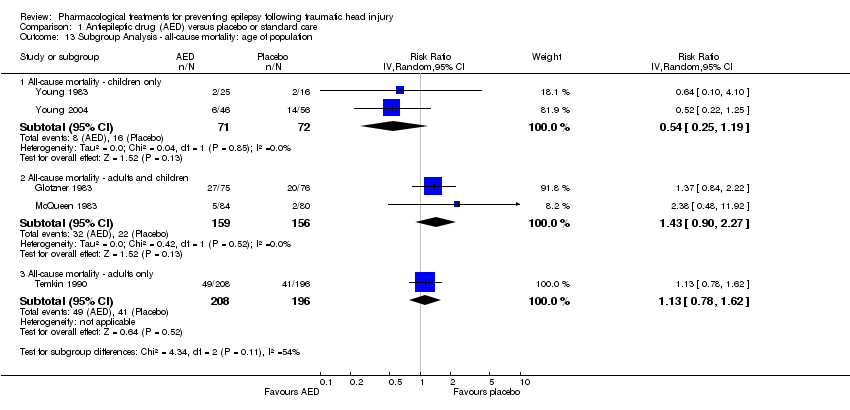
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 13 Subgroup Analysis ‐ all‐cause mortality: age of population.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 14 Subgroup analysis ‐ all‐cause mortality: treatment duration.
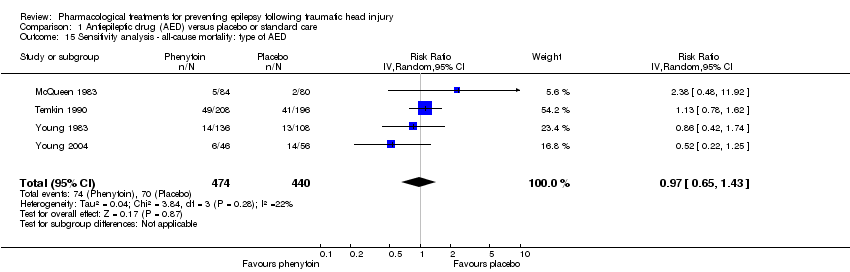
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 15 Sensitivity analysis ‐ all‐cause mortality: type of AED.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 16 Sensitivity analysis ‐ all‐cause mortality: study quality.
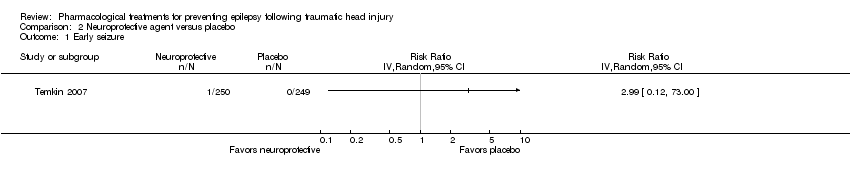
Comparison 2 Neuroprotective agent versus placebo, Outcome 1 Early seizure.

Comparison 2 Neuroprotective agent versus placebo, Outcome 2 Late seizure.

Comparison 2 Neuroprotective agent versus placebo, Outcome 3 All‐cause mortality.
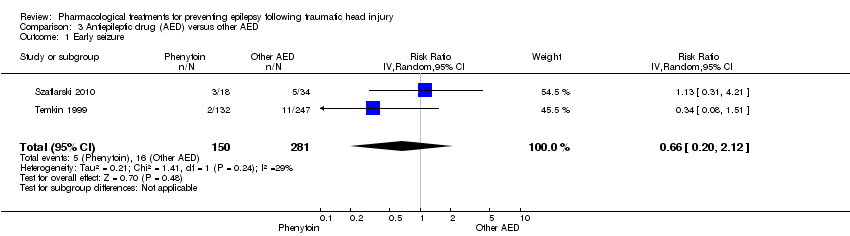
Comparison 3 Antiepileptic drug (AED) versus other AED, Outcome 1 Early seizure.
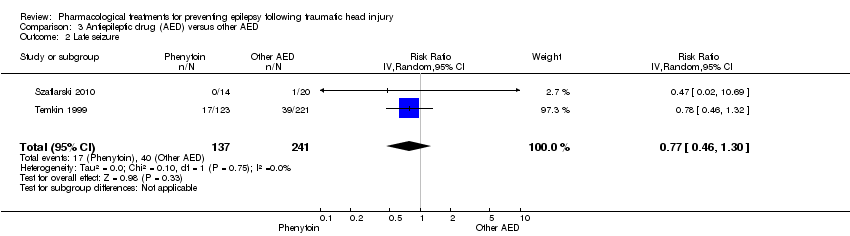
Comparison 3 Antiepileptic drug (AED) versus other AED, Outcome 2 Late seizure.

Comparison 3 Antiepileptic drug (AED) versus other AED, Outcome 3 All‐cause mortality.
| Antiepileptic drugs compared with placebo or standard care for people at risk of epilepsy following traumatic head injury | ||||||
| Patient or population: people with traumatic head injuries | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or standard care | Antiepilepticdrugs | |||||
| Early seizures | 139 per 1000 | 59 per 1000 | RR 0.42 | 987 | ⊕⊕⊝⊝ | Sensitivity analysis by quality of the study shows that RR for early seizures in low/unclear risk studies was no longer significant (RR 0.59, 95% CI 0.20 and 1.73) |
| Late seizures | 178 per 1000 | 162 per 1000 | RR 0.91 | 1029 | ⊕⊝⊝⊝ | RR of late seizures remained insignificant regardless of type of antiepileptic drug, treatment duration, age of population or quality of the study |
| All‐cause mortality | 174 per 1000 | 188 per 1000 | RR 1.08 | 1065 | ⊕⊝⊝⊝ | RR for all‐cause mortality remained insignificant regardless of treatment duration, age of population or quality of the study |
| Any serious adverse event of treatment count of events Follow up: 12 months | 94 per 1000 | 154 per 1000 (69 to 345) | RR 1.63 (0.73 to 3.66) | 568 (2 studies) | ⊕⊕⊝⊝ | |
| Time to first seizure from randomization | See comment | See comment | Not estimable | 0 | See comment | No study reported time to first seizure in an interpretable way |
| *The basis for the assumed risk is the event rate in the control (placebo or standard care) group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias: Two studies included in this outcome had instances of high risk of bias assessment. The remaining studies had a mix of low and unclear risk of bias. 5 Downgraded one level due to imprecision of results: wide 95% CI that includes both considerable harm and benefit. 6 Downgraded one level due to serious risk of bias: selection bias was likely in both trials | ||||||
| Neuroprotective agents compared with placebo for people at risk of epilepsy following traumatic head injury | ||||||
| Patient or population: people with traumatic head injuries | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Neuroprotective agents | |||||
| Early seizure Count of events Follow‐up: 7 days | 0 per 1000 | 0 per 1000 | RR 2.99 (0.12 to 73.00) | 499 | ⊕⊕⊝⊝ | No events occurred in the control group therefore corresponding risk is also zero |
| Late seizure Count of events Follow‐up: 6 months | 56 per 1000 | 60 per 1000 | RR 1.07 (0.53 to 2.17) | 498 | ⊕⊕⊕⊕ | |
| All‐cause mortality Follow‐up: 6 months | 150 per 1000 | 180 per 1000 | RR 1.20 (0.80 to 1.81) | 466 | ⊕⊕⊕⊕ | |
| Any serious adverse event of treatment | See comment | See comment | Not estimable | 0 | See comment | No study reported adverse event data |
| Time to first seizure from randomization | See comment | See comment | Not estimable | 0 | See comment | No study reported time to first seizure in an interpretable way |
| *The basis for the assumed risk is the event rate in the control (placebo or standard care) group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to risk of bias: As reported in the study paper, 96% of participants received phenytoin for the first week in both treatment groups. This may have resulted in a very low early seizure rate 2 Downgraded one level due to imprecision of results: wide 95% CI that includes both considerable harm and benefit. | ||||||
| Anti‐epileptic drugs compared to other anti‐epileptic drugs for people at risk of epilepsy following traumatic head injury | ||||||
| Patient or population: people with traumatic head injuries | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other AEDs | Phenytoin | |||||
| Early seizure Counts of events Follow up: 7 days | 57 per 1000 | 38 per 1000 | RR 0.66 (0.20 to 2.12) | 431 | ⊕⊕⊝⊝ | |
| Late seizure Counts of events Follow up: 6 months to 2 years | 166 per 1000 | 128 per 1000 | RR 0.77 (0.46 to 1.30) | 378 | ⊕⊕⊕⊝ | |
| All‐cause mortality Follow up: 6 months to 2 years | 164 per 1000 | 87 per 1000 | RR 0.53 (0.30 to 94) | 431 | ⊕⊕⊕⊝ | |
| Any serious adverse event of treatment | See comment | See comment | Not estimable | 0 | See comment | No study reported adverse event data |
| Time to first seizure from randomization | See comment | See comment | Not estimable | 0 | See comment | No study reported time to first seizure in an interpretable way |
| *The basis for the assumed risk is the event rate in the control (placebo or standard care) group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to risk of bias; unclear information reported in one study regarding study design (randomisation and blinding) and loss to follow up from the study 2 Downgraded one level due to imprecision of results: wide 95% CI that includes both considerable harm and benefit. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Early seizure Show forest plot | 5 | 987 | Risk Ratio (IV, Random, 95% CI) | 0.42 [0.23, 0.73] |
| 2 Late seizure Show forest plot | 6 | 1029 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.57, 1.46] |
| 3 All‐cause mortality Show forest plot | 5 | 1065 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.79, 1.46] |
| 4 Any serious event Show forest plot | 2 | 568 | Risk Ratio (IV, Random, 95% CI) | 1.63 [0.73, 3.66] |
| 5 Skin rash Show forest plot | 2 | 568 | Risk Ratio (IV, Random, 99% CI) | 1.65 [0.54, 5.04] |
| 6 Sensitivity analysis ‐ early seizure: age of population Show forest plot | 4 | 885 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.21, 0.60] |
| 7 Sensitivity analysis ‐ early seizure: study quality Show forest plot | 2 | 506 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.11, 2.18] |
| 8 Subgroup: late seizure: type of AED Show forest plot | 6 | 1029 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.57, 1.46] |
| 8.1 Late seizure ‐ phenytoin | 4 | 752 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.40, 1.70] |
| 8.2 Late seizure ‐ other AED | 2 | 277 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.46, 1.99] |
| 9 Subgroup ‐ late seizure: treatment duration Show forest plot | 6 | 1029 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.57, 1.46] |
| 9.1 Long treatment duration | 5 | 943 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.81, 1.46] |
| 9.2 Short treatment duration | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 0.14 [0.03, 0.55] |
| 10 Sensitivity analysis ‐ late seizure: age of population Show forest plot | 5 | 706 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.44, 1.48] |
| 11 Sensitivity analysis ‐ late seizure: comparison group Show forest plot | 5 | 903 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.48, 1.41] |
| 12 Sensitivity analysis ‐ late seizure: study quality Show forest plot | 1 | 323 | Risk Ratio (IV, Fixed, 95% CI) | 1.25 [0.79, 1.96] |
| 13 Subgroup Analysis ‐ all‐cause mortality: age of population Show forest plot | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 13.1 All‐cause mortality ‐ children only | 2 | 143 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.25, 1.19] |
| 13.2 All‐cause mortality ‐ adults and children | 2 | 315 | Risk Ratio (IV, Random, 95% CI) | 1.43 [0.90, 2.27] |
| 13.3 All‐cause mortality ‐ adults only | 1 | 404 | Risk Ratio (IV, Random, 95% CI) | 1.13 [0.78, 1.62] |
| 14 Subgroup analysis ‐ all‐cause mortality: treatment duration Show forest plot | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 14.1 All‐cause mortality ‐ short‐term treatment duration | 2 | 346 | Risk Ratio (IV, Random, 95% CI) | 0.69 [0.39, 1.24] |
| 14.2 All‐cause mortality ‐ long‐term treatment duration | 3 | 719 | Risk Ratio (IV, Random, 95% CI) | 1.24 [0.93, 1.65] |
| 15 Sensitivity analysis ‐ all‐cause mortality: type of AED Show forest plot | 4 | 914 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.65, 1.43] |
| 16 Sensitivity analysis ‐ all‐cause mortality: study quality Show forest plot | 2 | 506 | Risk Ratio (IV, Fixed, 95% CI) | 1.00 [0.72, 1.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Early seizure Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2 Late seizure Show forest plot | 1 | 498 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.53, 2.17] |
| 3 All‐cause mortality Show forest plot | 1 | 466 | Risk Ratio (IV, Random, 95% CI) | 1.2 [0.80, 1.81] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Early seizure Show forest plot | 2 | 431 | Risk Ratio (IV, Random, 95% CI) | 0.66 [0.20, 2.12] |
| 2 Late seizure Show forest plot | 2 | 378 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.46, 1.30] |
| 3 All‐cause mortality Show forest plot | 2 | 431 | Risk Ratio (IV, Random, 95% CI) | 0.53 [0.30, 0.94] |

