Magnesio para los calambres musculares esqueléticos
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Double‐blind, parallel group RCT | |
| Participants | 73 pregnant women (mean 29 wk gestation) with rest cramps and no previous cramp treatment. Recruitment from Swedish prenatal care clinics. | |
| Interventions | Either a chewable tablet containing 122 mg elemental magnesium ("primarily as Mg lactate or Mg citrate"), or matched placebo tablet, taken once each morning and twice each evening for 3 weeks | |
| Outcomes | Primary outcome unclear. Change in cramp frequency on a 5‐point ordinal scale. Time of day cramps occurred on a 4‐point nominal scale. Presence of symptoms the day after a night of cramping on a 3‐point ordinal scale. Global patient assessment of treatment effect on a 5‐point ordinal scale. Cramp intensity on a visual analog scale (VAS). Serum magnesium and calcium and 24‐h urinary magnesium and calcium excretion | |
| Notes | Published. Manufacturer sponsored. Did laboratory tests at only one of the two centres | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described ("The patients were then randomly allocated to either magnesium or placebo") |
| Allocation concealment (selection bias) | Unclear risk | Not described. See above for only quote |
| Blinding (performance bias and detection bias) | Low risk | Quote: “A magnesium‐placebo tablet batch of 90 numbered bottles was prepared by ACO Lakemedel...” Comment: Probably satisfactory, although pills were not described |
| Incomplete outcome data (attrition bias) | Low risk | 4/73 subjects dropped out of the study and were excluded from the analysis. Reasons for dropout were well described but treatment group was not identified. One placebo patient withdrew from treatment but appears (unclear) to have been included in the analysis. Comment: Probably adequate as total number of dropouts was small. |
| Selective reporting (reporting bias) | High risk | No description of outcomes by primary and secondary, and outcomes were incompletely described in methods, i.e. only in the results is it evident that before and after comparisons, mean differences and numbers attaining specific cut‐offs are used. Unclear how well outcomes were predefined. Inadequate reporting: no actual numbers for many P values. This study also reported a reduction in cramp frequency "from the initial average of every other day, to every 3 days in the placebo group and one to two times a week in the magnesium group (P < 0.05)". However, "every 3 days" and "one to two times a week" do not belong to the 5‐point ordinal scale used to measure this outcome (daily, every other day, twice a week, once a week, never). |
| Cramp diary (recall bias) | High risk | No diary used |
| Other bias | Unclear risk | Subjects treated differently at each site (one used laboratory testing, the other did not). |
| Methods | Double‐blind RCT of cross‐over design | |
| Participants | 45 non‐pregnant rest cramp sufferers > 18 years (mean age 61.6 years) having a normal neurologic exam and at least 6 leg cramps in a 4‐week placebo run‐in. Recruitment from a single large university‐based Argentinean family practice clinic. | |
| Interventions | Magnesium citrate 900 mg pill (approx. 100 mg elemental magnesium) twice daily or similar tasting and appearing placebo, each for 4 weeks. Four‐week placebo run‐in and 4‐week washout between treatments. | |
| Outcomes | Primary: Number of cramps in treatment period. Secondary: Cramp duration by 4 ordinal categories (< 5 minutes, 5 to 10 minutes, 10 to 30 minutes, > 30 minutes). Cramp intensity by “analog scale”. Sleep disturbance on a 0 to 10 scale with 0 = “no sleep disturbance” and 10 = “could not sleep because of the cramps”. Adverse events. | |
| Notes | Published. Independent funding. The 4‐week placebo run‐in was pre‐randomization. Unclear what the range for the analog scale of intensity is (assumed 0 to 10). Cramp duration was recorded by ordinal category but reported with a mean and standard deviation in minutes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients randomly received magnesium or placebo...” Comment: unclear how randomization was performed |
| Allocation concealment (selection bias) | Unclear risk | Quote: “The codes were inside a sealed envelope opened at the end of the analysis.” Comment: Unclear who allocated subjects and maintained the blinding |
| Blinding (performance bias and detection bias) | Low risk | Quote: “Each pill contained 900 mg of magnesium citrate or matched placebo (same appearance and taste).” Comment: Satisfactory blinding |
| Incomplete outcome data (attrition bias) | Low risk | 3/45 subjects withdrew with reasons given. It is not stated which intervention they were receiving at the time or how their data were dealt with. Comment: Probably satisfactory as the number of dropouts was small. |
| Selective reporting (reporting bias) | Unclear risk | No indication of selective reporting for clinical endpoints (although urine for magnesium was collected and not reported). Duration of cramps was measured on a 4‐point ordinal scale but results were reported with the mean duration and standard deviation measured in minutes as though they were a continuous variable |
| Cramp diary (recall bias) | Low risk | Diary used |
| Other bias | Low risk | No obvious other bias |
| Methods | Double‐blind, parallel group RCT | |
| Participants | 46 non‐pregnant rest cramp sufferers (mean age 69.3 yrs) with at least 8 cramps in a 30 day baseline diary. Recruitment from posters and pamphlets in 21 Canadian (Richmond BC) family practitioner offices and also by newspaper advertizement | |
| Interventions | 5 days consecutive 4 hour intravenous infusions of 250 ml D5W (5% dextrose in water) either with (treatment group) or without (control group) 20 mmol of magnesium sulfate added (20 mmol = 486 mg elemental magnesium). Indistinguishable. | |
| Outcomes | Primary: Change in the number of cramps per week from baseline at 30 days. Secondary: Change in the number of cramps per week from baseline at 90 days. Percentage change in cramps / wk. Cramp pain (1 to 10 interval scale). Cramp duration on a 3‐point ordinal scale (1 = < 1 minute, 2 = 1‐5 minutes, 3 = > 5 minutes). 24‐h urinary magnesium on days 1 and 5 to determine % retention of infused magnesium. | |
| Notes | Published. Independent funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization, using a computer generated random allocation sequence without any blocking or stratification was carried out by the hospital pharmacist dispensing the study drugs according to a series of opaque allocation envelopes kept in the pharmacy." Comment: Satisfactory randomization |
| Allocation concealment (selection bias) | Low risk | Quote: "All investigators, study nurses and subjects were blinded as to treatment allocation." Comment: Satisfactory allocation concealment |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: 1) "Active and Placebo solutions were indistinguishably clear and colorless." 2) "Subjects had been told that IV site discomfort was possible with both placebo and Mg infusions. While generally it was considered that blinding was reasonable, the sensation of burning at the IV site, coupled with the additional saline dilution in some Mg subjects, could have compromised the blind to some extent (presumably favouring the intervention)." |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs or losses to follow‐up. Analysis was intention‐to‐treat. |
| Selective reporting (reporting bias) | Low risk | Did not process urine samples for magnesium on those getting placebo (although did a reasonable job collecting urine samples from all patients to make sure the blinding was not broken). Severity and duration of cramps were described only as not being different (i.e. no numbers given), however, these data were made available by the authors. |
| Cramp diary (recall bias) | Low risk | Diary used |
| Other bias | Low risk | No obvious other bias |
| Methods | Double‐blind, parallel group RCT | |
| Participants | 45 pregnant women with rest cramps and no previous cramp treatment. Recruitment by pamphlets provided to pregnant Norwegian women undergoing 18 week ultrasound | |
| Interventions | Either a chewable tablet containing 122 mg elemental magnesium ("primarily as Mg lactate and Mg citrate"), or a matched placebo tablet, taken once each morning and twice each evening for 2 weeks | |
| Outcomes | Number of days or nights in which cramps occurred over 2 weeks. Degree of cramp pain on a 5‐point ordinal scale. Side effects. Serum magnesium and calcium and 24‐h urinary magnesium on days 1 and 15 | |
| Notes | Published. Source of funding not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The randomization program was provided by Medstat Research AS.” Comment: Probably adequate |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation method given |
| Blinding (performance bias and detection bias) | Low risk | Quote: “Both groups received a plastic container with the trial medication, 42 chewable tablets...”, containing either magnesium or placebo, both provided by the manufacturer Comment: Probably adequate |
| Incomplete outcome data (attrition bias) | Unclear risk | 7/45 women (15.6%) dropped out (2 from the treatment arm and 5 from control). Reasons were given and most were unrelated to potential drug effects. None of the 7 were included in the analysis because of a lack of data. |
| Selective reporting (reporting bias) | Low risk | Primary outcome assumed to be the number of days and nights with cramping but not explicitly stated. All outcomes reported. |
| Cramp diary (recall bias) | Low risk | Diary used |
| Other bias | Unclear risk | Frequency of cramping at baseline was not assessed, making it impossible to tell if the group was imbalanced in this important baseline characteristic |
| Methods | Double blind RCT of cross‐over design | |
| Participants | 73 non‐pregnant rest cramp sufferers (mean age 63 yrs), having at least 2 cramps per week. Recruitment by community advertizement in a UK population | |
| Interventions | Either 1830 mg of tri‐magnesium dicitrate powder (300 mg elemental magnesium) poured from a sachet into a glass of water, or matched placebo powder, taken orally each night for 6 weeks before switching to the alternate therapy. 2 week magnesium free run‐in and effectively a 2 week washout between treatments since only the last 4 weeks of each 6 weeks on treatment was used for outcome assessment | |
| Outcomes | Number of cramps during the last 4 weeks of each treatment period. Severity of cramps (mild, moderate, severe). Duration of cramps (short, medium, long). Self reported assessment of treatment effectiveness (yes, no) | |
| Notes | Published. Manufacturer sponsored. Only data from the first period have been used in this review because large differences in treatment effect are seen depending on the sequence in which treatment is given. Patient level data provided by the principle investigator. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The manufacturer provided centralized randomization for the trial in large blocks of 10. Specifics regarding the sequence generation were not given. The resulting allocation was unequal with more subjects included in the analysis receiving magnesium second (29 vs 17). |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation code was not known to the investigators who gave out the sachets. The code remained concealed from everyone except the pharmacist who prepared the sachets..." Comment: Satisfactory concealment |
| Blinding (performance bias and detection bias) | Unclear risk | No description of whether the magnesium and placebo suspensions tasted different |
| Incomplete outcome data (attrition bias) | High risk | Reasons for dropout documented, but 27 of 73 subjects (37%) did not complete the study |
| Selective reporting (reporting bias) | Low risk | Severity and duration of cramps were described only as not being different (i.e. no numbers given), however, these data were provided to us by the authors. |
| Cramp diary (recall bias) | Low risk | Diary used |
| Other bias | Unclear risk | Manufacturer played an active role in the trial. There was a large difference in treatment effect depending on the sequence of treatments (much greater benefit if treatment was received in the order placebo→magnesium). Unclear if this difference was due entirely to period effect or if noncompleters, the potential for carry‐over or unblinding contributed. This difference in benefit resulting from treatment order was important since the randomization was unbalanced (many more subjects receiving the placebo→magnesium sequence). |
| Methods | Double blind, parallel group RCT | |
| Participants | 40 non‐pregnant rest cramp sufferers (45 to 80 years of age) with normal renal function having at least 2 cramps per week that were rated 5 or more on a 0‐10 pain scale. Recruitment by radio advertisement in an American (State of Michigan) population. | |
| Interventions | Either 168 mg elemental magnesium from slow release magnesium lactate tablets (MagTabSR) or matching placebo tablets taken orally twice daily for 30 days. | |
| Outcomes | Frequency, duration and severity of leg cramps captured daily x 1 wk pre‐intervention and daily during the 30 days of intervention (via diary recording of cramps and sleep disturbance). Pittsburgh Sleep Quality questionnaire also administered pre‐ and post‐intervention. | |
| Notes | Unpublished. Sponsorship not provided. Incomplete results reporting. Patient level data for cramp frequency were kindly provided by study statistician. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomized but details not provided. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Masking: Double Blind (Subject, Investigator, Outcomes Assessor)". No details provided. Probably adequate. |
| Incomplete outcome data (attrition bias) | Low risk | Small number of dropouts, two from magnesium and one from placebo. Reasons not provided. |
| Selective reporting (reporting bias) | High risk | Patient level data were provided to us but were only available for a subset of the outcomes. |
| Cramp diary (recall bias) | Low risk | Diary used |
| Other bias | Low risk | No obvious other bias |
| Methods | Open label RCT with 4 parallel treatment groups | |
| Participants | 84 pregnant women. Recruitment method (Iranian women) not provided. | |
| Interventions | Group 1: 500 mg calcium carbonate tablet once daily Group 2: 7.5 mmol magnesium aspartate (182 mg elemental magnesium) twice daily Group 3: 100 mg of thiamine (vitamin B1) plus 40 mg of pyridoxine (vitamin B6) once daily Group 4: No treatment | |
| Outcomes | "Change in muscle spasms" on a 3‐point ordinal scale (no change, "relative improvement", or "absolute improvement") | |
| Notes | Unusual design. Each treatment was given over two weeks but efficacy was assessed at 4 weeks. Published as a "brief communication" (letter) only. Funding source not provided. No definition of relative and absolute improvement was given in the manuscript but this was confirmed with the author to mean partial and complete resolution of the overall cramp burden (which presumably takes into account both intensity and frequency). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding (performance bias and detection bias) | High risk | Open label trial |
| Incomplete outcome data (attrition bias) | Low risk | No details regarding flow of patients in the manuscript but author communication suggests no dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome not identified (though only one outcome reported). Table 2 showed statistical significance in total improvement for groups 2 and 3 compared to group 4 but in the text it stated groups 1 and 3 (which is supported by the CI results). |
| Cramp diary (recall bias) | High risk | Specifics were not given but there appeared to have only been a qualitative assessment of the change in cramps upon study completion |
| Other bias | High risk | Baseline characteristics were said to be not significantly different but they were not provided. Unclear who rated the degree of improvement (patient or physician).Trial was very under reported. Outcomes were grouped in an impractical way |
CI: confidence interval
IV: intravenous
RCT: randomized controlled trial
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| This RCT looked at muscle strength, muscle mass and muscle magnesium content. It did not look at measures of muscle cramping | |
| No control group. Article in German with English abstract. Methods translated | |
| Did not appear to be randomized. Evaluated serum magnesium levels in pregnant cramp sufferers before and after magnesium supplementation. Did not evaluate changes in muscle cramping. Article in German with English abstract. Methods translated | |
| No magnesium treatment arm | |
| No control group. Article in German. Abstract and Methods translated | |
| Uncontrolled. Article in German with English abstract | |
| This RCT looked at exercise performance and magnesium concentration in various tissues. It did not look at measures of muscle cramping |
RCT: randomized controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 % Change in cramp frequency from baseline at 4 weeks Show forest plot | 2 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐3.93 [‐21.12, 13.26] |
| Analysis 1.1  Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 1 % Change in cramp frequency from baseline at 4 weeks. | ||||
| 2 % Change in cramp frequency from baseline at 12 weeks Show forest plot | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐12.09 [‐40.22, 16.04] |
| Analysis 1.2  Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 2 % Change in cramp frequency from baseline at 12 weeks. | ||||
| 3 Proportion of subjects with a ≥ 25% reduction in cramp frequency at 4 weeks Show forest plot | 2 | 83 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.08 [‐0.28, 0.12] |
| Analysis 1.3  Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 3 Proportion of subjects with a ≥ 25% reduction in cramp frequency at 4 weeks. | ||||
| 4 Proportion of subjects with a ≥ 25% reduction in cramps at 12 weeks Show forest plot | 1 | 43 | Risk Difference (M‐H, Fixed, 95% CI) | 0.11 [‐0.19, 0.41] |
| Analysis 1.4  Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 4 Proportion of subjects with a ≥ 25% reduction in cramps at 12 weeks. | ||||
| 5 Number of cramps per week at 4 weeks Show forest plot | 4 | 213 | Mean Difference (Fixed, 95% CI) | 0.01 [‐0.52, 0.55] |
| Analysis 1.5  Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 5 Number of cramps per week at 4 weeks. | ||||
| 6 Number of cramps per week at 12 weeks Show forest plot | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.84 [‐3.23, 1.55] |
| Analysis 1.6  Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 6 Number of cramps per week at 12 weeks. | ||||
| 7 Cramp intensity (pain) on a 3 point scale (1 = mild, 2 = moderate, 3 = severe) at 4 weeks Show forest plot | 3 | 175 | Mean Difference (Fixed, 95% CI) | ‐0.04 [‐0.18, 0.11] |
| Analysis 1.7  Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 7 Cramp intensity (pain) on a 3 point scale (1 = mild, 2 = moderate, 3 = severe) at 4 weeks. | ||||
| 8 Cramp intensity (pain) on a 3 point scale (1 = mild, 2 = moderate, 3 = severe) at 12 weeks Show forest plot | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.55, 0.19] |
| Analysis 1.8  Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 8 Cramp intensity (pain) on a 3 point scale (1 = mild, 2 = moderate, 3 = severe) at 12 weeks. | ||||
| 9 Proportion of subjects rating their cramps as moderate or severe (i.e. ≥ 2 on the 3 point intensity scale) at 4 weeks Show forest plot | 2 | 91 | Risk Difference (M‐H, Fixed, 95% CI) | 0.09 [‐0.07, 0.25] |
| Analysis 1.9  Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 9 Proportion of subjects rating their cramps as moderate or severe (i.e. ≥ 2 on the 3 point intensity scale) at 4 weeks. | ||||
| 10 Proportion of subjects rating their cramps as moderate to severe (i.e. with mean cramp intensity ≥2 on the 3 point intensity scale) at 12 weeks Show forest plot | 1 | 43 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.06 [‐0.21, 0.10] |
| Analysis 1.10  Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 10 Proportion of subjects rating their cramps as moderate to severe (i.e. with mean cramp intensity ≥2 on the 3 point intensity scale) at 12 weeks. | ||||
| 11 Proportion of subjects with the majority of cramp durations ≥ 1 minute at 4 weeks Show forest plot | 1 | 46 | Risk Difference (M‐H, Fixed, 95% CI) | 0.19 [‐0.07, 0.45] |
| Analysis 1.11  Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 11 Proportion of subjects with the majority of cramp durations ≥ 1 minute at 4 weeks. | ||||
| 12 Proportion of subjects with majority of cramp durations ≥ 1 minute at 12 weeks Show forest plot | 1 | 43 | Risk Difference (M‐H, Fixed, 95% CI) | 0.14 [‐0.13, 0.42] |
| Analysis 1.12  Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 12 Proportion of subjects with majority of cramp durations ≥ 1 minute at 12 weeks. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Withdrawals due to adverse events Show forest plot | 4 | 201 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.03 [‐0.10, 0.03] |
| Analysis 2.1  Comparison 2 Adverse effects of treatment, magnesium versus placebo, Outcome 1 Withdrawals due to adverse events. | ||||
| 1.1 Idiopathic cramps (largely older adults) | 2 | 86 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.06, 0.11] |
| 1.2 Pregnancy‐associated leg cramps | 2 | 115 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.07 [‐0.17, 0.02] |
| 2 Number of subjects with major adverse events Show forest plot | 2 | 91 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.10, 0.05] |
| Analysis 2.2  Comparison 2 Adverse effects of treatment, magnesium versus placebo, Outcome 2 Number of subjects with major adverse events. | ||||
| 2.1 Idiopathic cramps (largely older adults) | 1 | 46 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.05 [‐0.16, 0.07] |
| 2.2 Pregnancy‐associated leg cramps | 1 | 45 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.08, 0.08] |
| 3 Number of subjects with minor adverse events Show forest plot | 1 | 45 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.27, 0.25] |
| Analysis 2.3  Comparison 2 Adverse effects of treatment, magnesium versus placebo, Outcome 3 Number of subjects with minor adverse events. | ||||
| 3.1 Idiopathic cramps (largely older adults) | 0 | 0 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Pregnancy‐associated leg cramps | 1 | 45 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.27, 0.25] |
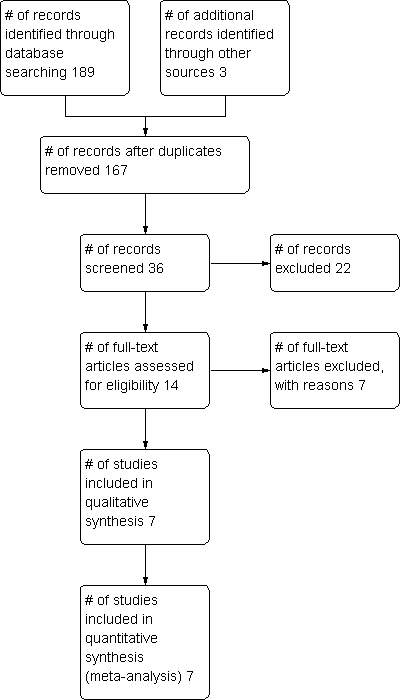
Study flow diagram.
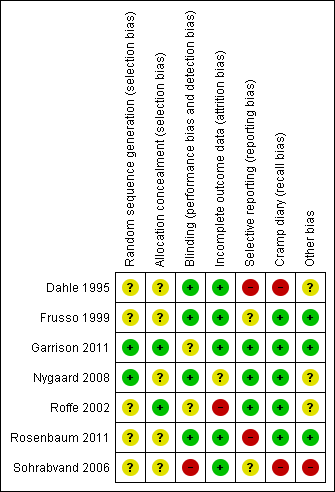
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
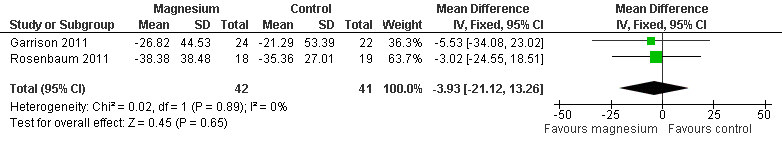
Forest plot of comparison: 1 Idiopathic rest cramps, magnesium versus placebo, outcome: 1.1 % Change in cramp frequency from baseline at 4 weeks.

Forest plot of comparison: 1 Idiopathic rest cramp efficacy, magnesium versus placebo, outcome: 1.3 Proportion of subjects with a ≥ 25% reduction in cramp frequency at 4 weeks.
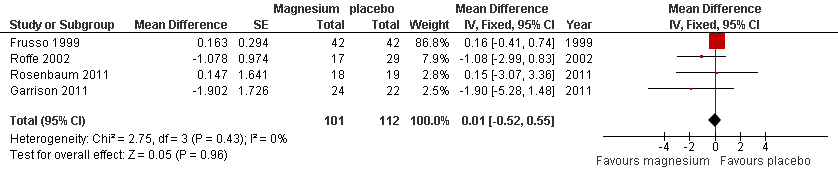
Forest plot of comparison: 1 Idiopathic rest cramps, magnesium versus placebo, outcome: 1.6 Number of cramps per week at 4 weeks.

Forest plot of comparison: 1 Idiopathic rest cramp efficacy, magnesium versus placebo, outcome: 1.7 Cramp intensity (pain) on a 3 point scale (1 = mild, 2 = moderate, 3 = severe) at 4 weeks.

Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 1 % Change in cramp frequency from baseline at 4 weeks.
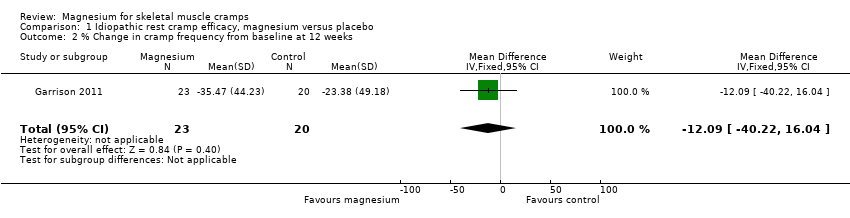
Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 2 % Change in cramp frequency from baseline at 12 weeks.

Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 3 Proportion of subjects with a ≥ 25% reduction in cramp frequency at 4 weeks.

Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 4 Proportion of subjects with a ≥ 25% reduction in cramps at 12 weeks.

Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 5 Number of cramps per week at 4 weeks.

Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 6 Number of cramps per week at 12 weeks.

Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 7 Cramp intensity (pain) on a 3 point scale (1 = mild, 2 = moderate, 3 = severe) at 4 weeks.

Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 8 Cramp intensity (pain) on a 3 point scale (1 = mild, 2 = moderate, 3 = severe) at 12 weeks.

Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 9 Proportion of subjects rating their cramps as moderate or severe (i.e. ≥ 2 on the 3 point intensity scale) at 4 weeks.

Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 10 Proportion of subjects rating their cramps as moderate to severe (i.e. with mean cramp intensity ≥2 on the 3 point intensity scale) at 12 weeks.

Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 11 Proportion of subjects with the majority of cramp durations ≥ 1 minute at 4 weeks.

Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 12 Proportion of subjects with majority of cramp durations ≥ 1 minute at 12 weeks.
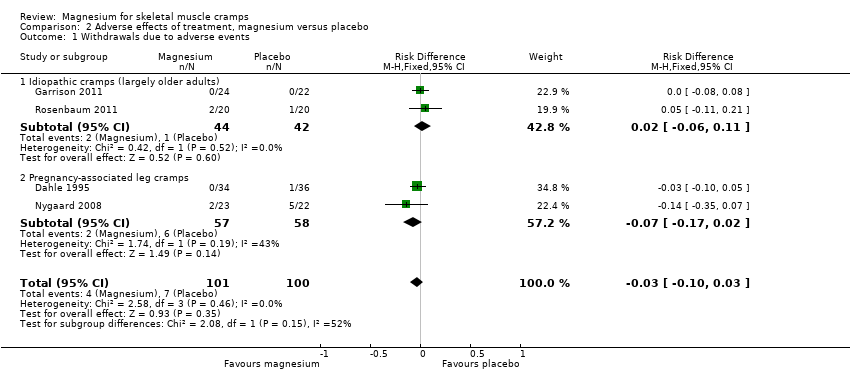
Comparison 2 Adverse effects of treatment, magnesium versus placebo, Outcome 1 Withdrawals due to adverse events.

Comparison 2 Adverse effects of treatment, magnesium versus placebo, Outcome 2 Number of subjects with major adverse events.
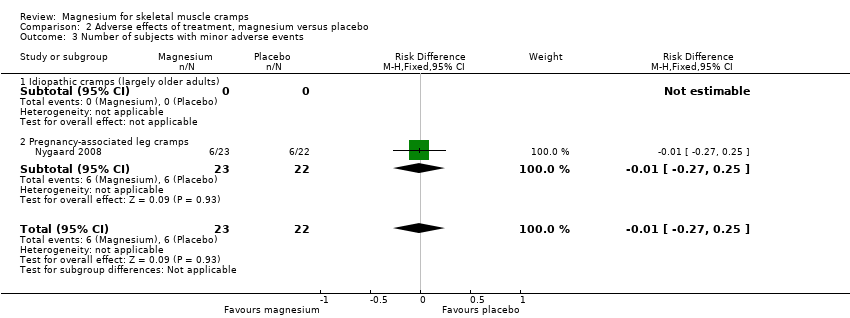
Comparison 2 Adverse effects of treatment, magnesium versus placebo, Outcome 3 Number of subjects with minor adverse events.
| Magnesium for skeletal muscle cramps | ||||||
| Patient or population: Nonpregnant patients with muscle cramps (largely older adults) Settings: Outpatients recruited through primary care clinics or community advertising Intervention: Magnesium supplements (oral or intravenous) Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Magnesium | |||||
| Percentage change in cramp frequency from baseline at 4 weeks | The mean percentage change in cramp frequency in the control groups was ‐27.8% (i.e. a 27.8% reduction) | The mean percentage change in cramp frequency in the magnesium groups was 3.9% lower | ‐3.9% (‐21.1 to 13.3) | 83 | ⊕⊕⊕⊝ | This difference was neither clinically nor statistically significant. The 95% confidence interval excludes a 25% reduction beyond placebo |
| Percentage of participants with a ≥ 25% reduction in their cramp frequency at 4 weeks | The mean percentage of placebo recipients achieving a 25% or better reduction in the frequency of their cramps was 65.9% | The mean percentage of magnesium recipients achieving a 25% or better reduction in the frequency of their cramps was 8% lower | ‐8% (‐28% to 12%) | 83 | ⊕⊕⊕⊝ | This difference was neither clinically nor statistically significant |
| Number of cramps per week at 4 weeks | The mean number of cramps per week in the placebo groups while on treatment was 4.35 | The mean number of cramps per week in the magnesium groups was 0.01 cramps per week higher | 0.01 cramps per week (‐0.52 to 0.55) | 213 | ⊕⊕⊕⊝ | This difference was neither clinically nor statistically significant. The 95% confidence interval excludes a 1 cramp per week reduction |
| Percentage of participants rating their cramps as moderate or severe (i.e. mean cramp intensity ≥ 2 on the 3 point intensity scale) at 4 weeks | The mean percentage of placebo recipients rating their cramps as moderate or severe was 30% | The mean percentage of magnesium recipients rating their cramps as moderate or severe was 9% greater | 9% (‐7% to 25%) | 91 (2 studies) | ⊕⊕⊕⊝ | This difference was neither clinically nor statistically significant |
| Percentage of participants with the majority of cramp durations ≥ 1 minute at 4 weeks | The mean percentage of placebo recipients with the majority of cramp durations ≥ 1 minute was 22.7% | The mean percentage of magnesium recipients with the majority of cramp durations ≥ 1 minute was 19% greater | 19% (‐7% to 45%) | 46 | ⊕⊕⊝⊝ | This difference was neither clinically nor statistically significant |
| Number of participants with major adverse events | 1 out of 22 | 0 out of 24 | ‐50 per 1000 (‐160 to 70) | 46 (1 study) | ⊕⊝⊝⊝ | This difference was neither clinically nor statistically significant |
| Number of participants with minor adverse events | Adverse events were not reported in a way that permitted the number of participants with minor adverse events to be determined. Each study of oral magnesium inferred that side effects were similar in frequency to placebo. Intravenous magnesium was associated with asymptomatic hypotension (3/24 magnesium versus 0/22 placebo recipients), transient light‐headedness (2/24 magnesium versus 0/22 placebo) and burning of the IV site (12/24 magnesium versus 0/22 placebo). | |||||
| CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence | ||||||
| Downgrading of the quality of evidence is based largely on the number of studies and participants contributing to each estimate. The quality of evidence for the number of participants with major adverse events is considered very low because such events are rare. | ||||||
| Study | Number/design/clinical Setting | Mean age (years) | % Female | Magnesium dose and route of administration | Frequency of administration | Treatment and assessment periods | Washout period | Comparator |
| N = 73 Parallel Pregnancy | Not given (child‐ bearing years) | 100% | 5 mmol combination Mg lactate + Mg citrate (122 mg elemental Mg) taken orally | Once each morning and twice each evening | Treatment 21 Assessment 21 | Not applicable | Matched placebo tablet | |
| N = 45 Cross‐over Idiopathic | 61.6 | 73.3% | Mg citrate 900 mg tablet (100 mg elemental Mg) taken orally | Twice daily | Treatment 28 Assessment 28 | 28 | Matched placebo tablet | |
| N = 46 Parallel Idiopathic | 69.3 | 69.6% | 20 mmol Mg sulfate (486 mg elemental Mg) given intravenously | Once daily over 4 hrs on 5 consecutive days | Treatment 5 Assessment 90 | Not applicable | Matched placebo solution | |
| N = 45 Parallel Pregnancy | 30.9 | 100% | Mg lactate and Mg citrate chewable tablets containing 122 mg elemental Mg taken orally | Once each morning and twice each evening | Treatment 14 Assessment 14 | Not applicable | Matched placebo tablet | |
| N = 73 Cross‐over Idiopathic | 62.9 | 54.3% | 1830 mg of tri‐magnesium dicitrate powder (300 mg elemental Mg) poured from a sachet into a glass of water taken orally | Once each evening | Treatment 42 Assessment during last 28 days of treatment | First 14 days of second treatment period considered as washout | Matched placebo powder | |
| N = 40 Parallel Idiopathic | 66.6 | 57.5% | Slow release tablet of Mg lactate containing 84 mg of elemental Mg taken orally | Two tablets twice daily | Treatment 30 Assessment 30 | Not applicable | Matched placebo tablet | |
| N = 84 Parallel Pregnancy | Not given (child‐ bearing years) | 100% | 7.5 mmol magnesium aspartate (182 mg elemental Mg) taken orally. Unclear if tablet or powder / solution | Twice daily | Treatment 14 Assessment 28 | Not applicable | 3 different comparators 1) No treatment 2) 500 mg calcium carbonate tablet once daily 3)100 mg of thiamine (vit B1) plus 40 mg of pyridoxine (vit B6) once daily | |
| vit B1: vitamin B1 | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 % Change in cramp frequency from baseline at 4 weeks Show forest plot | 2 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐3.93 [‐21.12, 13.26] |
| 2 % Change in cramp frequency from baseline at 12 weeks Show forest plot | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐12.09 [‐40.22, 16.04] |
| 3 Proportion of subjects with a ≥ 25% reduction in cramp frequency at 4 weeks Show forest plot | 2 | 83 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.08 [‐0.28, 0.12] |
| 4 Proportion of subjects with a ≥ 25% reduction in cramps at 12 weeks Show forest plot | 1 | 43 | Risk Difference (M‐H, Fixed, 95% CI) | 0.11 [‐0.19, 0.41] |
| 5 Number of cramps per week at 4 weeks Show forest plot | 4 | 213 | Mean Difference (Fixed, 95% CI) | 0.01 [‐0.52, 0.55] |
| 6 Number of cramps per week at 12 weeks Show forest plot | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.84 [‐3.23, 1.55] |
| 7 Cramp intensity (pain) on a 3 point scale (1 = mild, 2 = moderate, 3 = severe) at 4 weeks Show forest plot | 3 | 175 | Mean Difference (Fixed, 95% CI) | ‐0.04 [‐0.18, 0.11] |
| 8 Cramp intensity (pain) on a 3 point scale (1 = mild, 2 = moderate, 3 = severe) at 12 weeks Show forest plot | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.55, 0.19] |
| 9 Proportion of subjects rating their cramps as moderate or severe (i.e. ≥ 2 on the 3 point intensity scale) at 4 weeks Show forest plot | 2 | 91 | Risk Difference (M‐H, Fixed, 95% CI) | 0.09 [‐0.07, 0.25] |
| 10 Proportion of subjects rating their cramps as moderate to severe (i.e. with mean cramp intensity ≥2 on the 3 point intensity scale) at 12 weeks Show forest plot | 1 | 43 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.06 [‐0.21, 0.10] |
| 11 Proportion of subjects with the majority of cramp durations ≥ 1 minute at 4 weeks Show forest plot | 1 | 46 | Risk Difference (M‐H, Fixed, 95% CI) | 0.19 [‐0.07, 0.45] |
| 12 Proportion of subjects with majority of cramp durations ≥ 1 minute at 12 weeks Show forest plot | 1 | 43 | Risk Difference (M‐H, Fixed, 95% CI) | 0.14 [‐0.13, 0.42] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Withdrawals due to adverse events Show forest plot | 4 | 201 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.03 [‐0.10, 0.03] |
| 1.1 Idiopathic cramps (largely older adults) | 2 | 86 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.06, 0.11] |
| 1.2 Pregnancy‐associated leg cramps | 2 | 115 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.07 [‐0.17, 0.02] |
| 2 Number of subjects with major adverse events Show forest plot | 2 | 91 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.10, 0.05] |
| 2.1 Idiopathic cramps (largely older adults) | 1 | 46 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.05 [‐0.16, 0.07] |
| 2.2 Pregnancy‐associated leg cramps | 1 | 45 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.08, 0.08] |
| 3 Number of subjects with minor adverse events Show forest plot | 1 | 45 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.27, 0.25] |
| 3.1 Idiopathic cramps (largely older adults) | 0 | 0 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Pregnancy‐associated leg cramps | 1 | 45 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.27, 0.25] |

