Tratamientos farmacológicos para la prevención de la epilepsia después del traumatismo craneoencefálico
Resumen
Antecedentes
El traumatismo craneoencefálico es un evento frecuente y puede causar un espectro de discapacidades motoras y cognitivas. Una complicación frecuente son las crisis convulsivas. Los fármacos antiepilépticos (FAE) como la fenitoína se utilizan con frecuencia en la práctica clínica con la intención de prevenir la epilepsia postraumática. Actualmente se desconoce si la intervención médica inmediata después del traumatismo craneoencefálico con FAE o fármacos neuroprotectores puede alterar el proceso de epileptogénesis y dar lugar a un resultado más favorable. Esta revisión intentó analizar la efectividad de estas intervenciones de tratamientos. Esta revisión actualiza y amplía la revisión Cochrane anterior.
Objetivos
Comparar la eficacia de los fármacos antiepilépticos y los agentes neuroprotectores versus placebo, atención habitual u otros agentes farmacológicos para la prevención de la epilepsia postraumática en pacientes con diagnóstico de lesión cerebral traumática de cualquier gravedad.
Métodos de búsqueda
Se hicieron búsquedas en el registro especializado del Grupo Cochrane de Epilepsia (Cochrane Epilepsy Group), CENTRAL, MEDLINE, ClinicalTrials.gov y en la World Health Organization International Clinical Trials Registry Platform (ICTRP) en enero 2015. Se hicieron búsquedas en EMBASE, Biological Abstracts y en el National Research Register en septiembre 2014 y en SCOPUS en diciembre 2013. El Grupo Cochrane de Epilepsia realizó búsquedas manuales en revistas relevantes.
Criterios de selección
Se incluyeron los ensayos controlados aleatorios (ECA) que compararon FAE o agentes neuroprotectores con placebo, otro agente farmacológico o un grupo de atención habitual. Los resultados medidos incluyeron una crisis convulsiva que ocurrió en el transcurso de una semana desde el traumatismo (crisis convulsiva temprana), crisis convulsiva que ocurrió después de una semana postraumatismo (crisis convulsiva tardía), mortalidad y cualquier evento adverso.
Obtención y análisis de los datos
Dos autores de la revisión evaluaron de forma independiente la calidad de los estudios y extrajeron los datos. Se calcularon los cocientes de riesgos (CR) y los intervalos de confianza (IC) del 95% para cada resultado. Se utilizaron modelos de efectos aleatorios en los metanálisis y se realizaron análisis de subgrupos y de sensibilidad predefinidos.
Resultados principales
Esta revisión incluyó diez ECA (informados en 12 artículos) con 2326 participantes. La calidad metodológica de los estudios varió. El tipo de intervención se dividió en tres categorías; FAE versus placebo o atención estándar, agente neuroprotector alternativo versus placebo o atención estándar y FAE versus otro FAE. El tratamiento con un FAE (fenitoína o carbamazepina) disminuyó el riesgo de una crisis convulsiva temprana en comparación con placebo o atención estándar (CR 0,42; IC del 95%: 0,23 a 0,73; pruebas de muy baja calidad). No hubo pruebas de una diferencia en el riesgo de aparición de una crisis convulsiva tardía entre FAE y placebo o atención estándar (CR 0,91; IC del 95%: 0,57 a 1,46; pruebas de muy baja calidad). No hubo pruebas de una diferencia significativa en la mortalidad por todas las causas entre FAE y placebo o atención estándar (CR 1,08; IC del 95%: 0,79 a 1,46, pruebas de calidad muy baja). Solamente un estudio analizó otros agentes potencialmente neuroprotectores (sulfato de magnesio) en comparación con placebo. Los cocientes de riesgos fueron: crisis convulsiva tardía 1,07 (IC del 95%: 0,53 a 2,17) y mortalidad por todas las causas 1,20 (IC del 95%: 0,80 a 1,81). No fue posible calcular el cociente de riesgos para la aparición de una crisis convulsiva temprana.
Dos estudios analizaron la comparación de dos FAE (levetiracetam, valproato) y utilizaron fenitoína como el comparador principal en cada estudio. El cociente de riesgos para la mortalidad por todas las causas fue 0,53 (IC del 95%: 0,30 a 0,94). No hubo pruebas de efectos beneficiosos del tratamiento con fenitoína en comparación con otro FAE para las crisis convulsivas tempranas (CR 0,66; 95%: 0,20 a 2,12) o las crisis convulsivas tardías (CR 0,77; IC del 95%: 0,46 a 1,30).
Solo dos estudios informaron eventos adversos. El CR de cualquier evento adverso con FAE en comparación con placebo fue 1,65 (IC del 95%: 0,73 a 3,66; pruebas de baja calidad). No hubo datos suficientes sobre los eventos adversos en las otras comparaciones de tratamiento.
Conclusiones de los autores
Esta revisión encontró pruebas de baja calidad de que el tratamiento precoz con un FAE en comparación con placebo o atención estándar redujo el riesgo de crisis convulsivas postraumáticas tempranas. No hubo pruebas para apoyar una reducción en el riesgo de crisis convulsivas tardías o de mortalidad. No hubo pruebas suficientes para establecer cualquier conclusión con respecto a la efectividad o la seguridad de otros agentes neuroprotectores en comparación con placebo o para la comparación de fenitoína, un FAE tradicional, con otro FAE.
PICO
Resumen en términos sencillos
Fármacos para la prevención de la epilepsia después del traumatismo craneoencefálico
Antecedentes
El traumatismo craneoencefálico es un evento frecuente y puede dañar el cerebro. Esta lesión grave a menudo está seguida de crisis convulsivas (ataques), que pueden empeorar el daño y provocar epilepsia crónica, un trastorno neurológico caracterizado por crisis convulsivas recurrentes frecuentes. Los fármacos antiepilépticos generalmente se administran para suprimir las crisis convulsivas ya diagnosticadas. Su función para curar la enfermedad y prevenir la aparición de epilepsia en los pacientes que se consideran en riesgo de crisis convulsivas después de cualquier lesión cerebral, incluido el traumatismo craneoencefálico, no se conoce bien.
Características de los estudios
Se buscaron los estudios que evaluaron el efecto de la administración precoz de fármacos antiepilépticos u otros posibles agentes neuroprotectores (que actúan al proteger la estructura o la función de los nervios) sobre la epilepsia postraumática. Los resultados primarios de interés fueron las crisis convulsivas postraumáticas tempranas (en el transcurso de una semana desde el traumatismo) y las crisis convulsivas tardías (después de una semana postraumatismo). También se analizó la muerte, el tiempo hasta la crisis convulsiva tardía y los efectos secundarios. Las pruebas están actualizadas hasta enero 2015.
Resultados clave
Se encontraron 10 ensayos clínicos con 2326 pacientes informados en 12 artículos publicados. Las pruebas disponibles indicaron que el tratamiento precoz con un fármaco antiepiléptico tradicional (fenitoína o carbamazepina) puede reducir el riesgo de crisis convulsivas postraumáticas tempranas. Los fármacos antiepilépticos tradicionales no son más eficaces que el placebo (un comprimido simulado) o la atención estándar para reducir las crisis convulsivas tardías o la mortalidad. Hubo datos limitados disponibles para la comparación de un FAE con otro FAE y para la comparación de otros agentes potencialmente neuroprotectores con placebo. La mayoría de los estudios no informó efectos secundarios graves y otros efectos secundarios.
Calidad de la evidencia
La calidad general de las pruebas varió y los resultados deben interpretarse con precaución.
Conclusiones de los autores
Summary of findings
| Antiepileptic drugs compared with placebo or standard care for people at risk of epilepsy following traumatic head injury | ||||||
| Patient or population: people with traumatic head injuries | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or standard care | Antiepilepticdrugs | |||||
| Early seizures | 139 per 1000 | 59 per 1000 | RR 0.42 | 987 | ⊕⊕⊝⊝ | Sensitivity analysis by quality of the study shows that RR for early seizures in low/unclear risk studies was no longer significant (RR 0.59, 95% CI 0.20 and 1.73) |
| Late seizures | 178 per 1000 | 162 per 1000 | RR 0.91 | 1029 | ⊕⊝⊝⊝ | RR of late seizures remained insignificant regardless of type of antiepileptic drug, treatment duration, age of population or quality of the study |
| All‐cause mortality | 174 per 1000 | 188 per 1000 | RR 1.08 | 1065 | ⊕⊝⊝⊝ | RR for all‐cause mortality remained insignificant regardless of treatment duration, age of population or quality of the study |
| Any serious adverse event of treatment count of events Follow up: 12 months | 94 per 1000 | 154 per 1000 (69 to 345) | RR 1.63 (0.73 to 3.66) | 568 (2 studies) | ⊕⊕⊝⊝ | |
| Time to first seizure from randomization | See comment | See comment | Not estimable | 0 | See comment | No study reported time to first seizure in an interpretable way |
| *The basis for the assumed risk is the event rate in the control (placebo or standard care) group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias: Two studies included in this outcome had instances of high risk of bias assessment. The remaining studies had a mix of low and unclear risk of bias. 5 Downgraded one level due to imprecision of results: wide 95% CI that includes both considerable harm and benefit. 6 Downgraded one level due to serious risk of bias: selection bias was likely in both trials | ||||||
| Neuroprotective agents compared with placebo for people at risk of epilepsy following traumatic head injury | ||||||
| Patient or population: people with traumatic head injuries | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Neuroprotective agents | |||||
| Early seizure Count of events Follow‐up: 7 days | 0 per 1000 | 0 per 1000 | RR 2.99 (0.12 to 73.00) | 499 | ⊕⊕⊝⊝ | No events occurred in the control group therefore corresponding risk is also zero |
| Late seizure Count of events Follow‐up: 6 months | 56 per 1000 | 60 per 1000 | RR 1.07 (0.53 to 2.17) | 498 | ⊕⊕⊕⊕ | |
| All‐cause mortality Follow‐up: 6 months | 150 per 1000 | 180 per 1000 | RR 1.20 (0.80 to 1.81) | 466 | ⊕⊕⊕⊕ | |
| Any serious adverse event of treatment | See comment | See comment | Not estimable | 0 | See comment | No study reported adverse event data |
| Time to first seizure from randomization | See comment | See comment | Not estimable | 0 | See comment | No study reported time to first seizure in an interpretable way |
| *The basis for the assumed risk is the event rate in the control (placebo or standard care) group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to risk of bias: As reported in the study paper, 96% of participants received phenytoin for the first week in both treatment groups. This may have resulted in a very low early seizure rate 2 Downgraded one level due to imprecision of results: wide 95% CI that includes both considerable harm and benefit. | ||||||
| Anti‐epileptic drugs compared to other anti‐epileptic drugs for people at risk of epilepsy following traumatic head injury | ||||||
| Patient or population: people with traumatic head injuries | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other AEDs | Phenytoin | |||||
| Early seizure Counts of events Follow up: 7 days | 57 per 1000 | 38 per 1000 | RR 0.66 (0.20 to 2.12) | 431 | ⊕⊕⊝⊝ | |
| Late seizure Counts of events Follow up: 6 months to 2 years | 166 per 1000 | 128 per 1000 | RR 0.77 (0.46 to 1.30) | 378 | ⊕⊕⊕⊝ | |
| All‐cause mortality Follow up: 6 months to 2 years | 164 per 1000 | 87 per 1000 | RR 0.53 (0.30 to 94) | 431 | ⊕⊕⊕⊝ | |
| Any serious adverse event of treatment | See comment | See comment | Not estimable | 0 | See comment | No study reported adverse event data |
| Time to first seizure from randomization | See comment | See comment | Not estimable | 0 | See comment | No study reported time to first seizure in an interpretable way |
| *The basis for the assumed risk is the event rate in the control (placebo or standard care) group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to risk of bias; unclear information reported in one study regarding study design (randomisation and blinding) and loss to follow up from the study 2 Downgraded one level due to imprecision of results: wide 95% CI that includes both considerable harm and benefit. | ||||||
Antecedentes
Descripción de la afección
El traumatismo craneoencefálico es un evento frecuente y puede causar un espectro de discapacidades motoras y cognitivas. Una complicación frecuente son las crisis convulsivas. Aunque las "crisis convulsivas tempranas" con frecuencia se consideran reacciones difusas no específicas como resultado de una encefalopatía aguda y se resuelven de forma espontánea, las crisis convulsivas que continúan varias semanas o meses después del traumatismo craneoencefálico parecen reflejar un proceso subyacente de formación de cicatrices postraumáticas y epileptogénesis. Sin embargo, hay pruebas de estudios epidemiológicos de que las crisis convulsivas tempranas pueden ser variables predictivas de crisis convulsivas tardías (Wyllie 2010). Lo anterior indica que estas definiciones reflejan simplificaciones de la transformación continua tisular subyacente con el transcurso del tiempo. El riesgo de recurrencia de las crisis convulsivas tardías "no provocadas" aumenta con la gravedad de la lesión, el compromiso de la corteza cerebral, la presencia de penetración en la duramadre, la fractura de cráneo y el hematoma intracerebral, así como la aparición de crisis convulsivas tempranas (Jennett 1981; Annegers 1998). No se conoce el momento ni la interacción de los factores que pueden involucrados en el desarrollo de este proceso epileptogénico.
Descripción de la intervención
Detrás del concepto de prevención de la epilepsia postraumática se encuentra la esperanza de que el período silencioso de semanas y meses posteriores al traumatismo, antes de la aparición de las crisis convulsivas, sea un período de oportunidad para detener el proceso mediante estrategias de tratamiento de intervención apropiadas (Temkin 2009). Los fármacos antiepilépticos (FAE) pueden suprimir las crisis convulsivas; sin embargo, existe un debate polémico sobre si también son capaces de interferir de forma positiva en el proceso que da lugar a la epilepsia. Estudios experimentales que analizan los agentes neuroprotectores, como los antioxidantes y los radicales libres, también han sido prometedores, aunque históricamente no se han trasladado de forma adecuada al ambiente clínico (Slemmer 2008). Por lo tanto, esta revisión Cochrane evaluará de forma cuidadosa el impacto del uso temprano o tardío de FAE y agentes neuroprotectores en la aparición de crisis convulsivas no provocadas después del traumatismo.
De qué manera podría funcionar la intervención
Los estudios de investigación experimentales actuales sobre la epilepsia con modelos animales, como la estimulación inducida repetida (kindling) y la condición epiléptica posestado, indican que algunos FAE nuevos pueden tener la posibilidad de alterar el proceso epileptogénico subyacente y actuar como agentes que modifican la enfermedad (Löscher 2002; Brandt 2006). También hay algunas pruebas de que los agentes neuroprotectores pueden alterar el proceso epileptogénico. Por ejemplo, los antioxidantes podrían suprimir este proceso al interferir con las reacciones de los radicales libres iniciadas por la hemorragia asociada con las lesiones cerebrales (Willmore 2009).
Por qué es importante realizar esta revisión
Las crisis convulsivas postraumáticas son muy prevalentes. En su mayoría estos pacientes son sometidos a un cuidadoso algoritmo de diagnóstico funcional y estructural que incluye electroencefalografía (EEG) e imaginología de resonancia magnética (IRM) o al menos tomografía computarizada (TC). Por lo tanto, las crisis convulsivas postraumáticas se pueden considerar un modelo ideal para estudiar los cambios tisulares y la hiperexcitabilidad regional como parte de la cicatriz epileptogénica en evolución. Todavía no se conoce si la intervención médica inmediata luego del traumatismo craneoencefálico con FAE o fármacos neuroprotectores puede alterar el proceso de epileptogénesis y dar lugar a un resultado más favorable. Hay datos limitados sobre los FAE tradicionales como la fenitoína, el fenobarbital, el valproato y la carbamazepina.
Con el advenimiento desde mediados del año 2000 de muchos FAE nuevos y estudios de investigación sobre tratamientos alternativos como los agentes neuroprotectores, parece crucial y oportuno examinar cuidadosamente la experiencia humana y evaluar de qué forma estos resultados experimentales podrían traducirse en la prevención de la epilepsia postraumática en la práctica clínica. Por lo tanto, esta revisión dará lugar a una revisión sistemática actualizada de los ensayos controlados aleatorios (ECA) que examinan la efectividad y la seguridad de los FAE y de los agentes neuroprotectores, con un énfasis especial en los productos autorizados recientemente.
Objetivos
Comparar la eficacia de los fármacos antiepilépticos y los agentes neuroprotectores versus placebo, atención habitual u otros agentes farmacológicos para la prevención de la epilepsia postraumática en pacientes con diagnóstico de lesión cerebral traumática de cualquier gravedad.
Métodos
Criterios de inclusión de estudios para esta revisión
Tipos de estudios
ECA que incluyeran FAE o agentes neuroprotectores comparados con placebo, otro agente farmacológico o un grupo de atención habitual. Se incluyeron estudios publicados en cualquier idioma. Se excluyeron los estudios cuasialeatorios, los estudios de determinación de dosis y los ensayos con asignación al azar grupal o cruzados.
Tipos de participantes
Pacientes de todas las edades con diagnóstico de lesión cerebral traumática (LCT) aguda que recibieron tratamiento profiláctico con FAE o agentes neuroprotectores. La administración fue posterior a la lesión y anterior a la aparición de una primera crisis convulsiva postraumática (PCCP). Se excluyó a los pacientes con crisis convulsivas no provocadas previamente documentadas.
Tipos de intervenciones
Tratamiento
-
Administración de cualquier FAE convencional luego de la lesión y antes de la aparición de una PCCP. Los FAE tradicionales incluyeron, pero no estuvieron limitados a, carbamazepina, fenitoína y valproato, y los ejemplos de FAE nuevos incluyeron, pero no estuvieron limitados a, oxcarbazepina, lamotrigina, levetiracetam y topiramato.
-
Cualquier tratamiento farmacológico neuroprotector alternativo, incluida la administración de factores neurotróficos diferenciados, hormonas o antioxidantes posterior a la lesión y antes de la aparición de una PCCP.
Comparación
Otro agente farmacológico, placebo o atención habitual.
-
Agentes farmacológicos (FAE) versus placebo o atención habitual.
-
Agente neuroprotector versus placebo o atención habitual.
-
Agente farmacológico A (FAE) versus agente farmacológico B (FAE).
Se analizó cada comparación por separado.
Tipos de medida de resultado
Resultados primarios
-
Proporción de participantes que presentaron una crisis convulsiva temprana posterior al traumatismo, definida como la que ocurre en el transcurso de una semana desde el traumatismo.
-
Proporción de participantes que presentaron una crisis convulsiva tardía posterior al traumatismo, definida como la que ocurre transcurrida una semana desde el traumatismo.
Resultados secundarios
-
Mortalidad por cualquier causa durante el período de seguimiento.
-
Tiempo hasta la primera crisis convulsiva desde la asignación al azar.
-
Proporción de participantes que experimentaron eventos adversos graves relacionados con el tratamiento.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
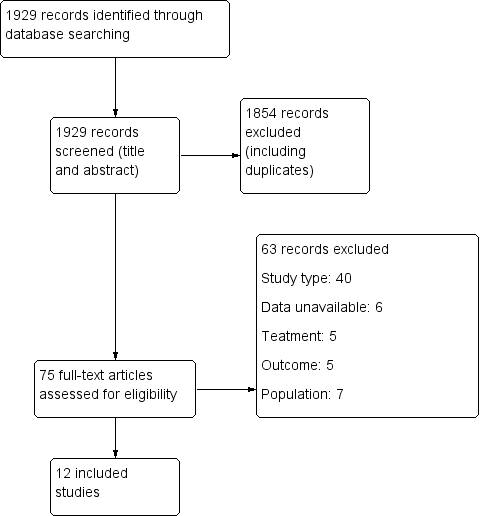
Of the 1929 initial citations identified, we screened 75 reports (See Figure 1).

Study flow diagram.
Included studies
Ten RCTs described in 12 published articles met review inclusion criteria and included 2326 randomized participants ages five years and older. All trials included participants with moderate and severe TBI and excluded people with pre‐existing epilepsy.
Five trials included children (McQueen 1983; Young 1983; Pechadre 1991; Manaka 1992; Young 2004). Young 2004 included only children under the age of 10 years.
Six trials reported on short‐term treatments (five to seven days to one month) (Young 1983; Pechadre 1991; Temkin 1999; Young 2004; Temkin 2007; Szaflarski 2010), three reported on mid‐term treatments (six to 12 months) (McQueen 1983; Temkin 1990; Temkin 1999), and three trials reported on long‐term treatments (18 months to two years) (Glotzner 1983; Young 1983; Manaka 1992). Most studied traditional AEDs versus placebo or usual care: phenytoin (McQueen 1983; Young 1983; Temkin 1990; Pechadre 1991; Young 2004), phenobarbital (Manaka 1992), carbamazepine (Glotzner 1983), and valproate (Temkin 1999); one studied a newly licensed agent: levetiracetam versus phenytoin (Szaflarski 2010), and one studied an 'other' agent, magnesium sulfate (MgSO4) versus placebo (Temkin 2007).
Six trials were conducted in the USA (Young 1983; Temkin 1990; Temkin 1999; Young 2004; Temkin 2007; Szaflarski 2010), three in Europe (Glotzner 1983; McQueen 1983; Pechadre 1991), and one in Japan (Manaka 1992).
We included nine trials in the meta‐analysis and assessed the primary and secondary outcomes of early seizures, late seizures, all‐cause mortality and adverse events (Glotzner 1983; McQueen 1983; Young 1983; Temkin 1990; Pechadre 1991; Manaka 1992; Temkin 1999; Young 2004; Szaflarski 2010). All but two trials reported incidence of early and late seizures; Manaka 1992 and McQueen 1983 reported only late seizures. All trials but Manaka 1992 and Pechadre 1991 reported mortality. The majority of trials primarily investigated whether AEDs (traditional or newly licensed) prevented early or late (or both) seizure occurrence in people with TBI. One trial primarily investigated safety and reported on adverse events (Szaflarski 2010). McQueen 1983 and Temkin 1990 also reported the occurrence of skin rashes. Temkin 2007 was not included in a meta‐analysis as it was the only study included in the review that studied an 'other' agent. See Characteristics of included studies table for details.
Excluded studies
We excluded 63 studies from the review. Forty were not RCTs or quasi‐randomized trials, in seven the data were unavailable (i.e. trial cancelled due to lack of enrolment or unable to acquire details from author), four studies were secondary publications of studies already included, which contained no further relevant information. Five studies did not report treatment of interest, seven did not include the population of interest. See Characteristics of excluded studies table for details.
Risk of bias in included studies
Figure 2 and Figure 3 summarize the risk of bias of included studies. We deemed no study to be at low risk of bias in all bias types. The majority of studies had a mix of low and unclear bias to varying degrees. Five trials had a number of bias types classified as high risk of bias (Glotzner 1983; McQueen 1983; Young 1983; Pechadre 1991; Manaka 1992).
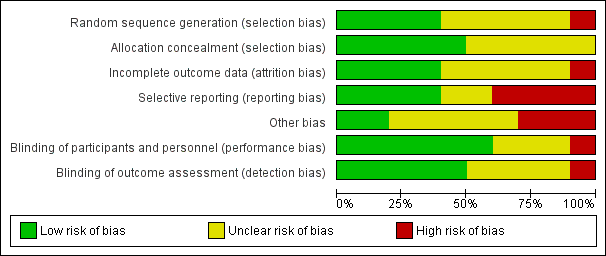
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
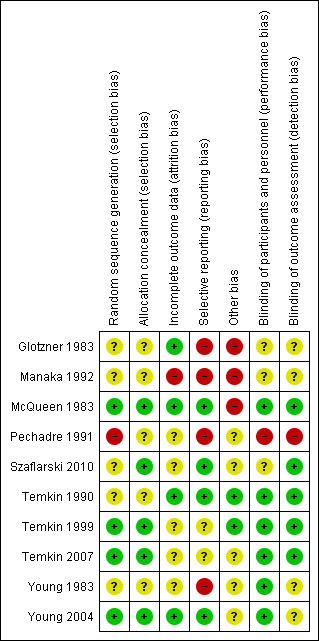
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Allocation
Only three trials adequately described the sequence generation and allocation process (Temkin 1999; Young 2004; Temkin 2007). Szaflarski 2010 indicated the participants were randomized by the pharmacy but did not describe the sequence generation and, therefore, the risk of selection bias was unclear. Risk of selection bias was unclear in McQueen 1983; Young 1983; Temkin 1990; Manaka 1992 , due to lack of clear description of the sequence generation and allocation process. The sequence generation in Glotzner 1983 and Pechadre 1991 was based on odd/even birthday or days of admission and, therefore, at high risk of predicting group allocation.
Blinding
Six of the 10 trials were low risk for performance bias as they adequately described blinding of participants and personnel (McQueen 1983; Young 1983; Temkin 1990; Temkin 1999; Young 2004; Temkin 2007). In Glotzner 1983; Manaka 1992; and Szaflarski 2010, risk of bias for performance bias was unclear as these trials did not report on blinding of participants and personnel. Risk of detection bias was low in five trials (McQueen 1983; Temkin 1990; Temkin 1999; Temkin 2007; Szaflarski 2010), and unclear in four publications as they did not describe blinding of the outcome assessment (Glotzner 1983; Young 1983; Manaka 1992; Young 2004). Pechadre 1991 was at high risk for performance and detection bias as it did not describe blinding of the participants, personnel or outcome assessment and the predictable randomization process suggested that assessors could easily determine which participants were allocated to treatment and control groups.
Incomplete outcome data
Three trials were low risk for attrition bias as they clearly described outcome data and attrition patterns (McQueen 1983; Temkin 1990; Young 2004). Six trials had unclear risk for attrition bias due to poor descriptions of reasons for attrition (Glotzner 1983; Young 1983; Pechadre 1991; Temkin 1999; Temkin 2007; Szaflarski 2010), and Manaka 1992 was high risk of bias for lack of description or details on 52 people who were excluded or dropped out from study.
Selective reporting
Only four of the 10 trials were low risk for reporting bias (McQueen 1983; Temkin 1990; Young 2004; Szaflarski 2010). Four trials were at high risk of selective reporting as they did not report adverse events (Glotzner 1983; Young 1983; Pechadre 1991; Manaka 1992). In addition, Manaka 1992 and Pechadre 1991 did not report mortality and Young 1983 reported mortality inconsistently across age groups. Young 1983 reported mortality as a count of events for all ages in the short‐term treatment; however, mortality in adults on long‐term treatment were not reported as a count of events and, therefore, overall deaths for the entire trial were underestimated. Manaka 1992 was high risk for reporting bias as the trial did not report baseline characteristics. The remaining two trials had unclear risk (Temkin 1999; Temkin 2007).
Other potential sources of bias
Three publications were at high risk for other types of bias (Glotzner 1983; McQueen 1983; Manaka 1992). Glotzner 1983 reported that the majority of participants received an AED in the first week regardless of allocation group resulting in potential contamination of controls. McQueen 1983 reported potential significant baseline differences between the control and treatment groups with more five to 15 year olds in the treatment group and below therapeutic levels of the phenytoin were reported. Manaka 1992 did not report baseline characteristics, so it was impossible to compare baseline characteristics between treatment groups. Several studies reported difficulties with compliance (McQueen 1983; Young 1983; Temkin 1990; Temkin 1999), and maintaining therapeutic levels particularly when evaluating late seizure outcome.
Effects of interventions
See: Summary of findings for the main comparison Antiepileptic drugs compared with placebo or standard care for people at risk of epilepsy following traumatic head injury; Summary of findings 2 Neuroprotective agent versus placebo for people at risk of epilepsy following traumatic head injury; Summary of findings 3 Anti‐epileptic drugs compared to other anti‐epileptic drugs for people at risk of epilepsy following traumatic head injury
1. Antiepileptic drug versus placebo or usual care
1.1 Occurrence of early seizure
Five trials involving 987 participants examined the occurrence of early seizures (Glotzner 1983; Pechadre 1991; Temkin 1990; Young 2004; Young 1983). All trials compared a traditional AED (carbamazepine or phenytoin) with a placebo or usual care. The trials included a range of ages from children to adult. Duration of treatment for this outcome varied from five to seven days. The proportion of participants experiencing an early seizure in the treatment group was 5.0% (25/499) compared with 13.9% (68/488) in the placebo group/usual care group. The pooled results favored the traditional AED treatment compared with the control group (RR 0.42, 95% CI 0.23 to 0.73; Analysis 1.1). Heterogeneity was low among the studies (I2 = 29%). We reanalyzed the outcome using fixed‐effect methods; results were consistent with those obtained using random‐effects models. We rated the quality of the evidence as low due to high selective reporting bias in 3 of the studies and inconsistency in RR results in the two studies with low risk of bias (Analysis 1.7).
1.2 Occurrence of late seizure
Six trials reported on late seizures in 1029 participants (Glotzner 1983; McQueen 1983; Young 1983; Temkin 1990; Pechadre 1991; Manaka 1992). Manaka 1992 compared phenobarbital with usual care, Glotzner 1983 compared carbamazepine with placebo, while the other four trials compared phenytoin with placebo. Duration of treatment varied from three months to two years. Five of the six trials included adults and children; Temkin 1999 was the only trial to assess adults exclusively. About 15.6% (81/518) of participants receiving AED treatment experienced a late seizure compared with 17.8% (91/511) receiving placebo/usual care. The six‐pooled studies showed no statistically significant effect for traditional AEDs compared with placebo or usual care on late seizure occurrence (RR 0.91, 95% CI 0.57 to 1.467; Analysis 1.2). There was evidence of heterogeneity among the studies (I2 = 54%). This result was rated as very low quality due to high risk of bias in 2 or more categories for several studies, imprecision of pooled RR estimate and moderate level of heterogeneity.
1.3 All‐cause mortality
Five trials reported mortality in 1065 participants (Glotzner 1983; McQueen 1983; Young 1983; Temkin 1990; Young 2004). They compared a traditional AED (carbamazepine or phenytoin) with placebo. Duration of treatments varied from five days to 24 months. About 18.4% (101/549) of participants in the AED group died compared with 17.4% (90/516) in the placebo group. The five pooled trials showed no statistically significant difference in the RR of death between participants treated with traditional AEDs compared with placebo (RR 1.08, 95% CI 0.79 to 1.46; Analysis 1.3). Heterogeneity was low among the studies (I2 = 19%). This result was rated as very low quality due to high risk of bias in 2 or more categories for several studies, imprecision of pooled RR estimate with confidence interval ranging from benefit to harm.
1.4 Any serious event
McQueen 1983 and Temkin 1990 looked at any serious events comparing phenytoin with placebo. About 14.4% (42/292) of participants in treatment group experienced an adverse event compared with 9.4% (26/276) in the placebo group. The pooled RR of an adverse event in treatment group compared with placebo was 1.63 (95% CI 0.73 to 3.66; 568 participants; Analysis 1.4). Heterogeneity was low among the studies (I2 = 18%). We performed no subgroup analysis due to too few studies.The result was rated as low based on imprecision of RR estimate with confidence intervals covering both benefit and harm and serious risk of bias in one study.
1.5 Skin rash
McQueen 1983 and Temkin 1990 reported skin rash comparing phenytoin with placebo. About 10.3% (30/292) of participants in the phenytoin group experienced skin rash compared with 6.5% (18/276) in the placebo group. The RR of skin rash in the phenytoin group compared with placebo was 1.65 (99% CI 0.54 to 5.04; 568 participants; Analysis 1.5). Heterogeneity was low among the studies (I2 = 17%). We performed no subgroup analysis due to too few studies. The result was rated as low based on imprecision of RR estimate with confidence intervals covering both benefit and harm and serious risk of bias in one study.
1.6 Sensitivity analysis
Occurrence of early seizure: age of population
Four of the five trials that reported on early seizures had a mean or median age that was greater than 18 years (Glotzner 1983; Young 1983; Temkin 1990; Pechadre 1991). Young 2004 consisted solely of children. We ran the analysis excluding Young 2004. About 4.9% (22/453) of participants treated with an AED experienced an early seizure compared with 15% (65/432) receiving placebo. The result still favored AED treatments compared with placebo; producing a marginally lower RR compared with the original analysis in Section 1.1 (RR 0.36, 95% CI 0.21 to 0.60, I2 = 12%; Analysis 1.6) (compare with Analysis 1.1).
1.7 Sensitivity analysis
Occurrence of early seizure: study quality
Three of the five trials that examined early seizures had high risk of bias in one or more category (Glotzner 1983; Young 1983; Pechadre 1991). We reran the analysis excluding Glotzner 1983; Young 1983; and Pechadre 1991. The pooled results of the two remaining studies no longer showed a benefit of AED treatment compared with placebo (RR 0.48, 95% CI 0.11 to 2.18, 506 participants; Analysis 1.7) (Temkin 1990; Young 2004). Heterogeneity (I2 = 68%) increased compared with the original analysis (see Analysis 1.1). Differences in participant populations likely contributed to the increase in heterogeneity; participants in the Temkin 1990 trial were adults, whereas Young 2004 studied exclusively children.
1.8 Subgroup analysis
Occurrence of late seizure: type of antiepileptic drug
Four of the six trials compared a traditional AED treatment, phenytoin, with placebo for late seizures (McQueen 1983; Young 1983; Temkin 1990; Pechadre 1991). The two remaining studies treated participants with other AEDs; carbamazepine compared with placebo (Glotzner 1983), and phenobarbital compared with usual care (Manaka 1992). In subgroup analysis, 15% (59/393) of participants treated with phenytoin experienced a late seizure compared with 17.5% (63/359) receiving placebo (RR 0.83, 95% CI 0.40 to 1.70; 752 participants; Analysis 1.8). About 17.6% (22/125) of participants receiving carbamazepine or phenobarbital experienced a late seizure compared with 18.4% (28/152) in the placebo or usual care group (RR 0.96, 95% CI 0.46 to 1.99, 277 participants; Analysis 1.8). There was no statistically significant subgroup difference between the types of antiepileptic drug (P=0.78, I2 =0.0%). The subgroup RRs did not differ substantially from the primary analysis results (see Analysis 1.2).
1.9 Subgroup analysis
Occurrence of late seizure: treatment duration
Five of the six trials that examined the occurrence of late seizures had a treatment duration ranging from 12 to 24 months (Glotzner 1983; McQueen 1983; Young 1983; Temkin 1990; Manaka 1992). One trial had a treatment duration less than one year (Pechadre 1991). In subgroup analysis, for treatment duration of 12 to 24 months, 16.3% (79/484) of participants in the AED group experienced late seizures compared with 15.1% (69/459) of participants in the control groups. In the Pechadre 1991 trial, 5.88% (2/34) of participants in the AED treatment group experienced late seizures compared with 42.3% (22/52) in the control group. Although there was no statistically significant subgroup difference between different treatment durations (P=0.87, I2 =0.004%) the results show greater risk in the AED treatment group for studies with longer treatment duration (12 to 24 months) (RR 1.08, 95% CI 0.81 to 1.46, 943 participants; Analysis 1.9) while the one study with treatment duration of less than one year showed reduced risk (RR 0.14, 95% CI 0.03 to 0.55, 86 participants; Analysis 1.9) (Pechadre 1991).
1.10 Sensitivity analysis
Occurrence of late seizure: age of population
Five of the six trials that examined occurrence of late seizures included both adults and children (Glotzner 1983; McQueen 1983; Young 1983; Pechadre 1991; Manaka 1992). Removing Temkin 1990, the only study that excluded children, from the analysis did not alter the results substantially. The pooled effect remained statistically non‐significant as per the original results (RR 0.81, 95% CI 0.44 to 1.48, 706 participants; Analysis 1.10) as per the original results (see Analysis 1.2).
1.11 Sensitivity analysis
Occurrence of late seizure: comparison group
Five trials of the six trials that examined the occurrence of late seizures compared an AED with placebo (Glotzner 1983; McQueen 1983; Young 1983; Temkin 1990; Pechadre 1991). Manaka 1992 compared an AED treatment with usual care. The pooled RR, excluding Manaka 1992, remained not statistically significant (RR 0.83, 95% CI 0.48 to 1.41, 903 participants; Analysis 1.11) (see Analysis 1.2).
1.12 Sensitivity analysis
Occurrence of late seizure: study quality
Five of the six trials that examined the occurrence of late seizures had a high risk of bias in one or more bias categories (Glotzner 1983; McQueen 1983; Young 1983; Pechadre 1991; Manaka 1992). Temkin 1990 was the only trial that did not have a high risk of bias in any category. The Temkin 1990 trial favored the placebo group (RR 1.25, 95% CI 0.79 to 1.96, 323 participants; Analysis 1.18), which differs from the original analysis, which favored the AED treatment (see Analysis 1.2). However, neither comparison was statistically significant.
1.13 Subgroup analysis
All‐cause mortality: age of population
Two studies examined all‐cause mortality in children only (ages under 17 years) (Young 1983; Young 2004). The pooled proportion of children that died in the AED treatment group was 8.5% (8/71) and 22.2% (16/72) died in the placebo group (RR 0.54, 95% CI 0.25 to 1.19, 143 participants; Analysis 1.13). Temkin 1990 was the only study that exclusively enrolled participants over 17 years of age. About 23.5% (49/208) of adults treated with AED died compared with 20.1% (41/196) of adults receiving placebo. The pooled RR was 1.13 (95% CI 0.78 to 1.62, 404 participants; Analysis 1.13). The remaining two studies examined all‐cause mortality in a predominantly adult population; although children were included (Glotzner 1983;McQueen 1983). The pooled proportion that died in the AED treatment group was 20.1% (32/159) and 14.1% (22/156) died in the placebo group (RR 1.43, 95% CI 0.90 to 2.27, 315 participants; Analysis 1.13). The studies including exclusively or predominately adults showed an increased risk of mortality in the AED group compared with placebo (Glotzner 1983; McQueen 1983; Temkin 1990), while the studies including only children showed a decreased risk with treatment. The test for subgroup differences showed moderate heterogeneity (P=0.11, I2 =53.9%).
1.14 Subgroup analysis
All‐cause mortality: treatment duration
Two of the five trials examining all‐cause mortality had a short‐term treatment duration of less than one week (Young 1983; Young 2004). 9.9% (18/182) of participants treated with an AED for one week or less died compared with 15.2% (25/164) of participants in the control groups. The pooled RR for these short‐term treatments was non‐significant and favored AED treatment (RR 0.69, 95% CI 0.39 to 1.24, 346 participants; Analysis 1.14). In comparison, the three trials that used a treatment duration of a 12 months or longer had a non‐significant pooled RR that favored the control group; 22.1% (81/367) of participants in the AED group died compared with 17.9% (63/352) of participants in the control groups (RR 1.24, (95% CI 0.93 to 1.65, 719 participants; Analysis 1.14) (Glotzner 1983; McQueen 1983; Temkin 1990). The test for subgroup differences between studies of different treatment duration was statistically significant and suggested moderate heterogeneity (P=0.08, I2 =67.4%). Duration of treatment was not further divided into mid‐term and long‐term duration due to low number of studies.
1.15 Sensitivity analysis
All‐cause mortality: type of antiepileptic drug
In four of the five trials that examined mortality, participants received phenytoin in the AED group (McQueen 1983; Young 1983; Temkin 1990; Young 2004). Glotzner 1983 compared carbamazepine with placebo. Excluding Glotzner 1983, 15.6% (74/474) of participants treated with phenytoin died compared with 15.9% (70/440) of participants who received placebo (RR 0.97, 95% CI 0.65 to 1.43, 914 participants; Analysis 1.15). The results remain consistent with the original analysis (see Analysis 1.3).
1.16 Sensitivity analysis
All‐cause mortality: study quality
Three of the five trials that examined mortality had a high risk of bias in one or more bias categories (Glotzner 1983; McQueen 1983; Young 1983). We reran the analysis excluding these studies. The pooled results for the remaining studies with low/unclear risk of bias showed no statistically significant difference between treatment groups ((RR 1.00, 95% CI 0.72 to 1.41) 506 participants; Analysis 1.16) (Temkin 1990; Young 2004). The original results were also statistically non‐significant, but favored the placebo group (see Analysis 1.3).
2. Neuroprotective agent versus placebo
Only one study compared a pharmacologic agent (magnesium sulfate; MgSO4) other than an AED with a placebo (Temkin 2007).
2.1 Occurrence of early seizure
Temkin 2007 reported on the occurrence of early seizures. About 0.4% (1/250) of participants in the neuroprotective agent group experienced an early seizure compared with 0% (0/249) in the placebo group (Analysis 2.1). However, as reported in the results section of their paper, 96% of participants received phenytoin for the first week in both treatment groups. This may have resulted in a very low early seizure rate.
2.2 Occurrence of late seizure
Temkin 2007 reported on the occurrence of late seizures. About 6% (15/250) of participants treated with neuroprotective agent experienced late seizures compared with 5.6% (14/249) of participants treated with a placebo (RR 1.07, 95% CI 0.53 to 2.17, 498 participants; Analysis 2.2). There was no evidence of effect of neuroprotective agents compared with placebo on late seizures.
2.3 All‐cause mortality
Only Temkin 2007 reported mortality. About 21% (52/250) of participants died in the neuroprotective agent group compared with 14% (35/240) of participants in the control group (RR 1.20, 95% CI 0.80 to 1.81, 466 participants; Analysis 2.3).
3. Antiepileptic drugs versus other antiepileptic drugs
Two trials compared phenytoin with another AED (Temkin 1999; Szaflarski 2010). Szaflarski 2010 compared phenytoin with levetiracetam, a newly licensed AED while Temkin 1999 compared phenytoin with valproate. Treatment duration was one week in the Szaflarski 2010 trial compared with up to six months in the valproate arm of the Temkin 1999 study. The age ranges were similar in both studies and neither study showed high bias in any category. We performed no subgroup analysis due to too few studies and low evidence of heterogeneity between the two studies.
3.1 Occurrence of early seizure
Szaflarski 2010 and Temkin 1999 reported on the occurrence of early seizures and compared phenytoin with another AED. About 3.3% (5/150) of participants treated with phenytoin had an early seizure compared with 5.7% (16/281) of participants treated with another AED. The pooled results of Szaflarski 2010 and Temkin 1999 showed no statistically significant effect of phenytoin compared with another AED (RR 0.66, 95% CI 0.20 to 2.12, 558 participants; Analysis 3.1). Heterogeneity between the two studies was low (I2= 29%).
3.2 Occurrence of late seizure
Szaflarski 2010 and Temkin 1999 reported on the occurrence of late seizures and compared phenytoin with another AED drug. About 12.4% (17/137) of participants treated with phenytoin experienced late seizures compared with 16.6% (40/241) of participants treated with another AED. The pooled RR of experiencing late seizures on phenytoin compared with another AED was not statistically significant (RR 0.77, 95% CI 0.46 to 1.30, 378 participants; Analysis 3.2). Heterogeneity between the two studies was low (I2 = 0%).
3.3 All‐cause mortality
Szaflarski 2010 and Temkin 1999 reported all‐cause mortality. About 8.7% (13/150) of participants in the phenytoin group died compared with 16.4% (46/281) of participants in the other AED group. The pooled RR for mortality in the phenytoin group compared with the other AED group was 0.53 (95% CI 0.30 to 0.94; Analysis 3.3). Heterogeneity between the two studies was low (I2 = 0%). We performed no subgroup analysis due to too few studies and low evidence of heterogeneity.
Discusión
Resumen de los resultados principales
La revisión incluyó 10 ECA descritos en 12 informes, que incluían a 2326 participantes. Las intervenciones se informaron en tres categorías; FAE tradicional versus placebo o atención habitual, fenitoína versus otro tratamiento con FAE, y agente neuroprotector alternativo versus placebo o atención habitual.
Cinco estudios con 987 participantes estudiaron la crisis convulsiva temprana en participantes tratados con un FAE tradicional en comparación con placebo o atención habitual. Hubo pruebas de baja calidad de que el tratamiento con un FAE tradicional (fenitoína o carbamazepina) disminuyó el riesgo de crisis convulsiva temprana en comparación con placebo o atención habitual (CR 0,42; IC del 95%: 0,23 a 0,73; valor de p = 0,003).
El riesgo de aparición de crisis convulsivas tardías se redujo con el tratamiento con FAE en comparación con placebo o atención habitual, aunque el beneficio no fue estadísticamente significativo (CR 0,91; IC del 95%: 0,57 a 1,46; 1029 participantes). El riesgo de crisis convulsiva tardía favoreció a placebo en el único ensayo que no tuvo alto riesgo de sesgo en las diferentes categorías (CR 1,25; IC del 95%: 0,79 a 1,96; 323 participantes), aunque las pruebas del efecto se mantuvieron no significativas (Temkin 1990). Se debe proceder con cuidado al considerar este análisis de sensibilidad debido a que se basó solamente en un estudio.
No hubo diferencias significativas en la mortalidad entre los participantes del grupo de FAE y los participantes del grupo de atención habitual o placebo (CR 1,08; IC del 95%: 0,79 a 1,46; valor de p = 0,64), aunque los resultados se basaron en pruebas de muy baja calidad debido a la imprecisión y la inconsistencia en los resultados.
La revisión solamente incluyó un estudio que examinó otros agentes potencialmente neuroprotectores en comparación con placebo o atención habitual (Temkin 2007). No hubo pruebas de un efecto del tratamiento sobre las crisis convulsivas tardías (CR 1,07; IC del 95%: 0,53 a 2,17) o la mortalidad por todas las causas (CR 1,20; IC del 95%: 0,80 a 1,81) en esta comparación. No hubo eventos en el brazo placebo para el resultado crisis convulsiva temprana y hubo una tasa de crisis convulsivas tempranas del 0,4% (1/250) en el grupo de tratamiento. Sin embargo, casi todos los participantes (96%) de este estudio también recibieron fenitoína durante la primera semana posterior a la lesión. En el artículo no se proporcionaron las dosis ni los detalles sobre los niveles de tratamiento con fenitoína.
Hubo pruebas de un efecto beneficioso del tratamiento con fenitoína en comparación con otro FAE (levetiracetam o valproato). La fenitoína redujo significativamente el riesgo de mortalidad en comparación con otro FAE (CR 0,53; IC del 95%: 0,30 a 0,94). Se debe proceder con cuidado debido a que este resultado solamente se basó en dos estudios. No se observó un efecto beneficioso del tratamiento con fenitoína en comparación con otro FAE para la crisis convulsiva temprana (CR 0,66; IC del 95%: 0,20 a 2,12) o la crisis convulsiva tardía (CR 0,77; IC del 95%: 0,46 a 1,3).
Solamente dos de los ensayos incluidos informaron cualquier evento adverso grave relacionado con el tratamiento. Ambos ensayos compararon un FAE tradicional con placebo. No hubo pruebas de un aumento en el riesgo de efectos adversos en el grupo de FAE (CR 1,63; IC del 95%: 0,73 a 3,66). Tampoco hubo pruebas de un aumento en el riesgo de erupción cutánea (CR 1,65; IC del 99%: 0,54 a 5,04).
Compleción y aplicabilidad general de las pruebas
Todos los participantes incluidos en la revisión presentaban un diagnóstico de LCT moderada a grave. Los métodos de medición de la gravedad de la LCT variaron entre los estudios. La mayoría de los participantes había ingresado a centros traumatológicos o a servicios de urgencias. Los participantes se asignaron al azar y recibieron tratamiento en el transcurso de las 24 horas desde el ingreso; sin embargo, un estudio informó que la mayoría de los participantes recibió tratamiento en los 14 días posteriores a la lesión. Tres estudios permitieron la inclusión de participantes con una crisis convulsiva inmediatamente posterior a la lesión, mientras que en otros estudios este hecho fue un criterio de exclusión. Un estudio incluyó a los participantes cuando habían tenido una crisis convulsiva previa a la lesión; sin embargo, estos participantes se excluyeron de los hallazgos de los resultados de las crisis convulsivas tempranas y tardías. El informe de los resultados no fue consistente entre los estudios y solamente dos estudios informaron sobre cualquier evento adverso grave y las erupciones cutáneas. Dos estudios tampoco documentaron la mortalidad y la mayoría de los estudios no consideró el tiempo hasta la primera crisis convulsiva ni el tiempo hasta la segunda crisis convulsiva desde la primera crisis convulsiva. El mantenimiento de los niveles terapéuticos de los FAE fue un reto en muchos de los ensayos y se informó que solamente el 40% al 80% de los participantes mantuvo los niveles terapéuticos en las visitas de seguimiento. Varios estudios no realizaron el seguimiento de los participantes más allá de la duración del tratamiento, lo que limitó la capacidad para determinar si el efecto del tratamiento se mantiene una vez que se interrumpe la medicación.
Calidad de la evidencia
La calidad general de las pruebas fue variable. Todos los ensayos incluidos fueron ECA, aunque la mayoría no describió adecuadamente la asignación al azar ni señaló claramente los procesos de asignación. La mayoría realizó el cegamiento de los participantes y el personal de forma adecuada, aunque el riesgo de sesgo para el cegamiento de la evaluación de resultado se consideró incierto o alto en cinco de los diez ensayos. El riesgo de sesgo de informe selectivo también fue incierto o alto en seis ensayos. Las potenciales fuentes de sesgo en esta revisión fueron: la incapacidad para evaluar de forma adecuada si los efectos del tratamiento difirieron entre los niños y los adultos, debido a que muchos de los ensayos incluyeron a niños y no los analizaron por separado; y los diferentes protocolos de tratamiento con respecto al momento adecuado y la duración del tratamiento, la dosis de los FAE, el mantenimiento de los niveles terapéuticos de los FAE, las diferencias en la gravedad del traumatismo y los diferentes métodos para evaluar la aparición de crisis convulsivas. Debido a la alta proporción de estudios en esta revisión que se categorizaron como con riesgo incierto o alto de sesgo, los hallazgos de esta revisión se deben interpretar con cuidado. La calidad de las pruebas se calificó como baja a muy baja para todas las comparaciones de resultado según el alto riesgo de sesgo considerado previamente, así como a la incertidumbre en las estimaciones, con muchos intervalos de confianza que muestran efectos beneficiosos y perjudiciales.
Sesgos potenciales en el proceso de revisión
El Grupo de Revisión Cochrane de epilepsia llevó a cabo una búsqueda exhaustiva de todos los datos publicados, así como búsquedas manuales en la bibliografía de estudios y revisiones seleccionados. Se examinaron los informes de texto completo y dos revisores (KT y HA) extrajeron los datos y resolvieron los desacuerdos mediante discusión para minimizar el sesgo. No se pudo obtener más información de algunos de los ensayos, ya que se publicaron hace muchos años o no se pudo contactar con los autores. No fue posible hacer observaciones sobre la posibilidad de sesgo de publicación en la revisión debido al número insuficiente de estudios para analizar el sesgo de publicación en gráficos en embudo.
Acuerdos y desacuerdos con otros estudios o revisiones
Esta revisión fue diferente de la revisión original Schierhout 2001 en que incluyó un estudio que consideró otros agentes potencialmente neuroprotectores y estudios con grupos de tratamiento dual. La comparación de los FAE tradicionales con placebo ahora incluye un estudio realizado exclusivamente en niños (Young 2004), que no se incluyó en la revisión anterior. Los resultados fueron consistentes en ambas revisiones, el tratamiento con FAE tradicionales redujo el riesgo de crisis convulsiva temprana en comparación con placebo o atención habitual. Al eliminar los estudios con sesgo alto en al menos una categoría, las pruebas del efecto del tratamiento dejaron de ser significativas. Sin embargo, la heterogeneidad entre este subgrupo de estudios aumentó, posiblemente debido a las diferencias en la duración del tratamiento y la mediana de edad de los participantes.
No hubo pruebas de un efecto del tratamiento sobre la aparición de crisis convulsivas tardías o la mortalidad. Este resultado fue consistente con los resultados de la aparición de crisis convulsivas tardías y la mortalidad publicados en la revisión anterior (Schierhout 2001).
Study flow diagram.

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
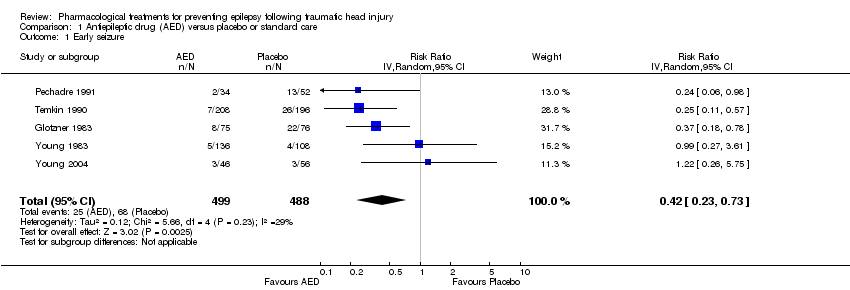
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 1 Early seizure.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 2 Late seizure.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 3 All‐cause mortality.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 4 Any serious event.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 5 Skin rash.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 6 Sensitivity analysis ‐ early seizure: age of population.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 7 Sensitivity analysis ‐ early seizure: study quality.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 8 Subgroup: late seizure: type of AED.
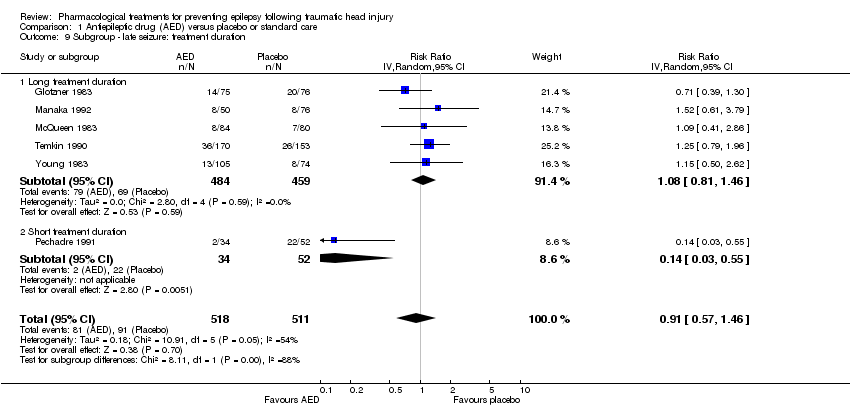
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 9 Subgroup ‐ late seizure: treatment duration.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 10 Sensitivity analysis ‐ late seizure: age of population.
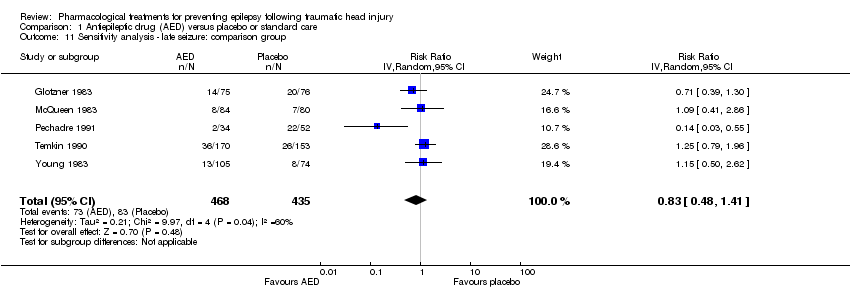
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 11 Sensitivity analysis ‐ late seizure: comparison group.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 12 Sensitivity analysis ‐ late seizure: study quality.
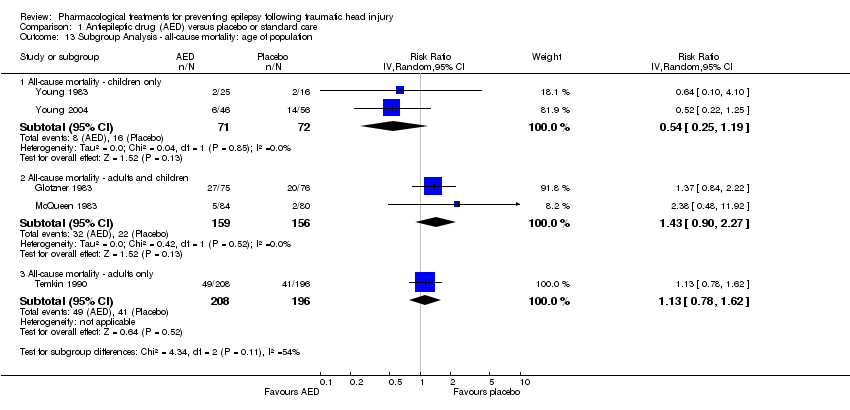
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 13 Subgroup Analysis ‐ all‐cause mortality: age of population.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 14 Subgroup analysis ‐ all‐cause mortality: treatment duration.
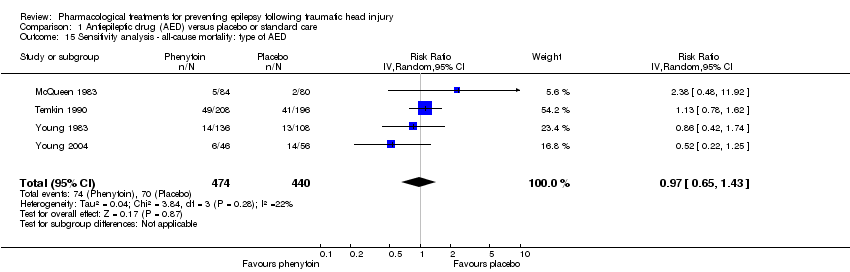
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 15 Sensitivity analysis ‐ all‐cause mortality: type of AED.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 16 Sensitivity analysis ‐ all‐cause mortality: study quality.
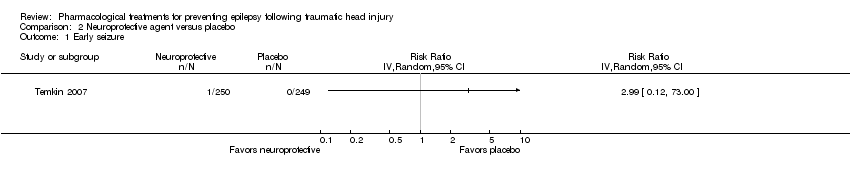
Comparison 2 Neuroprotective agent versus placebo, Outcome 1 Early seizure.

Comparison 2 Neuroprotective agent versus placebo, Outcome 2 Late seizure.

Comparison 2 Neuroprotective agent versus placebo, Outcome 3 All‐cause mortality.
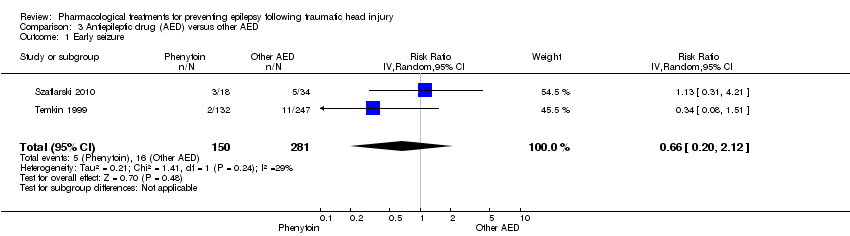
Comparison 3 Antiepileptic drug (AED) versus other AED, Outcome 1 Early seizure.
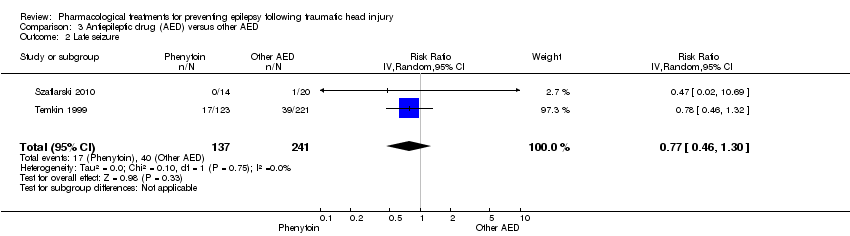
Comparison 3 Antiepileptic drug (AED) versus other AED, Outcome 2 Late seizure.

Comparison 3 Antiepileptic drug (AED) versus other AED, Outcome 3 All‐cause mortality.
| Antiepileptic drugs compared with placebo or standard care for people at risk of epilepsy following traumatic head injury | ||||||
| Patient or population: people with traumatic head injuries | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or standard care | Antiepilepticdrugs | |||||
| Early seizures | 139 per 1000 | 59 per 1000 | RR 0.42 | 987 | ⊕⊕⊝⊝ | Sensitivity analysis by quality of the study shows that RR for early seizures in low/unclear risk studies was no longer significant (RR 0.59, 95% CI 0.20 and 1.73) |
| Late seizures | 178 per 1000 | 162 per 1000 | RR 0.91 | 1029 | ⊕⊝⊝⊝ | RR of late seizures remained insignificant regardless of type of antiepileptic drug, treatment duration, age of population or quality of the study |
| All‐cause mortality | 174 per 1000 | 188 per 1000 | RR 1.08 | 1065 | ⊕⊝⊝⊝ | RR for all‐cause mortality remained insignificant regardless of treatment duration, age of population or quality of the study |
| Any serious adverse event of treatment count of events Follow up: 12 months | 94 per 1000 | 154 per 1000 (69 to 345) | RR 1.63 (0.73 to 3.66) | 568 (2 studies) | ⊕⊕⊝⊝ | |
| Time to first seizure from randomization | See comment | See comment | Not estimable | 0 | See comment | No study reported time to first seizure in an interpretable way |
| *The basis for the assumed risk is the event rate in the control (placebo or standard care) group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias: Two studies included in this outcome had instances of high risk of bias assessment. The remaining studies had a mix of low and unclear risk of bias. 5 Downgraded one level due to imprecision of results: wide 95% CI that includes both considerable harm and benefit. 6 Downgraded one level due to serious risk of bias: selection bias was likely in both trials | ||||||
| Neuroprotective agents compared with placebo for people at risk of epilepsy following traumatic head injury | ||||||
| Patient or population: people with traumatic head injuries | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Neuroprotective agents | |||||
| Early seizure Count of events Follow‐up: 7 days | 0 per 1000 | 0 per 1000 | RR 2.99 (0.12 to 73.00) | 499 | ⊕⊕⊝⊝ | No events occurred in the control group therefore corresponding risk is also zero |
| Late seizure Count of events Follow‐up: 6 months | 56 per 1000 | 60 per 1000 | RR 1.07 (0.53 to 2.17) | 498 | ⊕⊕⊕⊕ | |
| All‐cause mortality Follow‐up: 6 months | 150 per 1000 | 180 per 1000 | RR 1.20 (0.80 to 1.81) | 466 | ⊕⊕⊕⊕ | |
| Any serious adverse event of treatment | See comment | See comment | Not estimable | 0 | See comment | No study reported adverse event data |
| Time to first seizure from randomization | See comment | See comment | Not estimable | 0 | See comment | No study reported time to first seizure in an interpretable way |
| *The basis for the assumed risk is the event rate in the control (placebo or standard care) group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to risk of bias: As reported in the study paper, 96% of participants received phenytoin for the first week in both treatment groups. This may have resulted in a very low early seizure rate 2 Downgraded one level due to imprecision of results: wide 95% CI that includes both considerable harm and benefit. | ||||||
| Anti‐epileptic drugs compared to other anti‐epileptic drugs for people at risk of epilepsy following traumatic head injury | ||||||
| Patient or population: people with traumatic head injuries | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other AEDs | Phenytoin | |||||
| Early seizure Counts of events Follow up: 7 days | 57 per 1000 | 38 per 1000 | RR 0.66 (0.20 to 2.12) | 431 | ⊕⊕⊝⊝ | |
| Late seizure Counts of events Follow up: 6 months to 2 years | 166 per 1000 | 128 per 1000 | RR 0.77 (0.46 to 1.30) | 378 | ⊕⊕⊕⊝ | |
| All‐cause mortality Follow up: 6 months to 2 years | 164 per 1000 | 87 per 1000 | RR 0.53 (0.30 to 94) | 431 | ⊕⊕⊕⊝ | |
| Any serious adverse event of treatment | See comment | See comment | Not estimable | 0 | See comment | No study reported adverse event data |
| Time to first seizure from randomization | See comment | See comment | Not estimable | 0 | See comment | No study reported time to first seizure in an interpretable way |
| *The basis for the assumed risk is the event rate in the control (placebo or standard care) group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to risk of bias; unclear information reported in one study regarding study design (randomisation and blinding) and loss to follow up from the study 2 Downgraded one level due to imprecision of results: wide 95% CI that includes both considerable harm and benefit. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Early seizure Show forest plot | 5 | 987 | Risk Ratio (IV, Random, 95% CI) | 0.42 [0.23, 0.73] |
| 2 Late seizure Show forest plot | 6 | 1029 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.57, 1.46] |
| 3 All‐cause mortality Show forest plot | 5 | 1065 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.79, 1.46] |
| 4 Any serious event Show forest plot | 2 | 568 | Risk Ratio (IV, Random, 95% CI) | 1.63 [0.73, 3.66] |
| 5 Skin rash Show forest plot | 2 | 568 | Risk Ratio (IV, Random, 99% CI) | 1.65 [0.54, 5.04] |
| 6 Sensitivity analysis ‐ early seizure: age of population Show forest plot | 4 | 885 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.21, 0.60] |
| 7 Sensitivity analysis ‐ early seizure: study quality Show forest plot | 2 | 506 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.11, 2.18] |
| 8 Subgroup: late seizure: type of AED Show forest plot | 6 | 1029 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.57, 1.46] |
| 8.1 Late seizure ‐ phenytoin | 4 | 752 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.40, 1.70] |
| 8.2 Late seizure ‐ other AED | 2 | 277 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.46, 1.99] |
| 9 Subgroup ‐ late seizure: treatment duration Show forest plot | 6 | 1029 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.57, 1.46] |
| 9.1 Long treatment duration | 5 | 943 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.81, 1.46] |
| 9.2 Short treatment duration | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 0.14 [0.03, 0.55] |
| 10 Sensitivity analysis ‐ late seizure: age of population Show forest plot | 5 | 706 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.44, 1.48] |
| 11 Sensitivity analysis ‐ late seizure: comparison group Show forest plot | 5 | 903 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.48, 1.41] |
| 12 Sensitivity analysis ‐ late seizure: study quality Show forest plot | 1 | 323 | Risk Ratio (IV, Fixed, 95% CI) | 1.25 [0.79, 1.96] |
| 13 Subgroup Analysis ‐ all‐cause mortality: age of population Show forest plot | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 13.1 All‐cause mortality ‐ children only | 2 | 143 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.25, 1.19] |
| 13.2 All‐cause mortality ‐ adults and children | 2 | 315 | Risk Ratio (IV, Random, 95% CI) | 1.43 [0.90, 2.27] |
| 13.3 All‐cause mortality ‐ adults only | 1 | 404 | Risk Ratio (IV, Random, 95% CI) | 1.13 [0.78, 1.62] |
| 14 Subgroup analysis ‐ all‐cause mortality: treatment duration Show forest plot | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 14.1 All‐cause mortality ‐ short‐term treatment duration | 2 | 346 | Risk Ratio (IV, Random, 95% CI) | 0.69 [0.39, 1.24] |
| 14.2 All‐cause mortality ‐ long‐term treatment duration | 3 | 719 | Risk Ratio (IV, Random, 95% CI) | 1.24 [0.93, 1.65] |
| 15 Sensitivity analysis ‐ all‐cause mortality: type of AED Show forest plot | 4 | 914 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.65, 1.43] |
| 16 Sensitivity analysis ‐ all‐cause mortality: study quality Show forest plot | 2 | 506 | Risk Ratio (IV, Fixed, 95% CI) | 1.00 [0.72, 1.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Early seizure Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2 Late seizure Show forest plot | 1 | 498 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.53, 2.17] |
| 3 All‐cause mortality Show forest plot | 1 | 466 | Risk Ratio (IV, Random, 95% CI) | 1.2 [0.80, 1.81] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Early seizure Show forest plot | 2 | 431 | Risk Ratio (IV, Random, 95% CI) | 0.66 [0.20, 2.12] |
| 2 Late seizure Show forest plot | 2 | 378 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.46, 1.30] |
| 3 All‐cause mortality Show forest plot | 2 | 431 | Risk Ratio (IV, Random, 95% CI) | 0.53 [0.30, 0.94] |

