Intervenciones para la prevención de la erisipela y celulitis recurrentes
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | A randomised controlled, open‐label, parallel‐group trial | |
| Participants | 1. Setting: the trial recruited participants admitted to the infectious diseases service of the central university hospital in Monastir, Tunisia 3. Sex (men/women): 15/29 (14 participants were lost to follow‐up) 4. Mean age ± SD: 46.2 ± 19.4 5. Area of body involved: leg 6. Number of episodes of cellulitis prior to intervention: NR | |
| Interventions | Study groups:
Duration of treatment: between 1 month and 38 months (average = 11.6 months) Follow‐up: during treatment phase‐ every 3 months; after treatment phase‐ NF | |
| Outcomes | 1. The number of participants with repeat episodes of cellulitis 2. The number of repeat episodes of cellulitis 3. Costs | |
| Notes | Criteria for diagnosis of cellulitis: fever + signs of local inflammation confirmed by a single infectious diseases specialist Funding source and Declaration of interest: NC and NR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A la sortie du service, un tirage au sort est effactué afin de classer le patient dans l'un des 2 groupes suivants". Comment: Randomisation was done by drawing lots |
| Allocation concealment (selection bias) | Low risk | The author reported using sealed enveloped in a separate email correspondence (Table 2) |
| Blinding of participants and personnel (performance bias) | High risk | This was an open‐label study |
| Blinding of outcome assessment (detection bias) | High risk | This was an open‐label study |
| Incomplete outcome data (attrition bias) | High risk | 76% of participants were followed up (75% of the intervention group‐ 18/24, and 76% of the control group‐ 26/34) with low number of participants and events and 'per protocol' analysis (Table 1) |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information |
| Similarity of groups at baseline (baseline imbalance bias) | Low risk | Quote: "Les deux groupes sont statistiquement comparables pour les critères sus‐cités (tableau I)". Comment: Baseline characteristics were reported and balanced |
| Early termination (early stopping bias) | Unclear risk | Termination criteria or stopping rule were not reported |
| Other bias | Low risk | No other sources of potential bias were found |
| Methods | A randomised, double‐blind, placebo‐controlled parallel‐group trial | |
| Participants | 1. Setting: participants with lymphoedema following mastectomy admitted to Wittlinger's therapy centre in Walchsee, Austria (private rehabilitation clinic) 3. Sex (men/women): presumably all women 4. Mean age: 60.5 5. Area of body involved: upper limb 6. Number of episodes of cellulitis prior to intervention: ≥ 4 | |
| Interventions | Study groups:
Concomitant treatment: 3 weeks of inpatient care (Table 1) of congestion relief for both groups including daily treatment with manual lymph drainage; bandaging; exercise; skin care and high voltage therapy Duration of treatment:15 weeks: 3 weeks of intensive congestion relief treatment and 3 months of oral solution Follow‐up: during intensive treatment phase‐ inpatient care follow‐up, afterwards‐ NR | |
| Outcomes | 1.The number of participants with repeat episodes of cellulitis 2. Blood selenium levels before and after treatment | |
| Notes | 1. Criteria for diagnosis of cellulitis: prior to study enrolment diagnosis was made in general and university hospitals; after enrolment diagnosis was based on clinical examination and blood test markers of inflammation (erythrocyte sedimentation rate and CRP) but exact criteria are NR 2. No details reported on high voltage therapy Funding source and Declaration of interest: NR but on further examination we confirmed industrial sponsorship (Biosyn Arzneimittel GmbH ‐ Biosyn 2015, also in Table 2) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The paper did not detail the randomisation process |
| Allocation concealment (selection bias) | Unclear risk | The paper did not provide details |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "double‐blind study". Comment: the paper did not provide details on randomisation process, including similarity of treatment characteristics (possible different taste or colour of selenium solution versus physiological salt solution) or allocation schedule control (breaking of the code for analysis or for medical reasons) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "double‐blind study" Comment: the investigator did not identify those blinded in the trial or other necessary data (specified above) to allow judgement of blinding of the participants, care‐givers, outcome assessors or others |
| Incomplete outcome data (attrition bias) | High risk | Quote: "During the treatment, some of the patients were excluded from the study since they did not meet the criteria for study inclusion, namely, too short period of stay". Comment: 3 participants were omitted from analyses. The study did not specify exclusion criteria nor the group to which these participants belonged or the reason for their short period of stay in the trial |
| Selective reporting (reporting bias) | High risk | The study report failed to include results for outcomes that would be expected to have been reported for such a study, such as number of episodes of cellulitis, severity of lymphoedema, adverse events. In addition, the investigator reported measurements of selenium blood levels, which had not been proposed prior to the results |
| Similarity of groups at baseline (baseline imbalance bias) | Unclear risk | The investigator did not report on baseline differences between study groups including previous treatment with radiation or other oncological treatment |
| Early termination (early stopping bias) | Unclear risk | The trial ended after 15 weeks but the investigator did not prespecify termination criteria nor did he explain the study's duration |
| Other bias | High risk | 1. Criteria for the diagnosis of cellulitis are unclear, especially in the ambulatory (Table 1) phase of the trial (this might cause differences in diagnosis of cellulitis between the intensive care phase and the ambulatory phase) 2.The investigator did not answer queries (sent by emails, post and professional website ‐ drkasseroller.at (accessed February 2014)) |
| Methods | A randomised controlled, open‐label, parallel‐group trial | |
| Participants | 1. Setting: the trial was conducted in an outpatient (Table 1) setting in northern Israel. The recruitment process of participants was NR 3. Sex (men/women): 14/18 (8 participants were lost to follow‐up and their details are NR) 4. Mean age (range): treatment group‐ 63.2 (42 ‐ 75), control group‐ 65.5 (32 ‐ 75) 5. Area of body involved: leg. 2 participants from the control group suffered upper extremity infections 6. Number of episodes of cellulitis prior to intervention: ≥ 2 | |
| Interventions | Study groups:
Concomitant treatment: local antifungal treatment for tinea pedis Duration of treatment: 18 months Follow‐up:during treatment phase‐monthly, after treatment phase‐ NR | |
| Outcomes | 1. The number of participants with repeat episodes of cellulitis 2. The number of repeat episodes of cellulitis 3. The number of participants requiring hospitalisation 4. The number of adverse drug reactions | |
| Notes | Criteria for diagnosis of cellulitis: NR. Funding source and Declaration of interest: NR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The paper did not provide details |
| Allocation concealment (selection bias) | Unclear risk | The paper did not provide details |
| Blinding of participants and personnel (performance bias) | High risk | This was an open‐label study |
| Blinding of outcome assessment (detection bias) | High risk | This was an open‐label study |
| Incomplete outcome data (attrition bias) | High risk | 8 participants were lost to follow‐up (20% of participants in the study) and the paper did not detail to which groups they were assigned; we conducted a 'per protocol' analysis |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information |
| Similarity of groups at baseline (baseline imbalance bias) | Low risk | Baseline characteristics were reported and balanced (Table 1 in the article). |
| Early termination (early stopping bias) | High risk | Quote: "After I8 months' follow‐up the differences between the two groups were dramatic, and led us to conclude the study" Comment: the study was terminated based on results and did not report sample size calculation, formal interim analysis or a formal stopping rule |
| Other bias | Low risk | No other sources of potential bias were found |
| Methods | A randomised controlled, open‐label, parallel‐group trial | |
| Participants | 1. Setting: the trial recruited participants admitted to the infectious diseases department of Roslagstull hospital in Stockholm, Sweden 3. Sex (men/women): 20/20 4. Mean age (range): treatment group‐ 67.5 (36 ‐ 87), control group‐ 62.6 (25 ‐ 84) 5. Area of body involved: leg. 1 participant from the control group suffered upper limb lymphoedema after having a mastectomy 6. Number of episodes of cellulitis prior to intervention: ≥ 2 | |
| Interventions | Study groups:
5 participants that were allergic to penicillin received oral erythromycin 250 mg X 2/d; 250 mg + 500 mg/d and 500 mg X 2/d for the corresponding weight groups
Concomitant treatment:local skin care and compression stockings or elastic bandages Duration of treatment (mean (range), days) ‐ treatment group‐ 443 (50 ‐ 1047), control group‐ 436 (25 ‐ 84) Follow‐up: during treatment phase ‐ every 3 months, after treatment phase ‐ NF | |
| Outcomes | 1. The number of participants with repeat episodes of cellulitis 2. The number of participants with adverse drug reactions requiring the interruption of treatment and other adverse events of interest (changes in blood cell count, liver enzyme disturbances, gastrointestinal symptoms, e.g. nausea, diarrhoea and rash) 3. Colonisation with streptococci and staphylococci (from blood, skin, nasopharynx and throat cultures) | |
| Notes | Criteria for diagnosis of cellulitis:a febrile infection of acute onset with a sharply demarcated, warm, indurated and painful erythema (Table 1) accompanied by a temperature of at least 38° C. The diagnosis was made by 2 infectious diseases specialists Funding source and Declaration of interest: NC and NR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators described using stratified block randomisation (Table 1) in a separate email correspondence (Table 2) |
| Allocation concealment (selection bias) | Low risk | Quote: "The patients were randomly assigned to treatment or control groups using sealed envelopes". Comment: investigators mentioned in correspondence that envelopes were sequentially numbered |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "This was an open study" Comment: trial was not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "This was an open study" Comment: trial was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | 2 participants stopped treatment due to adverse drug reactions (95% follow‐up) and ITT analysis was performed |
| Selective reporting (reporting bias) | Low risk | The publication reported findings on all outcomes listed in the Methods section |
| Similarity of groups at baseline (baseline imbalance bias) | Low risk | Quote: "No major differences concerning predisposing factors were found between the groups". Comment: Baseline characteristics were reported and balanced |
| Early termination (early stopping bias) | Unclear risk | The trial stopped after a mean time of 14.4 months and the paper did not report sample size calculation, a formal stopping rule or results of an interim analysis (stated to be conducted every 6 months for at least 20 participants followed up for a minimum of 1 year) |
| Other bias | High risk | Randomisation included fixed‐size blocks (of 10) and the trial was open, thus allowing predictability |
| Methods | A multicentre, randomised, double‐blind, placebo‐controlled trial | |
| Participants | 1. Setting: the trial recruited participants from 20 hospitals in the UK and Ireland, either in hospital setting or retrospectively via medical coding in records or poster adverts 3. Sex (men/women): 42/81 4. Mean age (range): treatment group‐ 56.7 (18 ‐ 81), placebo group‐ 59.5 (23 ‐ 84) 5. Area of body involved: leg 6. Number of episodes of cellulitis prior to intervention: ≥ 1 | |
| Interventions | Study groups:
Duration of treatment: 6 months Follow‐up: on‐prophylaxis phase‐ telephone calls from co‐ordinating centre every 3 months , post‐prophylaxis phase‐ phone calls at 6‐month intervals. In addition participants were asked to complete a study diary and to immediately inform the trial staff of a repeat episode | |
| Outcomes | Primary outcomes: 1. Time to next episode of cellulitis Secondary outcomes: 1. The proportion of participants with repeat episodes of cellulitis at the end of the treatment phase, and at the end of the non‐intervention follow‐up phase 2. The number of repeat episodes of cellulitis 4. The number of nights in hospital for the treatment of repeat episodes of cellulitis 5. The number of adverse drug reactions and/or adverse events of interest (death, nausea, diarrhoea, thrush, rash) 6. Cost effectiveness 7. Predictors of response model to explore the impact of known risk factors in predicting the efficacy of prophylaxis (stated to be conducted only if a significant treatment effect was found) | |
| Notes | 1. Criteria for diagnosis of cellulitis: ‐ Criteria for an episode of cellulitis for study eligibility (index episode): a confirmed diagnosis of cellulitis by the recruiting dermatologist; if the patient was not seen by the recruiting clinician, validation of diagnosis was sought from the medical records and interview with the patient. In this case specific criteria were required consistent with clinical signs and symptoms of cellulitis (specified in the report). Any doubt over the certainty of the diagnosis was grounds for exclusion ‐ Criteria for a repeat episode of cellulitis during treatment or follow‐up phases: next episode of cellulitis in either leg that had been reported by the participant and confirmed by a medical practitioner (sensitivity analysis was performed for patient‐reported cases that required antibiotic treatment and were not confirmed as stated) 2. The paper reported good and balanced adherence in both groups: "From self‐reported tablet counts, 97 (79%) patients fully adhered to treatment, defined as at least 75% of tablets taken.This was similar across treatment groups". Funding source and Declaration of interest: NC (The BUPA Health Foundation) and no conflicts of interests. The Thomas 2012 and Thomas 2013 report on 2 studies that were led by the same group of researchers under the title of: Prophylactic Antibiotics for the Treatment of Cellulitis at Home ‐ The PATCH trials (The PATCH II and PATCH I studies, respectively). Similar study designs were reported for both trials including randomisation process, allocation, blinding, definitions of outcomes, monitoring and analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants were randomised by staff at the coordinating centre using a web‐based randomisation service provided by the Clinical Trials Unit (CTU)...". Comment: investigators used computer‐generated random list |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment allocations were concealed from all members of the trial team". Comments: central block randomisation was conducted with varying block sizes |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Participants and all members of the study team were blinded to treatment allocation throughout the trial, and analysis was performed prior to breaking of the randomization code...Although the treatments were packaged in an identical way, and the placebo tablets were of the same size and shape as penicillin V, the tablets were not identical due to difficulties in obtaining a matched placebo product. Nevertheless, there was a low risk of unblinding...". Comment: The trial included placebo, randomisation list was held by the CTU, breaking of the allocation code was allowed according to decisions by the data monitoring committee and as prespecified in the protocol of the trial |
| Blinding of outcome assessment (detection bias) | Low risk | As detailed for blinding of the participants and personnel: there was blinding, and it was unlikely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Of the 123 participants randomized, 20 (16%) participants (11 penicillin V and nine placebo) did not reach the end of the study... Participants in both groups had a similar study time experience, and approximately 80% had at least 2 years of follow‐up". Comment: most of participants completed follow‐up (84%); their number is balanced between groups; the reasons for withdrawal from study are probably not related to treatment or outcome;and ITT was performed |
| Selective reporting (reporting bias) | Low risk | Changes to outcomes, as prespecified in the protocol, were explained. Other outcomes were reported as mentioned in the protocol, that had been registered and available online (via ongoing trials registries and the trial website ‐Thomas 2012) |
| Similarity of groups at baseline (baseline imbalance bias) | Low risk | Quote:"...these stratification factors and other baseline variables were broadly similar across the two treatment groups". Comment: Baseline characteristics were reported and balanced |
| Early termination (early stopping bias) | Low risk | Quote:"...the identification of suitable participants was much slower than anticipated, and recruitment was therefore stopped after 2 years due to funding limitations. The possible reasons for this failure to recruit have been reported elsewhere" (Thomas 2010). Comment: Sample size calculation was stated (a sample of 400 participants) and the goal of recruitment was not achieved but carefully examined. Nevertheless, the study ended according to the protocol (see Thomas 2012) |
| Other bias | Low risk | No other sources of potential bias were found |
| Methods | A multicentre, randomised, double‐blind, placebo‐controlled trial | |
| Participants | 1. Setting:the trial recruited participants from 28 hospitals in the UK and Ireland, either in hospital setting or retrospectively via medical coding in records or poster adverts 3. Sex (men/women):109/165 4. Mean age ± SD: treatment group ‐ 58.1 ± 12.6, placebo group ‐ 57.4 ± 14.4 5. Area of body involved: leg 6. Number of episodes of cellulitis prior to intervention: ≥ 2 | |
| Interventions | Study groups:
Concomitant treatment: "normal clinical practice" for predisposing factors such as tinea pedis Duration of treatment‐ 12 months Follow‐up:on‐prophylaxis phase‐ telephone calls from co‐ordinating centre every 3 months, post‐prophylaxis phase‐ phone calls at 6‐month intervals. In addition participants were asked to complete a study diary and to immediately inform the trial staff on a repeat episode | |
| Outcomes | Primary outcomes: 1. Time to next episode of cellulitis Secondary outcomes: 1. The proportion of participants with repeat episodes of cellulitis at the end of the treatment phase, and at the end of the non‐intervention follow‐up phase 2. The number of repeat episodes of cellulitis 4. The number of nights in hospital for the treatment of repeat episodes of cellulitis 5. The number of adverse drug reactions and/or adverse events of interest (death, nausea, diarrhoea, thrush, rash, severe skin reactions, sepsis, and renal failure) 6. Cost effectiveness 7. Predictors of response model to explore the impact of known risk factors in predicting the efficacy of prophylaxis | |
| Notes | 1. Criteria for diagnosis of cellulitis: ‐ Criteria for an episode of cellulitis for study eligibility (index episode):a confirmed diagnosis of cellulitis by the recruiting dermatologist; if the patient was not seen by the recruiting clinician, validation of diagnosis was sought from the medical records and interview with the patient. In this case specific criteria were required consistent with clinical signs and symptoms of cellulitis (specified in the report). Any doubt over the certainty of the diagnosis was grounds for exclusion ‐ Criteria for a repeat episode of cellulitis during treatment or follow‐up phases: next episode of cellulitis in either leg that had been reported by the participant and confirmed by a medical practitioner (sensitivity analysis was performed for patient‐reported cases that required antibiotic treatment and were not confirmed as stated). 2. The paper reported good and balanced adherence in both groups: "A total of 214 participants (78%) reported taking at least 75% of the study tablets; the proportion of patients who reported taking at least 75% of the tablets was similar in the two groups". Funding source and Declaration of interest: NC (Action Medical Research‐ medical research charity) and no conflicts of interests The Thomas 2012 and Thomas 2013 report on 2 studies that were led by the same group of researchers under the title of: Prophylactic Antibiotics for the Treatment of Cellulitis at Home ‐ The PATCH trials (The PATCH II and PATCH I studies, respectively). Similar study designs were reported for both trials including randomisation process, allocation, blinding, definitions of outcomes, monitoring and analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The coordinating center randomly assigned the participants with the use of the Nottingham Clinical Trials Unit (NCTU) Web‐based randomization service". Comment: Investigators used computer‐generated random list |
| Allocation concealment (selection bias) | Low risk | Quote: "The computer‐generated randomization list was produced before the start of recruitment, with the use of randomly varying block sizes, and was held by the NCTU". Comment: Central block randomisation was conducted with varying block sizes |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Participants and all members of the study team were unaware of the treatment assignments throughout the trial, and the analysis was performed before the breaking of the randomization code". Comment: The trial included placebo, randomisation list was held by the CTU, breaking of the allocation code was allowed according to decisions by the data monitoring committee and as prespecified in the protocol of the trial |
| Blinding of outcome assessment (detection bias) | Low risk | As detailed for blinding of the participants and personnel: there was blinding, and it was unlikely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "A total of 247 patients (90%) underwent at least 18 months of follow‐up (median, 25)" Comment: attrition was low |
| Selective reporting (reporting bias) | Low risk | All the outcomes prespecified in the protocol were reported and any changes to its plan were explained (see Thomas 2013) |
| Similarity of groups at baseline (baseline imbalance bias) | Low risk | Quote: "The baseline characteristics of the participants were well balanced between the groups". Comment: Baseline characteristics were reported and balanced |
| Early termination (early stopping bias) | Low risk | Sample size calculation was stated (a sample of 260 participants), the goal of recruitment was achieved (274 participants randomised), and the study did not terminate prematurely |
| Other bias | Low risk | No other sources of potential bias were found. |
Abbreviations:
d ‐ Day
g ‐ Microgram
ITT ‐ Intention‐to‐treat (Table 1)
NC ‐ Non‐commercial
NF ‐ No follow‐up
NR ‐ Not reported
SD ‐ Standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| This was a retrospective cohort study (Table 1) | |
| Participants were children (ages 1 to 14) | |
| The study mainly investigated recurrent purulent abscesses in children | |
| This was a retrospective cohort study | |
| This study investigated prophylaxis for recurrent skin abscesses | |
| Participants were children (ages 2 to 5) | |
| This study investigated prophylaxis for recurrent furunculosis and folliculitis (Table 1) | |
| This was a non‐randomised study |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Interventional study, exact methods are unclear |
| Participants | 66 participants with recurrent erysipelas, probably from Russia |
| Interventions | Study groups:
|
| Outcomes | Not reported |
| Notes |
|
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of cellulitis Show forest plot | 5 | 513 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.13, 0.72] |
| Analysis 1.1 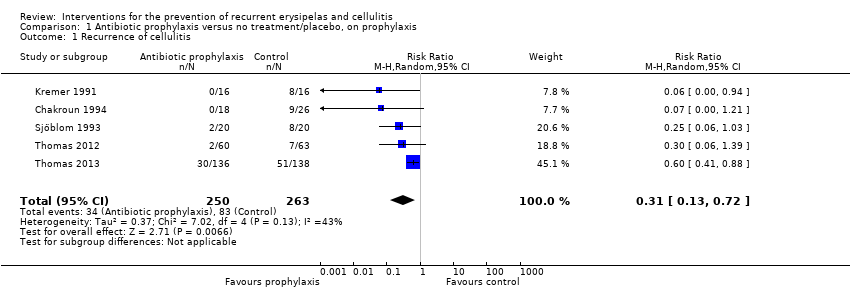 Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 1 Recurrence of cellulitis. | ||||
| 2 Incidence rate of recurrence of cellulitis Show forest plot | 4 | 4375 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.22, 0.89] |
| Analysis 1.2  Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 2 Incidence rate of recurrence of cellulitis. | ||||
| 3 Time to next episode of cellulitis Show forest plot | 3 | Hazard Ratio (Random, 95% CI) | 0.51 [0.34, 0.78] | |
| Analysis 1.3 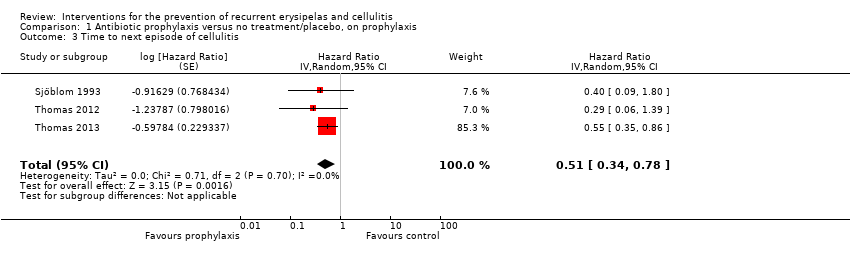 Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 3 Time to next episode of cellulitis. | ||||
| 4 Hospitalisation Show forest plot | 3 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.37, 1.57] |
| Analysis 1.4  Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 4 Hospitalisation. | ||||
| 5 Any adverse reactions Show forest plot | 4 | 469 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.58, 1.30] |
| Analysis 1.5  Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 5 Any adverse reactions. | ||||
| 5.1 Penicillin | 3 | 437 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.60, 1.10] |
| 5.2 Erythromycin | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.39, 125.44] |
| 6 Mortality Show forest plot | 3 | 437 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.32, 3.91] |
| Analysis 1.6  Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 6 Mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of cellulitis Show forest plot | 2 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.59, 1.31] |
| Analysis 2.1  Comparison 2 Antibiotic prophylaxis versus no treatment/placebo, post‐prophylaxis, Outcome 1 Recurrence of cellulitis. | ||||
| 2 Incidence rate of recurrence of cellulitis Show forest plot | 2 | 4566 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.65, 1.36] |
| Analysis 2.2 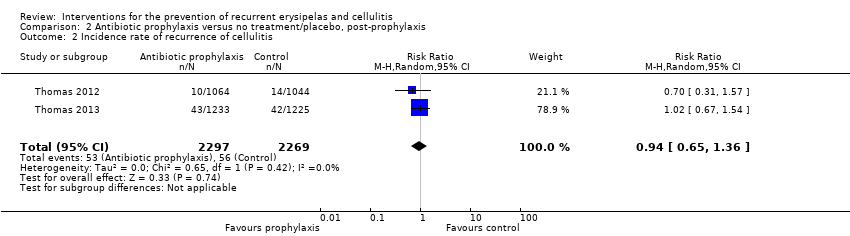 Comparison 2 Antibiotic prophylaxis versus no treatment/placebo, post‐prophylaxis, Outcome 2 Incidence rate of recurrence of cellulitis. | ||||
| 3 Time to next episode of cellulitis Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 0.78 [0.39, 1.56] | |
| Analysis 2.3  Comparison 2 Antibiotic prophylaxis versus no treatment/placebo, post‐prophylaxis, Outcome 3 Time to next episode of cellulitis. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of cellulitis Show forest plot | 2 | 397 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.59, 0.95] |
| Analysis 3.1 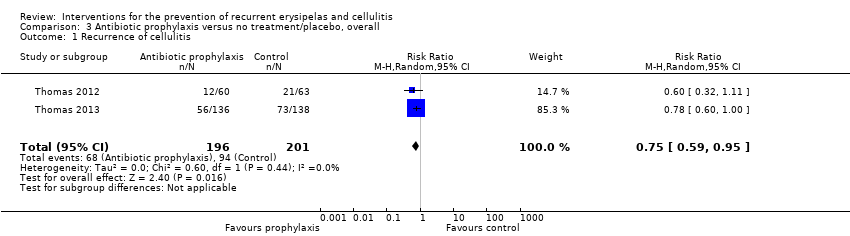 Comparison 3 Antibiotic prophylaxis versus no treatment/placebo, overall, Outcome 1 Recurrence of cellulitis. | ||||
| 2 Incidence rate of recurrence of cellulitis Show forest plot | 2 | 7854 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.56, 0.85] |
| Analysis 3.2 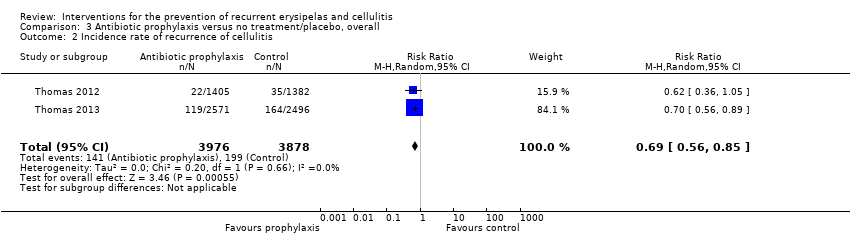 Comparison 3 Antibiotic prophylaxis versus no treatment/placebo, overall, Outcome 2 Incidence rate of recurrence of cellulitis. | ||||

Study selection flow diagram.
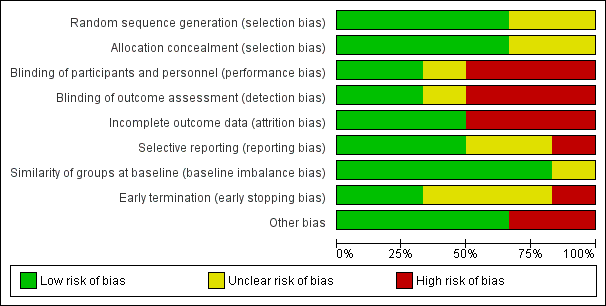
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 1 Recurrence of cellulitis.
Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 2 Incidence rate of recurrence of cellulitis.

Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 3 Time to next episode of cellulitis.
Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 4 Hospitalisation.

Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 5 Any adverse reactions.

Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 6 Mortality.

Comparison 2 Antibiotic prophylaxis versus no treatment/placebo, post‐prophylaxis, Outcome 1 Recurrence of cellulitis.

Comparison 2 Antibiotic prophylaxis versus no treatment/placebo, post‐prophylaxis, Outcome 2 Incidence rate of recurrence of cellulitis.
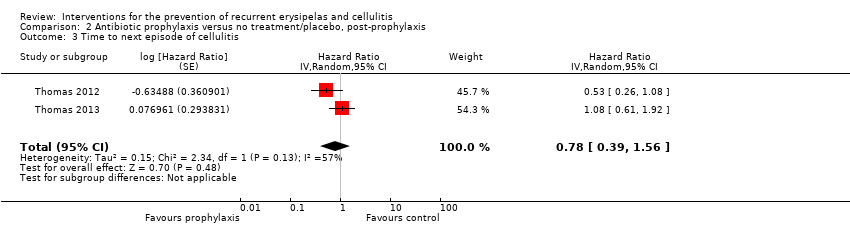
Comparison 2 Antibiotic prophylaxis versus no treatment/placebo, post‐prophylaxis, Outcome 3 Time to next episode of cellulitis.

Comparison 3 Antibiotic prophylaxis versus no treatment/placebo, overall, Outcome 1 Recurrence of cellulitis.

Comparison 3 Antibiotic prophylaxis versus no treatment/placebo, overall, Outcome 2 Incidence rate of recurrence of cellulitis.
| Antibiotic prophylaxis compared to no treatment/placebo for the prevention of recurrent erysipelas and cellulitis ‐ on prophylaxis | ||||||
| Patient or population: People with recurrent erysipelas or cellulitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| no treatment/placebo | Antibiotic prophylaxis | |||||
| Recurrence of cellulitis | Study population | RR 0.31 | 513 | ⊕⊕⊕⊝ | Number needed to treat for 1 additional beneficial outcome (NNTB) is 6 | |
| 316 per 1000 | 98 per 1000 | |||||
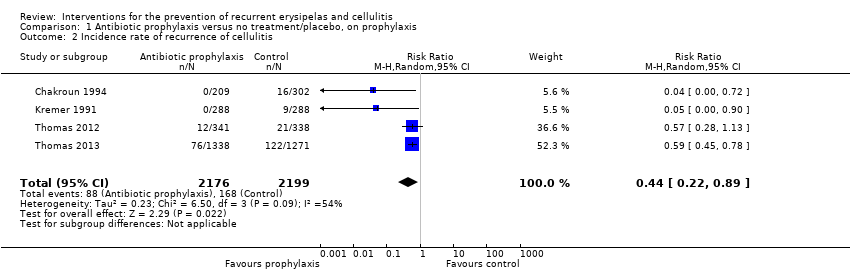
| Incidence rate of cellulitis | Study population | RR 0.44 (0.22 to 0.89) | 473 (4 RCTs) | ⊕⊕⊕⊝ | ‐ | |
| 43 fewer episodes of cellulitis per 1000 person‐months in treatment group (from 8 fewer to 60 fewer) | ||||||
| Time to next episode of cellulitis | Not estimable | HR 0.51 | 437 | ⊕⊕⊕⊝ | ‐ | |
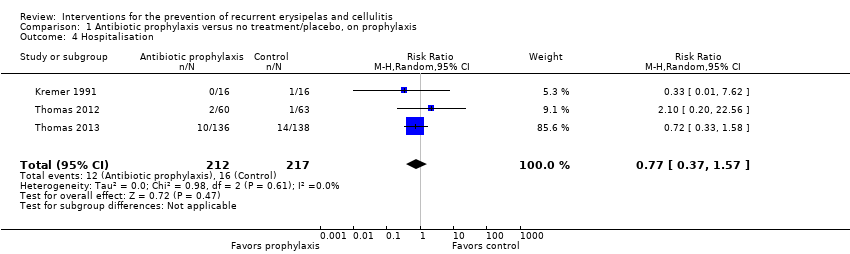
| Hospitalisation | Study population | RR 0.77 | 429 | ⊕⊕⊝⊝ | ‐ | |
| 74 per 1000 | 57 per 1000 | |||||
| Any adverse reactions | Study population | RR 0.87 | 469 | ⊕⊕⊝⊝ | ‐ | |
| 287 per 1000 | 250 per 1000 | |||||
| Quality of life | ‐ | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded by one level because of imprecision due to the low number of events and the small sample size (optimal information size ‐ OIS). 2 We downgraded by two levels because of imprecision due to the low number of events and the small sample size (OIS) and the 95% confidence interval overlapping the line of no effect and ranging from benefit to harm. 3We downgraded by two levels because of imprecision due to the considerable low number of events and the small sample size (OIS). We decided not to downgrade any of the outcomes for risk of bias as we decided it was not serious enough to affect the overall quality of the outcome. | ||||||
| Medical term | Explanation |
| Ambulatory | Ambulatory is when the patient can walk and is not bedridden. When referring to medical care it means that it is being provided to patients that are not hospitalised (outpatients) |
| Block randomisation | A method of randomisation that ensures allocation of participants into roughly equal sizes of comparison groups |
| Clostridium difficile | A bacterium that causes inflammation of the colon (colitis), typically resulting in diarrhoea, and is strongly associated with the use of antibiotics |
| Comorbidity | The presence of one or more diseases or conditions other than those of primary interest |
| Contralateral | On the opposite side of the body (e.g. a repeat episode of leg cellulitis can recur in the same leg [see 'ipsilateral'] or the other, contralateral leg |
| Control event rate (CER) | The rate at which events of interest (i.e. episodes of cellulitis in our review) occur in the control group of the study |
| Diuretics | Commonly known as "water pills" these are drugs that help the body to eliminate unneeded water and salt through the urine |
| Epidemiology | The study of the health of populations and communities, not just particular individuals |
| Erythema | Redness of the skin caused by increased blood flow. Often a sign of inflammation or infection |
| Filariasis | A disease caused by infection with worms, usually in tropical and subtropical areas of the world. The worms reside in the lymphatic system of the affected person and interfere with the drainage of the lymph, subsequently causing a significant swelling of the involved limb |
| Filarial lymphoedema | see Filiariasis |
| Folliculitis | Inflammation of hair follicles |
| Furunculosis | Deep form of inflammation of the hair follicles resulting in lumps caused by the accumulation of pus (boils) |
| Gastrointestinal | Relating to the stomach and the intestines |
| Incidence rate/Incidence rate ratio | The number of new occurrences of events in a population divided by its time period at risk; Incidence rate ratio is the ratio of two incidence rates |
| Ipsilateral | On the same side of the body; as opposed to 'contralateral' |
| Mastectomy | Surgical removal of one or both breasts |
| Outpatient/Inpatient | Outpatient is a person that is being treated without being hospitalised overnight and visits the physician in the clinic, hospital or other facility; compared with an inpatient who requires an overnight stay in hospital for medical treatment |
| Person‐months | The sum of the number of months each participant in the trial has been under observation (treated/followed) |
| 'Per protocol'/Intention‐to‐treat (ITT) analyses | 'Per protocol' analysis compares participants in a study based on the treatment they actually took and includes only those patients who completed the treatment originally allocated, as opposed to intention‐to‐treat analysis that compares participants on the basis of their random assignment to groups (treatment or placebo), regardless of adherence to treatment |
| Prophylaxis | Preventive treatment for disease |
| Retrospective cohort study | An observational study in which a defined group of people (the cohort) is followed over time. A retrospective cohort study identifies persons from past medical records and follows them from the time of those records to the present |
| Sensitivity analysis | An analysis used to determine how sensitive the results of a study or systematic review are to changes in how it was done |
| Tinea pedis | Fungal infection of the foot (athlete's foot) |
| Tonsillectomy | Surgical removal of tonsils |
| Study | Way of communication | Date | Information provided | Notes |
| | 12/2013 | ‐ Allocation concealment ‐ Participants follow‐up ‐ Criteria for diagnosis ‐ Adherence ‐ Adverse reactions ‐ Informed consent ‐ Ethical committee approval ‐ Source of funding | Full information was not available for all queries, but investigators responded to all of them | |
| airmail, email, website | 2013 ‐ 2014 | ‐ | Investigator did not reply to our queries We also contacted a potential sponsor, not reported by the author, who confirmed their financial support for the conduct of this study (email correspondence with the head of medical‐scientific department of 'biosyn Arzneimittel GmbH' from January 2015) | |
| email and telephone | 12/2013 and 1/2014 | ‐ | Data were not available and the investigator did not remember any details | |
| | 12/2013 | ‐ Allocation concealment ‐ Participants follow‐up ‐ By whom cellulitis was diagnosed ‐ Adherence ‐ Source of funding | Full information was not available for all queries, but investigators responded to all of them | |
| | 1/2014 | ‐ Episodes of recurrent cellulitis per person‐months (incidence rate) ‐Time to next episode ‐ Adverse events by study arm ‐ Duration of hospitalisation ‐ Quality of life | ‐ | |
| | 1/2014 | ‐ Episodes of recurrent cellulitis per person‐months (incidence rate) ‐ Quality of life | Hospitalisation and quality of life were not evaluated directly in this trial but were reported by indirect evaluation in Mason 2014 |
| Guideline | Organisation | Recommended antibiotic | Duration of Px | No of episodes to initiate Px | Adjunctive Tx | Quality of evidence † |
| BLS | penicillin by mouth; alternatives: cephalexin, erythromycin, clarithromycin, clindamycin, doxycycline | 2 y; life‐long Px if recurrence after Px stopped | 2 ≥/y | skin care, decongestive Tx, antifungal Tx, alcohol wipes; SIT | NS | |
| ALA | penicillin by mouth; alternatives: cephalexin, erythromycin, clindamycin | 2 y; life‐long Px if recurrence after Px stopped | 2 ≥/y | skin care, decongestive Tx, bacterial decolonisation Tx; SIT | NS | |
| IDSA | penicillin by mouth/IM; alternatives:erythromycin | as long as RF persist | 3 – 4 /y | skin care, Tx of oedema, weight reduction | antibiotic Px ‐weak, moderate ‡ duration of Px ‐ strong, moderate ‡ skin care ‐ strong, moderate ‡ | |
| penicillin by mouth/IM; alternatives:erythromycin | NS | frequent | skin care, Tx of oedema compression stockings, diuretics; SIT | grade IIB § | ||
| ISL | penicillin; alternatives: broad spectrum antibiotic | NS | repeat despite physical Tx | skin care, antifungal Tx | NS | |
| SIMIT and ISC | penicillin or macrolide | NS | recurrent | skin hygiene and compression stockings | NS | |
| NHG | penicillin by mouth/IM | 1 ‐ 2 y | 2 ≥/y | skin care, compression stockings; SIT | NS | |
| ILF | penicillin by mouth; alternatives:erythromycin, clindamycin, clarithromycin | 2 y; life‐long Px if recurrence after Px stopped | 2 ≥/y | skin care, decongestive Tx, antifungal Tx; SIT | NS | |
| CREST | penicillin or erythromycin by mouth | 2 y | 2 ≥/y | SIT may be preferable | weak and inconclusive | |
| NICE | a trial should be considered | NS | > 2/y | skin care, Tx of oedema, compression stockings, weight reduction | weak and inconclusive | |
| Other* | antibiotic may be needed; type of antibiotic NS | long term | NS | skin care, Tx of oedema, antifungal Tx; SIT | NS | |
| SFD | penicillin by mouth/IM; alternatives: macrolide | prolonged, probably indefinitely | several/poorly controlled RF | skin care, Tx of oedema | NS | |
| FMSD | antibiotic should be considered; type of antibiotic NS | long term | frequent | skin care | NS | |
| † Assessement of quality of evidence as defined and graded by the authors of the document. ‡ Strong recommendation, moderate quality ‐ desirable effects clearly outweigh undesirable effects; evidence from RCTs with important limitations or exceptionally strong evidence from unbiased observational studies; recommendation can apply to most patients in most circumstances and further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. Weak recommendation, moderate quality ‐ desirable effects closely balanced with undesirable effects; evidence from RCTs with important limitations;recommendation may change when higher‐quality evidence becomes available; and further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. § Moderate evidence ‐ should generally be offered; II ‐ evidence from one well‐designed clinical trial. Abbreviations IM ‐ injections into the muscle (intramuscular) Medical organisations BLS ‐ British Lymphology Society * 5 of 6 experts in this consensus paper were from North America; published in the Journal of the British Society for Antimicrobial Chemotherapy | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of cellulitis Show forest plot | 5 | 513 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.13, 0.72] |
| 2 Incidence rate of recurrence of cellulitis Show forest plot | 4 | 4375 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.22, 0.89] |
| 3 Time to next episode of cellulitis Show forest plot | 3 | Hazard Ratio (Random, 95% CI) | 0.51 [0.34, 0.78] | |
| 4 Hospitalisation Show forest plot | 3 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.37, 1.57] |
| 5 Any adverse reactions Show forest plot | 4 | 469 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.58, 1.30] |
| 5.1 Penicillin | 3 | 437 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.60, 1.10] |
| 5.2 Erythromycin | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.39, 125.44] |
| 6 Mortality Show forest plot | 3 | 437 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.32, 3.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of cellulitis Show forest plot | 2 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.59, 1.31] |
| 2 Incidence rate of recurrence of cellulitis Show forest plot | 2 | 4566 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.65, 1.36] |
| 3 Time to next episode of cellulitis Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 0.78 [0.39, 1.56] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of cellulitis Show forest plot | 2 | 397 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.59, 0.95] |
| 2 Incidence rate of recurrence of cellulitis Show forest plot | 2 | 7854 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.56, 0.85] |

