Estimulación eléctrica neuromuscular para la debilidad muscular en adultos con enfermedades avanzadas
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | 2‐arm parallel RCT (n = 17) | |
| Participants | Inclusion criteria: acute exacerbation of COPD, age < 75 years, body mass index < 30 kg/m2 Exclusion criteria: locomotor or neurological condition or disability that could limit ability to exercise, implanted cardiac pacemaker Gender: 13 male, 2 female (2 unknown due to attrition) Age: median (IQR) 59 (57, 69) and 67 (59, 72) years Illness severity: median (IQR) FEV1 15 (10, 27) and 25 (17, 41) % predicted | |
| Interventions | NMES: bilateral quadriceps and hamstrings stimulation (35 Hz, 400 µs, duty cycle 33%) for 1 hour, 5 times each week for 6 weeks Amplitude set to elicit visible contraction to maximum tolerated intensity Control: parameters as per NMES arm, amplitude set to avoid visible or palpable muscle contraction | |
| Outcomes | Isometric quadriceps strength (hand‐held dynamometry), sub‐maximal exercise capacity (6MWT) | |
| Notes | Standard deviations for laboratory outcomes derived from standard errors reported in original report and from authors by request | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation used |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes prepared independently |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo/sham model used |
| Blinding of outcome assessment (detection bias) | High risk | Assessors not blinded to group allocation |
| Incomplete outcome data (attrition bias) | Low risk | All appropriate patients included in analysis, all attrition accounted for, similar across groups (1 patient each) and not related to study intervention (disease‐ related readmission and family refusal) |
| Selective reporting (reporting bias) | Low risk | Full results provided in online supplement |
| Methods | 2‐arm parallel RCT (n = 18) | |
| Participants | Inclusion criteria: moderate to severe COPD FEV1< 65% predicted, age < 70 years, limited exercise tolerance Exclusion criteria: cardiovascular or neurological condition, active or debilitating joint disease, pulmonary rehabilitation previous 2 years Gender: 10 male, 8 female Age: mean (SD) 59 (2) and 62 (2) years Illness severity: GOLD stage III/IV | |
| Interventions | NMES: bilateral quadriceps, hamstrings and calve stimulation (50 Hz, 200 µs, duty cycle 13%) for 1 hour (20 min each muscle), 3 times each week for 6 weeks. Amplitude set to maximum tolerated intensity Control: set up as per NMES arm but no active stimulation | |
| Outcomes | Isokinetic quadriceps and hamstring strength (dynamometry), maximal exercise capacity (incremental shuttle walk test) | |
| Notes | Standard deviation derived from standard errors reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo/sham model used |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors blinded to group allocation |
| Incomplete outcome data (attrition bias) | Low risk | All patients included in analysis |
| Selective reporting (reporting bias) | Low risk | Full results provided |
| Methods | 2‐arm cross‐over RCT (n = 17) | |
| Participants | Inclusion criteria: COPD FEV1:FEV < 70%, MRC breathlessness score II/III, stable medication previous 3 months Exclusion criteria: locomotor or neurological condition, malignancy, severe endocrine, hepatic or renal disease, cardiac failure, implanted cardiac pacemaker, distal arteriopathy, recent surgery, use of anticoagulant medication Gender: 16 male, 1 female Age: mean (SD) 66 (9) years Illness severity: GOLD stage III/IV | |
| Interventions | NMES: bilateral quadriceps stimulation (50 Hz, 400 µs, duty cycle 16% to 33%) for 1 hour, 5 times each week for 6 weeks. Amplitude set to elicit visible contraction to maximum tolerated intensity Control: bilateral quadriceps stimulation (10 Hz, 50 µs, duty cycle 16% to 33%) for 1 hour, 5 times each week for 6 weeks. Amplitude limited to 10 mA set to avoid muscle contraction | |
| Outcomes | Isokinetic quadriceps strength (dynamometry), sub‐maximal exercise capacity (6MWT), body composition (DEXA) | |
| Notes | Patients included in Nápolis 2011 clinical outcomes (excluded from meta‐analysis to avoid multiplicity). Laboratory outcomes included separately. The wash‐out period was deemed sufficient to include both study phases in the meta‐analysis. Results from paired analyses were used as recommended by Elbourne 2002. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo/sham model used |
| Blinding of outcome assessment (detection bias) | High risk | Muscle biopsies only taken in NMES arm |
| Incomplete outcome data (attrition bias) | Low risk | All patients included in analysis |
| Selective reporting (reporting bias) | Low risk | Full results provided |
| Methods | 2‐arm parallel RCT (n = 16) | |
| Participants | Inclusion criteria: non‐small cell lung cancer, Eastern Co‐operative Oncology Group performance status 0 to 1, < 10% weight loss Exclusion criteria: chemotherapy or radiotherapy previous 4 weeks, change in medication previous week, ischaemic heart disease, implanted cardiac pacemaker Gender: 9 male, 7 female Age: mean (SD) 64 (5) and 56 (9) years Illness severity: locally advanced or metastatic, stage III/IV | |
| Interventions | NMES: bilateral quadriceps stimulation (50 Hz, 350 µs, duty cycle 11% to 25%) for 30 min, daily for 4 weeks. Amplitude set to elicit visible contraction to maximum tolerated intensity Control: no intervention | |
| Outcomes | Isokinetic quadriceps strength (dynamometry), sub‐maximal exercise capacity (endurance shuttle walk test), physical activity level (accelerometer) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block generated independently |
| Allocation concealment (selection bias) | Low risk | Using sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) | High risk | No placebo/sham model |
| Blinding of outcome assessment (detection bias) | High risk | Assessors not blinded to group allocation |
| Incomplete outcome data (attrition bias) | Low risk | All patients included in analysis. Data on 1 participant (NMES group) missing for each quadriceps strength and physical activity level due to technical problems. |
| Selective reporting (reporting bias) | Low risk | Full results provided |
| Methods | 2‐arm parallel RCT (n = 15) | |
| Participants | Inclusion criteria: severe COPD FEV1 < 50% predicted, MRC breathlessness score IV/V Exclusion criteria: locomotor or neurological condition, change in medication or exacerbation in previous 4 weeks Gender: 9 male, 6 female Age: mean (SD) 67 (8) and 65 (5) years Illness severity: GOLD stage IV | |
| Interventions | NMES: bilateral quadriceps stimulation (50 Hz, 300 to 400 µs, duty cycle 11% to 25%) for 30 min, 5 times each week for 6 weeks Amplitude set to elicit visible contraction to maximum tolerated intensity Control: no intervention | |
| Outcomes | Isokinetic and isometric quadriceps strength (dynamometry), quadriceps endurance (constant load), maximal exercise capacity (CPET cycle ergometry), quality of life (Chronic Respiratory Questionnaire) | |
| Notes | Control patients received NMES after the first study period and pre‐post changes reported. These data were not used in meta‐analysis. Change score for the meta‐analysis for quadriceps strength and exercise capacity were estimated using the difference between pre‐ and post‐intervention groups means the widest standard deviations as per a previous review (Roig 2009) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised |
| Allocation concealment (selection bias) | Low risk | Referers blinded to sequence allocation |
| Blinding of participants and personnel (performance bias) | High risk | No placebo/sham model used |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | All patients included in analysis |
| Selective reporting (reporting bias) | Low risk | Full results provided |
| Methods | 2‐arm parallel RCT (n = 34) | |
| Participants | Inclusion criteria: symptomatic left ventricular fraction < 35%, optimised medication Exclusion criteria: acute heart failure, angina, arrhythmia, implanted cardiac pacemaker Gender: 29 male, 5 female Age: mean (SD) 53 (10) years Illness severity: NYHA stage II to IV | |
| Interventions | NMES: bilateral quadriceps and hamstrings stimulation (15 Hz, 500 µs, duty cycle 33%) for 4 hours, daily for 10 weeks. Amplitude set to elicit visible contraction to maximum tolerated intensity Control: parameters as per NMES arm, amplitude set to avoid visible or palpable muscle contraction | |
| Outcomes | Maximal exercise capacity (CPET cycle ergometry), sub‐maximal exercise capacity (6MWT), quality of life (Minnesota living with heart failure questionnaire) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo/sham model used |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | All attrition accounted for, small number of patients (n = 2) and not related to study intervention (urgent heart transplantation) |
| Selective reporting (reporting bias) | High risk | Under adverse events sub‐heading "maximum voluntary strength of the stimulated muscle groups did not differ from baseline data" |
| Methods | 2‐arm cross‐over RCT (n = 30) | |
| Participants | Inclusion criteria: COPD FEV1:FEV < 70%, MRC breathlessness score I/III Exclusion criteria: locomotor or neurological condition, malignancy, severe endocrine, hepatic or renal disease, cardiac failure, implanted cardiac pacemaker, distal arteriopathy, recent surgery, use of anticoagulant medication, change in medication or exacerbation in previous 4 weeks, regular physical activity, previous pulmonary rehabilitation Gender: 26 male, 4 female Age: mean (SD) 64 (7) years Illness severity: GOLD stage II/III | |
| Interventions | NMES: bilateral quadriceps stimulation (50 Hz, 300 to 400 µs, duty cycle 16% to 33%) for up to 1 hour, 5 times each week for 6 weeks. Amplitude set to elicit visible contraction to maximum tolerated intensity Control: bilateral quadriceps stimulation (50 Hz, 200 µs, duty cycle 16%) for 15 minutes, 3 times each week for 6 weeks. Amplitude limited to 10 mA set to avoid muscle contraction | |
| Outcomes | Isokinetic quadriceps strength (dynamometry), maximal exercise capacity (CPET cycle ergometry) sub‐maximal exercise capacity (6MWT) | |
| Notes | Patients from Dal Corso 2007 were included in this study. For clinical outcomes Nápolis 2011 data were used in meta‐analysis to avoid multiplicity). The wash‐out period was deemed sufficient to include both study phases in the meta‐analysis. Results from paired analyses were used as recommended by Elbourne 2002. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After randomisation |
| Allocation concealment (selection bias) | Low risk | As per Dal Corso 2007 |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo/sham model used |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors blinded to patient treatment sequence |
| Incomplete outcome data (attrition bias) | Low risk | All patients included in analysis. Data on 2 and 4 participants were missing for maximal and sub‐maximal exercise capacity respectively due to technical problems (group allocation unknown). |
| Selective reporting (reporting bias) | Low risk | Full results provided |
| Methods | 2‐arm parallel RCT (n = 42) | |
| Participants | Inclusion criteria: severe chronic heart failure, optimised drug therapy Exclusion criteria: unstable disease, peripheral oedema, implanted cardiac pacemaker Gender: 21 male, 12 female Age: mean (SD) 59 (6) and 57 (8) years Illness severity: NYHA stage II to IV | |
| Interventions | NMES: bilateral quadriceps and hamstrings stimulation (50 Hz, 700 µs, duty cycle 25%) for up to 1 hour, 5 times each week for 8 weeks. Amplitude set to elicit visible contraction to maximum tolerated intensity Control: encouraged to continue engagement in usual activities of daily living recorded in diary | |
| Outcomes | Isokinetic and isometric quadriceps and hamstrings strength (dynamometry), quadriceps endurance (interval fixed load), body composition (computed tomography), lower limb functional activities (stair climb, rise from chair, rise from supine), quality of life (SF‐36) | |
| Notes | Standard deviations for outcomes of quadriceps and hamstrings strength, quadriceps endurance and body composition were derived from reported 95% confidence intervals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block‐wise randomisation using list provided by independent staff |
| Allocation concealment (selection bias) | Low risk | Randomisation code locked until the end of the study |
| Blinding of participants and personnel (performance bias) | High risk | No placebo/sham model |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were not aware of the patients' group allocation |
| Incomplete outcome data (attrition bias) | Low risk | All attrition accounted for, similar numbers across groups (NMES n = 2, control n = 5) and not related to study intervention (urgent heart transplantation n = 6, pacemaker implanted n = 1, renal failure n = 1, died (control) n = 1) |
| Selective reporting (reporting bias) | Low risk | Full results provided |
| Methods | 2‐arm parallel RCT (n = 17) | |
| Participants | Inclusion criteria: severe COPD, COPD FEV1:FEV < 70%, FEV1< 50% predicted, body mass index < 22 kg/m2 , quadriceps maximum voluntary strength < 50% predicted Exclusion criteria: cardiovascular, renal or hepatic disease, acute respiratory failure Gender: 11 male, 6 female Age: mean (SD) age 59 (15) and 68 (12) years Illness severity: GOLD stage IV | |
| Interventions | NMES: bilateral quadriceps stimulation (35 Hz, 400 µs, duty cycle 47%) for 30 minutes, 4 times each week for 4 weeks. Amplitude set to elicit visible contraction to maximum tolerated intensity. Additional usual rehabilitation as described below Control: usual rehabilitation limb mobilisations, slow treadmill walking, light upper limb resistance training for ˜30 minutes, 4 times each week for 4 weeks | |
| Outcomes | Isometric quadriceps strength (dynamometry), sub‐maximal exercise capacity (6MWT), body composition (anthropometry), quality of life (Maugeri Foundation Respiratory Failure Questionnaire) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised into 2 groups |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | No placebo/sham model used |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Body composition assessments optional |
| Selective reporting (reporting bias) | Low risk | Full results provided, body composition assessments optional, similar numbers across groups (NMES n = 6, control n = 5) |
| Methods | 2‐arm parallel RCT (n = 22) | |
| Participants | Inclusion criteria: severe COPD FEV1:FEV < 70%, FEV1 < 50% predicted, 6‐minute walking distance < 400 metres, > 20 year smoking pack‐year history, sedentary lifestyle, < 1 hour from hospital Exclusion criteria: acute exacerbation or systemic steroids in previous 4 weeks, condition associated with muscle wasting including active inflammatory illness, heart failure or diabetes Gender: 13 male, 7 female Age: mean (SD) 68 (9) and 70 (3) years Illness severity: GOLD stage IV | |
| Interventions | NMES: bilateral quadriceps and calve stimulation (50 Hz, 400 µs, duty cycle 27%) for 1 hour (35 minutes quadriceps and 25 minutes calves), 5 times each week for 6 weeks. Amplitude set to elicit visible contraction to maximum tolerated intensity Control: bilateral quadriceps stimulation (5 Hz, 100 µs, continuous) for 1 hour (35 minutes quadriceps and 25 minutes calves), 5 times each week for 6 weeks | |
| Outcomes | Isometric quadriceps strength (dynamometry), quadriceps endurance (constant load test), body composition (computed tomography), sub‐maximal exercise capacity (endurance shuttle walk test) | |
| Notes | Standard deviations for all outcomes derived from standard errors reported in original report and from authors by request | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Blinding of participants and personnel (performance bias) | Low risk | |
| Incomplete outcome data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Methods | 2‐arm parallel RCT (n = 24) | |
| Participants | Inclusion criteria: chronic hypercapnic respiratory failure, COPD FEV1:FEV < 70%, mechanically ventilated, severe peripheral muscle atrophy, bed‐bound > 30 days Exclusion criteria: condition or disease other than COPD, change in medication previous 4 weeks, corticosteroid use > 5 days whilst on intensive care unit Gender: 17 male, 7 female Age: mean (SD) 68 (8) and 65 (4) years Illness severity: respiratory failure due to COPD | |
| Interventions | NMES: bilateral quadriceps and glutei stimulation (35 Hz, 350 µs, duty cycle not reported) for 30 minutes, 5 times each week for 4 weeks. Amplitude not reported. Used as adjunct to active limb mobilisation described below Control: active limb mobilisation of upper and lower limbs for up to 30 minutes within patient tolerance, 5 times each week for 4 weeks | |
| Outcomes | Peripheral muscle strength (manual muscle testing), number of days from bed to chair | |
| Notes | Peripheral muscle strength outcome not clearly limited to quadriceps and excluded from meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | No placebo/sham model used |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors blinded to group allocation |
| Incomplete outcome data (attrition bias) | Low risk | All patients included in analysis |
| Selective reporting (reporting bias) | Low risk | Full results provided |
Abbreviations: 6MWT = six minute walk test, COPD = chronic obstructive pulmonary disease, CPET = cardiopulmonary exercise testing, DEXA = dual energy x‐ray absorptiometry, FEV1 forced expiratory volume in 1 second, FVC = forced vital capacity, Hz = hertz, IQR = interquartile range, MRC = Medical Research Council, NMES = neuromuscular electrical stimulation, NYHA = New York Heart Association, RCT = randomised controlled trial, SD = standard deviation, SF‐36 = short form 36, µs = microseconds
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| The majority of patients (9/10) had early‐stage (NYHA II) disease | |
| The intervention studied involved magnetic rather than electrical stimulation to elicit a muscular contraction | |
| The majority of patients (18/24) had early‐stage (NYHA II) disease | |
| The majority of patients (22/30) had early‐stage (NYHA II) disease | |
| Group allocation reportedly occurred according to level of illness severity and muscle dysfunction: "due to illness severity and muscle dysfunction 8 patients were included in NMES and 11 patients were included in endurance program" | |
| Randomisation occurred at the level of the limb with one leg stimulated and the other acting as a control | |
| The majority of patients (35/46) had early‐stage (NYHA II) disease | |
| The majority of patients (28/35) had early‐stage (NYHA II) disease | |
| The majority of patients (99/101) had early‐stage disease |
NMES = neuromuscular electrical stimulation, NYHA = New York Heart Association
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Quadriceps muscle strength Show forest plot | 8 | 195 | Std. Mean Difference (IV, Random, 95% CI) | 0.90 [0.33, 1.46] |
| Analysis 1.1  Comparison 1 NMES versus control, Outcome 1 Quadriceps muscle strength. | ||||
| 2 Exercise performance Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 NMES versus control, Outcome 2 Exercise performance. | ||||
| 2.1 6MWT | 4 | 125 | Mean Difference (IV, Random, 95% CI) | 40.05 [‐4.14, 84.24] |
| 2.2 ISWT | 1 | 18 | Mean Difference (IV, Random, 95% CI) | 68.8 [18.54, 119.06] |
| 2.3 ESWT | 2 | 36 | Mean Difference (IV, Random, 95% CI) | 160.22 [33.73, 286.70] |

Review flow diagram.
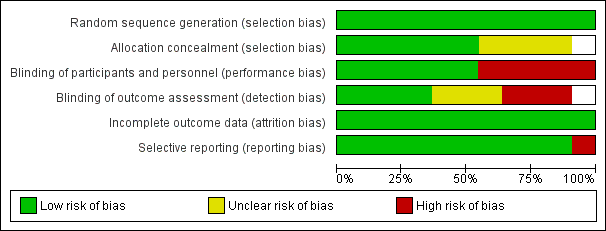
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of quadriceps muscle strength for NMES versus control.

Forest plot of exercise performance for NMES versus control.

Comparison 1 NMES versus control, Outcome 1 Quadriceps muscle strength.
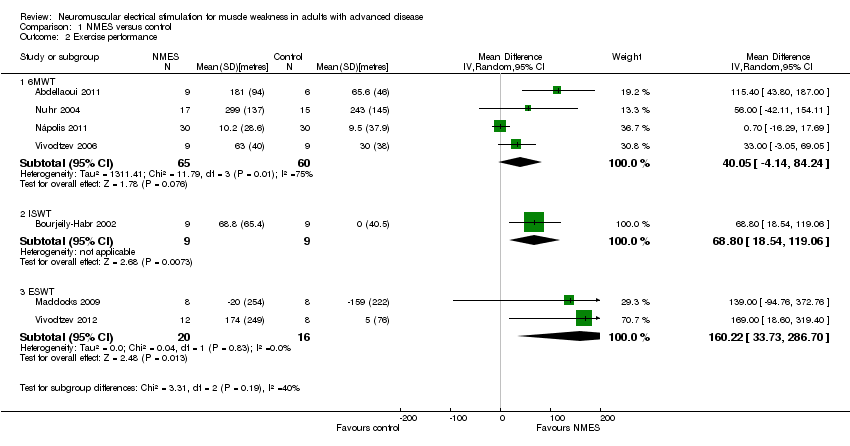
Comparison 1 NMES versus control, Outcome 2 Exercise performance.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Quadriceps muscle strength Show forest plot | 8 | 195 | Std. Mean Difference (IV, Random, 95% CI) | 0.90 [0.33, 1.46] |
| 2 Exercise performance Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 6MWT | 4 | 125 | Mean Difference (IV, Random, 95% CI) | 40.05 [‐4.14, 84.24] |
| 2.2 ISWT | 1 | 18 | Mean Difference (IV, Random, 95% CI) | 68.8 [18.54, 119.06] |
| 2.3 ESWT | 2 | 36 | Mean Difference (IV, Random, 95% CI) | 160.22 [33.73, 286.70] |

