Inhibidores de la enzima convertidora de angiotensina o bloqueadores de los receptores de angiotensina II tipo 1 perioperatorios para la prevención de la mortalidad y la morbilidad en adultos
Información
- DOI:
- https://doi.org/10.1002/14651858.CD009210.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 27 enero 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Anestesia
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Zui Zou (ZZ), Hong B Yuan (HBY), Bo Yang (BY), Fengying Xu (FYX), Xiao Y Chen (XYC), Guan J Liu (GJL), Xue Y Shi (XYS)
Joint first authors: Zui Zou, Hong B Yuan, Bo Yang
Conceiving the review: ZZ, XYS
Co‐ordinating the review: ZZ, XYS
Undertaking manual searches: XYC
Screening search results: ZZ, XYC
Organizing retrieval of papers: ZZ, HBY
Screening retrieved papers against inclusion criteria: ZZ
Appraising quality of papers: ZZ, XYS
Abstracting data from papers: ZZ, XYC
Writing to authors of papers for additional information: XYS
Providing additional data about papers: HBY, BY
Obtaining and screening data on unpublished studies: HBY
Data management for the review: ZZ, XYS
Entering data into Review Manager (RevMan 5.3): BY
RevMan statistical data: ZZ, XYS
Other statistical analysis not using RevMan: ZZ, GJL
Double entry of data: ZZ, HBY
Interpretation of data: XYS
Statistical inferences: GJL
Writing the review: ZZ, BY, XYS
Revising the review: FYX, BY
Securing funding for the review: XYS
Performing previous work that was the foundation of the present study: ZZ, XYS
Guarantor for the review (one author): XYS
Person responsible for reading and checking review before submission: ZZ, XYS
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Nature Science Foundation of China (81372103), China.
-
Key Program of Medical Science Development of PLA (BWS12J027), China.
-
Program of Shanghai Municipal Health Planning Commission (2013SY025), China.
-
Shanghai Rising‐Star Program (15QA1405000), China.
-
Natural Science Foundation of Shanghai (14ZR1413700), China.
Declarations of interest
See: Sources of support.
Zui Zou: none known
Hong B Yuan: none known
Bo Yang: none known
Fengying Xu: none known
Xiao Y Chen: none known
Guan J Liu: none known
Xue Y Shi: none known
Acknowledgements
We would like to thank Javier Eslava‐Schmalbach (content editor), Nathan Pac (statistical editor), Pierre Foex, Claudio Bravo (peer reviewers), and Patricia Tong (consumer referee) for their help and editorial advice during the preparation of this systematic review.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Jan 27 | Perioperative angiotensin‐converting enzyme inhibitors or angiotensin II type 1 receptor blockers for preventing mortality and morbidity in adults | Review | Zui Zou, Hong B Yuan, Bo Yang, Fengying Xu, Xiao Y Chen, Guan J Liu, Xue Y Shi | |
| 2011 Jul 06 | Perioperative angiotensin‐converting enzyme inhibitors or angiotensin II type 1 receptor blockers for preventing surgery‐related mortality and morbidity | Protocol | Zui Zou, Hong B Yuan, Xiao Y Chen, Guan J Liu, Xue Y Shi | |
Differences between protocol and review
-
Two new authors joined the team: Bo Yang and Fengying Xu.
-
We changed the original title of the protocol, 'Perioperative angiotensin‐converting enzyme inhibitors or angiotensin II type 1 receptor blockers for preventing surgery‐related mortality and morbidity in adults', to 'Perioperative angiotensin‐converting enzyme inhibitors or angiotensin II type 1 receptor blockers for preventing mortality and morbidity in adults', as suggested by referee Pierre Foex.
-
We changed the original objectives of the protocol, 'to systematically assess the benefits and harms of administration (prophylaxis or treatment or both) of angiotensin‐converting enzyme inhibitors (ACEIs) and angiotensin II type I receptors blockers (ARBs) in the short‐term perioperative period for the prevention of surgery related mortality and morbidity', to 'to systematically assess the benefits and harms of administration of ACEIs or ARBs perioperatively for the prevention of mortality and morbidity in adults (aged 18 years and above) undergoing any type of surgery under general anaesthesia' to keep coincident with the revised title and ensure the precision of the objectives description.
-
We rearranged the criteria for considering studies for this review and refined the presentation but did not change the exact meaning.
-
We added length of hospital stay as a secondary outcome to enrich the results.
-
We also added treatment related adverse effects to the secondary outcomes to enrich the results
-
We added description in the Data synthesis section 'SDchange were unavailable in the original report, we imputed standard deviations for changes from baseline with the following technique: SDchange = (SDbaseline2 + SDfinal2 ‐ 2*Corr*SDbaseline*SDfinal)0.5. Default value (0.8) imputed for the correlation value' to provide more detailed methodology.
-
We added the following to the Sensitivity analysis section: 'when we came across studies where the standard deviation of changes from baseline was missing, we imputed the missing standard deviation using an imputed value, Corr, for the correlation coefficient. Besides the default value (0.8), we used different hypothesized values of Corr based on reasoned argument to determine whether the overall result of the analysis was robust to the use of imputed correlation coefficients', to provide more detailed methodology.
-
We did not perform funnel plots because there were no more than three trials in each analysis.
-
Since all the included studies had high risk of bias, we did not perform sensitivity analysis. It was only possible to perform subgroup analysis according to the types of surgery and interventions because of the sparse data in other groups.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Anesthesia, General;
- Angiotensin II Type 1 Receptor Blockers [*therapeutic use];
- Angiotensin‐Converting Enzyme Inhibitors [*therapeutic use];
- Cardiac Surgical Procedures [adverse effects, *mortality];
- Cause of Death;
- Cerebrovascular Disorders [prevention & control];
- Heart Failure [prevention & control];
- Hypertension [*drug therapy];
- Hypotension [prevention & control];
- Length of Stay;
- Myocardial Infarction [prevention & control];
- Perioperative Care [*methods, mortality];
- Randomized Controlled Trials as Topic;
- Renal Insufficiency [prevention & control];
- Surgical Procedures, Operative [mortality];
- Vascular Surgical Procedures [adverse effects, *mortality];
Medical Subject Headings Check Words
Adult; Humans;
PICO
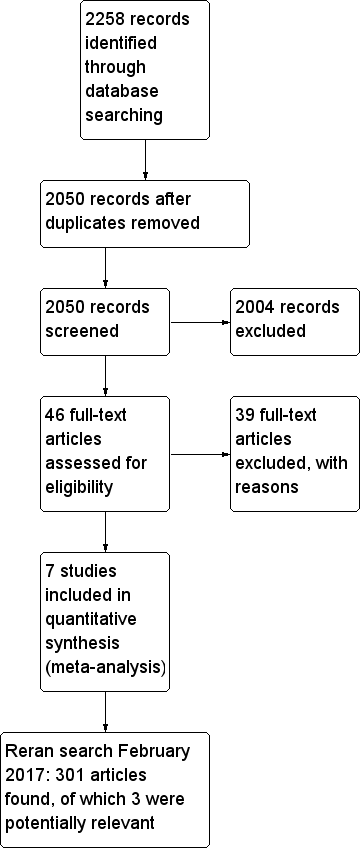
Flow diagram of study selection process.
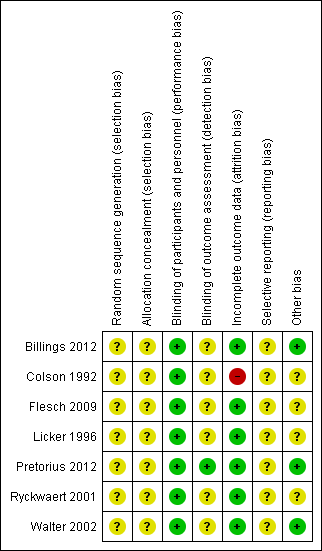
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
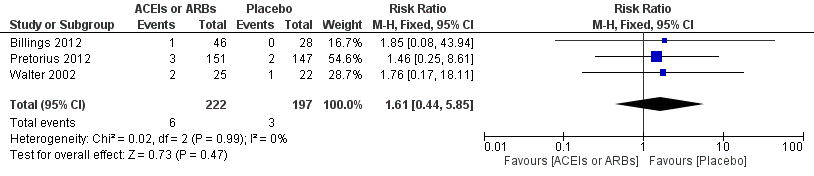
Forest plot of comparison: 1 All‐cause mortality, outcome: 1.1 All‐cause mortality.
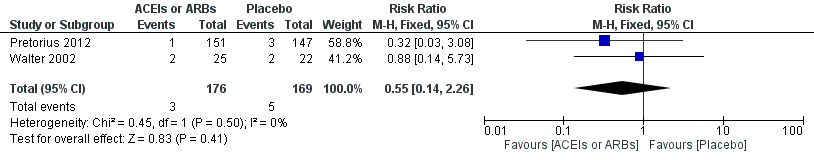
Forest plot of comparison: 1 ACEIs or ARBs versus placebo, outcome: 1.2 ST‐elevation or new Q wave in ECG test.

Forest plot of comparison: 1 ACEIs or ARBs versus placebo, outcome: 1.3 Cardiac index.

Forest plot of comparison: 1 ACEIs or ARBs versus placebo, outcome: 1.4 Rate of perioperative cerebrovascular complications.

Forest plot of comparison: 1 ACEIs or ARBs versus placebo, outcome: 1.5 Length of hospital stay.

Comparison 1 ACEIs or ARBs versus placebo, Outcome 1 All cause mortality.
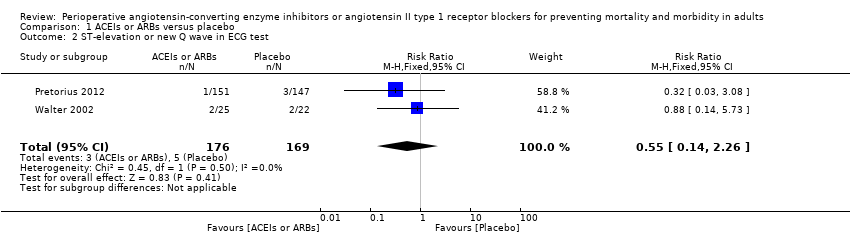
Comparison 1 ACEIs or ARBs versus placebo, Outcome 2 ST‐elevation or new Q wave in ECG test.

Comparison 1 ACEIs or ARBs versus placebo, Outcome 3 Cardiac index.
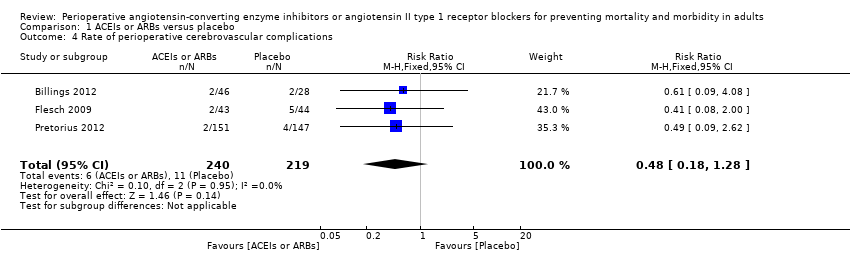
Comparison 1 ACEIs or ARBs versus placebo, Outcome 4 Rate of perioperative cerebrovascular complications.

Comparison 1 ACEIs or ARBs versus placebo, Outcome 5 Length of hospital stay.
| ACEIs or ARBs compared to placebo for preventing surgery‐related mortality and morbidity in adults | ||||||
| Patient or population: Patients undergoing any type of surgery under general anaesthesia receiving ACEIs or ARBs perioperatively | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | ACEIs or ARBs | |||||
| All‐cause mortality | Study population | RR 1.61 | 419 | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Total sample size is lower than the calculated. Duration of follow‐up: until discharge from hospital | |
| 16 per 1000 | 25 per 1000 | |||||
| Moderate | ||||||
| 7 per 1000 | 11 per 1000 | |||||
| Risk of acute myocardial ischaemia | Study population | RR 0.55 | 345 | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Total sample size is lower than the calculated. Duration of follow‐up: until discharge from hospital | |
| 30 per 1000 | 16 per 1000 | |||||
| Moderate | ||||||
| 56 per 1000 | 31 per 1000 | |||||
| Congestive heart failure | The mean cardiac index in the intervention groups was | ‐ | 34 | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Total population size is less than 400. Duration of follow‐up: not specified | |
| Hypotension | ‐ | RR 1.95 (0.86 to 4.41) | 298 (1 study) | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Total population size is less than 400. Duration of follow‐up: not specified | |
| Rate of perioperative cerebrovascular complications | Study population | RR 0.48 | 459 | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Duration of follow‐up: until discharge from hospital (Billings 2012; Pretorius 2012); 90 days after surgery (Flesch 2009) | |
| 50 per 1000 | 24 per 1000 | |||||
| Moderate | ||||||
| 71 per 1000 | 34 per 1000 | |||||
| Length of hospital stay | The mean length of hospital stay in the intervention groups was | ‐ | 372 | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Total population size is less than 400. Duration of follow‐up: until discharge from hospital | |
| Treatment related adverse events | ‐ | ‐ | ‐ | 385 (2 studies) | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Total population size is less than 400. Authors did not provided detailed information on adverse events, which made the synthesis of the results less clinically relevant. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by three levels due to very serious study limitations (all the trials included were at high risk of bias) and serious imprecision (total population size is less than 400). | ||||||
| Outcome or subgroup | Studies | Participants | Statistical method | Effect estimate |
| Rate of hypotension | 1 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.86, 4.41] |
| Risk ratio < 1 favours angiotensin‐converting enzyme inhibitors and angiotensin II type 1 receptor blockers group. Risk ratio > 1 favours control group. | ||||
| Outcome or subgroup | Studies | Participants | Statistical method | Effect estimate |
| Glomerular filtration rate | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐10.30, 7.50] |
| IV ‐ inverse variance IV: intravenous | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All cause mortality Show forest plot | 3 | 419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.44, 5.85] |
| 2 ST‐elevation or new Q wave in ECG test Show forest plot | 2 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.14, 2.26] |
| 3 Cardiac index Show forest plot | 2 | 34 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.70, ‐0.50] |
| 4 Rate of perioperative cerebrovascular complications Show forest plot | 3 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.18, 1.28] |
| 5 Length of hospital stay Show forest plot | 2 | 372 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐0.93, ‐0.16] |

