Intervenciones para el tratamiento de la enfermedad de las alturas aguda
Appendices
Appendix 1. Risk categories for acute high altitudes
| Risk categories | Description |
| Low | Individuals with no prior history of altitude illness and ascending to |
| Low | Individuals taking ≥2 days to arrive at 2500 to 3000 m/ 8202 to 9842 feet |
| Moderate | Individuals with prior history of AMS and ascending to 2500 to 2800 m |
| Moderate | No history of AMS and ascending to > 2800 m (9186 feet) in one day. |
| Moderate | All individuals ascending > 500 m/d (1640 feet) (increase in sleeping |
| High | History of AMS and ascending to ? 2800 m / 9186 feet in one day. |
| High | All individuals with a prior history of HAPE or HACE. |
| High | All individuals ascending to > 3500 m/ 11,482 feet in one day. |
| High | All individuals ascending >500 m/ 1640 feet /d increase in sleeping |
| High | Very rapid ascents (e.g., Mt. Kilimanjaro). |
Appendix 2. Medical terms glossary
| Term | Definition |
| Anorexia | The lack or loss of appetite accompanied by an aversion to food and the inability to eat |
| Ataxia | Impairment of the ability to perform smoothly coordinated voluntary movements |
| Brian herniation | Protrusion of tissue, structure, or part of an organ through the bone, muscular tissue, or the membrane by which it is normally contained |
| Dyspnoea | Difficult or laboured breathing |
| Dizziness | An imprecise term which may refer to a sense of spatial disorientation, motion of the environment, or lightheadedness |
| Endothelium | A layer of epithelium that lines the heart, blood vessels (endothelium vascular), lymph vessels (endothelium lymphatic), and the serous cavities of the body |
| Fatigue | The state of weariness following a period of exertion, mental or physical, characterized by a decreased capacity for work and reduced efficiency to respond to stimuli |
| Hallucination | Subjectively experienced sensations in the absence of an appropriate stimulus, but which are regarded by the individual as real |
| Headache | The symptom of pain in the cranial region |
| Hypoxia | A disorder characterized by a reduction of oxygen in the blood |
| Insomnia | Disorders characterized by impairment of the ability to initiate or maintain sleep |
| Lightheadedness | See dizziness |
| Nausea | An unpleasant sensation in the stomach usually accompanied by the urge to vomit |
| Pulmonary oedema | An unpleasant sensation in the stomach usually accompanied by the urge to vomit |
| Pulmonary alveoli | Small polyhedral outpouchings along the walls of the alveolar sacs, alveolar ducts and terminal bronchioles through the walls of which gas exchange between alveolar air and pulmonary capillary blood takes place |
| Seizures | Clinical or subclinical disturbances of cortical function due to a sudden, abnormal, excessive, and disorganized discharge of brain cells.Clinicalmanifestations include abnormal motor, sensory and psychic phenomena |
Source: http://www.ncbi.nlm.nih.gov/mesh
Appendix 3. The most frequents adverse effects of the pharmacological interventions.
| Drug | Description and contraindications | Adverse events |
| Paracetamol | It is a non‐steroidal anti‐inflammatory drug | Paracetamol may cause liver damage |
| Acetazolamide | Acetazolamide, an inhibitor of the enzyme carbonic anhydrase. | Adverse reactions, occurring most often early in therapy, include paraesthesias, particularly a “tingling” feeling in the extremities, hearing dysfunction or tinnitus, loss of appetite, taste alteration and gastrointestinal disturbances such as nausea, vomiting and diarrhoea; polyuria, and occasional instances of drowsiness and confusion |
| Aspirin | It is a non‐steroidal anti‐inflammatory drug. | Reye’s syndrome (a rare but serious illness). |
| Dexamethasone | Glucocorticoids, naturally occurring and synthetic, are adrenocortical steroids that are readily absorbed from the gastrointestinal tract. Glucocorticoids cause varied metabolic effects. In addition, they modify the body’s immune responses to diverse stimuli. | Several adverse events (e.g. hyperglycaemia, fluid retention, hypokalaemic alkalosis, potassium loss, sodium retention) |
| Furosemide | It is potent diuretic. Furosemide is contraindicated in patients with anuria and in patients with a history of hypersensitivity to furosemide | The principal signs and symptoms of overdose with furosemide are dehydration, blood volume reduction, hypotension, electrolyte imbalance, hypokalaemia and hypochloraemic alkalosis, and are extensions of its diuretic action |
| Gabapentin | Gabapentin is an anticonvulsant. Gabapentin is contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients | Somnolence, dizziness, ataxia, fatigue, and nystagmus |
| Ibuprofen | It is a nonsteroidal anti‐inflammatory drug (NSAID) | Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. It is a an NSAID, which may cause severe stomach bleeding |
| Magnesium | Magnesium should not be administered if there is renal impairment, marked myocardial disease or to comatose patients | The usual precautions for parenteral administration should be observed. Administer with caution if flushing and sweating occurs. Respiration and blood pressure should be carefully observed during and after administration of magnesium chloride injection |
| Medroxyprogesterone | It is a derivative of progesterone. | Fluid retention, and several others related with the prolonged use |
| Methazolamide | Methazolamide is a potent inhibitor of carbonic anhydrase. Methazolamide therapy is contraindicated in situations in which sodium and/or potassium serum levels are depressed, in cases of marked kidney or liver disease or dysfunction, in adrenal gland failure, | Adverse reactions, occurring most often early in therapy, include paraesthesias, particularly a “tingling” feeling in the extremities; hearing dysfunction or tinnitus; fatigue; malaise; loss of appetite; taste alteration; gastrointestinal disturbances such as nausea, vomiting, and diarrhoea; polyuria; and occasional instances of drowsiness and confusion |
| Nifedipine | It is a calcium channel blocker. Nifedipine must not be used in cases of cardiogenic shock. It is contraindicated in patients with a known hypersensitivity to any component of the tablet | Headache, flushing/heat sensation, dizziness, fatigue/asthenia, nausea |
| Temazepam | It is a benzodiazepine hypnotic agent. It is contraindicated in women who are or may become pregnant | Drowsiness |
| Theophylline | Theophylline is classified as amethylxanthine. Theophylline should be used with extreme caution in patients with the following clinical conditions due to the increased risk of exacerbation of the concurrent condition: active peptic ulcer disease, seizure disorders and cardiac arrhythmias (not including bradyarrhythmias) | Nausea, vomiting, headache, and insomnia |
Source: DailyMed. dailymed.nlm.nih.gov/dailymed/about.cfm
Appendix 4. Search strategy
Search strategy for CENTRAL, in the Cochrane Library
#1 MeSH descriptor Altitude Sickness explode all trees
#2 MeSH descriptor Pulmonary Edema explode all trees
#3 MeSH descriptor Altitude, this term only
#4 (illnes* or sicknes* or ((cerebral or pulmonary) and (oedema or edema)))
#5 altitude
#6 (#5 AND #4)
#7 (mountain near (sickness or illness)) or (AMS or HACE or HAPE or HAI):ti,ab
#8 (#1 OR #2 OR #3 OR #6 OR #7)
#9 (nifedipine or dexamethasone or theophylline or acetazolamide or medroxyprogesterone or aspirin or ibuprofen or acetaminophen or sumatriptan or gabapentin or furosemide or spironolactone or calcium channel blocker* or selective inhibitor* of phosphodiesterase type 5 or nonsteroidal anti‐inflammatory drug* or steroid* or glucocorticosteroid* or corticosteroid* or non‐selective phosphodiesterase‐inhibitor* or carbonic anhydrase inhibitor* or 5‐HT1 receptor agonist*or N‐methyl‐D‐aspartate antagonist* or oxygen or descent* or hyperbaric chamber or portable pressure bag* or Gamow bag* or breathing system* or positive airway pressure) or (therapy or treat*):ti,ab
#10 (#8 AND #9)
Search strategy for MEDLINE (Ovid SP)
1. exp Altitude Sickness/ or exp Pulmonary Edema/ or Altitude/ or (high‐altitude adj5 (illnes*or sicknes* or ((cerebral or pulmonary) and (oedema or edema)))).mp. or (high altitude adj5 (illnes*or sicknes* or ((cerebral or pulmonary) and (oedema or edema)))).mp. or (highaltitude adj5 (illnes*or sicknes* or ((cerebral or pulmonary) and (oedema or edema)))).mp. or (mountain adj3 (sickness or illness)).af. or (AMS or HACE or HAPE or HAI).ti,ab.
2. (nifedipine or dexamethasone or theophylline or acetazolamide or medroxyprogesterone or aspirin or ibuprofen or acetaminophen or sumatriptan or gabapentin or furosemide or spironolactone or calcium channel blocker* or selective inhibitor* of phosphodiesterase type 5 or nonsteroidal anti‐inflammatory drug* or steroid* or glucocorticosteroid* or corticosteroid* or non‐selective phosphodiesterase‐inhibitor* or carbonic anhydrase inhibitor* or 5‐HT1 receptor agonist*or N‐methyl‐D‐aspartate antagonist* or oxygen or descent* or hyperbaric chamber or portable pressure bag* or Gamow bag* or breathing system* or positive airway pressure).mp. or (therapy or treat*).ti,ab
3. 1 and 2
4. ((randomised controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh
5. 3 and 4
Search strategy for Embase (www.embase.com)
1. 'altitude disease ' or altitude NEAR/3 (illnes* OR sicknes*) or mountain NEAR/3 (sickness or illness) or ((altitude or mountain) AND cerebral:ab,ti OR pulmonary:ab,ti OR lung:ab,ti AND (oedema:ab,ti OR edema:ab,ti)) or ams:ab,ti OR have:ab,ti OR hape:ab,ti OR hai:ab,ti
2. nifedipine or dexamethasone or theophylline or acetazolamide or medroxyprogesterone or aspirin or ibuprofen or acetaminophen or sumatriptan or gabapentin or furosemide or spironolactone or calcium channel blocker* or selective inhibitor* of phosphodiesterase type 5 or nonsteroidal anti‐inflammatory drug* or steroid* or glucocorticosteroid* or corticosteroid* or non‐selective phosphodiesterase‐inhibitor* or carbonic anhydrase inhibitor* or 5‐HT1 receptor agonist*or N‐methyl‐D‐aspartate antagonist* or oxygen or descent* or hyperbaric chamber or portable pressure bag* or Gamow bag* or breathing system* or positive airway pressure or (therapy or treat*):ab,ti
3. 1 and 2
4. placebo:ab,ti or 'controlled study':ab,ti or random*:ab,ti or trial*:ab,ti or ((singl* or doubl* or trebl* or tripl*) NEAR/3 (blind* or mask*))
5. 3 and 4
Search strategy for LILACS via BIREME interface
"EDEMA CEREBRAL" or "edema pulmonary$" or "mountain sickness" or "high‐altitude sickness" or ?montaña enfermedad? or ?o mal da montanha? or ?doença de alta altitude? or ?mal de altura?
tw:("mountain sickness") OR ("high‐altitude sickness") OR ("enfermedad de montaña") or ("mal da montanha") or ("doença de alta altitude") or mh:("Mal de Altura")
Search strategy for ISI Web of Science
#1 TS= ("high altitude" NEAR illnes*) or TS= ("high altitude" NEAR sicknes*) or TS= ("high altitude" NEAR "cerebral *edema") or TS= ("high altitude" NEAR "pulmonar* *edema") or TS=(mountain NEAR (sicknes* or illnes*)) or TS=(AMS or HACE or HAPE or HAI)
#2 TS=(nifedipine or dexamethasone or theophylline or acetazolamide or medroxyprogesterone or aspirin or ibuprofen or acetaminophen or sumatriptan or gabapentin or furosemide or spironolactone or calcium channel blocker* or selective inhibitor* of phosphodiesterase type 5 or nonsteroidal anti‐inflammatory drug* or steroid* or glucocorticosteroid* or corticosteroid* or non‐selective phosphodiesterase‐inhibitor* or carbonic anhydrase inhibitor* or 5‐HT1 receptor agonist*or N‐methyl‐D‐aspartate antagonist* or oxygen or descent* or hyperbaric chamber or portable pressure bag* or Gamow bag* or breathing system* or positive airway pressure) or TI=(therapy or treat*)
#3 #2 and #1
#4 TS=((random* or controlled or clinical or multicent* or prospective*) NEAR trial*) or TS=((single or double or triple or treble) NEAR trial*)
#5 #3 and #4
Search strategy for CINAHL (EBSCO host)
S1 ( (MM "Altitude Sickness") OR (MH "Pulmonary Edema") ) OR ( (high?altitude and (illnes*or sicknes* or ((cerebral or pulmonary) and (oedema or edema)))) ) OR ( (mountain and (sickness or illness)) or (AMS or HACE or HAPE or HAI) )
S2 ( nifedipine or dexamethasone or theophylline or acetazolamide or medroxyprogesterone or aspirin or ibuprofen or acetaminophen or sumatriptan or gabapentin or furosemide or spironolactone or calcium channel blocker* or selective inhibitor* of phosphodiesterase type 5 or nonsteroidal anti‐inflammatory drug* or steroid* or glucocorticosteroid* or corticosteroid* or non‐selective phosphodiesterase‐inhibitor* or carbonic anhydrase inhibitor* or 5‐HT1 receptor agonist*or N‐methyl‐D‐aspartate antagonist* or oxygen or descent* or hyperbaric chamber or portable pressure bag* or Gamow bag* or breathing system* or positive airway pressure ) OR AB ( prevent* or therapy or treat* )
S3 S1 and S2
S4 ( (MM "Randomized Controlled Trials") OR (MM "Random Assignment") OR (MH "Clinical Trials") OR (MH "Multicenter Studies") OR (MH "Double‐Blind Studies") OR (MH "Single‐Blind Studies") OR (MH "Triple‐Blind Studies") ) OR ( random* or ((controlled or clinical) and trial*) )
S5 S3 and S4
Search strategy for Wanfang (Wanfangdata.com)
"Acute Mountain Sickness" OR "High Altitude Pulmonary Edema" OR "High Altitude Cerebral Edema"
Also in Chinese (高山病、高原肺水肿、高原脑水肿)
Appendix 5. WHO International Trials Registry Portal search
Advanced search: Altitude Sickness OR Altitude illness OR acute mountain sickness OR High‐altitude edema OR high‐altitude oedema (in the title field)
Appendix 6. Data collection form
Notes on using a data extraction form:
· Be consistent in the order and style you use to describe the information for each report.
· Record any missing information as unclear or not described, to make it clear that the information was not found in the study report(s), not that you forgot to extract it.
| Review title or ID |
| Interventions for Treating High Altitude Illness |
| Study ID(surname of first author and year first full report of study was published e.g. Smith 2001) |
| Report ID(if different to Study ID) | Report IDs of other reports of this study(e.g. duplicate publications, follow‐up studies) |
| Notes: |
General Information
| Date form completed(dd/mm/yyyy) | |
| Name/ID of person extracting data | |
| Reference citation | |
| Study author contact details | |
| Publication type (e.g. full report, abstract, letter) | |
| Notes: | |
Study eligibility
| Study Characteristics | Eligibility criteria (Insert inclusion criteria for each characteristic as defined in the Protocol) | Eligibility criteria met? | Location in text or source(pg & /fig/table/other) | ||
| Yes | No | Unclear | |||
| Type of study | Randomized Controlled Trial | ||||
| Participants | Were they people with HAI (AMS/HACE and HAPE, or both). | ||||
| Types of intervention | Did one group receive A) Non‐pharmacological interventions
B) Pharmacological interventions
| ||||
| Types of comparison | Did the comparison group receive a Placebo, monotherapy or any combination (non‐pharmacological plus pharmacological; pharmacological interventions). | ||||
| INCLUDE EXCLUDE | |||||
| Reason for exclusion | DO NOT PROCEED IF STUDY IS EXCLUDED FROM REVIEW | ||||
| Notes: | |||||
Characteristics of included studies
Methods
| Descriptions as stated in report/paper | Location in text or source(pg &/fig/table/other) | ||
| Country(where the study was conducted) | |||
| Design(e.g. parallel, cluster) | |||
| Was the study multicentre?(if yes, state No. of centres) | |||
| Funders of the trial | |||
| Duration of trial(state start date and end date of trial) | |||
| Duration of participation (from start of recruitment to last follow‐up) | |||
| Ethical approval needed/ obtained for study | Yes No Unclear | ||
| Notes: | |||
Participants
| Description Include comparative information for each intervention or comparison group if available | Location in text or source(pg & /fig/table/other) | |
| Population description (describe any risk factors, and criteria for diagnosing high‐altitude pulmonary edema ) | ||
| Setting (from where were participants enrolled?) | ||
| Inclusion criteria | ||
| Exclusion criteria | ||
| Method of recruitment of participants(e.g. phone, mail, clinic patients) | ||
| Total no. randomized | ||
| Withdrawals and exclusions (if not provided below by outcome) | ||
| Age | ||
| Sex | ||
| Race/Ethnicity | ||
| Notes: | ||
Intervention groups
| Description as stated in report/paper | Location in text or source(pg & /fig/table/other) | |
| Drug name | ||
| No. randomized to group (specify whether no. people or clusters) | ||
| Details of the drug/intervention (e.g. brand, look, taste) | ||
| Dosing regimen(e.g. dose, frequency, duration) | ||
| Mode of Delivery(e.g. oral) | ||
| Co‐interventions(any additional interventions given) | ||
| Notes: | ||
Placebo Group
| Description as stated in report/paper | Location in text or source(pg & /fig/table/other) | |
| Comparison name | ||
| No. randomized to group (specify whether no. people or clusters) | ||
| Details of placebo(e.g. similarity to intervention) | ||
| Dosing regimen(e.g. dose, frequency, duration) | ||
| Mode of Delivery(e.g. oral) | ||
| Co‐interventions(any additional interventions given) | ||
| Notes: | ||
Outcomes
| Description as stated in report/paper | Location in text or source(pg & /fig/table/other) | ||
| Outcome name | All‐cause mortality:
| ||
| Time points measured (specify whether from start or end of intervention) | |||
| Time points reported | |||
| Person measuring/ reporting | |||
| How was pain assessed?(measurement scale) | |||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear | ||
| Imputation of missing data | |||
| Notes: | |||
| Description as stated in report/paper | Location in text or source(pg & /fig/table/other) | ||
| Outcome name | Complete relief of HAPE symptoms (in terms of course duration) | ||
| What adverse events were assessed? | |||
| Time points measured (specify whether from start or end of intervention) | |||
| Time points reported | |||
| Person measuring/ reporting | |||
| How were adverse events assessed?(measurement scale, diaries, healthcare notes, participant recall) | |||
| Scales: upper and lower limits – if applicable(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear | ||
| Imputation of missing data | Not reported | ||
| Notes: | |||
| Description as stated in report/paper | Location in text or source(pg & /fig/table/other) | ||
| Outcome name | Reduction in illness severity scores of AMS (headache, nausea, insomnia and dizziness; alone or in any combination) evaluated by the Lake Louise Questionnaire, Environmental Symptoms Questionnaire or any other validated scale. Because these different scales are not directly comparable, we will analyse the results for each scale separately. Any pooled analysis will be carefully justified. | ||
| What adverse events were assessed? | |||
| Time points measured (specify whether from start or end of intervention) | |||
| Time points reported | |||
| Person measuring/ reporting | |||
| How were adverse events assessed?(measurement scale, diaries, healthcare notes, participant recall) | |||
| Scales: upper and lower limits – if applicable(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear | ||
| Imputation of missing data | |||
| Notes: | |||
| Description as stated in report/paper | Location in text or source(pg & /fig/table/other) | ||
| Outcome name | Adverse events:
| ||
| What adverse events were assessed? | |||
| Time points measured (specify whether from start or end of intervention) | |||
| Time points reported | |||
| Person measuring/ reporting | |||
| How were adverse events assessed?(measurement scale, diaries, healthcare notes, participant recall) | |||
| Scales: upper and lower limits – if applicable(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear | ||
| Imputation of missing data | |||
| Notes: | |||
Data and analysis
Dichotomous/Continuous outcome:
| Description as stated in report/paper | Location in text or source(pg & /fig/table/other) | ||
| Comparison | |||
| Outcome | |||
| Subgroup | |||
| Time point | |||
| Results | |||
| Any other results reported(e.g. odds ratio, risk difference, CI or P value) | |||
| No. missing participants | |||
| Reasons missing | |||
| No. participants moved from other group | |||
| Reasons moved | |||
| Unit of analysis(by individuals, cluster/groups or body parts) | |||
| Statistical methods used and appropriateness of these(e.g. adjustment for correlation) | |||
| Reanalysis required?(specify, e.g. correlation adjustment) | Yes No Unclear | ||
| Reanalysis possible? | Yes No Unclear | ||
| Reanalysed results | |||
| Notes: | |||
Risk of Bias assessment
| Domain | Risk of bias | Support for judgement (include direct quotes where available with explanatory comments) | Location in text or source(pg & /fig/table/other) | ||
| Low risk | High risk | Unclear | |||
| Random sequence generation (selection bias) | |||||
| Allocation concealment (selection bias) | |||||
| Blinding of participants and personnel (performance bias)Outcome group: HAPE symptoms and course duration | |||||
| Outcome group: Adverse events | |||||
| Blinding of outcome assessment (detection bias) Outcome group: HAPE symptoms and course duration | |||||
| Outcome group: Adverse events | |||||
| Incomplete outcome data (attrition bias) Outcome group: HAPE symptoms and course duration (short term: 24hrs) | |||||
| Outcome group: HAPE symptoms and course duration (long term: 2‐7 days) | |||||
| Outcome group: Adverse events | |||||
| Selective outcome reporting? (reporting bias) | |||||
| Notes: | |||||
Other information
| Correspondence required for further study information(from whom, what and when) | |
| Any additional comments you would like to make about this study: | |
Definitions
| Clusters | A group of participants who have been allocated to the same intervention arm together, as in a cluster‐randomized trial, e.g. a whole family, town, school or patients in a clinic may be allocated to the same intervention rather than separately allocating each individual to different arms. |
| Co‐morbidities | The presence of one or more diseases or conditions other than those of primary interest. In a study looking at treatment for one disease or condition, some of the individuals may have other diseases or conditions that could affect their outcomes. |
| Compliance | Participant behaviour that abides by the recommendations of a doctor, other health care provider or study investigator (also called adherence or concordance). |
| Exclusions | Participants who were excluded from the study or the analysis by the investigators. |
| Imputation | Assuming a reasonable value for a measure where the true value is not available (e.g. assuming last observation carried forward for missing participants). |
| Reanalysis | Additional analysis of a study's results by a review author (e.g. to introduce adjustment for correlation that was not done by the study authors). |
| Report ID | A unique ID code given to a publication or other report of a study by the review author (e.g. first author's name and year of publication). If a study has more than one report (e.g. multiple publications or additional unpublished data) a separate Report ID can be allocated to each to help review authors keep track of the source of extracted data. |
| Sociodemographics | Social and demographic information about a study or its participants, including economic and cultural information, location, age, gender, ethnicity, etc. |
| Study ID | A unique ID code given to an included or excluded study by the review author (e.g. first author's name and year of publication from the main report of the study). Although a study may have multiple reports or references, it should have one single Study ID to help review authors keep track of all the different sources of information for a study. |
| Unit of allocation | The unit allocated to an intervention arm. In most studies individual participants will be allocated, but in others it may be individual body parts (e.g. different teeth or joints may be allocated separately) or clusters of multiple people. |
| Unit of analysis | The unit used to calculate N in an analysis, and for which the result is reported. This may be the number of individual people, or the number of body parts or clusters of people in the study. |
| Unit of measurement | The unit in which an outcome is measured, e.g. height may be measured in cm or inches; depression may be measured using points on a particular scale. |
| Validated | A process to test and establish that a particular measurement tool or scale is a good measure of that outcome. |
| Withdrawals | Participants who voluntarily withdrew from participation in a study before the completion of outcome measurement. |
Appendix 7. 'Summary of findings' tables 1 and 2. Optimal information size calculations (performed with STATA 15)
'Summary of findings' table number 1
Reduction in symptom score severity at 12 hours
| Estimated sample sizes for a two‐sample means test Satterthwaite's t test assuming unequal variances |
| Ho: m2 = m1 versus Ha: m2 ≠ m1 |
| Study parameters: |
| alpha = 0.0500 |
| power = 0.8000 |
| delta = −0.6000 |
| m1 = 3.1000 |
| m2 = 2.5000 |
| sd1 = 2.3000 |
| sd2 = 2.0000 |
| Estimated sample sizes: |
| N = 408 |
| N per group = 204 |
Adverse effects during treatment
| Estimated sample sizes for a two‐sample proportions test Pearson's chi‐squared test |
| Ho: p2 = p1 versus Ha: p2 ≠ p1 |
| Study parameters: |
| alpha = 0.0500 |
| power = 0.8000 |
| delta = 0.0010 (difference) |
| p1 = 0.0010 |
| p2 = 0.0020 |
| Estimated sample sizes: |
| N = 47,022 |
| N per group = 23,511 |
'Summary of findings' table number 2
Reduction in symptom score severity
Gabapentin versus placebo
| Estimated sample sizes for a two‐sample means test Satterthwaite's t test assuming unequal variances |
| Ho: m2 = m1 versus Ha: m2 ≠ m1 |
| Study parameters: |
| alpha = 0.0500 |
| power = 0.8000 |
| delta = ‐1.8300 |
| m1 = 4.7500 |
| m2 = 2.9200 |
| sd1 = 3.1100 |
| sd2 = 2.9100 |
| Estimated sample sizes: |
| N = 88 |
| N per group = 44 |
Magnesium versus placebo
| Estimated sample sizes for a two‐sample means test Satterthwaite's t test assuming unequal variances |
| Ho: m2 = m1 versus Ha: m2 ≠ m1 |
| Study parameters: |
| alpha = 0.0500 |
| power = 0.8000 |
| delta = ‐1.3000 |
| m1 = 10.3000 |
| m2 = 9.0000 |
| sd1 = 2.8000 |
| sd2 = 3.5000 |
| Estimated sample sizes: |
| N = 190 |
| N per group = 95 |
Adverse effects
Acetazolamide versus placebo
| Estimated sample sizes for a two‐sample proportions test |
| Pearson's chi‐squared test |
| Ho: p2 = p1 versus Ha: p2 ≠ p1 |
| Study parameters: |
| alpha = 0.0500 |
| power = 0.8000 |
| delta = 0.0010 (difference) |
| p1 = 0.0010 |
| p2 = 0.0020 |
| Estimated sample sizes: |
| N = 47,022 |
| N per group = 23,511 |
Appendix 8. Scores used in the included studies to measure symptoms and signs in acute mountain illness patients
| Lake Louise Score (0 to 16) Roach 1993 | |
| Headache | No headache (0) Mild headache (1) Moderate headache (2) Severe headache (3) |
| Gastrointestinal symptoms | None (0) Poor appetite or nausea (1) Moderate nausea &/or vomiting (2) Severe nausea &/or vomiting (3) |
| Fatigue &/or weakness | Not tired or weak (0) |
| Dizziness/lightheadedness | Not dizzy (0) Mild dizziness (1) Moderate dizziness (2) Severe dizziness, incapacitating (3) |
| Enviromental Symptoms Questionnaire | Used in Sampson 1983 |
|
| 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 0‐1‐2‐3‐4‐5 |
| Clinical Score: used in Bärtsch 1990 and Bärtsch 1993 |
| "a score of 1 point each was given for headache, nausea, dizziness, insomnia, and facial oedema and 2 points each for headache resistant to mild analgesics taken within the previous 12 hours, nausea with vomiting, and ataxia documented by abnormal heel‐to‐toe walking or Romberg test." |
| Acute Muntain Syndrome Questionnaire used in Grissom 1992 |
| Headache: transient or relieved with analgesic (1), severe or not relieved with analgesics (2) |
| Clinical assessment used in Keller 1995 |
| Change in mental state
Ataxia (heel to toe walking)
Peripheral oedema
|
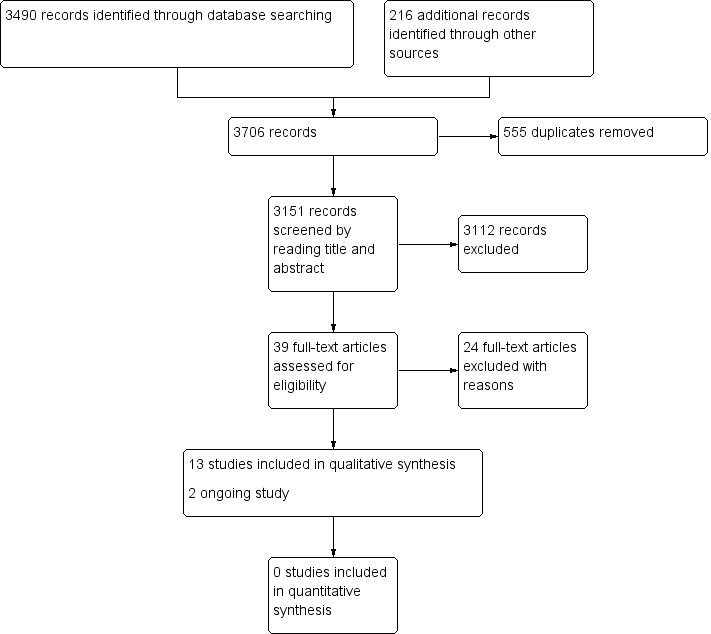
Study flow diagram.
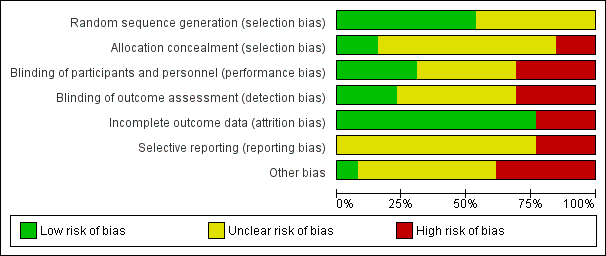
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
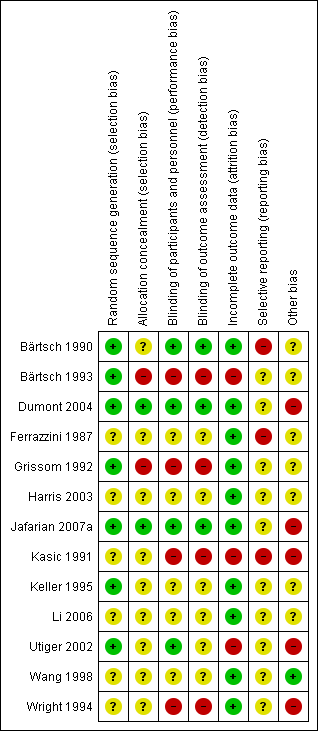
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Acetazolamide versus placebo, Outcome 1 AMS symptoms (standardized).
| Non‐pharmacological interventions for treating acute high altitude illness | ||||||
| Patient or population: people suffering from high altitude illness | ||||||
| Outcomes and intervention | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with various interventions | Risk with non‐pharmacological interventions | |||||
| All‐cause mortality | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Complete relief of AMS symptoms | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Reduction in symptom score severity at 12 hours (Clinical score: ranged from 0 to 11 (worse)) Intervention: Simulated descent of 193 millibars versus 20 millibars | The mean score in the control group was 3.1 | The mean score in the intervention group was 2.5 | 0.6 points lower with intervention | 64 (1 RCT) | ⊕⊕⊝⊝ | |
| Adverse effects during treatment Intervention: Hyperbaric chamber/ 160 millibars versus supplementary oxygen | 0 per 1000 | 0 per 1000 | Nil | 29 | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Quality of evidence downgraded by two levels due to serious risk of bias (performance bias (blinding was not specified), attrition bias and selective reporting bias) and serious imprecision (optimal information size criteria not achieved) | ||||||
| Pharmacological interventions for treating acute high altitude illness | |||||||
| Patient or population: people suffering from high altitude illness | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | ||
| Risk with various interventions | Risk with pharmacological interventions | ||||||
| All‐cause mortality | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported | |
| Complete relief of AMS symptoms (12 to 16 hours after treatment) Scale used: Acute Mountain Sickness score (ranged from 0 to 9 (worse)) | Dexamethasone versus placebo | 0 per 1000 | 471 per 1000 | No estimable | 35 | ⊕⊕⊝⊝ | |
| Reduction in symptom score severity Time of measurement: 1 to 48 hours after treatment, end of treatment Scale of measurement: Self‐administered AMS questionnaires (ranged from 0 to 90 (worse)), AMS Symptom Questionnaire (ranged from 0 to 22 (worse)), Acute Mountain Sickness score (ranged from 0 to 9 (worse)), HAH Visual analogue score (VAS) (range no stated), Lake Louise Score (from 0 to 15 (worse)), | Acetazolamide versus placebo | Standardized Mean Difference 1.15 lower | 25 | ⊕⊕⊝⊝ | |||
| Dexamethasone versus placebo | Mean change from baseline: 0.4 units | Mean change from baseline: 4.1 units | Difference of 3.7 units (reported by trial authors) | 35 | ⊕⊕⊕⊝ | ||
| Gabapentin versus placebo | Mean VAS score: 4.75 | Mean VAS score: 2.92 | Not stated | 24 | ⊕⊕⊝⊝ | ||
| Magnesium versus placebo | Mean score: 10.3 units | Mean score: 9 units | Not stated | 25 | ⊕⊕⊝⊝ | ||
| Adverse effects Time of measurement: 1 to 48 hours after treatment, end of treatment Scale of measurement:not stated | Acetazolamide versus placebo | No reported | 0 per 1000 | Not estimable | 25 | ⊕⊕⊝⊝ | |
| Gabapentin versus placebo | 0 per 1000 | 0 per 1000 | Not stated | 24 | ⊕⊕⊝⊝ | ||
| Magnesium sulphate versus placebo | 77 per 1000 | 750 per 1000 | Not stated | 25 | ⊕⊕⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1 Quality of evidence downgraded by two levels due to very serious risk of bias (multiple unclear biases and high risk of selective reporting bias) 2 Quality of evidence downgraded by two levels due to serious risk of bias (selection bias) and serious inconsistency (I² = 58%). 3 Quality of evidence downgraded by one level due to serious risk of bias (selection, performance and detection bias). 4 Quality of evidence downgraded by two levels due to serious risk of bias and serious imprecision. | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 AMS symptoms (standardized) Show forest plot | 2 | 25 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐2.56, 0.27] |

