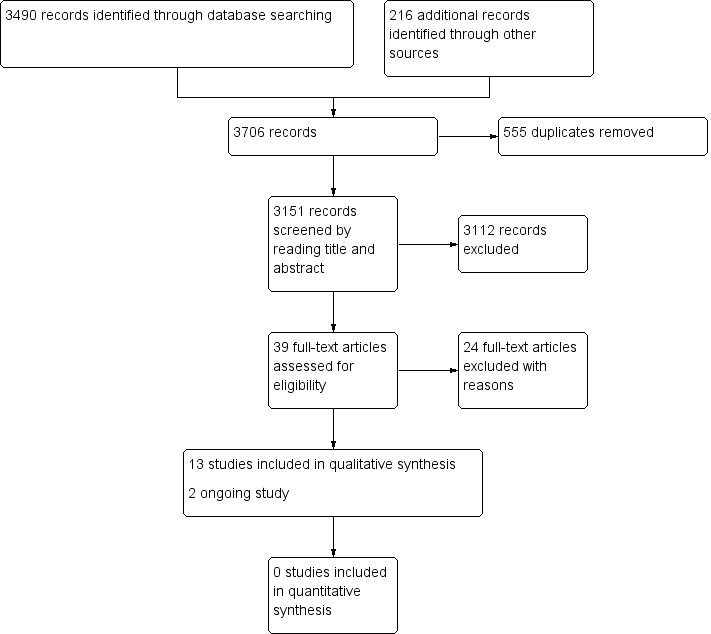
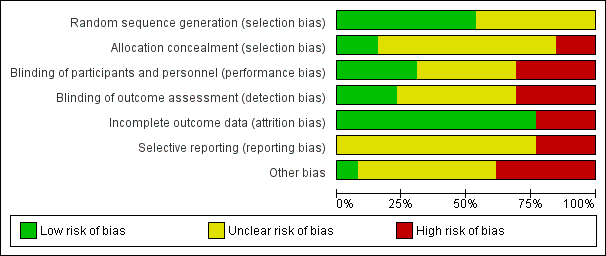
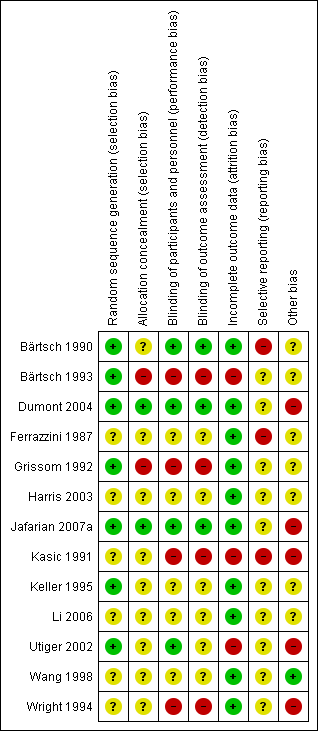
| Pharmacological interventions for treating acute high altitude illness |
| Patient or population: people suffering from high altitude illness
Setting: Alaska, borders between China, India and Pakistan, Iran, Nepal, Tibet, Swiss‒Italian border.
Intervention: pharmacological interventions (dexamethasone, acetazolamide, gabapentin)
Comparison: placebo |
| All‐cause mortality | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Complete relief of AMS symptoms (12 to 16 hours after treatment) Scale used: Acute Mountain Sickness score (ranged from 0 to 9 (worse)) | Dexamethasone versus placebo | 0 per 1000 | 471 per 1000 | No estimable | 35
(1 RCT) | ⊕⊕⊝⊝
Low 1 | |
| Reduction in symptom score severity Time of measurement: 1 to 48 hours after treatment, end of treatment Scale of measurement: Self‐administered AMS questionnaires (ranged from 0 to 90 (worse)), AMS Symptom Questionnaire (ranged from 0 to 22 (worse)), Acute Mountain Sickness score (ranged from 0 to 9 (worse)), HAH Visual analogue score (VAS) (range no stated), Lake Louise Score (from 0 to 15 (worse)), | Acetazolamide versus placebo | | | Standardized Mean Difference 1.15 lower
(2.56 lower to 0.27 higher) | 25
(2 RCTs) | ⊕⊕⊝⊝
Low 2 | |
| Dexamethasone versus placebo | Mean change from baseline: 0.4 units | Mean change from baseline: 4.1 units | Difference of 3.7 units (reported by trial authors) | 35
(1 RCT) | ⊕⊕⊕⊝
Moderate 3 | |
| Gabapentin versus placebo | Mean VAS score: 4.75 | Mean VAS score: 2.92 | Not stated | 24
(1 RCT) | ⊕⊕⊝⊝
Low 4 | |
| Magnesium versus placebo | Mean score: 10.3 units | Mean score: 9 units | Not stated | 25
(1 RCT) | ⊕⊕⊝⊝
Low 4 | |
| Adverse effects Time of measurement: 1 to 48 hours after treatment, end of treatment Scale of measurement:not stated | Acetazolamide versus placebo | No reported | 0 per 1000 | Not estimable | 25
(1 RCT) | ⊕⊕⊝⊝
Low 4 | |
| Gabapentin versus placebo | 0 per 1000 | 0 per 1000 | Not stated | 24
(1 RCT) | ⊕⊕⊝⊝
Low 4 | |
| Magnesium sulphate versus placebo | 77 per 1000 | 750 per 1000 | Not stated | 25
(1 RCT) | ⊕⊕⊝⊝
Low 4 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: Confidence interval; RR: Risk ratio; OR: Odds ratio |
| GRADE Working Group grades of evidence
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect
Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect
Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect |