Intervenciones para la prevención de la erisipela y celulitis recurrentes
Resumen
Antecedentes
La erisipela y la celulitis (de aquí en adelante denominadas “celulitis”) son infecciones bacterianas comunes de la piel que generalmente afectan las extremidades inferiores. A pesar de la carga de morbilidad, la evidencia sobre las diferentes estrategias de prevención es poco clara.
Objetivos
Evaluar los efectos beneficiosos y adversos de la profilaxis con antibióticos u otras intervenciones profilácticas para la prevención de los episodios recurrentes de celulitis en adultos mayores de 16 años de edad.
Métodos de búsqueda
Se hicieron búsquedas en las siguientes bases de datos hasta junio de 2016: el Registro Especializado del Grupo Cochrane de la Piel (Cochrane Skin Group), el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials, CENTRAL), MEDLINE, Embase y LILACS. También se realizaron búsquedas en cinco bases de datos de registros de ensayos, y se verificaron las listas de referencias de los estudios y revisiones incluidos para obtener referencias adicionales a ensayos controlados aleatorizados (ECA) relevantes. Se realizaron búsquedas en dos series de actas de congresos de dermatología y BIOSIS Previews.
Criterios de selección
Ensayos controlados aleatorizados que evaluaban cualquier tratamiento para la prevención de la celulitis recurrente.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, realizaron la selección de estudios, la extracción de datos, la evaluación de los riesgos de sesgo y los análisis. El resultado primario predeterminado fue la recurrencia de la celulitis durante la administración del tratamiento y después del tratamiento. Los resultados secundarios incluyeron la tasa de incidencia, el tiempo hasta el próximo episodio, la hospitalización, la calidad de vida, el desarrollo de resistencia a los antibióticos, las reacciones adversas y la mortalidad.
Resultados principales
Se incluyeron seis ensayos, con un total de 573 participantes evaluables, que tenían una edad promedio entre 50 y 70 años. Hubo pocos episodios anteriores de celulitis en los pacientes incluidos en los ensayos, que variaron entre uno y cuatro episodios por estudio.
Cinco de los seis ensayos incluidos evaluaron la prevención con antibióticos en participantes con celulitis de las piernas, y uno evaluó el selenio en los participantes con celulitis de los brazos. Entre los estudios que evaluaron los antibióticos, un estudio evaluó la eritromicina oral (n = 32) y cuatro estudios evaluaron la penicilina (n = 481). La duración del tratamiento varió de seis a 18 meses, y dos estudios continuaron el seguimiento de los participantes después de la interrupción de la profilaxis, con un período de seguimiento de hasta un año y medio a dos años. Cuatro estudios fueron de centro único y dos multicéntricos; se llevaron a cabo en cinco países: el Reino Unido, Suecia, Túnez, Israel y Austria.
Sobre la base de cinco ensayos, la profilaxis con antibióticos (al final de la fase de tratamiento ("en profilaxis")) redujo el riesgo de recurrencia de la celulitis en un 69%, en comparación con la ausencia de tratamiento o el placebo (riesgos relativos (RR) 0,31, intervalo de confianza (IC) del 95%: 0,13 a 0,72; n = 513; p = 0,007), número necesario a tratar para un resultado beneficioso adicional (NNTB) seis, (IC del 95%: 5 a 15), y se calificó la certeza de la evidencia para este resultado como moderada.
Bajo tratamiento profiláctico y en comparación con ningún tratamiento o placebo, la profilaxis con antibióticos redujo la tasa de incidencia de la celulitis en un 56% (RR 0,44; IC del 95%: 0,22 a 0,89; cuatro estudios; n = 473; valor de P = 0,02; evidencia de certeza moderada) y disminuyó significativamente la tasa hasta el siguiente episodio de celulitis (riesgos relativos (RR) 0,51; IC del 95%: 0,34 a 0,78; tres estudios; n = 437; P = 0,002; evidencia de certeza moderada).
Los efectos protectores del antibiótico no duraron después de que se interrumpió la profilaxis ("postprofilaxis") por el riesgo de recurrencia de la celulitis (RR 0,88; IC del 95%: 0,59 a 1,31; dos estudios; n = 287; P = 0,52), tasa de incidencia de celulitis (RR 0,94; IC del 95%: 0,65 a 1,36; dos estudios; n = 287; P = 0,74), y tasa hasta el siguiente episodio de celulitis (CRI 0,78; IC del 95%: 0,39 a 1,56; dos estudios; n = 287). La evidencia fue de certidumbre baja.
Los efectos son relevantes principalmente para los pacientes después de al menos dos episodios de celulitis de la pierna que se presentan en un período de hasta tres años.
No se encontró ninguna diferencia significativa en los efectos adversos ni la hospitalización entre los antibióticos y ningún tratamiento o placebo; para los efectos adversos: RR 0,87, IC del 95%: 0,58 a 1,30; cuatro estudios; n = 469; P = 0,48; para la hospitalización: RR 0,77, IC del 95%: 0,37 a 1,57; tres estudios; n = 429; P = 0,47, con certeza de la evidencia calificada como baja para estos resultados. Los datos existentes no permitieron explorar plenamente su impacto en la duración de la estancia hospitalaria.
Las reacciones adversas más comunes fueron síntomas gastrointestinales, principalmente náuseas y diarrea; sarpullido (no se comunicaron reacciones adversas cutáneas graves); y muguet. Tres estudios informaron efectos adversos que dieron lugar a la interrupción del tratamiento asignado. En un estudio (eritromicina), tres participantes informaron dolor abdominal y náuseas, por lo que su tratamiento se cambió a penicilina. En otro estudio, dos participantes tratados con penicilina se retiraron del tratamiento debido a la diarrea o las náuseas. En un estudio, alrededor del 10% de los participantes interrumpieron el tratamiento debido al dolor en el sitio de inyección (el grupo de tratamiento activo recibió inyecciones intramusculares de penicilina benzatínica).
Ninguno de los estudios incluidos evaluó el desarrollo de resistencia a los antimicrobianos ni las medidas de la calidad de vida.
Con respecto a los riesgos de sesgo, dos estudios incluidos estuvieron en riesgo bajo de sesgo y otros tres se consideraron como en riesgo alto de sesgo, principalmente debido a la ausencia de cegamiento.
Conclusiones de los autores
En cuanto a la recurrencia, la incidencia, y el tiempo hasta el siguiente episodio, los antibióticos probablemente son un tratamiento preventivo efectivo para la celulitis recurrente de los miembros inferiores en los pacientes bajo tratamiento profiláctico, en comparación con placebo o ningún tratamiento (evidencia de certidumbre moderada). Sin embargo, estos efectos preventivos de los antibióticos parecen disminuir después de su interrupción (evidencia de certidumbre baja). El tratamiento con antibióticos no desencadena eventos adversos graves, y los eventos asociados son menores, como náuseas y erupciones cutáneas (evidencia de certidumbre baja). La evidencia está limitada a los pacientes con al menos dos episodios pasados de celulitis de la pierna en un plazo de hasta tres años, y ninguno de los estudios investigó otras intervenciones comunes como los métodos de reducción del linfedema o el cuidado adecuado de la piel. Se justifica la realización de estudios más amplios, de alta calidad, que incluyan un seguimiento a largo plazo y otras medidas profilácticas.
PICO
Resumen en términos sencillos
Tratamientos preventivos para los episodios repetidos de celulitis y erisipela
Antecedentes
La celulitis y la erisipela son infecciones bacterianas de la piel que afectan más comúnmente las piernas. La erisipela afecta las capas superiores de la piel, y la celulitis afecta las partes más profundas, aunque en la práctica, a menudo es difícil establecer la diferencia entre las mismas, de manera que se las consideró juntas para esta revisión (y se las denomina “celulitis”). Hasta un 50% de los pacientes con celulitis experimentan episodios repetidos. A pesar de la carga de este trastorno, hay una falta de información de alta certidumbre basada en evidencia acerca del tratamiento aconsejable para la prevención de la celulitis recurrente.
Pregunta de la revisión
¿Cuáles son los mejores tratamientos disponibles para prevenir los episodios repetidos de celulitis en adultos a partir de los 16 años de edad en comparación con ningún tratamiento, placebo, otra intervención o la misma intervención con un plan de tratamiento diferente y cuáles son sus efectos secundarios?
Características de los estudios
Se buscó en bases de datos y registros relevantes hasta junio de 2016. Se identificaron seis ensayos, con 573 participantes, que tenían una edad promedio entre 50 y 70 años. Se incluyeron ambos sexos, aunque hubo casi el doble de mujeres. Cinco ensayos administraron tratamiento con antibióticos (cuatro penicilina y uno eritromicina), en comparación con ningún tratamiento o placebo, y un ensayo administró selenio en comparación con solución salina fisiológica. Los tratamientos duraron de seis a 18 meses.
El contexto más común fue el hospital, y dos estudios fueron multicéntricos. Los estudios se realizaron en el Reino Unido, Suecia, Túnez, Israel y Austria. Hubo un número pequeño de episodios previos de celulitis en los pacientes incluidos en los ensayos, que varió entre uno y cuatro episodios en cada estudio. Los ensayos de los antibióticos evaluaron la prevención con antibióticos en pacientes con celulitis de las piernas, y el ensayo del selenio evaluó a pacientes con celulitis de los brazos.
Resultados clave
El resultado principal fue la prevención de los episodios repetidos de celulitis. Los otros resultados incluyeron el número de ataques repetidos de celulitis, el tiempo hasta el próximo ataque, la hospitalización, la calidad de vida, el desarrollo de resistencia a los antibióticos, las reacciones adversas y la muerte.
Al combinar los resultados de los cinco ensayos que administraron antibióticos, se encontró evidencia de certidumbre moderada de que para los pacientes bajo tratamiento preventivo, el tratamiento con antibióticos en general, y la penicilina en particular, probablemente es tan efectivo como seguro para la prevención de los episodios repetidos de celulitis de la pierna en comparación con ningún tratamiento o placebo.
Los análisis demostraron que, en comparación con ningún tratamiento o placebo, la administración de antibióticos redujo el riesgo de episodios futuros en un 69%, redujo su número en más de un 50%, y redujo significativamente la tasa del tiempo hasta el próximo ataque (evidencia de certidumbre moderada). Sin embargo, se encontró evidencia de certidumbre baja de que el efecto protector de los antibióticos para estos tres resultados se redujo con el transcurso del tiempo después de la interrupción del tratamiento. Además, el efecto beneficioso de los antibióticos fue relevante para los pacientes con al menos dos episodios pasados de celulitis en un plazo de hasta tres años.
Se encontró evidencia de certidumbre baja de que no hay ninguna diferencia entre los antibióticos y ningún tratamiento/placebo para los efectos secundarios y la hospitalización. La evidencia no permitió la exploración completa del efecto del tratamiento sobre la duración de la estancia hospitalaria.
No se informó ningún efecto adverso grave, y los efectos secundarios comunes incluyeron diarrea, náuseas, erupciones cutáneas (no se informaron reacciones adversas severas en la piel) y candidiasis bucal. En tres estudios, los efectos adversos causaron la interrupción de los antibióticos en los participantes. Tres pacientes que recibieron eritromicina presentaron dolor abdominal y náuseas, lo cual causó la interrupción del tratamiento y que recibieran penicilina en su lugar. En un estudio, dos pacientes se retiraron del tratamiento con penicilina debido a la diarrea o las náuseas. En otro estudio, debido al dolor en el sitio de inyección, alrededor de un 10% de los participantes interrumpieron las inyecciones intramusculares de penicilina benzatínica.
Ninguno de los estudios incluidos midió la calidad de vida ni el desarrollo de resistencia a los antibióticos.
Certeza de la evidencia
La evidencia de los efectos de los antibióticos en comparación con ningún tratamiento o placebo sobre la recurrencia, la tasa de incidencia y el tiempo hasta el siguiente episodio de celulitis bajo tratamiento preventivo fue de certidumbre moderada, y fue limitada por el número pequeño de participantes y eventos. La evidencia sobre los resultados restantes informados fue de certidumbre baja por las mismas razones, así como los resultados imprecisos.
Authors' conclusions
Summary of findings
| Antibiotic prophylaxis compared to no treatment/placebo for the prevention of recurrent erysipelas and cellulitis ‐ on prophylaxis | ||||||
| Patient or population: People with recurrent erysipelas or cellulitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| no treatment/placebo | Antibiotic prophylaxis | |||||
| Recurrence of cellulitis | Study population | RR 0.31 | 513 | ⊕⊕⊕⊝ | Number needed to treat for 1 additional beneficial outcome (NNTB) is 6 | |
| 316 per 1000 | 98 per 1000 | |||||
| Incidence rate of cellulitis | Study population | RR 0.44 (0.22 to 0.89) | 473 (4 RCTs) | ⊕⊕⊕⊝ | ‐ | |
| 43 fewer episodes of cellulitis per 1000 person‐months in treatment group (from 8 fewer to 60 fewer) | ||||||
| Time to next episode of cellulitis | Not estimable | HR 0.51 | 437 | ⊕⊕⊕⊝ | ‐ | |
| Hospitalisation | Study population | RR 0.77 | 429 | ⊕⊕⊝⊝ | ‐ | |
| 74 per 1000 | 57 per 1000 | |||||
| Any adverse reactions | Study population | RR 0.87 | 469 | ⊕⊕⊝⊝ | ‐ | |
| 287 per 1000 | 250 per 1000 | |||||
| Quality of life | ‐ | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded by one level because of imprecision due to the low number of events and the small sample size (optimal information size ‐ OIS). 2 We downgraded by two levels because of imprecision due to the low number of events and the small sample size (OIS) and the 95% confidence interval overlapping the line of no effect and ranging from benefit to harm. 3We downgraded by two levels because of imprecision due to the considerable low number of events and the small sample size (OIS). We decided not to downgrade any of the outcomes for risk of bias as we decided it was not serious enough to affect the overall quality of the outcome. | ||||||
Background
Description of the condition
Soft‐tissue infections are a common type of infection causing considerable disease. They account for up to 10% of all hospital admissions in western countries (Nathwani 2001; Vinh 2005) and are associated with high use of healthcare resources (Ostermann 2014). While these infections have many different causes, they are all the result of bacterial invasion through the skin barrier with a variable level of involvement of the soft tissue. Impetigo, boils, and erysipelas are superficial infections of the skin, while cellulitis involves deeper skin tissues (Stevens 2014). More advanced infections are myositis (muscle inflammation) and necrotising fasciitis (commonly known as the 'flesh‐eating disease') (Stevens 2014). Necrotising fasciitis is a severe, rapidly progressive bacterial inflammation that reaches the depth of the muscles and their surrounding tissues, leading to severe tissue destruction, which can be fatal (Stevens 2005; Stevens 2014).
Definition
Erysipelas is an infection that involves the superficial layer of the skin with significant inflammation of the lymphatic vessels (lymphangitis) (Bisno 1996). The resulting clinical picture is usually a skin area that is red, raised, and well‐demarcated from the surrounding normal skin (Raff 2016; Swartz 2004).
Cellulitis is a soft‐tissue infection that involves the deep subcutaneous (underlying) layer of the skin (Swartz 2004). The infected area, most commonly the leg, is characterised by local warmth, redness, swelling, and tenderness. Forty per cent of people with this infection can develop fever and illness (Morris 2008). Nevertheless, the lack of strict criteria or an optimal test for the diagnosis ('gold standard') of cellulitis and erysipelas can result in diagnostic errors, especially by non‐specialists (Arakaki 2014; David 2011; Levell 2011; Strazzula 2015; Weng 2016).
Although the two conditions are considered by some to be distinct, distinguishing erysipelas from cellulitis in daily clinical practice is challenging. Difficulties arise from a significant overlap between these two clinical patterns with regard to infective agents, risk factors, the areas of the body that are involved, and the depth of skin involvement (Bisno 1996). Some physicians use the terms 'erysipelas' and 'cellulitis' interchangeably, rendering this distinction even more problematic (Stevens 2005). Hence, in this review we will refer to both conditions as 'cellulitis', in accordance with the Cochrane Review on 'Interventions for cellulitis and erysipelas' (Kilburn 2010).
Epidemiology
Despite being a common medical problem, few studies provide good data on the incidence of cellulitis. Epidemiological surveys report an incidence that ranges from 0.2/1000 person‐years to 24.6/1000 person‐years in different populations (Ellis 2006; Lum 2002).
Although cellulitis affects the lower limbs in most cases, it can also affect other areas, such as the upper limbs, face, ears, and trunk (Ellis 2006). Upper limb cellulitis mostly occurs in women after surgical treatment for breast cancer following lymphatic system damage (Simon 1992). In this group, cellulitis affects up to 24% of women (Harris 2001). As more conservative breast surgery has been performed, this type of cellulitis tends to be more localised to the breast, rather than the entire upper limb (Mertz 1998; Miller 1998).
Microbiology
The most common causative agents of cellulitis are part of the skin's microbial flora (natural bacterial inhabitants). It is usually caused by streptococcal infection (Bruun 2015), but an infection by Staphylococcus aureus is also possible, and more commonly seen after surgery, penetrating trauma, or in wounds (Bernard 1987; Eriksson 1996; Jorup‐Rönström 1986; Siljander 2008; Swartz 2004). Unlike cellulitis, erysipelas is chiefly caused by Group A streptococci, infrequently involving other species of streptococci (groups B, C, or G) or Staphylococcus aureus (Mandell 2010).
Less common infective organisms include Streptococcus pneumoniae, Haemophilus influenzae, gram negative bacilli, and anaerobes. These pathogens are more likely to be involved in specific epidemiological settings, such as following injury, burns, and other co‐existing diseases (e.g. diabetes, cancer, malnutrition) (Bisno 1996; Brook 1995; Carratala 2003). Pseudomonas aeruginosa is involved in many types of soft tissue infections, frequently targeting vulnerable populations, and also those who have been exposed to contaminated hot tubs, loofah sponges, or nail punctures (Bowler 2001; Eron 2003). Pseudomonas aeruginosa has been shown to be the pathogen associated with the development of shock and death in some people with cellulitis, probably due to a combination of its high virulence and their poor health status (Carratala 2003).
Other cases involve unique types of bacteria. Vibrio vulnificus is associated with exposure to seawater, and Aeromonas hydrophila with freshwater exposure (Swartz 2004). Several organisms are characteristic of animal and human bites: Pasteurella multocida and Capnocytophaga canimorsus have been implicated following animal bites, and Eikenella corrodens following human bites or fist injuries (Stevens 2005).
The value of microbiological culture in the management of cellulitis is limited. Needle aspirations (samples), taken from the infected skin areas and then cultured, are positive for bacterial growth in only 10% of cases (CREST 2005). A higher yield is noticed in surgically‐removed full‐thickness skin biopsies or other tissue specimens from the lesion, with successful bacterial growth in 20% to 30% of cases (Duvanel 1989; Hook 1986). Blood cultures are even less productive, with only 2% to 4% of cultures showing positive bacterial growth. Furthermore, blood culture samples are often contaminated by irrelevant commensals (bacteria living at the same site, but with no harmful or beneficial effects) (Swartz 2004). The diagnosis of cellulitis is therefore based primarily on clinical features. Blood cultures might be useful when the risk of bacteraemia (bacteria in the blood) is high, such as in cases of severe infection or sepsis, or in cellulitis secondary to lymphoedema (an accumulation of lymph in the tissues) (Woo 2000).
Risk factors
Retrospective studies exploring risk factors for cellulitis have reported its association with several factors, including obesity, diabetes mellitus, immunosuppression, and alcoholism (Bartholomeeusen 2007; Koutkia 1999; Lazzarini 2005; Quirke 2016).
The physical condition of a person's skin may play a central role in predisposition to cellulitis, especially on the lower limb. Toe web intertrigo (skin maceration and inflammation, often due to a fungal infection), cracks on the soles of the feet, oedema (swelling that may be due to a damaged lymphatic system, e.g. lymphoedema), leg ulcers, prior trauma (injury), history of venous surgery, and venous insufficiency (caused by a malfunctioning of the venous system of the legs due to valves that are no longer able to pump blood back to the heart) have all been associated with an increased risk of cellulitis (Bjornsdottir 2005; Dupuy 1999; Halpern 2008; Mokni 2006; Pavlotsky 2004; Roujeau 2004). A previous episode of cellulitis is another important risk factor for leg cellulitis (Dupuy 1999; Halpern 2008; Roujeau 2004).
There is a strong association between cellulitis and foot dermatomycosis (fungal infection of the foot). A fungal infection causes breaching of the epidermal layer and fosters bacterial overgrowth, thus facilitating the entry of bacteria to cause skin infection (Roldan 2000; Semel 1996). This pathogenesis probably underlies the strong association between cellulitis and clinical and microscopically‐proven tinea pedis (athlete's foot) (Dupuy 1999; Roujeau 2004). This relationship, together with the high prevalence of tinea pedis in the general population, highlights the significant impact of fungal foot infection on the occurrence of cellulitis (Dupuy 1999; Roujeau 2004).
Recurrent cellulitis
Recurrent cellulitis is a frequent complication of single‐episode cellulitis (Cox 2006; Stoberl 1987). The incidence of recurrent cellulitis varies between studies. It has been noted that 10% to 30% of people who suffer an episode of cellulitis experience repeated attacks across different time intervals (Cox 1998; Dupuy 1999; Ellis 2006; Eriksson 1996; Jorup‐Rönström 1987). In more recent studies with a longer follow‐up period, there was a considerably higher percentage of recurrence. A retrospective analysis by Pavlotsky 2004 carried out on hospitalised patients during a three‐year period showed that more than 45% of admissions of people with cellulitis were due to recurrent episodes. Similar results were also reported by Cox 2006 in a community‐based survey. It is therefore remarkable that only a small number of studies have examined the cause of recurrent cellulitis.
Largely a disease of the lower limb, recurrent cellulitis is thought to be caused by repeated bacterial invasion of the skin through breaches in its protective barrier (Pavlotsky 2004). Accordingly, the most important risk factors for recurrent cellulitis are the local physical factors listed above (Cox 2006; Dupuy 1999; Jorup‐Rönström 1987; Karppelin 2010; Lewis 2006; McNamara 2007; Pavlotsky 2004; Wang 1997).
The primary general risk factors for recurrent cellulitis are lymphoedema, obesity, and a history of cancer (Cox 2006; Dupuy 1999; Karppelin 2010; Lewis 2006; McNamara 2007; Pavlotsky 2004). Contradictory results were obtained for the influence of smoking on recurrent cellulitis (Karppelin 2010; Lewis 2006; Pavlotsky 2004). Previously thought to encourage cellulitis and its recurrence, a weak association has been noted between diabetes as well as alcoholism and recurrent cellulitis (Karppelin 2010; Lewis 2006; Pavlotsky 2004). Nevertheless, a recent report by Karppelin 2013 underscores the significance of diabetes in recurrent cellulitis and also suggests an association between psoriasis and recurrence, as well as surgical removal of tonsils and recurrence of this infection.
Description of the intervention
Small case series and case reports have advocated the use of antibiotic treatment for cellulitis prophylaxis (Table 1) (Babb 1966; Bitnun 1985; Duvanel 1985; Ferrieri 1973; Thind 1985). According to these reports, the use of a long‐term preventive therapy with penicillin was effective in reducing the rate of cellulitis recurrence. While acknowledging the value of antibiotic prophylaxis, different experts have called for the rigorous control of predisposing factors (Baddour 2000; Cox 2006; Lewis 2006; McNamara 2007; Pavlotsky 2004; Swartz 2004). Scrupulous skin care, oedema reduction using compression stockings and diuretics (Table 1), antifungal therapy, and proper footwear have all been proposed as part of prophylactic regimens for recurrent cellulitis (Baddour 2000; Pauszek 1991; Pierce 1992; Stalbow 2004; Swartz 2004). Prophylaxis based on the anti‐inflammatory effects of selenium have been proposed by Kasseroller (Kasseroller 1996; Kasseroller 1998), who demonstrated the benefit of selenium in a randomised controlled trial among women following gynaecological cancer treatment, in most cases following mastectomy (Table 1).
| Medical term | Explanation |
| Ambulatory | Ambulatory is when the patient can walk and is not bedridden. When referring to medical care it means that it is being provided to patients that are not hospitalised (outpatients) |
| Block randomisation | A method of randomisation that ensures allocation of participants into roughly equal sizes of comparison groups |
| Clostridium difficile | A bacterium that causes inflammation of the colon (colitis), typically resulting in diarrhoea, and is strongly associated with the use of antibiotics |
| Comorbidity | The presence of one or more diseases or conditions other than those of primary interest |
| Contralateral | On the opposite side of the body (e.g. a repeat episode of leg cellulitis can recur in the same leg [see 'ipsilateral'] or the other, contralateral leg |
| Control event rate (CER) | The rate at which events of interest (i.e. episodes of cellulitis in our review) occur in the control group of the study |
| Diuretics | Commonly known as "water pills" these are drugs that help the body to eliminate unneeded water and salt through the urine |
| Epidemiology | The study of the health of populations and communities, not just particular individuals |
| Erythema | Redness of the skin caused by increased blood flow. Often a sign of inflammation or infection |
| Filariasis | A disease caused by infection with worms, usually in tropical and subtropical areas of the world. The worms reside in the lymphatic system of the affected person and interfere with the drainage of the lymph, subsequently causing a significant swelling of the involved limb |
| Filarial lymphoedema | see Filiariasis |
| Folliculitis | Inflammation of hair follicles |
| Furunculosis | Deep form of inflammation of the hair follicles resulting in lumps caused by the accumulation of pus (boils) |
| Gastrointestinal | Relating to the stomach and the intestines |
| Incidence rate/Incidence rate ratio | The number of new occurrences of events in a population divided by its time period at risk; Incidence rate ratio is the ratio of two incidence rates |
| Ipsilateral | On the same side of the body; as opposed to 'contralateral' |
| Mastectomy | Surgical removal of one or both breasts |
| Outpatient/Inpatient | Outpatient is a person that is being treated without being hospitalised overnight and visits the physician in the clinic, hospital or other facility; compared with an inpatient who requires an overnight stay in hospital for medical treatment |
| Person‐months | The sum of the number of months each participant in the trial has been under observation (treated/followed) |
| 'Per protocol'/Intention‐to‐treat (ITT) analyses | 'Per protocol' analysis compares participants in a study based on the treatment they actually took and includes only those patients who completed the treatment originally allocated, as opposed to intention‐to‐treat analysis that compares participants on the basis of their random assignment to groups (treatment or placebo), regardless of adherence to treatment |
| Prophylaxis | Preventive treatment for disease |
| Retrospective cohort study | An observational study in which a defined group of people (the cohort) is followed over time. A retrospective cohort study identifies persons from past medical records and follows them from the time of those records to the present |
| Sensitivity analysis | An analysis used to determine how sensitive the results of a study or systematic review are to changes in how it was done |
| Tinea pedis | Fungal infection of the foot (athlete's foot) |
| Tonsillectomy | Surgical removal of tonsils |
To the best of our knowledge, 12 guidelines for cellulitis prophylaxis have so far been issued (ALA 2015; BLS 2016; CREST 2005; Draijer 2008; Duodecim 1999; Eron 2003; Esposito 2011; ILF 2006; ISL 2013; NICE 2005; SFD 2000; Stevens 2005).
All documents call for reducing predisposing conditions. Avoiding dry and cracked skin, treating macerated skin and fungal infections, and using compressive dressings are agreed. Antibiotic prophylaxis is also advocated by the different groups. Long‐term antibiotic prophylaxis with oral or intramuscular penicillin or macrolides is recommended, while the British Lymphology Society (BLS 2016), the Australasian Lymphology Association (ALA 2015), the International Lymphoedema Framework (ILF 2006), the Dutch College of General Practitioners (Draijer 2008), the Clinical Resource Efficiency Support Team (CREST 2005) and NICE (National Institute for Health and Clinical Excellence) (NICE 2005) groups advise initiating treatment after the second cellulitis episode. The International Society of Lymphology (ISL 2013), the Infectious Diseases Society of America (Stevens 2005), the French Society of Dermatology (SFD 2000) and the working group set up by the Finnish Medical and the Finnish Dermatological Societies (Duodecim 1999) indicate that repeated, frequent or several episodes of treatment are necessary. The American guidelines are classified as grade B‐II recommendation (B refers to moderate evidence to support a recommendation for use that should generally be offered, and II means the evidence is available from well‐conducted non‐randomised studies). Seven groups propose an alternative to antibiotic prophylaxis with early self‐initiated antibiotic treatment (ALA 2015; BLS 2016; CREST 2005; Draijer 2008; Eron 2003; ILF 2006; Stevens 2005).
These recommendations are mostly supported by observational studies and expert opinion, and lack a systematic analysis of high‐quality research.
How the intervention might work
A plausible strategy aiming to prevent recurrent attacks of cellulitis is likely to rely on targeting the causative bacterial agents of cellulitis, i.e. streptococci and Staphylococcus aureus, or on controlling risk factors for recurrence that are amenable to change.
Antibiotic treatment is aimed at eradicating and preventing the growth of bacteria. However, the consistent difficulty isolating bacteria from the infected skin (Crisp 2015) might indicate that once cellulitis is initiated, it is promoted and perpetuated by bacterial toxins, pointing to another possible preventive mechanism: inhibition of bacterial toxins and their production. Such a mode of action is attributed to clindamycin (Goscinski 2006; Sawai 2007), an antibiotic that is often used in infections that are considered as toxin‐mediated. A cellulitis vaccine would naturally be the ultimate preventive measure for people at high risk for recurrent cellulitis, providing an acquired immunity against the disease.
Another preventive approach for recurrent cellulitis is to treat its underlying risk factors. The primary mechanism of action of different moisturisers and the treatment of fungal foot infection is probably by maintaining the skin barrier and preventing its breach by pathogenic bacteria.
Chronic lymphoedema and oedema, characterised by the accumulation of fluid in the tissues (usually of the limbs), foster the growth of bacteria and fungi and impair the body's ability to produce an appropriate local immune response (Mallon 1997; Mortimer 2014). Different methods have been described to treat lymphoedema and oedema, most of which are non‐operative and act principally by mechanical compression and increasing of blood and lymphatic flow: compressive bandages, elastic stockings, physical therapy and exercise. Diuretic treatment generally works through the production of urine and shifting of the body's fluids from the swollen tissues into the blood vessels, and weight loss works by reducing limb volume and the facilitation of vascular flow and lympathic drainage (Arsenault 2011; Ezzo 2015; Szolnoky 2014). Surgical techniques to treat lymphoedema have slowly been introduced, aiming to reconstruct a lymphatic drainage system and to remove overgrowing tissue, including the removal of fat tissue (liposuction) (Campisi 2015; Szolnoky 2014).
Why it is important to do this review
Cellulitis is a common disease treated in primary‐care settings, emergency rooms, and hospital departments by physicians of various specialties, including internal medicine, infectious diseases, dermatology, oncology, and different surgical subspecialties. Many people with cellulitis require hospitalisation with a relatively long length of stay for what can be regarded, in most cases, as a non‐fatal, simple‐to‐treat disease.
A significant proportion of all hospital admissions for cellulitis are for people experiencing recurrent episodes (Pavlotsky 2004). This population tends to occupy more bed days compared with the non‐relapsing group, which adds considerably to the costs and burden of this disease (Karppelin 2010). The economic burden of cellulitis was determined by a Dutch study, with an estimated total direct cost of more than EUR 17 million in 2001 (Goettsch 2006).
Despite this considerable burden, there is a lack of high‐quality, evidence‐based information regarding optimal treatments for the prevention of recurrent cellulitis. The purpose of this review is to summarise high‐quality research, giving clinicians the tools to provide more evidence‐based treatment for people with recurrent cellulitis, as well as delineating areas of focus for future research.
The plans for this review were published as a protocol 'Interventions for the prevention of recurrent erysipelas and cellulitis' (Dalal 2012).
Objectives
To assess the beneficial and adverse effects of antibiotic prophylaxis or other prophylactic interventions for the prevention of recurrent episodes of cellulitis in adults aged over 16.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Adults older than 16 years after an episode of erysipelas and cellulitis (hereafter referred to as 'cellulitis').
We excluded people with cellulitis or erysipelas secondary to filarial lymphoedema.
When a study included participants with various types of skin infections, we included the study only if it had reported separately on cellulitis or if authors provided data on the subgroup with cellulitis. In cases where results had not been given separately, we excluded the study if more than 30% of participants had infections other than cellulitis.
Types of interventions
Interventions
Any intervention aimed at preventing cellulitis. Specifically, we aimed to assess the following interventions:
-
antibiotic prophylaxis;
-
anti‐inflammatory prophylaxis;
-
compression stockings;
-
treatments for toe web intertrigo (including antifungal treatments for tinea pedis);
-
treatments for venous insufficiency;
-
other interventions to reduce leg oedema.
If we found other relevant interventions, we also included them. We accepted any duration of intervention.
Control
No treatment, placebo, or another intervention as defined above, or the same intervention with a different treatment schedule (dose, frequency, timing, duration).
We contacted authors for results relating to cellulitis if they were not reported separately.
Types of outcome measures
Primary outcomes
-
Recurrence of cellulitis (number of participants with at least one recurrent episode within a follow‐up period of at least three months after randomisation) under prophylactic treatment. When specified, we considered a recurrence as a repeat episode of cellulitis in the same limb.
-
For all outcomes, we extracted data on physician‐diagnosed cellulitis, preferably that of a dermatologist. However, if the diagnoses were based on the assessment of other physicians, we accepted these diagnoses and documented the person assessing the outcomes. We accepted the study definitions of cellulitis and documented the differences between studies.
-
The primary time point for analysis of all outcomes was at the end of the treatment phase ('on prophylaxis'). Secondary time points for analysis were: after prophylaxis discontinuation ('post‐prophylaxis') and at the end of follow‐up ('overall').
Secondary outcomes
-
Incidence rate of cellulitis, i.e. episodes of recurrent cellulitis per person‐months of follow‐up in the trial under prophylactic treatment; also assessed after prophylaxis discontinuation ('post‐prophylaxis') and at the end of follow‐up.
-
Time to next episode of cellulitis among all trial participants under prophylactic treatment; also assessed after prophylaxis discontinuation ('post‐prophylaxis') and at end of follow‐up.
-
Number of participants requiring hospitalisation and number of hospital days.
-
Quality‐of‐life measures, using the score or scale reported in the study.
-
Development of resistance to antibiotics. In studies that used preventive antibiotic treatment, we evaluated this outcome by assessing laboratory‐proven growth of resistant bacteria to the study drug/s and infections (cellulitis or other) caused by such bacteria.
-
Adverse reactions, including allergic reactions, skin reactions, and pseudomembranous colitis (mainly for antibiotic prophylaxis interventions).
-
Mortality.
Search methods for identification of studies
We aimed to identify all relevant RCTs regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
We searched the following databases up to 23 June 2016:
-
the Cochrane Skin Group Specialised Register using the following terms: (erysipelas or cellulitis or "ignis sacer" or "holy fire" or "st anthony's fire" or impetigo or "soft tissue infection*" or staphylococc* or streptococc*);
-
the Cochrane Central Register of Controlled Trials (CENTRAL) 2015, Issue 2, in the Cochrane Library using the search strategy in Appendix 1;
-
MEDLINE via Ovid (from 1946) using the strategy in Appendix 2;
-
Embase via Ovid (from 1974) using the strategy in Appendix 3; and
-
Latin American and Caribbean Health Science Information database (LILACS) (from 1982) using the strategy in Appendix 4.
Trials registers
We searched the following trials registers up to 22 August 2016, using the terms “cellulitis” or “erysipelas”:
-
the ISRCTN registry (www.isrctn.com);
-
the World Health Organization International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/);
-
the Australian New Zealand Clinical Trials Registry (www.anzctr.org.au);
-
ClinicalTrials.gov (www.clinicaltrials.gov); and
-
the EU Clinical Trials Register (www.clinicaltrialsregister.eu/).
Searching other resources
Reference lists
We checked the reference lists of included studies and review articles for further references to relevant trials.
Conference proceedings
We searched the following major dermatological conference proceedings for relevant studies by searching the formal electronic journal of each academy up to 22 August 2016:
-
the American Academy of Dermatology annual conference proceedings (from 1990); and
-
the European Academy of Dermatology and Venereology annual conference proceedings (from 1991).
Grey literature
We searched BIOSIS Previews (from 1990) for relevant studies up to 10 March 2015, using the terms “cellulitis” or “erysipelas”.
Correspondence
We contacted the authors of potentially relevant and unpublished trials to obtain full trial results.
Data collection and analysis
Selection of studies
Two authors (AD and SR) independently reviewed the titles and abstracts identified by the literature search. The same authors later reviewed the full‐text versions of the eligible studies. We evaluated the studies according to our preset criteria, resolving disagreements by referral to a third author (MP).
Data extraction and management
AD and MS independently extracted data from the included studies onto data extraction forms, discussing any differences with a third author (MP). AD checked and entered data into Review Manager 5. We extracted the following data:
(1) Trial characteristics
-
publication status (published, published as abstract, unpublished);
-
year (defined as recruitment initiation year) and country/s of study;
-
setting (hospital/outpatient);
-
design (method of allocation generation and concealment, blinding);
-
intention‐to‐treat analysis (performed, possible to extract, efficacy analysis);
-
cellulitis case definition (diagnosis and by whom);
-
exclusion criteria (age of participants, comorbidities, risk factors or their severity, infective agents, previous use of antibiotics);
-
duration of study follow‐up (from intervention and from the end of intervention);
-
funding;
-
ethical committee approval and informed consent.
(2) Baseline participant characteristics
-
number of participants eligible;
-
number of participants randomised;
-
mean age and sex distribution;
-
area of body involved;
-
number of cellulitis attacks and observation period prior to intervention;
-
possible risk factors for cellulitis and estimation of their severity:
-
-
percentage of participants with diabetes and its level of control ‐ HbA1C at baseline and during the trial
-
percentage of participants with oedema of any cause at baseline and during the trial. Severity was extracted descriptively or by any other score
-
percentage of participants with venous drainage impairment, diagnosed clinically or by imaging studies. Severity was recorded for clinical findings and imaging studies
-
percentage of participants with diagnosis of peripheral vascular disease or arterial insufficiency. Severity was recorded by the ankle brachial pressure index
-
mean body mass index (weight in kilograms, divided by height in metres squared) at baseline and during the trial. Alternatively, we extracted the percentage of participants with a diagnosis of overweight/obesity
-
any type of skin injury (i.e. surgery, ulcers, insect bites, burns, etc) prior to and during the trial follow‐up
-
diagnosis of toe web intertrigo or tinea pedis and antifungal treatment
-
co‐existing skin diseases
-
immunodeficiency ‐ hereditary or acquired, secondary to medications, chronic infections, malignancies, or chronic diseases
-
smoking status and alcohol consumption
-
(3) Interventions
-
type of intervention (if antibiotics or other medications) ‐ drug, dosage, schedule, way of administration;
-
concomitant medical advice, by any means, regarding diet, weight loss, physical activity, skin care, oedema reduction, or any other recommendations;
-
adherence.
(4) Outcomes
As defined above in our Methods section. If they were not reported numerically, we extracted outcomes from graphs or figures presented in the publications (by counting pixels).
Assessment of risk of bias in included studies
In the quality assessment, AD and MS independently evaluated the following components individually, since there is evidence that these are associated with biased estimates of treatment effect (Jüni 2001). We discussed any differences with a third author (MP). We used the criteria described in theCochrane Handbook for Systematic Reviews of Interventions (Table 8.5.d) (Higgins 2011) for quality assessment, categorising each study as high risk of bias, low risk of bias, or unclear risk of bias.
(a) Randomisation generation (method of allocation generation): we defined this as low risk of bias when the allocation procedure protects against biased allocation to the comparison groups
(b) Randomisation concealment (method of allocation concealment): we defined this as low risk of bias when neither clinicians nor participants were aware of future allocation
(c) Masking as blinding of participants/investigator and of the outcome assessor: we defined this as low risk of bias when events were as follows:
-
-
Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken.
-
There was no blinding or incomplete blinding, but the review authors judged that the outcome was not likely to be influenced by lack of blinding.
-
Blinding of outcome assessment was ensured, and it is unlikely that the blinding could have been broken.
-
There was no blinding of outcome assessment, but the review authors judged that the outcome measurement was not likely to be influenced by lack of blinding.
-
(d) Loss to follow‐up (information about dropouts and withdrawals, and the analysis of these): we defined this as low risk of bias when it was clear that all of the participants in the trial were analysed (i.e. 0% lost to follow‐up). Additionally, when participants had been analysed based on the intention‐to‐treat principle (where participants were analysed based on the treatment to which they were randomised).
(e) Selective reporting (reporting bias due to selective outcome reporting): we defined this as low risk of bias when the study protocol was available, and all of the study's prespecified (primary and secondary) outcomes that were of interest to the review had been reported in the prespecified way. Or if the study protocol was not available, but it was clear that the published reports included all expected outcomes, and these were reported numerically (rather than as "significant" or "non‐significant").
(f) We also assessed the following items:
-
-
Baseline imbalance: we defined this as low risk when the baseline characteristics of the treatment groups were adequately addressed and there was no systematic imbalance between groups;
-
Early termination: we defined this as low risk when the trial ended by achieving a predefined sample size or stopped early according to predefined stopping rules. We defined high risk of bias as trials with premature termination of the trial, contrary to predefined stopping rules. In other cases (no sample size definition) we defined early termination as unclear risk of bias;
-
Other potential bias: we defined this as low risk of bias when the study appeared to be free of other sources of bias.
-
Measures of treatment effect
For dichotomous outcomes, we calculated risk ratios (RRs) and corresponding 95% confidence intervals (CIs) from individual studies. When the outcome to be assessed was episodes (outcomes occurring more than once per participant) we calculated rate ratios, defined as episodes/person‐month, and the corresponding 95% CI.
For time‐to‐event outcomes, we attempted to extract the hazard ratio (HRs) with a 95% CI from included trials. If this had not been reported, we estimated it from Kaplan‐Meier curves or other statistics reported in the study (Parmar 1998; Tierney 2007).
Unit of analysis issues
We considered participants as the unit of analysis and not limbs; i.e. recurrence included both ipsilateral and contralateral episodes of cellulitis. We did not expect cross‐over trials to be carried out, since this design would not be suitable for the evaluation of prophylactic treatment (mainly because the first treatment may significantly influence the course of the disease).
Dealing with missing data
We performed an intention‐to‐treat (ITT; see Table 1) analysis when possible, and only used per protocol data, documenting it accordingly, if information was not specified in the study or retrieved from trial investigators. One author (AD) contacted trials authors to ask for missing data.
Assessment of heterogeneity
Where we identified enough studies assessing the same intervention and performing the same comparison to combine them, we assessed heterogeneity visually by inspecting the forest plot and looking at the magnitude and direction of the study results; if relevant, we reported on the I² statistic value (Higgins 2011).
Assessment of reporting biases
The capacity of funnel plots to detect bias in a small number of included studies is limited (Egger 1997). As expected, we were unable to assess reporting bias using funnel plots, due to the small number of studies in each comparison.
Data synthesis
We performed a meta‐analysis if we found more than one study assessing the same intervention and outcome. We pooled data for dichotomous outcomes using the Mantel‐Haenszel fixed‐effect model to calculate a treatment effect across trials, when heterogeneity was low and the beneficial effects of small studies would be overestimated by the random‐effects analysis. If heterogeneity between trials was significant (I² > 50%) we used the random‐effects model. We expressed results as the RR with 95% CI. We pooled HRs with 95% CIs for time‐to‐event outcomes, using the inverse variance method in a fixed‐effect or random‐effects model, according to our previously‐mentioned evaluation.
Subgroup analysis and investigation of heterogeneity
We planned to explore reasons for substantial heterogeneity (I² > 50%) in any meta‐analyses we performed, using the following subgroups:
-
lower versus upper limb cellulitis;
-
participants with versus those without limb oedema at baseline (randomisation);
-
participants with a single episode of cellulitis versus those with at least two episodes at baseline (randomisation); and
-
different types of antibiotic.
When the planned analyses were irrelevant or data were scarce, we discuss narratively the potential factors contributing to heterogeneity.
Sensitivity analysis
Where we found substantial heterogeneity in the meta‐analyses, we planned to explore the reasons by sensitivity analyses, including the quality of included studies.
For all outcomes, we primarily extracted data on physician‐diagnosed cellulitis, and as a secondary analysis, we used the data on participant‐reported episodes.
'Summary of findings' table
We created a 'Summary of findings' table that reported the following main outcomes at the primary time point for analysis (end of prophylaxis phase ('on prophylaxis')): recurrence of cellulitis; incidence rate of cellulitis; time to next episode of cellulitis; hospitalisation; any adverse reactions; and quality of life.
We adopted the GRADE approach to assess the quality of evidence using five factors: study limitations, indirectness, inconsistency, imprecision, and publication bias. If we found a reason to change the grading of the quality of evidence, we detailed it in the footnotes.
We used methods and guidance described in chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and in the GRADE handbook (Schünemann 2013), and we used the GRADEproGDT web‐based software (available at www.gradepro.org) to produce the 'Summary of findings' table.
Results
Description of studies
Results of the search
We identified 6139 records, using an inclusive and comprehensive search strategy. After removing duplicates, we had 5995 records. We excluded 5979 records based on titles and abstracts, and sought the full text of 16 studies. We retrieved the full text of 15 studies. For one (Ratnikova 1991) we had only an abstract, and we have listed the details of this study in Characteristics of studies awaiting classification. We hope to examine its full text in a future update of this review. Of the remaining 15 papers, we excluded eight (see Characteristics of excluded studies). We included six studies reported in seven publications (two reports represent one study and we included them under a single trial ID: Kasseroller 1998). We have summarised the screening process in the 'Study selection flow diagram' (Figure 1).

Study selection flow diagram.
Included studies
The six studies cover 595 participants, of which 573 were evaluated.
Design
All of the studies were randomised controlled trials using a two‐arm, parallel‐group design. Three studies had a placebo arm (Kasseroller 1998; Thomas 2012; Thomas 2013) and none of the included studies was a cross‐over trial.
Of the six included studies, four were single‐centre (Chakroun 1994; Kasseroller 1998; Kremer 1991; Sjöblom 1993) and two were multicentre with 20 (Thomas 2012) and 28 sites (Thomas 2013). The sample sizes of the studies ranged from 32 to 274 participants.
Setting
The included studies were carried out in five countries: UK (two studies: Thomas 2012; Thomas 2013), Sweden (Sjöblom 1993), Tunisia (Chakroun 1994), Israel (Kremer 1991) and Austria (Kasseroller 1998).
Four studies were hospital‐based (Chakroun 1994; Sjöblom 1993; Thomas 2012; Thomas 2013 ), one implied it was secondary‐care‐based (Kremer 1991), and one was conducted within a private clinic (Kasseroller 1998).
Most of the included studies were published in English. One study was printed in French (Chakroun 1994), and one report of a single trial (Kasseroller 1998) was published in German (Kasseroller 1996).
The Thomas 2012 and Thomas 2013 studies were led by the same research team.
Participants
Of the evaluable participants, 200 were men and 373 women.
The mean age of participants in the included studies was between 50 and 70 (Kasseroller 1998; Kremer 1991; Sjöblom 1993; Thomas 2012; Thomas 2013). In one study the mean age of participants was 46.2 (Chakroun 1994).
The number of previous episodes of cellulitis at recruitment to trial was at minimum: four episodes in one study (Kasseroller 1998), two episodes in three studies (Kremer 1991; Sjöblom 1993; Thomas 2013) and one episode in one study (Thomas 2012). The time interval to recurrence of cellulitis before trial entry was three years in two studies (Sjöblom 1993; Thomas 2013) and in other studies two years (Kasseroller 1998) and one year (Kremer 1991). Thomas 2012 included participants with one previous episode within 12 weeks from inclusion.These data were not reported in the Chakroun 1994 study.
In five of the included studies the vast majority of participants had past episodes of leg cellulitis at baseline (Chakroun 1994; Kremer 1991; Sjöblom 1993; Thomas 2012; Thomas 2013). In two of these studies three participants in the control group had upper limb cellulitis before entering the trial (two participants in the Kremer 1991 study and one participant in the Sjöblom 1993 study). Kasseroller 1998 investigated upper limb cellulitis in women after mastectomy (Table 1).
Four out of six studies described the clinical criteria for the diagnosis of past episodes of cellulitis at inclusion: in two studies the diagnosis of cellulitis was based on fever and local signs of skin infection/inflammation (Chakroun 1994;Sjöblom 1993); in Thomas 2012 and Thomas 2013 the diagnosis was either made by a physician or validated according to detailed clinical criteria of skin infection/inflammation, and any doubt over the certainty of the diagnosis was grounds for exclusion. One study mentioned parameters for diagnosis (physical examination and blood test markers) but it is unclear whether these were assigned to past episodes (Kasseroller 1998), and one study did not state criteria for the definition of cellulitis (Kremer 1991).
The baseline comorbidities of participants were reported in five out of six studies (Chakroun 1994; Kremer 1991; Sjöblom 1993; Thomas 2012; Thomas 2013), with Kasseroller 1998 not reporting any comorbidity data. The comorbidity profile of participants within the same study was similar. Variable data were reported for comorbidities from different studies: 5% to 25% of participants had diabetes mellitus (Chakroun 1994; Kremer 1991; Sjöblom 1993; Thomas 2012; Thomas 2013), 5.5% to 90% had venous insufficiency (Chakroun 1994; Sjöblom 1993; Thomas 2012; Thomas 2013), 10% to 68% had leg oedema (Chakroun 1994; Sjöblom 1993; Thomas 2012; Thomas 2013), 30% to 50% of participants had fungal foot infection (Chakroun 1994; Kremer 1991; Thomas 2012; Thomas 2013), and most participants were overweight (Chakroun 1994; Thomas 2012; Thomas 2013).
Interventions
Five studies evaluated antibiotic treatment (Chakroun 1994; Kremer 1991; Sjöblom 1993; Thomas 2012; Thomas 2013) and one study evaluated treatment with oral selenium (Kasseroller 1998).
1. Antibiotic therapy
-
Penicillin was used in four studies (Chakroun 1994; Sjöblom 1993; Thomas 2012; Thomas 2013) and erythromycin was used in one study (Kremer 1991). Three studies evaluated oral ingestion of penicillin (penicillin V) (Sjöblom 1993; Thomas 2012; Thomas 2013), at a dose of 250 mg twice a day in two studies (Thomas 2012; Thomas 2013) and 2 grams to 4 grams a day in one study (depending on participant's weight: 1 gr twice a day if < 90 kg; 1 gr + 2 gr a day if 90 kg to 120 kg; 2 gr twice a day if > 120 kg) (Sjöblom 1993). In Chakroun 1994 penicillin (benzathine penicillin) was injected into the muscle at a dose of 1.2 million units every 15 days. Kremer 1991 used erythromycin at a dose of 250 mg twice a day given by mouth. In two studies the control group received placebo (Thomas 2012; Thomas 2013).
-
In three out of four studies assessing penicillin, participants who were allergic to the drug were excluded from the trial (Chakroun 1994; Thomas 2012; Thomas 2013) and in one these participants received an alternative treatment with erythromycin at a dose of 250 mg to 500 mg twice a day, depending on participant's weight (Sjöblom 1993). Kremer 1991 did not refer participants with known allergy to erythromycin.
-
The duration of antibiotic therapy varied from six to 18 months across studies (Chakroun 1994; Kremer 1991; Sjöblom 1993; Thomas 2012; Thomas 2013). Treatment periods varied significantly among participants in the Chakroun 1994 study and lasted one to 38 months, with a mean duration of 11.6 months.
-
In four studies medical advice or treatment were given to both treatment and control arms: participants with fungal foot infection were treated with antifungal agents in one study (Kremer 1991), local skin care and the use of compression stockings/elastic bandages were recommended in one study (Sjöblom 1993), and treatment of predisposing factors, such as fungal foot infection, was given in two studies (stated as "normal clinical practice") (Thomas 2012; Thomas 2013).
-
Among the six included studies, two studies reported follow‐up, with follow‐up periods for the majority of participants of 18 months to two years after the treatment stopped (Thomas 2012; Thomas 2013).
2. Selenium therapy
Kasseroller 1998 used oral ingestion of sodium selenite solution given at daily doses of 1000 μg (micrograms) in the first week, 300 μg in the second and third weeks and 100 to 200 μg (depending on participant's weight) from the fourth to the 15th week. The participants in the control group were given physiological salt solution. In the first three weeks all participants were admitted to the medical centre for an intensive "congestive relief" therapy that consisted of manual lymph drainage, compression bandage, meticulous skin care, therapeutic exercises and high‐voltage therapy. No follow‐up period was reported after 15 weeks.
Outcomes
Primary outcome
-
All included studies reported the number of participants with recurrent episodes of cellulitis. Of the six studies, two reported on cellulitis recurrence after the discontinuation of treatment ('post‐prophylaxis') (Thomas 2012; Thomas 2013).
-
Five out of six studies mentioned the clinical findings for the diagnosis of cellulitis or its confirmation (Chakroun 1994; Kasseroller 1998; Sjöblom 1993; Thomas 2012; Thomas 2013).
-
In two studies the diagnosis of cellulitis was based on fever and local signs of skin infection/inflammation, and was established by an infectious diseases specialist (Chakroun 1994; Kremer 1991).
-
In Thomas 2012 and Thomas 2013 diagnosis of cellulitis before trial entry was established by a dermatologist, either by examination of the participant or by validation of the diagnosis from medical records according to prespecified criteria that indicated a skin infection/inflammation (such as pain, local warmth, tenderness, swelling, redness); during the trial period a new episode of cellulitis was defined as reported by the participant and confirmed by a medical practitioner. New episodes that were only self‐reported were included in sensitivity analysis (Table 1).
-
In Kasseroller 1998 diagnosis of cellulitis prior to enrolment was carried out in general or university hospitals; after enrolment the diagnosis was based on findings of the physical examination and blood test markers of inflammation. The report did not state who made the diagnosis or provide further data on its establishment.
-
One study (Kremer 1991) did not provide any details on how the diagnosis was made.
Secondary outcomes
-
Four studies reported incidence rate (Table 1) of cellulitis during treatment ('on prophylaxis') (Chakroun 1994; Kremer 1991; Thomas 2012; Thomas 2013); two reported the incidence rate after the treatment was stopped ('post‐prophylaxis') (Thomas 2012; Thomas 2013).
-
Three studies reported time to next episode of cellulitis 'on prophylaxis' (Sjöblom 1993; Thomas 2012; Thomas 2013); in Sjöblom 1993 these data were extracted from a survival curve; two of these three studies reported time to next episode of cellulitis 'post‐prophylaxis' (Thomas 2012; Thomas 2013).
-
Three studies provided data regarding hospitalisation of participants (Kremer 1991; Thomas 2012; Thomas 2013): all three reported the number of participants hospitalised, but only two stated the length of stay in hospital (Kremer 1991; Thomas 2013). A combined analysis of Thomas 2012 and Thomas 2013 for this outcome was described in a separate publication (Mason 2014).
-
Quality of life was not reported separately for any of the included studies. Quality‐of‐life measures were used in a combined analysis of Thomas 2012 and Thomas 2013 (Mason 2014).
-
The development of resistance to antibiotics was not examined in any of the included trials.
-
Adverse reactions were reported in five included studies (Chakroun 1994; Kremer 1991; Sjöblom 1993; Thomas 2012; Thomas 2013), but not in Kasseroller 1998.
Excluded studies
We excluded eight studies, reporting the reasons for their exclusion in the Characteristics of excluded studies tables. Three studies were not RCTs (Duvanel 1986; Haustein 1989; Wang 1997), two studies investigated preventive treatment for other skin infections (skin abscesses in Klempner 1988 and folliculitis/furunculosis (Table 1) in Raz 1996) and two studies were in children (Ferrieri 1973; Maddox 1985). After email communication with trial investigators we confirmed that another study focused on recurrent skin abscesses in children (more than 70% of participants were children) (Fritz 2011).
Studies awaiting classification
One study, Ratnikova 1991, is awaiting classification. For details, please see the Characteristics of studies awaiting classification table.
Risk of bias in included studies
We provide summaries of the risks of bias across the included studies in Figure 2 , Figure 3, and the Characteristics of included studies tables.
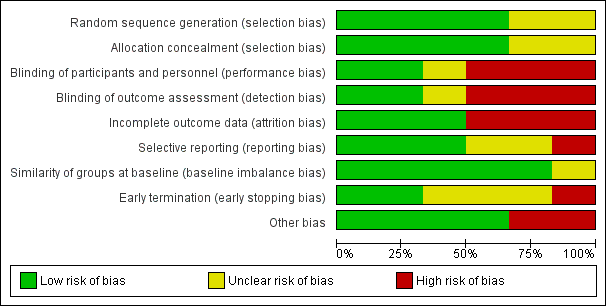
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
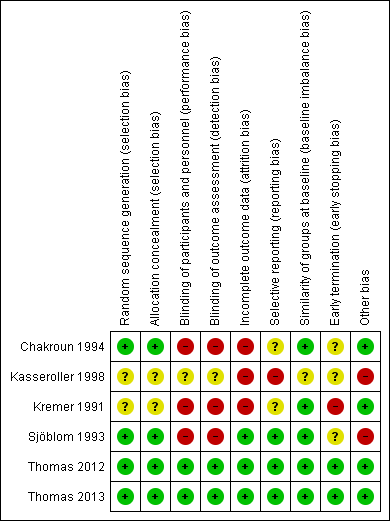
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
When the risk of bias information was not available in the study, we sought further data by correspondence with study investigators. We were answered by the investigators of five out of six studies (Chakroun 1994; Kremer 1991; Sjöblom 1993; Thomas 2012; Thomas 2013) (Table 2). For one of these studies the investigator could not provide details due to the age of the study, as the relevant data were not available (Kremer 1991). We sent emails, a letter and also attempted to contact the sole author of the Kasseroller 1998 study through his professional website but received no reply.
| Study | Way of communication | Date | Information provided | Notes |
| | 12/2013 | ‐ Allocation concealment ‐ Participants follow‐up ‐ Criteria for diagnosis ‐ Adherence ‐ Adverse reactions ‐ Informed consent ‐ Ethical committee approval ‐ Source of funding | Full information was not available for all queries, but investigators responded to all of them | |
| airmail, email, website | 2013 ‐ 2014 | ‐ | Investigator did not reply to our queries We also contacted a potential sponsor, not reported by the author, who confirmed their financial support for the conduct of this study (email correspondence with the head of medical‐scientific department of 'biosyn Arzneimittel GmbH' from January 2015) | |
| email and telephone | 12/2013 and 1/2014 | ‐ | Data were not available and the investigator did not remember any details | |
| | 12/2013 | ‐ Allocation concealment ‐ Participants follow‐up ‐ By whom cellulitis was diagnosed ‐ Adherence ‐ Source of funding | Full information was not available for all queries, but investigators responded to all of them | |
| | 1/2014 | ‐ Episodes of recurrent cellulitis per person‐months (incidence rate) ‐Time to next episode ‐ Adverse events by study arm ‐ Duration of hospitalisation ‐ Quality of life | ‐ | |
| | 1/2014 | ‐ Episodes of recurrent cellulitis per person‐months (incidence rate) ‐ Quality of life | Hospitalisation and quality of life were not evaluated directly in this trial but were reported by indirect evaluation in Mason 2014 |
Allocation
Sequence generation
We judged four studies to be at low risk of bias for this domain (Chakroun 1994; Sjöblom 1993; Thomas 2012; Thomas 2013). In two of them investigators used an independent party to provide randomised codes, which were generated by computer programmes (Thomas 2012; Thomas 2013), one used stratified block randomisation (Table 1) (Sjöblom 1993) and in Chakroun 1994 the investigators drew lots to assign participants to treatment or control groups. Two studies did not state how they generated allocation sequences and we rated them at unclear risk of bias (Kasseroller 1998; Kremer 1991).
Allocation
In two studies allocation concealment was verified using a computer‐based allocation system by a central co‐ordinating team, and we judged them to be at low risk of bias (Thomas 2012; Thomas 2013). Following communication with investigators, we rated two additional studies at low risk of bias for this domain (Chakroun 1994; Sjöblom 1993). In Kasseroller 1998 and Kremer 1991 the method used to conceal the allocation sequence was not reported and we therefore judged them to be at unclear risk of bias for this domain.
Blinding
Three studies did not blind participants or personnel to the intervention being studied so we rated them at high risk of bias (Chakroun 1994; Kremer 1991; Sjöblom 1993).
We judged two studies to be at low risk for performance bias and detection bias (Thomas 2012; Thomas 2013). In these studies treatment allocation was concealed throughout the trial, with the randomisation code held by the trial centre and analysis of the results performed prior to breaking the code. In addition, participants in the control group were given placebo tablets that were of the same size and shape as the penicillin tablets. The investigators noted that the penicillin and placebo tablets were not identical, due to technical reasons, but this was balanced by the wide geographic area from which participants were recruited, thus minimising the possibility of comparison of the different kinds of tablets. The study also included an assessment of blinding effectiveness, as participants were asked to guess which treatment they had received. Thomas 2012 reported that only 13% of participants correctly guessed what treatment they were on, based on the smell, taste or look of the penicillin (or absence, for placebo). The potential for detection bias was further reduced by confirmation of cellulitis cases on the basis of medical records.
Kasseroller 1998 was reported to be a 'double‐blind study', yet did not provide information on the blinding of participants, personnel or assessors. In this study the participants in the control arm received physiological salt solution and in the treatment arm selenium dissolved in physiological salt solution. It is unclear whether there were differences between these two solutions by appearance, taste, smell or adverse reactions, and we rated the risk of bias due to blinding for this study as unclear.
Incomplete outcome data
We judged three studies to be at low risk of bias, with data presented for all participants and ITT analyses (Table 1) performed (Sjöblom 1993; Thomas 2012; Thomas 2013). Two of them provided complete flow charts of participants during the trial (CONSORT flow diagram) (Thomas 2012; Thomas 2013). There was high risk of attrition bias, associated with withdrawals or dropouts, in three studies (Chakroun 1994; Kasseroller 1998; Kremer 1991) and we therefore conducted a 'per protocol' analysis (Table 1).
Selective reporting
We judged three studies to be at low risk of bias: in Thomas 2012 and Thomas 2013 outcomes were reported as mentioned in the protocol, that had been registered and was available online, and any changes to the prespecified outcomes were explained. In Sjöblom 1993 a protocol was not available, but based on the data in the Methods section of the report, it appears to have reported all prespecified outcomes and we therefore judged it to be free of selective reporting. We judged that the Kasseroller 1998 study could have introduced an element of bias through selective outcome reporting, as it failed to include results that would have been expected to have been reported for such a study, i.e. adverse events. In addition, the investigator reported measurements of selenium blood levels but this outcome had not been prespecified in the Methods section or sufficiently explained.
Two studies did not provide enough information to determine if the prespecified outcomes had been adequately reported; we therefore judged them to be at an unclear risk of bias (Chakroun 1994; Kremer 1991).
Other potential sources of bias
Baseline imbalance
We judged the risk of bias as low in five out of six studies (Chakroun 1994; Kremer 1991; Sjöblom 1993; Thomas 2012; Thomas 2013). Kasseroller 1998 did not report on important baseline characteristics of the treatment groups (comorbidities, prior oncological treatment) and we rated it at unclear risk of bias.
Early termination
We judged three of the studies to be at unclear risk of bias (Chakroun 1994; Kasseroller 1998; Sjöblom 1993), as prespecified stopping rules or sample size calculations were not reported and the duration/termination of the trial was not explained. Two studies addressed the sample size calculation methods (Thomas 2012; Thomas 2013); in one of them recruitment was stopped before the target number was attained due to slow recruitment of participants (Thomas 2012), with reasons provided and well analysed (Thomas 2010); we therefore rated these studies at low risk of bias. Kremer 1991 reported termination of the trial based on the apparent efficacy of the intervention group, and was assessed as being at high risk of bias because its early stopping for benefit might have overestimated the treatment effect.
Other potential bias
We rated bias associated with other causes as low in four studies (Chakroun 1994; Kremer 1991; Thomas 2012; Thomas 2013), and high in two studies (Kasseroller 1998; Sjöblom 1993).
Kasseroller 1998 reported two periods of intervention for all the participants in the trial: the first three weeks of inpatient intensive therapy was followed by three months of outpatient follow‐up, both in parallel with the consumption of selenium or physiological salt solution. The article did not report the method of follow‐up after the first three weeks of inpatient care, having stated that many of the clinic's patients came from abroad. We thought that possible differences in follow‐up methods might introduce considerable bias, and deemed the available data on the method of follow‐up as insufficient. We therefore evaluated the study as being at high risk of bias.
In Sjöblom 1993 the investigators used fixed and known block sizes for randomisation which might allow prediction of treatment assignment, and so we classed this as being at high risk of bias.
Effects of interventions
Please see our Glossary (Table 1) for an explanation of terms used in this section. For the exact definition of outcomes please see Types of outcome measures.
Antibiotics versus no treatment/placebo
See: summary of findings Table for the main comparison 'Antibiotic prophylaxis compared to no treatment/placebo'
Primary outcome
1. Recurrence
All five studies, evaluating 513 participants, reported on recurrence of cellulitis, i.e. number of participants with at least one repeat episode of cellulitis during the study period/number of evaluable participants. Pooling of the studies showed that antibiotic prophylaxis significantly reduced the recurrence of cellulitis during treatment: risk ratio (RR) 0.31 (95% confidence interval (CI) 0.13 to 0.72; P = 0.007; moderate‐certainty evidence) with moderate heterogeneity (I² = 43%; Analysis 1.1). The number of participants needed to treat to prevent one episode of cellulitis when on treatment ('on prophylaxis') was 6 (95% CI 5 to 15) with a control event rate (CER) of 83/263 (32%).
Only two studies (287 participants) continued to follow up participants after the cessation of treatment ('post‐prophylaxis') (Thomas 2012; Thomas 2013). Pooling the data did not show significant differences in recurrence after the treatment was stopped (RR 0.88, 95% CI 0.59 to 1.31; P = 0.52; I² = 0%; low‐certainty evidence; Analysis 2.1). We downgraded the evidence by two levels for imprecision (small sample size and low event rate, and a wide confidence interval including benefit and harm).
Similarly, evaluation of the overall effect of antibiotic prophylaxis ('overall trial') on cellulitis recurrence was available for Thomas 2012 and Thomas 2013, including 397 participants, with their results pooled into meta‐analysis that showed that antibiotic prophylaxis significantly reduced the recurrence of cellulitis: RR 0.75, 95% CI 0.59 to 0.95; P = 0.02; the number of participants needed to treat to prevent one episode of cellulitis was 8, 95% CI 5 to 42 (CER of 94/201 (47%)) with no heterogeneity (I² = 0%; Analysis 3.1).
Only one study included participants with a single episode of cellulitis at trial entry (Thomas 2012), and we were therefore unable to perform the planned analysis of participants with a single compared with at least two episodes of cellulitis at baseline. This study reported a reduction of recurrent episodes of cellulitis with six months treatment of antibiotic prophylaxis: 13/49 (27%) participants suffered a recurrent event in the control group and 8/48 (17%) participants in the treatment group. This result (RR 0.55, 95% CI 0.21 to 1.49; P = 0.24) was not statistically significant.
Secondary outcomes
1. Incidence rate
Data for this outcome were available from four studies (Chakroun 1994; Kremer 1991; Thomas 2012; Thomas 2013), including 256 episodes of cellulitis and 4375 person‐months. Antibiotic prophylaxis ('on prophylaxis') significantly reduced the incidence rate of cellulitis: RR 0.44, 95% CI 0.22 to 0.89; n = 473; P = 0.02; moderate‐certainty evidence, with moderate heterogeneity between studies (I² = 54%; Analysis 1.2). However, all studies indicated a consistent direction of benefit for use of antibiotics.
Subgroup analysis according to our predetermined subgroups (Subgroup analysis and investigation of heterogeneity) for this outcome was limited both by its irrelevance for the included studies and the small number of studies. Nevertheless, inspection of the forest plot for this analysis (Analysis 1.2) indicates a clear difference between the smaller trials (Chakroun 1994; Kremer 1991, including 44 and 40 evaluable participants, respectively) and the larger PATCH trials (Thomas 2012; Thomas 2013, including 123 and 274 participants, respectively), with the first group showing only few recurrent episodes in the control arm and no recurrence in the treatment arm. Possible reasons for this difference are the small number of participants in Chakroun 1994 and Kremer 1991, variable prophylactic regimens (including type, route and dosage of antibiotics) and methodological limitations of Chakroun 1994 and Kremer 1991 (mainly the lack of blinding and selective reporting).
As only two studies followed up participants after the treatment had stopped (Thomas 2012; Thomas 2013), a meta‐analysis of their results showed that incidence rates did not significantly change between groups after the cessation of antibiotics ('post‐prophylaxis'): RR 0.94, 95% CI 0.65 to 1.36; P = 0.74; I² = 0%; including 4566 person‐months and 109 episodes of cellulitis, low‐certainty evidence (Analysis 2.2). We downgraded the evidence by two levels for imprecision (small sample size and low event rate, and a wide confidence interval including benefit and harm). However, its overall benefit (both on and post‐prophylaxis) was still statistically significant (RR 0.69, 95% CI 0.56 to 0.85; P = 0.0005; I² = 0%; including 7854 person‐months and 340 episodes of cellulitis; Analysis 3.2), in agreement with the meta‐analyses for the primary outcome.
2. Time to next episode
Time to next episode of cellulitis was reported in two studies (Thomas 2012; Thomas 2013) and extracted from a survival curve of another study (Sjöblom 1993). The Thomas 2013 article provided exact figures with median time to first recurrence of cellulitis of 1 year and 8.4 months in the treatment group and 1 year and 5.4 months in the control group. Overall, three studies, including 437 participants, contributed to this outcome with a 49% lower risk of an episode in the antibiotic prophylaxis group 'on prophylaxis' (pooled hazard ratio (HR) of 0.51, 95% CI 0.34 to 0.78; P = 0.002; I² = 0%; moderate‐certainty evidence; Analysis 1.3). Similar to other outcomes, the beneficial effects of antibiotics ceased after the treatment stopped ('post‐prophylaxis'), with a pooled HR of 0.78, 95% CI 0.39 to 1.56; P = 0.48; I² = 57%; low‐certainty evidence (Analysis 2.3), from two studies (Thomas 2012; Thomas 2013), including 287 participants. We downgraded the evidence by two levels for imprecision (small sample size and low event rate, and a wide confidence interval including benefit and harm). As with other outcomes, subgroup analysis was limited by the small number of trials included in the analysis.
3. Hospitalisation
Three studies (429 participants) reported on the number of participants who required hospital admission (Kremer 1991; Thomas 2012; Thomas 2013). Overall, those on antibiotic prophylaxis were less likely to be hospitalised, but this was not statistically significant: RR 0.77, 95% CI 0.37 to 1.57; P = 0.47; I² = 0%; low‐certainty evidence (Analysis 1.4).
Only one study reported on the length of hospitalisation (Thomas 2013), finding no difference in duration of stay, with an overall mean of 10 days in the prophylaxis group (standard error of ± 7.1 days) compared with 9.2 days (standard error of ± 6.7 days) in the placebo group. A combined analysis of Thomas 2012 and Thomas 2013 did not identify a significant reduction in the number of hospital admissions or their duration (Mason 2014).
4. Quality of life
None of the six included studies reported directly on quality of life (QoL).
Mason 2014, which presented combined analysis of data from Thomas 2012 and Thomas 2013, indirectly assessed the influence of cellulitis on QoL using the European Quality of Life 5‐Dimensions questionnaire and the Dermatitis Quality of Life Index (EQ‐5D and DQLI, respectively).
The EQ‐D5 is a five‐dimension questionnaire used to measure health‐related QoL, consisting of two parts: self‐assessment questionnaire in five dimensions, i.e. mobility, self‐care, usual activities, pain/discomfort and anxiety/depression, and a self‐rated health status using a visual analogue scale. The DQLI is a 10‐item questionnaire used to measure QoL in people with a skin condition. The questions concern the influence of the disease on different aspects of life, including symptoms, self‐perception, social, interpersonal and daily activities. Each of the 10 questions is scored with a maximum of four points, which are then summed to a total score (0 to 30); higher scores mean greater impairment of quality of life (Finlay 1994).
In Mason 2014 the EQ‐D5 and the DQLI were used by participants during and 10 days after an episode of cellulitis, but before the start of trial prophylaxis, and were later used to approximate the detrimental effect of a recurrence of cellulitis on QoL. On average, an episode of cellulitis caused 26.3% reduction in QoL as measured by EQ‐5D (95% CI 18% to 35%, P < 0.001) and a deterioration of nearly 10 points as measured by DQLI (95% CI 7.83 to 11.5, P < 0.001). The investigators noted that they attempted to collect QoL data during the trials (Thomas 2012; Thomas 2013), but this was unsuccessful due to a time lag in being notified of the recurrent event.
5. Development of antibiotic resistance
We planned to evaluate the development of bacterial resistance according to the results of microbial cultures and antibiotic sensitivity testing in studies that used antibiotic prophylaxis. However, no trial properly examined the development of antibiotic resistance. Bacteriological surveillance cultures were performed in one study (Sjöblom 1993), but they were not taken from all the participants and no details of bacterial resistance were reported.
6. Adverse reactions
Four trials reported on the occurrence of any adverse reactions (Kremer 1991; Sjöblom 1993; Thomas 2012; Thomas 2013). These studies included 469 participants. There was no significant difference in adverse events between the two groups, with an overall pooled estimate of RR 0.87 (95% CI 0.58 to 1.30; P = 0.48; I² = 19%; low‐certainty evidence; Analysis 1.5). Synthesising the studies for adverse reactions did not show considerable heterogeneity, but we considered possible differences in adverse drug reaction profiles between studies, influenced by factors such as the type of antibiotic, its dosage and unblinding of trial participants to their treatment.
Assessing the clinical applicability of our findings, we performed a post hoc subgroup analysis on the type of antibiotic, including three studies that used oral penicillin (Sjöblom 1993; Thomas 2012; Thomas 2013), which demonstrated similar results, albeit with higher precision and consistency (RR 0.81, 95% CI 0.60 to 1.10; n = 437; P = 0.18; I² = 0%; low‐certainty evidence; Analysis 1.5).The P value for the test for subgroup differences was 0.15 when comparing penicillin versus erythromycin.
The common adverse reactions were: gastrointestinal symptoms, mainly nausea and diarrhoea, rash (severe cutaneous adverse reactions were not reported) and thrush. The authors of Thomas 2013 noted that no clostridium difficile colitis infections were reported, but none of the four studies actively tested participants with gastrointestinal symptoms for clostridium difficile.
Adverse effects that required discontinuation of the assigned therapy were reported in three studies (Kremer 1991; Sjöblom 1993; Thomas 2012): in Kremer 1991 three participants developed abdominal pain and nausea while treated with erythromycin and their treatment was changed to penicillin; in Sjöblom 1993 two participants stopped treatment with penicillin because of diarrhoea or nausea; and in Thomas 2012 five participants stopped treatment (three in the placebo group and two in the penicillin group) because of adverse reactions that were not further described. Another study did not report on adverse reactions, but the investigator noted in an email correspondence that approximately 10% of participants stopped treatment due to pain at the injection site (the active treatment group was given intramuscular injections of benzathine penicillin) (Chakroun 1994).
7. Mortality
Three studies including 437 participants reported on mortality (Sjöblom 1993; Thomas 2012; Thomas 2013). Overall 20 deaths occurred: nine in the control group and 11 in the penicillin group, none of which was related to treatment, and when data were pooled there was no statistically significant difference in mortality between the two groups (RR 1.12, 95% CI 0.32 to 3.91; P = 0.86; I² = 37%; Analysis 1.6).
Selenium versus physiological salt solution
Primary outcome
1. Recurrence
Only one study was included in this comparison (Kasseroller 1998). The study author reported that in the selenium group, 0 out of 29 participants had recurrent episodes of cellulitis. In the group of participants who received physiological salt solution, 15 out of 28 participants suffered recurrence (54%): one participant in the early inpatient‐care phase of the trial, followed by 14 participants in the ambulatory‐care phase.
Secondary outcomes
This study did not measure or report any of the relevant secondary outcomes: incidence rate, time to next episode, hospitalisation, quality of life, adverse reactions or mortality.
Sensitivity analysis
We had planned to explore heterogeneity through a sensitivity analysis; however, paucity of data for the relevant interventions made sensitivity analysis impractical.
Discussion
Summary of main results
The objective of this review was to summarise all available interventions for the prevention of cellulitis or erysipelas. A comprehensive search yielded six studies (573 evaluable participants), of which five assessed antibiotics versus no treatment (three studies) or placebo (two studies) in people with leg cellulitis, and one study evaluated prophylactic treatment with selenium solution against physiological salt solution in women after mastectomy.
A meta‐analysis of results from five studies, based on four studies evaluating penicillin and one evaluating erythromycin, demonstrated clear effectiveness of antibiotics compared to no treatment or placebo for the prevention of recurrent leg cellulitis, with a 69% reduction in the number of participants who were under prophylactic treatment (moderate‐certainty evidence; summary of findings Table for the main comparison). However, when treatment stopped ('post‐prophylaxis'), the difference was no longer statistically significant (low‐certainty evidence).
Antibiotics were also shown to reduce the incidence rate of leg cellulitis by 56% when compared with no treatment or placebo (256 episodes of leg cellulitis over 4375 person‐months, n = 473, four studies; moderate‐quality evidence; summary of findings Table for the main comparison) and significantly decreased the rate until the next episode under prophylactic treatment (n = 437, three studies, moderate‐certainty evidence; summary of findings Table for the main comparison). Nevertheless, the protective effect of antibiotics seems to wane after the treatment stops ('post‐prophylaxis') for these two outcomes (low‐certainty evidence).
There was insufficient information to determine the role of antibiotic prophylaxis after a single episode of leg cellulitis, which was not statistically significant (one study). The effects are relevant mainly for people after at least two episodes of leg cellulitis occurring within a period up to three years.
With regard to other secondary outcomes, a meta‐analysis of results from three studies showed there was no difference in hospitalisation between participants on antibiotic prophylaxis and those given no treatment or placebo (three studies, n = 429, low‐certainty evidence; summary of findings Table for the main comparison), but the current data did not allow us to explore its impact on length of hospital stay.
None of the studies reported severe side effects, with the main reactions being gastrointestinal symptoms, mainly nausea and diarrhoea; rash (no severe cutaneous adverse reactions were reported); and thrush. Participants discontinued treatment in three studies due to adverse events. Due to abdominal pain and nausea, three participants stopped treatment with erythromycin and instead took penicillin. A further two participants treated with penicillin stopped treatment due to diarrhoea or nausea. In one study, around 10% of participants stopped treatment because of pain at the injection site (the active treatment group was given intramuscular injections of benzathine penicillin).
Meta‐analyses looking at adverse reactions did not find a difference in tolerability or safety between antibiotic and no treatment or placebo (n = 469, four studies; low‐certainty evidence; summary of findings Table for the main comparison).
None of the included studies reported quality of life or development of resistance to antibiotics.
Overall completeness and applicability of evidence
Included studies recruited participants that are representative of the target population in western countries, mostly people over the age of 50, who are overweight and suffer local predisposing conditions for recurrent cellulitis (e.g. leg oedema, fungal foot infection, etc). Although the interventions seem to be suitable for other health systems, the characteristics of people who suffer recurrent cellulitis (excluding filariasis) might be different in other parts of the world, such as in Africa, Asia and South America. In the these areas, HIV carrier rates, nutrition status, sanitation and hygiene conditions, possibly different comorbidity profiles of the population (with regard to weight, diabetes, vascular disease) and access to quality medical services may hamper generalisation of our conclusions. The six studies referred to all relate to the use of antibiotics or selenium; although we had planned to include non‐drug preventive treatments, we found no studies that sought to measure the effectiveness of these interventions.
Ascertaining the diagnosis of cellulitis is essential, as incorrect diagnoses might both introduce bias into the studies and limit the applicability of the evidence when studies are combined in a meta‐analysis.The importance of an accurate diagnosis of cellulitis is highlighted by recent publications, suggesting that it is commonly misdiagnosed both inside and outside the hospital (Arakaki 2014; Levell 2011; Strazzula 2015). Despite caveats, the vast majority of participants were diagnosed by specialists, either in dermatology or infectious disease clinics; the two larger studies in the review (Thomas 2012; Thomas 2013), including 77% of all evaluable participants, used consistent and rigorous criteria to diagnose cellulitis, enabling the extrapolation of data to the general population affected.
The period of cellulitis recurrence prior to trial entry varied among studies and ranged from one to three years, with most of the participants suffering at least two prior episodes of cellulitis. Thus, although pertinent to many cases, it is possible that the beneficial impact of antibiotic prophylaxis or its magnitude on the recurrence of cellulitis is not readily applicable for people at the extremes, either after a single or multiple episodes of cellulitis. Furthermore, most of the evidence relates to people with cellulitis of the leg.
It was disappointing to note that only two studies included in this review followed up participants after treatment was stopped. The follow‐up period lasted up to two years, during which the effectiveness of prophylaxis diminished. The duration of prophylactic therapy was also limited, with the longest mean duration of treatment up to 18 months. Considering the long‐lasting nature of local and systemic risk factors for recurrent cellulitis and increasing life expectancy, the duration of preventive treatment and its enduring effect, during and possibly after treatment, ought to be more adequately addressed.
We noted a clinically important heterogeneity in the type of antibiotic, dosage and route of administration. The PATCH trial team introduced low‐dose oral penicillin as preventive therapy, gathering an unprecedented number of almost 400 participants with leg cellulitis into carefully‐designed and scrupulously‐analysed RCTs. Higher doses of oral penicillin (2 grams to 4 grams a day) (Sjöblom 1993) or penicillin injections (Chakroun 1994) were used in two other studies, but with small numbers of participants and less stringent study designs limiting high‐quality evidence‐based selection of an alternative prophylaxis. Only one study (Kremer 1991), enrolling 32 participants, explored an alternative to penicillin by using erythromycin; however, it did not allow us to determine the role of antimicrobial prophylaxis for people with penicillin allergy.
Intramuscular injection of penicillin is commonly used in current practice, but this intervention was only assessed in a single small study. Moreover, a recent report of three deaths following benzathine penicillin injections (Israeli Ministry of Health 2015) together with previous cases of death associated with its intramuscular delivery (Arumugam 2012; WHO 2000) raise concerns about the safety of this practice. However, RCTs are not the optimal platform to assess rare adverse events.
With regard to secondary outcomes, a meta‐analysis of results from three studies showed there was no difference in hospitalisation between participants on antibiotic prophylaxis and those given no treatment or placebo (three studies, n = 429, P value = 0.47, low‐certainty evidence; summary of findings Table for the main comparison), but the existing data did not allow us to explore its impact on length of hospital stay. A report by the PATCH trial team (Mason 2014) for their studies does not corroborate this analysis, and in this publication the preventive effect of antibiotic did not translate into a reduction in hospitalisations or the length of stay. The available studies did not permit assessment of the development of QoL or antimicrobial resistance.
Overall and without change with time, cellulitis is predominantly caused by group A streptococci and other β‐haemolytic streptococci (Jeng 2010). Staphylococcus aureus less frequently causes cellulitis, and is often associated with penetrating trauma and open wounds (Mandell 2010; Stevens 2014). Methicillin‐resistant Staphylococcus aureus (MRSA) is an unusual cause of typical cellulitis, and antibiotic coverage for this organism is usually unnecessary (Stevens 2014). The variable incidence of community‐acquired MRSA infections should therefore not limit the generalisability of our findings across countries with different incidence of MRSA‐associated skin infections. However, it would be prudent for future studies to examine the effectiveness of penicillin prophylaxis in geographical and other settings with a significant burden of MRSA infections.
Despite decades of use group A streptococci remained susceptible to penicillin (Gerber 1995; Horn 1998), making it an ideal antibiotic prophylaxis for cellulitis. Nonetheless, other bacteria are also known to be implicated in cellulitis (Chaniotakis 2016; Chira 2010; Garau 2015), raising a concern about the development of antimicrobial resistance. In addition to the possible development of resistant strains of bacteria in treated individuals, the introduction of antibiotic preventive therapy to a large population might further facilitate the evolution of antibiotic resistance and its relevance for public health must be addressed (Kunkel 2015). The high‐quality PATCH trials' preventive regimen (Thomas 2012; Thomas 2013) included low‐dose penicillin for a long treatment duration, which had been shown to induce the emergence of strains of drug‐resistant Streptococcus pneumoniae in another study (Guillemot 1998). However, the effect of any prophylactic regimen (with respect to dosage, duration or continuity) can not be easily predicted and should be based on future evidence (Kouyos 2014; Read 2011).
A number of attempts were made to develop a streptococcal vaccine but these were halted after serious safety concerns emerged (Massell 1969). Preliminary reports of later published trials indicating safe and effective results with newer streptococcal vaccines have revitalised efforts to develop a vaccine against group A streptococci (Kotloff 2004; McNeil 2005; Moreland 2014). We identified a single study assessing the use of streptococcal vaccine for cellulitis prevention (Haustein 1989) with a marked reduction in recurrence rate, but this was not a randomised controlled trial; larger well‐designed studies are necessary to establish the safety and effectiveness of a vaccine strategy.
Successful adherence to prophylactic treatment is a key element of any prevention strategy. The PATCH trial team reported good compliance, with more than 75% of participants adhering to treatment with penicillin tablets (see Included studies). Chakroun 1994 used penicillin injections into the muscle and mentioned a 10% dropout rate due to pain at the injection site. The benefits of antibiotic prophylaxis reported in these RCTs will only be replicated if people with cellulitis adhere to the prophylactic treatment. Possible barriers to adherence, except for adverse reactions, are the increasing age of those with recurrent cellulitis and the long‐term need for prophylaxis, as adherence would probably decrease with time. This emphasises the need to identify prophylactic regimens that are more convenient to both the person with recurrent cellulitis and the physician. These could include exploring other determinants of adherence, such as simplifying medication regimens, tailoring, and later monitoring the choice of prophylaxis to individuals, creating education and support programmes, and involving people with cellulitis and their families in clinical decision‐making as well as in research plans.
This review clearly demonstrates that current evidence falls far short of establishing the benefit of universally‐accepted treatments that mainly target local risk factors for recurrent cellulitis, including various methods to reduce lower or upper limb lymphoedema and oedema (Arsenault 2011; Brorson 2000; Campisi 2015; Damstra 2009; Didem 2005; Granzow 2014; Ko 1998; Szolnoky 2014; Yamamoto 2015), venous insufficiency and good local skin care, mainly antifungal treatment for tinea pedis (athlete's foot), but also proper local hygiene and maintenance of skin integrity.
Recurrent cellulitis of the upper limb was investigated by only one study evaluating preventive treatment with selenium, but data scarcity hinders its external validity.
Quality of the evidence
The five studies included in the quantitative analysis of the review can be classified into two groups of methodological quality: Thomas 2012 and Thomas 2013, conducted by the PATCH trial team, represent very high‐quality research characterised by a well‐drafted study protocol, meticulous study design with the use of placebo, and a complete report of predefined outcomes. The PATCH trial's data integrity proved to be solid, critically appraised by experts in several publications (Arasaratnam 2013; Durand 2013; Inghammar 2014; Oh 2014; Van Zuuren 2014) and with remarkable transparency and sharing of research data by the investigators. We judged these trials accordingly to be free of bias for all domains.
The other group of studies (Chakroun 1994; Kremer 1991; Sjöblom 1993) include small numbers of participants (range of 32 to 44 participants compared with 123 and 274 participants in the PATCH trials) with less stringent study design and reporting. They primarily lack blinding which might introduce bias, but they also raise concerns of incomplete reporting as detailed in the Risk of bias in included studies section and presented in Figure 3.
We graded the certainty of evidence for our primary outcome, the recurrence of leg cellulitis under preventive treatment, as moderate, reflecting the consistently clear beneficial effect of antibiotic prophylaxis on leg cellulitis recurrence across studies, and in particular the significant magnitude and large weight contributed by Thomas 2013 to the meta‐analysis. Similar reasons led to a moderate grading of other important comparisons: the incidence rate of cellulitis, and the time to next episode of cellulitis under treatment. However, we downgraded these outcomes due to imprecision because of a relatively low number of participants (small sample size).
Low certainty of evidence was attributed to the effect of antibiotic prophylaxis on hospital stay, adverse reactions and recurrence of cellulitis following the cessation of treatment, downgraded by imprecision of results, as indicated by the low number of participants and events (hospitalisations, adverse events and participants with recurrent episodes of cellulitis, respectively) and the 95% confidence interval overlapping the line of no effect (hospitalisations).
Three outcomes were assessed at 'post‐prophylaxis' (recurrence, incidence rate of recurrence, and time to next episode of cellulitis), and we rated them as low‐certainty evidence. We downgraded them two levels, due to serious imprecision from a small sample size/low event rate, wide confidence intervals and the confidence interval including both benefit and harm.
Potential biases in the review process
We conducted a highly inclusive search for published studies, and we further sought unpublished trials and abstracts submitted to major dermatological conferences and circulated in the informal grey literature. We scanned reference lists of all included studies, reviews and most of the other studies cited in the reference list of this review. We scrutinised many diverse guideline papers regarding recurrent cellulitis, from different countries and medical disciplines and published in different languages. We did not, however, search foreign‐language databases other than the Latin American and Caribbean Health Sciences Literature database (LILACS) (Appendix 4), so we cannot discount the possibility that relevant publications are to be found in the Chinese, Japanese or other non‐western medical literature. We planned in the protocol to search BIOSIS Previews from 1969 but only searched from 1990 in the review due to technical reasons and database availability.
We looked for all types of interventions, including local therapy, lifestyle modifications, surgery and systemic treatments. We placed no language restrictions and subsequently screened German‐published papers, a French‐published article, and we anticipate the evaluation of a Russian‐published study in a future update of this review. We included unpublished data for bias evaluation and further incorporation into meta‐analyses obtained from researchers (see Table 2).
We were not able to properly assess a single study that investigated the effectiveness of bemitil for cellulitis prophylaxis, for which the full text was not retrievable (Ratnikova 1991). This study is listed under studies awaiting classification. Despite incompleteness, we would not expect the missing information from this trial to influence the review's main findings on the value of antibiotic prophylaxis.
We cannot entirely rule out the risk of publication bias, especially when all studies exploring antibiotic prophylaxis point towards a positive effect of the intervention, but the small number of studies prevented us from formally assessing its probability. Nevertheless, the results of the recently‐published high‐quality PATCH trials (Thomas 2012; Thomas 2013), joining the other non‐industry‐funded RCTs in this review, and the fact that commercial investment in low‐cost preventive strategy with antibiotic are not likely to be profitable, minimise this risk.
Data from this review were insufficient to perform the subgroup analyses we had planned; we were therefore unable to investigate heterogeneity, due to the small number of included studies.
Agreements and disagreements with other studies or reviews
Two earlier systematic reviews evaluated the evidence for the prevention of recurrent cellulitis: Morris 2008 and Oh 2014.
Morris 2008 identified two RCTs (Kremer 1991; Sjöblom 1993), also included in our review. Examining these studies, the author of the review concluded that only limited evidence would support the use of prophylactic antibiotics for reduction of future attacks of cellulitis, and rated the certainty of evidence for the intervention as low, based on the GRADE classification. Our search retrieved four additional studies; two preceding the Morris 2008 review (Chakroun 1994; Kasseroller 1998), which were later followed by the two PATCH trials (Thomas 2012; Thomas 2013), which provide higher‐certainty evidence to further support the conclusion that prophylactic antibiotics reduce the incidence of future attacks of cellulitis.
Oh 2014 reviewed antibiotic prophylaxis for the prevention of recurrent cellulitis and found similar results to this review.
A number of guidance documents referring to the prevention of recurrent cellulitis have been issued up to this time and retrieved by our systematic search. These clinical practice guidelines and their major recommendations on antibiotic prophylaxis, its duration and adjunctive therapy are summarised in Table 3. Overall, most guidelines advocate antibiotic prophylaxis with penicillin, but differ widely on the best time to start treatment and its duration. There are also inconsistencies in alternatives to penicillin, as well as the means of delivery and dosage. Of note, only three groups, the Clinical Resources Efficiency Support Team, the National Institute for Health and Clinical Excellence and the Infectious Diseases Society of America, refer to the strength of their recommendations or the quality of evidence for the use prophylactic antibiotics (CREST 2005; NICE 2005; Stevens 2005; Stevens 2014).
| Guideline | Organisation | Recommended antibiotic | Duration of Px | No of episodes to initiate Px | Adjunctive Tx | Quality of evidence † |
| BLS | penicillin by mouth; alternatives: cephalexin, erythromycin, clarithromycin, clindamycin, doxycycline | 2 y; life‐long Px if recurrence after Px stopped | 2 ≥/y | skin care, decongestive Tx, antifungal Tx, alcohol wipes; SIT | NS | |
| ALA | penicillin by mouth; alternatives: cephalexin, erythromycin, clindamycin | 2 y; life‐long Px if recurrence after Px stopped | 2 ≥/y | skin care, decongestive Tx, bacterial decolonisation Tx; SIT | NS | |
| IDSA | penicillin by mouth/IM; alternatives:erythromycin | as long as RF persist | 3 – 4 /y | skin care, Tx of oedema, weight reduction | antibiotic Px ‐weak, moderate ‡ duration of Px ‐ strong, moderate ‡ skin care ‐ strong, moderate ‡ | |
| penicillin by mouth/IM; alternatives:erythromycin | NS | frequent | skin care, Tx of oedema compression stockings, diuretics; SIT | grade IIB § | ||
| ISL | penicillin; alternatives: broad spectrum antibiotic | NS | repeat despite physical Tx | skin care, antifungal Tx | NS | |
| SIMIT and ISC | penicillin or macrolide | NS | recurrent | skin hygiene and compression stockings | NS | |
| NHG | penicillin by mouth/IM | 1 ‐ 2 y | 2 ≥/y | skin care, compression stockings; SIT | NS | |
| ILF | penicillin by mouth; alternatives:erythromycin, clindamycin, clarithromycin | 2 y; life‐long Px if recurrence after Px stopped | 2 ≥/y | skin care, decongestive Tx, antifungal Tx; SIT | NS | |
| CREST | penicillin or erythromycin by mouth | 2 y | 2 ≥/y | SIT may be preferable | weak and inconclusive | |
| NICE | a trial should be considered | NS | > 2/y | skin care, Tx of oedema, compression stockings, weight reduction | weak and inconclusive | |
| Other* | antibiotic may be needed; type of antibiotic NS | long term | NS | skin care, Tx of oedema, antifungal Tx; SIT | NS | |
| SFD | penicillin by mouth/IM; alternatives: macrolide | prolonged, probably indefinitely | several/poorly controlled RF | skin care, Tx of oedema | NS | |
| FMSD | antibiotic should be considered; type of antibiotic NS | long term | frequent | skin care | NS |
† Assessement of quality of evidence as defined and graded by the authors of the document.
‡ Strong recommendation, moderate quality ‐ desirable effects clearly outweigh undesirable effects; evidence from RCTs with important limitations or exceptionally strong evidence from unbiased observational studies; recommendation can apply to most patients in most circumstances and further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate.
Weak recommendation, moderate quality ‐ desirable effects closely balanced with undesirable effects; evidence from RCTs with important limitations;recommendation may change when higher‐quality evidence becomes available; and further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate.
§ Moderate evidence ‐ should generally be offered; II ‐ evidence from one well‐designed clinical trial.
Abbreviations
IM ‐ injections into the muscle (intramuscular)
No ‐ number
NS ‐ not specified
Px ‐ preventive treatment (prophylaxis)
RF‐ risk factors
SIT ‐ self‐initiated treatment
Tx ‐ treatment
y ‐ year/s
Medical organisations
BLS ‐ British Lymphology Society
ALA ‐ Australasian Lymphology Association
IDSA ‐ Infectious Diseases Society of America
ISL ‐ International Society of Lymphology
SIMIT ‐ Società Italiana di Malattie Infettive e Tropicali ‐ Italian Society of Infectious Diseases
ISC ‐ International Society of Chemotherapy
NHG ‐ Nederlands Huisartsen Genootschap ‐ The Dutch College of General Practitioners
ILF ‐ International Lymphoedema Framework
CREST ‐ Clinical Resources Efficiency Support Team (UK)
NICE ‐ National Institute for Health and Clinical Excellence (England)
SFD ‐ La Société Française de Dermatologie ‐ The French Society of Dermatology
MSD ‐ The Finnish Medical Society Duodecim
* 5 of 6 experts in this consensus paper were from North America; published in the Journal of the British Society for Antimicrobial Chemotherapy
Findings from this systematic review are in agreement with most of the aforementioned guidelines supporting the use of antibiotic prophylaxis (penicillin in particular) for people with recurrent lower limb cellulitis.
Authors of many of the major guidelines point out that prolonged or even life‐long antibiotic prophylaxis is warranted, in accordance with our meta‐analyses, showing that the preventive effects of antibiotics gradually wane after prophylaxis is stopped, corresponding with a similar observation reported as early as 1985 (Duvanel 1985). However, the evidence for this is lacking.

Study selection flow diagram.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 1 Recurrence of cellulitis.
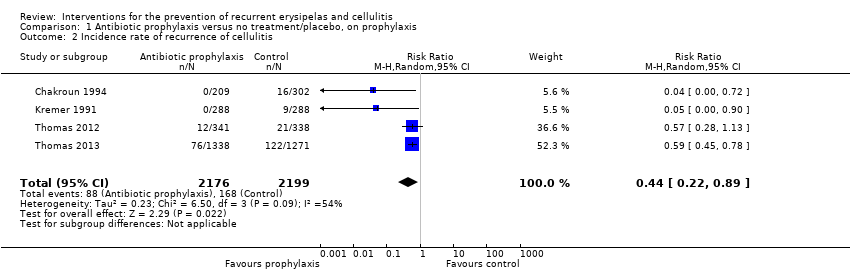
Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 2 Incidence rate of recurrence of cellulitis.

Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 3 Time to next episode of cellulitis.
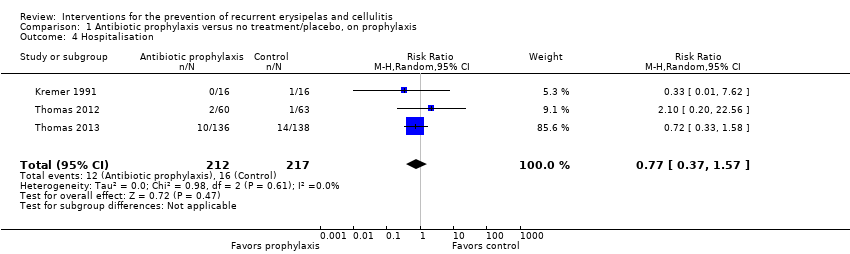
Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 4 Hospitalisation.

Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 5 Any adverse reactions.

Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 6 Mortality.

Comparison 2 Antibiotic prophylaxis versus no treatment/placebo, post‐prophylaxis, Outcome 1 Recurrence of cellulitis.

Comparison 2 Antibiotic prophylaxis versus no treatment/placebo, post‐prophylaxis, Outcome 2 Incidence rate of recurrence of cellulitis.
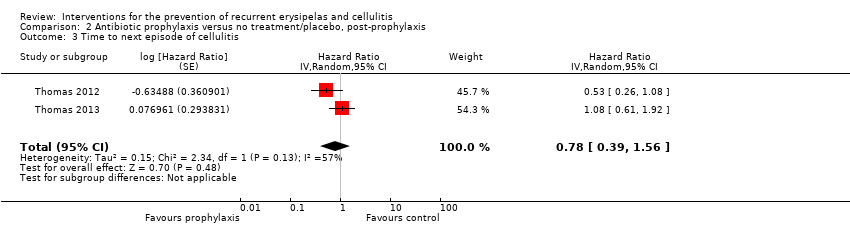
Comparison 2 Antibiotic prophylaxis versus no treatment/placebo, post‐prophylaxis, Outcome 3 Time to next episode of cellulitis.

Comparison 3 Antibiotic prophylaxis versus no treatment/placebo, overall, Outcome 1 Recurrence of cellulitis.

Comparison 3 Antibiotic prophylaxis versus no treatment/placebo, overall, Outcome 2 Incidence rate of recurrence of cellulitis.
| Antibiotic prophylaxis compared to no treatment/placebo for the prevention of recurrent erysipelas and cellulitis ‐ on prophylaxis | ||||||
| Patient or population: People with recurrent erysipelas or cellulitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| no treatment/placebo | Antibiotic prophylaxis | |||||
| Recurrence of cellulitis | Study population | RR 0.31 | 513 | ⊕⊕⊕⊝ | Number needed to treat for 1 additional beneficial outcome (NNTB) is 6 | |
| 316 per 1000 | 98 per 1000 | |||||
| Incidence rate of cellulitis | Study population | RR 0.44 (0.22 to 0.89) | 473 (4 RCTs) | ⊕⊕⊕⊝ | ‐ | |
| 43 fewer episodes of cellulitis per 1000 person‐months in treatment group (from 8 fewer to 60 fewer) | ||||||
| Time to next episode of cellulitis | Not estimable | HR 0.51 | 437 | ⊕⊕⊕⊝ | ‐ | |
| Hospitalisation | Study population | RR 0.77 | 429 | ⊕⊕⊝⊝ | ‐ | |
| 74 per 1000 | 57 per 1000 | |||||
| Any adverse reactions | Study population | RR 0.87 | 469 | ⊕⊕⊝⊝ | ‐ | |
| 287 per 1000 | 250 per 1000 | |||||
| Quality of life | ‐ | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded by one level because of imprecision due to the low number of events and the small sample size (optimal information size ‐ OIS). 2 We downgraded by two levels because of imprecision due to the low number of events and the small sample size (OIS) and the 95% confidence interval overlapping the line of no effect and ranging from benefit to harm. 3We downgraded by two levels because of imprecision due to the considerable low number of events and the small sample size (OIS). We decided not to downgrade any of the outcomes for risk of bias as we decided it was not serious enough to affect the overall quality of the outcome. | ||||||
| Medical term | Explanation |
| Ambulatory | Ambulatory is when the patient can walk and is not bedridden. When referring to medical care it means that it is being provided to patients that are not hospitalised (outpatients) |
| Block randomisation | A method of randomisation that ensures allocation of participants into roughly equal sizes of comparison groups |
| Clostridium difficile | A bacterium that causes inflammation of the colon (colitis), typically resulting in diarrhoea, and is strongly associated with the use of antibiotics |
| Comorbidity | The presence of one or more diseases or conditions other than those of primary interest |
| Contralateral | On the opposite side of the body (e.g. a repeat episode of leg cellulitis can recur in the same leg [see 'ipsilateral'] or the other, contralateral leg |
| Control event rate (CER) | The rate at which events of interest (i.e. episodes of cellulitis in our review) occur in the control group of the study |
| Diuretics | Commonly known as "water pills" these are drugs that help the body to eliminate unneeded water and salt through the urine |
| Epidemiology | The study of the health of populations and communities, not just particular individuals |
| Erythema | Redness of the skin caused by increased blood flow. Often a sign of inflammation or infection |
| Filariasis | A disease caused by infection with worms, usually in tropical and subtropical areas of the world. The worms reside in the lymphatic system of the affected person and interfere with the drainage of the lymph, subsequently causing a significant swelling of the involved limb |
| Filarial lymphoedema | see Filiariasis |
| Folliculitis | Inflammation of hair follicles |
| Furunculosis | Deep form of inflammation of the hair follicles resulting in lumps caused by the accumulation of pus (boils) |
| Gastrointestinal | Relating to the stomach and the intestines |
| Incidence rate/Incidence rate ratio | The number of new occurrences of events in a population divided by its time period at risk; Incidence rate ratio is the ratio of two incidence rates |
| Ipsilateral | On the same side of the body; as opposed to 'contralateral' |
| Mastectomy | Surgical removal of one or both breasts |
| Outpatient/Inpatient | Outpatient is a person that is being treated without being hospitalised overnight and visits the physician in the clinic, hospital or other facility; compared with an inpatient who requires an overnight stay in hospital for medical treatment |
| Person‐months | The sum of the number of months each participant in the trial has been under observation (treated/followed) |
| 'Per protocol'/Intention‐to‐treat (ITT) analyses | 'Per protocol' analysis compares participants in a study based on the treatment they actually took and includes only those patients who completed the treatment originally allocated, as opposed to intention‐to‐treat analysis that compares participants on the basis of their random assignment to groups (treatment or placebo), regardless of adherence to treatment |
| Prophylaxis | Preventive treatment for disease |
| Retrospective cohort study | An observational study in which a defined group of people (the cohort) is followed over time. A retrospective cohort study identifies persons from past medical records and follows them from the time of those records to the present |
| Sensitivity analysis | An analysis used to determine how sensitive the results of a study or systematic review are to changes in how it was done |
| Tinea pedis | Fungal infection of the foot (athlete's foot) |
| Tonsillectomy | Surgical removal of tonsils |
| Study | Way of communication | Date | Information provided | Notes |
| | 12/2013 | ‐ Allocation concealment ‐ Participants follow‐up ‐ Criteria for diagnosis ‐ Adherence ‐ Adverse reactions ‐ Informed consent ‐ Ethical committee approval ‐ Source of funding | Full information was not available for all queries, but investigators responded to all of them | |
| airmail, email, website | 2013 ‐ 2014 | ‐ | Investigator did not reply to our queries We also contacted a potential sponsor, not reported by the author, who confirmed their financial support for the conduct of this study (email correspondence with the head of medical‐scientific department of 'biosyn Arzneimittel GmbH' from January 2015) | |
| email and telephone | 12/2013 and 1/2014 | ‐ | Data were not available and the investigator did not remember any details | |
| | 12/2013 | ‐ Allocation concealment ‐ Participants follow‐up ‐ By whom cellulitis was diagnosed ‐ Adherence ‐ Source of funding | Full information was not available for all queries, but investigators responded to all of them | |
| | 1/2014 | ‐ Episodes of recurrent cellulitis per person‐months (incidence rate) ‐Time to next episode ‐ Adverse events by study arm ‐ Duration of hospitalisation ‐ Quality of life | ‐ | |
| | 1/2014 | ‐ Episodes of recurrent cellulitis per person‐months (incidence rate) ‐ Quality of life | Hospitalisation and quality of life were not evaluated directly in this trial but were reported by indirect evaluation in Mason 2014 |
| Guideline | Organisation | Recommended antibiotic | Duration of Px | No of episodes to initiate Px | Adjunctive Tx | Quality of evidence † |
| BLS | penicillin by mouth; alternatives: cephalexin, erythromycin, clarithromycin, clindamycin, doxycycline | 2 y; life‐long Px if recurrence after Px stopped | 2 ≥/y | skin care, decongestive Tx, antifungal Tx, alcohol wipes; SIT | NS | |
| ALA | penicillin by mouth; alternatives: cephalexin, erythromycin, clindamycin | 2 y; life‐long Px if recurrence after Px stopped | 2 ≥/y | skin care, decongestive Tx, bacterial decolonisation Tx; SIT | NS | |
| IDSA | penicillin by mouth/IM; alternatives:erythromycin | as long as RF persist | 3 – 4 /y | skin care, Tx of oedema, weight reduction | antibiotic Px ‐weak, moderate ‡ duration of Px ‐ strong, moderate ‡ skin care ‐ strong, moderate ‡ | |
| penicillin by mouth/IM; alternatives:erythromycin | NS | frequent | skin care, Tx of oedema compression stockings, diuretics; SIT | grade IIB § | ||
| ISL | penicillin; alternatives: broad spectrum antibiotic | NS | repeat despite physical Tx | skin care, antifungal Tx | NS | |
| SIMIT and ISC | penicillin or macrolide | NS | recurrent | skin hygiene and compression stockings | NS | |
| NHG | penicillin by mouth/IM | 1 ‐ 2 y | 2 ≥/y | skin care, compression stockings; SIT | NS | |
| ILF | penicillin by mouth; alternatives:erythromycin, clindamycin, clarithromycin | 2 y; life‐long Px if recurrence after Px stopped | 2 ≥/y | skin care, decongestive Tx, antifungal Tx; SIT | NS | |
| CREST | penicillin or erythromycin by mouth | 2 y | 2 ≥/y | SIT may be preferable | weak and inconclusive | |
| NICE | a trial should be considered | NS | > 2/y | skin care, Tx of oedema, compression stockings, weight reduction | weak and inconclusive | |
| Other* | antibiotic may be needed; type of antibiotic NS | long term | NS | skin care, Tx of oedema, antifungal Tx; SIT | NS | |
| SFD | penicillin by mouth/IM; alternatives: macrolide | prolonged, probably indefinitely | several/poorly controlled RF | skin care, Tx of oedema | NS | |
| FMSD | antibiotic should be considered; type of antibiotic NS | long term | frequent | skin care | NS | |
| † Assessement of quality of evidence as defined and graded by the authors of the document. ‡ Strong recommendation, moderate quality ‐ desirable effects clearly outweigh undesirable effects; evidence from RCTs with important limitations or exceptionally strong evidence from unbiased observational studies; recommendation can apply to most patients in most circumstances and further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. Weak recommendation, moderate quality ‐ desirable effects closely balanced with undesirable effects; evidence from RCTs with important limitations;recommendation may change when higher‐quality evidence becomes available; and further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. § Moderate evidence ‐ should generally be offered; II ‐ evidence from one well‐designed clinical trial. Abbreviations IM ‐ injections into the muscle (intramuscular) Medical organisations BLS ‐ British Lymphology Society * 5 of 6 experts in this consensus paper were from North America; published in the Journal of the British Society for Antimicrobial Chemotherapy | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of cellulitis Show forest plot | 5 | 513 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.13, 0.72] |
| 2 Incidence rate of recurrence of cellulitis Show forest plot | 4 | 4375 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.22, 0.89] |
| 3 Time to next episode of cellulitis Show forest plot | 3 | Hazard Ratio (Random, 95% CI) | 0.51 [0.34, 0.78] | |
| 4 Hospitalisation Show forest plot | 3 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.37, 1.57] |
| 5 Any adverse reactions Show forest plot | 4 | 469 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.58, 1.30] |
| 5.1 Penicillin | 3 | 437 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.60, 1.10] |
| 5.2 Erythromycin | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.39, 125.44] |
| 6 Mortality Show forest plot | 3 | 437 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.32, 3.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of cellulitis Show forest plot | 2 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.59, 1.31] |
| 2 Incidence rate of recurrence of cellulitis Show forest plot | 2 | 4566 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.65, 1.36] |
| 3 Time to next episode of cellulitis Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 0.78 [0.39, 1.56] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of cellulitis Show forest plot | 2 | 397 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.59, 0.95] |
| 2 Incidence rate of recurrence of cellulitis Show forest plot | 2 | 7854 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.56, 0.85] |

