Intervenciones para la prevención de la erisipela y celulitis recurrentes
Información
- DOI:
- https://doi.org/10.1002/14651858.CD009758.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 20 junio 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Piel
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
AD was the contact person with the editorial base, AD and MP co‐ordinated contributions from the co‐authors, and wrote the final draft of the review.
AD and SR screened papers against eligibility criteria.
AD obtained data on ongoing and unpublished studies.
AD, MS and MP appraised the quality of papers.
AD, MS and MP extracted data for the review and sought additional information about papers.
AD entered data into Review Manager 5.
AD, MS, DM, SR, WD, EH, LL and EH analysed and interpreted data.
AD, LL and MP worked on the Methods sections.
AD and MP drafted the clinical sections of the Background and responded to the clinical comments of the referees.
AD, LL and MP responded to the methodology and statistics comments of the referees.
WD was the consumer co‐author and checked the review for readability and clarity, as well as ensuring outcomes are relevant to consumers.
AD is the guarantor of the update.
Disclaimer
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Skin Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
Adam Dalal: nothing to declare.
Marina Eskin‐Shwartz: nothing to declare.
Daniel Mimouni: nothing to declare.
Sujoy Ray: nothing to declare.
Walford Days: nothing to declare.
Emmilia Hodak: nothing to declare.
Leonard Leibovici: nothing to declare.
Mical Paul: nothing to declare.
Oh Choon Chiat, who refereed this review, is the author/co‐author of two papers (Oh 2014; Tay 2015) cited in this review.
Acknowledgements
We are grateful to the Cochrane Skin Group editorial team for their assistance and guidance.
We wish to thank the following investigators of the included studies, who answered our queries and provided additional details: Mohamed Chakroun and Christina Jorup‐Rönström. We particularly thank Kim Thomas, together with Angela Crook, James Mason and their team for providing us access to the primary data of the PATCH trials and kindly replying to our requests.
The Cochrane Skin editorial base wishes to thank Sam Gibbs, Cochrane Dermatology Editor for this review; Ben Carter, Statistical Editor; Esther van Zuuren, Methods Editor; the clinical referees, Laurence Le Cleach and Oh Choon Chiat; and the consumer referee, Peter Smart; as well as Kate Cahill, who copy edited the review.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Jun 20 | Interventions for the prevention of recurrent erysipelas and cellulitis | Review | Adam Dalal, Marina Eskin‐Schwartz, Daniel Mimouni, Sujoy Ray, Walford Days, Emmilia Hodak, Leonard Leibovici, Mical Paul | |
| 2012 Apr 18 | Interventions for the prevention of recurrent erysipelas and cellulitis | Protocol | Adam Dalal, Marina Eskin‐Shwartz, Daniel Mimouni, Sujoy Ray, Walford Days, Emmilia Hodak, Leonard Leibovici, Mical Paul | |
Differences between protocol and review
Background: we updated the Background as we found other official guidelines during the search (the Australasian Lymphology Association consensus guideline, the International Society of Lymphology consensus document, the Italian Society of Infectious Diseases and International Society of Chemotherapy's consensus statement, the Dutch College of General Practitioners' practice guideline, the English National Institute for Health and Clinical Excellence (NICE) guideline, the International Lymphoedema Framework consensus document and the Finnish Dermatological Society's care guideline).
Objectives: the main objective of the review was changed and rephrased to reflect the importance of both the beneficial as well as the adverse effects of the intervention (therefore "effectiveness" was changed to "beneficial and adverse effects"). We also clarified the types of included participants.
Types of interventions: we contacted authors for results relating to cellulitis if they were not reported separately.
Types of outcome measures: we defined the relevant time points for extraction of data as it was not stated in original version of the protocol. The primary time point for analysis was the end of treatment phase ('on prophylaxis) and the secondary time points: after the cessation of treatment ('post‐prophylaxis') and at the end of follow‐up ('overall').
Types of outcome measures > Primary outcomes: we clarified that when data were available we defined recurrence as a repeat episode of cellulitis in the same limb.
Types of outcome measures > Secondary outcomes: we added another outcome of 'Mortality'. We intended to include this outcome under 'adverse reactions' but decided to separate mortality and morbidity outcomes for the reader's convenience.
Types of outcome measures > Secondary outcomes: for clarification, we slightly reworded and added extra information with regards to time points for secondary outcomes 1 and 2.
Electronic searches: we had planned in the protocol to search ISI Web of Science and NLM gateway but we chose not to search through the NLM Gateway as it had transitioned to a new pilot project. We did not search ISI Web of Science because of technical issues and database availability. We also updated the metaRegister of Controlled Trials website address
Data collection and analysis > Unit of analysis issues: we specifically referred to cross‐over trials and why their design would not be expected to be used.
Data collection and analysis > Dealing with missing data: we added the planned analysis of missing data that was omitted from the original protocol.
Data collection and analysis > Data synthesis: in our protocol we specified that we would use a Mantel‐Haenszel fixed‐effect model to calculate the treatment effect across trials. However, we decided instead to use a random‐effects models throughout, as we predicted there would be clinical and methodological heterogeneity between studies.
Data collection and analysis > Measures of treatment effect: we calculated the number needed to treat for an additional beneficial outcome (NNTB) for the primary outcome when the result was significant.
Data collection and analysis > 'Summary of findings' table: to adhere to Cochrane standards, we included a 'Summary of findings' table and described the basis on which it was created.
Searching other resources > Grey literature: we planned in the protocol to search BIOSIS Previews from 1969 but only searched from 1990 in the review, due to technical reasons and database availability.
Assessment of risk of bias in included studies: in the review we addressed other risks of bias under several headings which we had not planned and failed to include in the protocol. We included these because we thought them important: Baseline imbalance; Early termination; Other potential bias.
Subgroup analysis and investigation of heterogeneity: We planned to explore reasons for substantial heterogeneity (I² > 50%) in any meta‐analyses we performed but there were insufficient studies for the subgroups we planned. We performed a post hoc subgroup analysis with respect to the type of antibiotic because side effects are a critical outcome for the intervention assessed and side effects are drug‐specific.
Sensitivity analyses: We planned to explore heterogeneity through a sensitivity analysis; however, paucity of data for the relevant interventions made sensitivity analysis impractical.
Tables > 'Summary of findings' table: we included a 'Summary of findings' table as recommended by Cochrane guidelines. In addition, we also used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Antibiotic Prophylaxis [adverse effects];
- Anti‐Bacterial Agents [adverse effects, *therapeutic use];
- Arm;
- Cellulitis [*prevention & control];
- Erysipelas [*prevention & control];
- Erythromycin [adverse effects, therapeutic use];
- Hospitalization [statistics & numerical data];
- Leg Dermatoses [prevention & control];
- Penicillin G Benzathine [adverse effects, therapeutic use];
- Penicillin V [adverse effects, therapeutic use];
- Randomized Controlled Trials as Topic;
- Recurrence;
- Secondary Prevention [*methods];
- Selenium [*therapeutic use];
Medical Subject Headings Check Words
Aged; Humans; Middle Aged;
PICO

Study selection flow diagram.
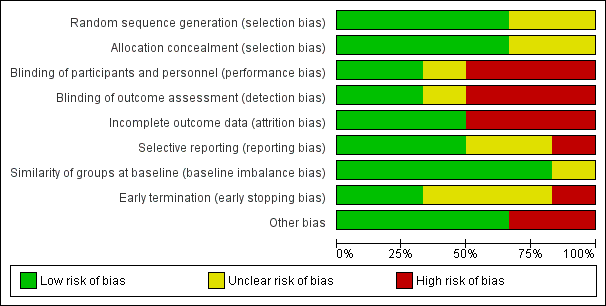
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 1 Recurrence of cellulitis.
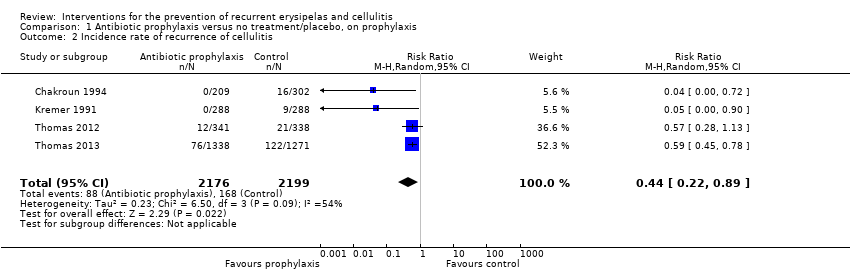
Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 2 Incidence rate of recurrence of cellulitis.

Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 3 Time to next episode of cellulitis.
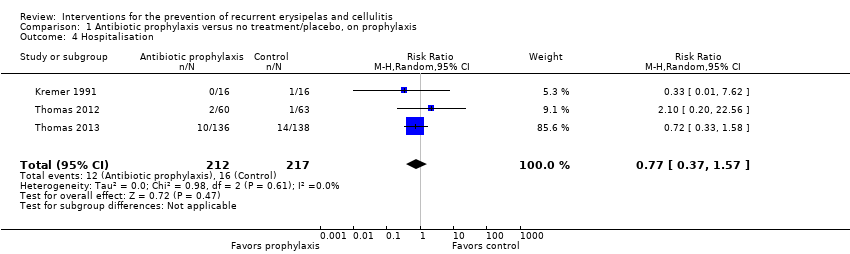
Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 4 Hospitalisation.

Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 5 Any adverse reactions.

Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 6 Mortality.

Comparison 2 Antibiotic prophylaxis versus no treatment/placebo, post‐prophylaxis, Outcome 1 Recurrence of cellulitis.

Comparison 2 Antibiotic prophylaxis versus no treatment/placebo, post‐prophylaxis, Outcome 2 Incidence rate of recurrence of cellulitis.
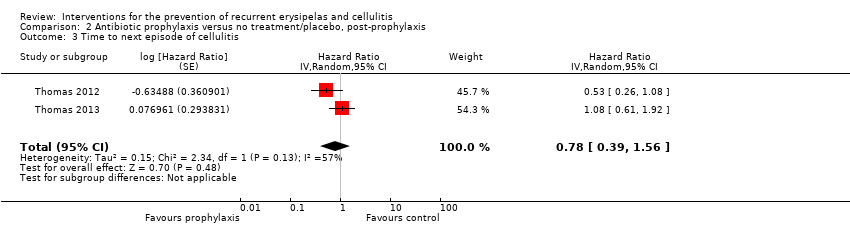
Comparison 2 Antibiotic prophylaxis versus no treatment/placebo, post‐prophylaxis, Outcome 3 Time to next episode of cellulitis.

Comparison 3 Antibiotic prophylaxis versus no treatment/placebo, overall, Outcome 1 Recurrence of cellulitis.

Comparison 3 Antibiotic prophylaxis versus no treatment/placebo, overall, Outcome 2 Incidence rate of recurrence of cellulitis.
| Antibiotic prophylaxis compared to no treatment/placebo for the prevention of recurrent erysipelas and cellulitis ‐ on prophylaxis | ||||||
| Patient or population: People with recurrent erysipelas or cellulitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| no treatment/placebo | Antibiotic prophylaxis | |||||
| Recurrence of cellulitis | Study population | RR 0.31 | 513 | ⊕⊕⊕⊝ | Number needed to treat for 1 additional beneficial outcome (NNTB) is 6 | |
| 316 per 1000 | 98 per 1000 | |||||
| Incidence rate of cellulitis | Study population | RR 0.44 (0.22 to 0.89) | 473 (4 RCTs) | ⊕⊕⊕⊝ | ‐ | |
| 43 fewer episodes of cellulitis per 1000 person‐months in treatment group (from 8 fewer to 60 fewer) | ||||||
| Time to next episode of cellulitis | Not estimable | HR 0.51 | 437 | ⊕⊕⊕⊝ | ‐ | |
| Hospitalisation | Study population | RR 0.77 | 429 | ⊕⊕⊝⊝ | ‐ | |
| 74 per 1000 | 57 per 1000 | |||||
| Any adverse reactions | Study population | RR 0.87 | 469 | ⊕⊕⊝⊝ | ‐ | |
| 287 per 1000 | 250 per 1000 | |||||
| Quality of life | ‐ | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded by one level because of imprecision due to the low number of events and the small sample size (optimal information size ‐ OIS). 2 We downgraded by two levels because of imprecision due to the low number of events and the small sample size (OIS) and the 95% confidence interval overlapping the line of no effect and ranging from benefit to harm. 3We downgraded by two levels because of imprecision due to the considerable low number of events and the small sample size (OIS). We decided not to downgrade any of the outcomes for risk of bias as we decided it was not serious enough to affect the overall quality of the outcome. | ||||||
| Medical term | Explanation |
| Ambulatory | Ambulatory is when the patient can walk and is not bedridden. When referring to medical care it means that it is being provided to patients that are not hospitalised (outpatients) |
| Block randomisation | A method of randomisation that ensures allocation of participants into roughly equal sizes of comparison groups |
| Clostridium difficile | A bacterium that causes inflammation of the colon (colitis), typically resulting in diarrhoea, and is strongly associated with the use of antibiotics |
| Comorbidity | The presence of one or more diseases or conditions other than those of primary interest |
| Contralateral | On the opposite side of the body (e.g. a repeat episode of leg cellulitis can recur in the same leg [see 'ipsilateral'] or the other, contralateral leg |
| Control event rate (CER) | The rate at which events of interest (i.e. episodes of cellulitis in our review) occur in the control group of the study |
| Diuretics | Commonly known as "water pills" these are drugs that help the body to eliminate unneeded water and salt through the urine |
| Epidemiology | The study of the health of populations and communities, not just particular individuals |
| Erythema | Redness of the skin caused by increased blood flow. Often a sign of inflammation or infection |
| Filariasis | A disease caused by infection with worms, usually in tropical and subtropical areas of the world. The worms reside in the lymphatic system of the affected person and interfere with the drainage of the lymph, subsequently causing a significant swelling of the involved limb |
| Filarial lymphoedema | see Filiariasis |
| Folliculitis | Inflammation of hair follicles |
| Furunculosis | Deep form of inflammation of the hair follicles resulting in lumps caused by the accumulation of pus (boils) |
| Gastrointestinal | Relating to the stomach and the intestines |
| Incidence rate/Incidence rate ratio | The number of new occurrences of events in a population divided by its time period at risk; Incidence rate ratio is the ratio of two incidence rates |
| Ipsilateral | On the same side of the body; as opposed to 'contralateral' |
| Mastectomy | Surgical removal of one or both breasts |
| Outpatient/Inpatient | Outpatient is a person that is being treated without being hospitalised overnight and visits the physician in the clinic, hospital or other facility; compared with an inpatient who requires an overnight stay in hospital for medical treatment |
| Person‐months | The sum of the number of months each participant in the trial has been under observation (treated/followed) |
| 'Per protocol'/Intention‐to‐treat (ITT) analyses | 'Per protocol' analysis compares participants in a study based on the treatment they actually took and includes only those patients who completed the treatment originally allocated, as opposed to intention‐to‐treat analysis that compares participants on the basis of their random assignment to groups (treatment or placebo), regardless of adherence to treatment |
| Prophylaxis | Preventive treatment for disease |
| Retrospective cohort study | An observational study in which a defined group of people (the cohort) is followed over time. A retrospective cohort study identifies persons from past medical records and follows them from the time of those records to the present |
| Sensitivity analysis | An analysis used to determine how sensitive the results of a study or systematic review are to changes in how it was done |
| Tinea pedis | Fungal infection of the foot (athlete's foot) |
| Tonsillectomy | Surgical removal of tonsils |
| Study | Way of communication | Date | Information provided | Notes |
| | 12/2013 | ‐ Allocation concealment ‐ Participants follow‐up ‐ Criteria for diagnosis ‐ Adherence ‐ Adverse reactions ‐ Informed consent ‐ Ethical committee approval ‐ Source of funding | Full information was not available for all queries, but investigators responded to all of them | |
| airmail, email, website | 2013 ‐ 2014 | ‐ | Investigator did not reply to our queries We also contacted a potential sponsor, not reported by the author, who confirmed their financial support for the conduct of this study (email correspondence with the head of medical‐scientific department of 'biosyn Arzneimittel GmbH' from January 2015) | |
| email and telephone | 12/2013 and 1/2014 | ‐ | Data were not available and the investigator did not remember any details | |
| | 12/2013 | ‐ Allocation concealment ‐ Participants follow‐up ‐ By whom cellulitis was diagnosed ‐ Adherence ‐ Source of funding | Full information was not available for all queries, but investigators responded to all of them | |
| | 1/2014 | ‐ Episodes of recurrent cellulitis per person‐months (incidence rate) ‐Time to next episode ‐ Adverse events by study arm ‐ Duration of hospitalisation ‐ Quality of life | ‐ | |
| | 1/2014 | ‐ Episodes of recurrent cellulitis per person‐months (incidence rate) ‐ Quality of life | Hospitalisation and quality of life were not evaluated directly in this trial but were reported by indirect evaluation in Mason 2014 |
| Guideline | Organisation | Recommended antibiotic | Duration of Px | No of episodes to initiate Px | Adjunctive Tx | Quality of evidence † |
| BLS | penicillin by mouth; alternatives: cephalexin, erythromycin, clarithromycin, clindamycin, doxycycline | 2 y; life‐long Px if recurrence after Px stopped | 2 ≥/y | skin care, decongestive Tx, antifungal Tx, alcohol wipes; SIT | NS | |
| ALA | penicillin by mouth; alternatives: cephalexin, erythromycin, clindamycin | 2 y; life‐long Px if recurrence after Px stopped | 2 ≥/y | skin care, decongestive Tx, bacterial decolonisation Tx; SIT | NS | |
| IDSA | penicillin by mouth/IM; alternatives:erythromycin | as long as RF persist | 3 – 4 /y | skin care, Tx of oedema, weight reduction | antibiotic Px ‐weak, moderate ‡ duration of Px ‐ strong, moderate ‡ skin care ‐ strong, moderate ‡ | |
| penicillin by mouth/IM; alternatives:erythromycin | NS | frequent | skin care, Tx of oedema compression stockings, diuretics; SIT | grade IIB § | ||
| ISL | penicillin; alternatives: broad spectrum antibiotic | NS | repeat despite physical Tx | skin care, antifungal Tx | NS | |
| SIMIT and ISC | penicillin or macrolide | NS | recurrent | skin hygiene and compression stockings | NS | |
| NHG | penicillin by mouth/IM | 1 ‐ 2 y | 2 ≥/y | skin care, compression stockings; SIT | NS | |
| ILF | penicillin by mouth; alternatives:erythromycin, clindamycin, clarithromycin | 2 y; life‐long Px if recurrence after Px stopped | 2 ≥/y | skin care, decongestive Tx, antifungal Tx; SIT | NS | |
| CREST | penicillin or erythromycin by mouth | 2 y | 2 ≥/y | SIT may be preferable | weak and inconclusive | |
| NICE | a trial should be considered | NS | > 2/y | skin care, Tx of oedema, compression stockings, weight reduction | weak and inconclusive | |
| Other* | antibiotic may be needed; type of antibiotic NS | long term | NS | skin care, Tx of oedema, antifungal Tx; SIT | NS | |
| SFD | penicillin by mouth/IM; alternatives: macrolide | prolonged, probably indefinitely | several/poorly controlled RF | skin care, Tx of oedema | NS | |
| FMSD | antibiotic should be considered; type of antibiotic NS | long term | frequent | skin care | NS | |
| † Assessement of quality of evidence as defined and graded by the authors of the document. ‡ Strong recommendation, moderate quality ‐ desirable effects clearly outweigh undesirable effects; evidence from RCTs with important limitations or exceptionally strong evidence from unbiased observational studies; recommendation can apply to most patients in most circumstances and further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. Weak recommendation, moderate quality ‐ desirable effects closely balanced with undesirable effects; evidence from RCTs with important limitations;recommendation may change when higher‐quality evidence becomes available; and further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. § Moderate evidence ‐ should generally be offered; II ‐ evidence from one well‐designed clinical trial. Abbreviations IM ‐ injections into the muscle (intramuscular) Medical organisations BLS ‐ British Lymphology Society * 5 of 6 experts in this consensus paper were from North America; published in the Journal of the British Society for Antimicrobial Chemotherapy | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of cellulitis Show forest plot | 5 | 513 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.13, 0.72] |
| 2 Incidence rate of recurrence of cellulitis Show forest plot | 4 | 4375 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.22, 0.89] |
| 3 Time to next episode of cellulitis Show forest plot | 3 | Hazard Ratio (Random, 95% CI) | 0.51 [0.34, 0.78] | |
| 4 Hospitalisation Show forest plot | 3 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.37, 1.57] |
| 5 Any adverse reactions Show forest plot | 4 | 469 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.58, 1.30] |
| 5.1 Penicillin | 3 | 437 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.60, 1.10] |
| 5.2 Erythromycin | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.39, 125.44] |
| 6 Mortality Show forest plot | 3 | 437 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.32, 3.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of cellulitis Show forest plot | 2 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.59, 1.31] |
| 2 Incidence rate of recurrence of cellulitis Show forest plot | 2 | 4566 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.65, 1.36] |
| 3 Time to next episode of cellulitis Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 0.78 [0.39, 1.56] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of cellulitis Show forest plot | 2 | 397 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.59, 0.95] |
| 2 Incidence rate of recurrence of cellulitis Show forest plot | 2 | 7854 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.56, 0.85] |

