Agonis dopamine untuk mencegah sindrom terlebih merangsang ovari
Abstract
Background
Ovarian hyperstimulation syndrome (OHSS) is a potentially serious complication of ovarian stimulation in assisted reproduction technology (ART). It is characterised by enlarged ovaries and an acute fluid shift from the intravascular space to the third space, resulting in bloating, increased risk of venous thromboembolism and decreased organ perfusion. Most cases are mild, but forms of moderate or severe OHSS appear in 3% to 8% of in vitro fertilisation (IVF) cycles. The dopamine agonist cabergoline was introduced as a secondary prevention intervention for OHSS in women at high risk of OHSS undergoing ART treatment. As cabergoline seemed to be effective in preventing OHSS, other types of dopamine agonists, such as quinagolide and bromocriptine, have since been studied in ART to prevent OHSS.
Objectives
To assess the effectiveness and safety of dopamine agonists in preventing OHSS in high‐risk women undergoing ART treatment.
Search methods
We searched several databases from inception to August 2016 (Cochrane Gynaecology and Fertility Specialised Register of trials, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, CINAHL, PsycINFO, Clinicaltrials.gov and the World Health Organization International Trials Registry Platform (ICTRP)) for randomised controlled trials (RCTs) assessing the effect of dopamine agonist in preventing OHSS. We handsearched the reference lists of relevant studies.
Selection criteria
We considered RCTs which compared dopamine agonists with placebo/no intervention or another intervention for preventing OHSS in high‐risk women for inclusion. Primary outcome measures were incidence of moderate or severe OHSS and live birth rate. Secondary endpoints were clinical pregnancy rate, multiple pregnancy rate, miscarriage rate and any other adverse effects of the treatment.
Data collection and analysis
Two authors independently screened titles, abstracts and full texts of publications, selected studies, extracted data and assessed risk of bias. We resolved any disagreements by consensus. We reported pooled results as odds ratios (OR) and 95% confidence interval (95% CI) by the Mantel‐Haenszel method. In addition, we graded the overall quality of the evidence using GRADE criteria.
Main results
The search identified 14 new RCTs since the last published version of this review, resulting in 16 included RCTs involving 2091 high‐risk women for this updated review. They evaluated three types of dopamine agonists: cabergoline, quinagolide and bromocriptine.
When compared with placebo or no intervention, dopamine agonists seemed effective in the prevention of moderate or severe OHSS (OR 0.27, 95% CI 0.19 to 0.39; 1022 participants; 8 studies; I2 = 0%; moderate quality evidence). This suggests that if 29% of women undergoing ART experience moderate or severe OHSS, the use of dopamine agonists will lower this to 7% to 14% of women. There was no evidence of a difference in live birth rate, clinical pregnancy rate, multiple pregnancy rate or miscarriage rate (very low to moderate quality evidence). However, taking dopamine agonists (especially quinagolide) may increase the incidence of adverse events such as gastrointestinal adverse effects (OR 4.54, 95% CI 1.49 to 13.84; 264 participants; 2 studies; I2 = 49%, very low quality evidence).
When we compared dopamine agonist plus co‐intervention with co‐intervention, there was no evidence of a difference in the outcomes of moderate or severe OHSS, live birth rate, clinical pregnancy rate, miscarriage rate or adverse events. The co‐interventions were hydroxyethyl starch (two RCTs) and albumin (one RCT).
Cabergoline was associated with a lower risk of moderate or severe OHSS compared with human albumin (OR 0.21, 95% CI 0.12 to 0.38; 296 participants; 3 studies; I2 = 72%). However, there was no evidence of a difference between cabergoline and hydroxyethyl starch, coasting (withholding any more ovarian stimulation for a few days) or prednisolone. There was an increased clinical pregnancy rate in the cabergoline group when cabergoline was compared with coasting (OR 2.65, 95% CI 1.13 to 6.21; 120 participants; 2 studies; I2 = 0%). In other respects, there was no evidence of a difference in clinical pregnancy rate, multiple pregnancy rate or miscarriage rate between cabergoline and other active interventions.
The quality of the evidence between dopamine agonist and placebo or no intervention ranged from very low to moderate, mainly due to poor reporting of study methods (mostly a lack of details on randomisation or blinding) and serious imprecision for some comparisons.
Authors' conclusions
Dopamine agonists appear to reduce the incidence of moderate or severe OHSS in women at high risk of OHSS (moderate quality evidence). If a fresh embryo transfer is performed, the use of dopamine agonists does not affect the pregnancy outcome (live birth rate, clinical pregnancy rate and miscarriage rate) (very low to moderate quality evidence). However, dopamine agonists might increase the risk of adverse events, such as gastrointestinal symptoms. Further research should focus on dose‐finding, comparisons with other effective treatments and consideration of combination treatments. Therefore, large, well‐designed and well‐executed RCTs that involve more clinical endpoints (e.g., live birth rate) are necessary to further evaluate the role of dopamine agonists in OHSS prevention.
PICO
Ringkasan bahasa mudah
Agonis dopamine untuk mencegah sindrom terlebih merangsang ovari di kalangan wanita yang sedang menjalani bantuan rawatan teknologi reproduktif
Soalan ulasan
Adakah agonis dopamine berkesan dan selamat untuk mencegah sindrom terlebih merangsang ovari (OHSS) di kalangan wanita yang berisiko tinggi untuk OHSS (contohnya wanita dengan ovari polikistic atau hasil daripada rangsangan yang menyebabkan banyak oocyte)? Bagaimanakah keberkesanan mereka berbanding rawatan aktif lain (misalnya albumin manusia)?
Latar belakang
OHSS berlaku disebabkan ovari terlebih rangsang (organ‐organ reproduktif perempuan yang menghasilkan telur dan hormon seks) dalam rawatan kesuburan (dipanggil bantuan rawatan teknologi reproduktif ). Ia bercirikan pembesaran ovari dan penyerapan cecair dari saluran darah ke rongga badan lain, menyebabkan kembung perut (perut), peningkatan risiko pembekuan darah dan pengurangan bekalan darah ke organ‐organ penting. Dalam kebanyakan kes, keadaan ini ringan dan diselesaikan sendiri tanpa rawatan, tetapi sesetengah wanita boleh mendapat OHSS yang sederhana atau teruk, yang memerlukan kemasukan ke hospital. Tidak terdapat penawar untuk OHSS selain daripada menunggu ia hilang sendiri dan mengurangkan simptom semasa di hospital. Ubat‐ubatan yang dikenali sebagai agonis dopamine telah diperkenalkan untuk cuba menghalang OHSS.
Ciri‐ciri ulasan
Ulasan ini melibatkan 16 kajian terkawal rawak yang melibatkan 2091 wanita yang berisiko tinggi untuk OHSS, yang menilai tiga agonis dopamine yang berbeza (cabergoline, bromocriptine dan quinagolide). Ukuran‐ukuran hasil utama adalah bilangan kes baru (kejadian) OHSS yang sedarhana atau buruk dan kadar kelahiran hidup. Bukti‐bukti adalah terkini hingga Ogos 2016.
Keputusan utama
Agonis dopamine dapat mengurangkan kejadian OHSS yang sederhana atau teruk di kalangan wanita berisiko tinggi OHSS (bukti‐bukti kualiti sederhana) berbanding dengan plasebo atau tiada rawatan. Ini mencadangkan bahawa jika 29% daripada wanita yang mengambil plasebo atau tiada rawatan mempunyai OHSS sederhana atau teruk, antara 7% hingga 14% wanita yang mengambil agonis dopamine akan mempunyai OHSS sederhana atau teruk. Bagi wanita yang menjalani pemindahan embrio segar sebagai sebahagian daripada kitaran rawatan mereka, tiada bukti bahawa agonis dopamine dapat mempengaruhi hasil kehamilan, tetapi mereka mungkin meningkatkan risiko kesan sampingan seperti sakit perut. Tidak bukti mengenai perbezaan antara agonis dopamine ditambah dengan satu lagi rawatan aktif berbanding dengan satu lagi rawatan aktif dalam kejadian OHSS sederhana atau teruk dan kadar kelahiran hidup.
Tiada bukti mengenai perbezaan dalam kadar OHSS antara cabergoline dan plasebo rawatan (cth: hydroxyethyl kanji, prednisolone atau 'coasting' (penangguhan rangsangan ovari selama beberapa hari)). Cabergoline berkait dengan penambahan kadar kehamilan klinikal berbanding dengan coasting.
Kualiti bukti
Kualiti bukti adalah diantara sangat rendah hingga sederhana. Batasan termasuk pelaporan kajian kaedah yang lemah dan ketidaktepatan (kekurangan kejadian) untuk setengah perbandingan.
Authors' conclusions
Summary of findings
| Dopamine agonist vs placebo/no intervention | ||||||
| Patient or population: women of reproductive age undergoing any ART therapy Settings: ART unit Intervention: dopamine agonist Comparison: placebo/no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with placebo/no intervention | Risk with dopamine agonist | |||||
| Incidence of moderate or severe OHSS | 286 per 1000 | 97 per 1000 (71 to 135) | OR 0.27 (0.19 to 0.39) | 1022 (8 studies) | ⊕⊕⊕⊝ | ‐ |
| Live birth rate | 509 per 1000 | 512 per 1000 (355 to 665) | OR 1.01 (0.53 to 1.91) | 182 | ⊕⊕⊝⊝ | |
| Clinical pregnancy rate | 401 per 1000 | 352 per 1000 (266 to 450) | OR 0.81 (0.54 to 1.22) | 432 (4 studies) | ⊕⊕⊕⊝ | |
| Multiple pregnancy | 50 per 1000 | 17 per 1000 (1 to 303) | OR 0.32 (0.01 to 8.26) | 40 | ⊕⊝⊝⊝ | |
| Miscarriage pregnancy rate | 72 per 1000 | 49 per 1000 (15 to 151) | OR 0.66 (0.19 to 2.28) | 168 (2 studies) | ⊕⊕⊝⊝ | |
| Adverse events | 43 per 1000 | 168 per 1000 (62 to 381) | OR 4.54 (1.49 to 13.84) | 264 | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ART: assisted reproductive technology; CI: confidence interval; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for serious risk of bias associated with poor reporting of study methods. 2 Downgraded one level for serious risk of imprecision: confidence interval compatible with benefit in either arm or with no difference between the groups. 3 Downgraded two levels for very serious risk of imprecision: only one event. 4 Downgraded one level for serious risk of imprecision: only 10 events. 5 Downgraded one level for serious risk of imprecision: only 29 events. | ||||||
Background
Description of the condition
Ovarian hyperstimulation syndrome (OHSS) is a complication of assisted reproduction technology (ART) treatment. It can occur following exposure of the ovaries of susceptible women to human chorionic gonadotrophin (hCG) or luteinising hormone (LH) during controlled ovarian stimulation with follicle‐stimulating hormone (FSH). Women at risk of OHSS are generally young and have polycystic ovary syndrome (PCOS) (Costello 2012). OHSS is characterised by enlarged ovaries and an acute fluid shift from the intravascular space to the third space (mainly to the abdominal or thoracic cavity), which may result in an accumulation of fluid in the peritoneal cavity and pleura, an elevation of haematocrit and a decrease in organ perfusion (Aboulghar 2003; Soares 2008; Vloeberghs 2009). Its symptoms range from abdominal bloating and a feeling of fullness to shortness of breath (Vloeberghs 2009). OHSS was classified as mild, moderate or severe by Golan and colleagues (Golan 1989), modified from Rabau and colleagues (Rabau 1967) by incorporating ultrasonographic measurement of the stimulated ovaries. Despite measures adopted by physicians to prevent these sequelae, mild OHSS may affect up to 33% of in vitro fertilisation (IVF) cycles. Moderate or severe OHSS arises in 3% to 8% of IVF cycles (RCOG 2006). Young women with low body mass index and polycystic ovaries are at particular risk of OHSS and the only way to entirely avoid the condition for women with fallopian tube compromise or whose partner has impaired semen parameters is to undergo in vitro oocyte maturation which is an approach that is not available in most centres (Walls 2015).
The pathophysiology of OHSS is not yet completely elucidated. Increased vascular permeability causing the loss of fluid into the third space (abdominal and pleural cavity) is the central feature of clinically significant OHSS, which triggers events that result in the associated symptoms (such as abdominal pain and distension) (Ata 2009). Most cases of OHSS have been associated with the use of hCG to trigger oocyte maturation prior to oocyte retrieval, however it is recognised that hCG has no direct effect on the vascular system (Gómez 2002). Vasoactive substances are released by the ovaries in response to hCG administration. It is almost certain that vascular endothelial growth factor (VEGF) is a key substance that induces vascular hyperpermeability, leading to a shift of fluids from the intravascular system to the third space (Busso 2009; Soares 2008). Higher production of VEGF from the many follicles during stimulation by ovarian steroids and hCG appears to be the specific key process leading to the development of OHSS in high‐risk women.
Description of the intervention
Severe OHSS is a potentially life‐threatening condition that occurs in women undergoing ART cycles. Several measures have been introduced to prevent OHSS (Prakash 2009). These include cycle cancellation or 'coasting' (D'Angelo 2002; Delvigne 2002), use of intravenous fluids (Aboulghar 2002; Youssef 2010), cryopreservation of embryos rather than immediate fresh embryo transfer (D'Angelo 2007), and the use of progesterone as luteal phase support (van der Linden 2015). More recent treatments include 'minimal stimulation IVF' (using a combination of medications to gently stimulate the ovaries), in vitro maturation of oocytes (letting oocytes mature in vitro) (Walls 2012), the use of 'natural cycle' IVF (collecting and fertilising one egg released during the normal monthly cycle and without the use of fertility drugs) (Edwards 2007), the use of metformin in women with PCOS (Tso 2014), the use of gonadotropin‐releasing hormone (GnRH) antagonist, as opposed to GnRH agonist for ovarian downregulation (a prerequisite to assist in the timing of oocyte retrieval), adjusting stimulation protocols (Al‐Inany 2011), and the use of an agonist trigger prior to oocyte retrieval in an antagonist cycle (Casper 2015). Despite their availability, there is no consensus on what would be the most favourable strategy to prevent OHSS, and none of these strategies have led to the eradication of OHSS (Aboulghar 2009). Research suggests that the use of dopamine agonists may be a promising strategy for the prevention and treatment of OHSS (Busso 2009; Castelo‐Branco 2009).
How the intervention might work
With a better understanding of the pathophysiology of OHSS and recognition of the important role of VEGF in the development of OHSS, a series of blockers, such as SU5416 (a potent and selective inhibitor of the vascular endothelial growth factor receptor (VEGFR)), were introduced to reverse the hCG action on vascular permeability by targeting VEGFR‐2 expressed on human ovaries (Gómez 2002). However, these anti‐angiogenic drugs could not be used clinically to prevent or treat OHSS due to their adverse effect profile (such as thromboembolism) (Glade‐Bender 2003; Kuenen 2003), and the possibility of affecting embryo implantation (Alvarez 2007a). Another approach is to consider the use of a dopamine agonist, which show similar effects to anti‐angiogenic drugs on vascular permeability and appear not to exert undesirable adverse effects (Castelo‐Branco 2009; Soares 2012). Moreover, dopamine agonists have been used for many years in other fields of medicine, for example to treat elevated serum prolactin levels. However, since the dopamine agonist cabergoline has been associated with fibrotic valvular heart disease when used chronically, other types of dopamine agonists are now being examined for use in OHSS. Possible advantages are the different pharmacokinetic profiles (e.g. shorter half‐life of the drugs (about 17 hours for quinagolide versus about 65 hours for cabergoline)) thereby reducing exposure of embryos to possible teratogenic effects (Busso 2010), and in case of bromocriptine, lower costs and longer experience in use during pregnancy (Beltrame 2013).
Research findings in animal models of OHSS, as well as in humans, have shown that cabergoline can prevent the increase in vascular permeability (Gómez 2006). Several clinical trials have also evaluated the clinical value of cabergoline and showed that prophylactic use of cabergoline was associated with a decrease in the severity of OHSS (Manno 2005). Dopamine agonists may therefore provide a new, specific and non‐toxic approach to the prevention and treatment of OHSS (Alvarez 2007a; Knoepfelmacher 2006).
Why it is important to do this review
Though short‐term use of dopamine agonists for preventing OHSS represents no significant risk for women, long‐term data on its effectiveness and safety requires corroboration. An increased incidence of cardiac valve regurgitation is suggested when women took cabergoline or pergolide for treating Parkinson's disease or hyperprolactinaemia (Kars 2008; Martin 2009; Schade 2007; Zanettini 2007). Clinical studies have increasingly suggested that cabergoline can be safely administered in ART for preventing OHSS without influencing pregnancy outcomes. However, the role of other dopamine agonists (e.g. quinagolide and bromocriptine) for preventing OHSS remain uncertain due to lack of robust evidence for their efficacy and safety. This updated review broadened the scope from only cabergoline to include all other dopamine agonists. This review aimed to summarise the available evidence from randomised controlled trials (RCTs) to determine whether dopamine agonists can reduce the incidence of moderate or severe OHSS in high‐risk women undergoing ART and identify any safety concerns.
Objectives
To assess the effectiveness and safety of dopamine agonists in preventing OHSS in high‐risk women undergoing ART treatment.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished RCTs investigating the effectiveness and safety of dopamine agonists compared with placebo/no intervention or another intervention. We handled conference abstracts in the same way as full publications. We excluded quasi‐randomised trials and, in the case of cross‐over trials, included only pre‐crossover data.
Types of participants
High‐risk women of reproductive age undergoing any ART therapy (as defined by the separate studies).
Types of interventions
Trials were eligible for inclusion when they evaluated any dose of dopamine agonist alone or as an add‐on therapy versus placebo, no intervention or other active treatments.
Types of outcome measures
Primary outcomes
-
Incidence of moderate or severe OHSS (as determined by study authors) per woman randomised.
-
Live birth rate (as a result of an embryo transferred in a fresh cycle using fertilised oocytes from the same menstrual cycle) defined as a live infant born after 20 weeks' gestation per woman randomised.
Secondary outcomes
-
Clinical pregnancy rate (as a result of an embryo transferred in a fresh cycle using fertilised oocytes from the same menstrual cycle) per woman randomised.
-
Multiple pregnancy rate (as a result of an embryo transferred in a fresh cycle using fertilised oocytes from the same menstrual cycle) per woman randomised.
-
Miscarriage rate (following an embryo transferred in a fresh cycle using fertilised oocytes from the same menstrual cycle) per woman randomised.
-
Any other adverse events of the treatment per woman randomised.
Search methods for identification of studies
See: Cochrane Gynaecology and Fertility (formerly Menstrual Disorders and Subfertility Group, MDSG) methods used in reviews (CGF).
We searched for published and unpublished articles in any language, that described or might describe RCTs of dopamine agonists (and more specifically cabergoline, quinagolide or bromocriptine) for preventing OHSS, in consultation with the Cochrane Gynaecology and Fertility Information Specialist.
Electronic searches
We searched:
-
the Cochrane Gynaecology and Fertility Group's (formerly Menstrual Disorders and Subfertility Group) Specialised Register using key terms on a Procite platform (from inception to 15 August 2016, see Appendix 1). This register also contains unpublished trial abstracts;
-
the following databases were also searched:
-
Cochrane CENTRAL Register of studies Online (CRSO), Web platform (from inception to 15 Aug 2016), see Appendix 2;
-
MEDLINE, Ovid platform (from 1946 to 15 August 2016), see Appendix 3;
-
Embase, Ovid platform (from 1974 to 15 August 2016), see Appendix 4;
-
PsycINFO, Ovid platform (from 1806 to 15 August 2016), see Appendix 5.
-
CINAHL through the EBSCO platform (from 1982 to 15 August 2016) see Appendix 6;
-
the World Health Organization (WHO) International Trials Registry Platform (ICTRP), Web platform (from inception up to 15 August 2016), see Appendix 7;
-
Clinicaltrials.gov, Web platform (from inception up to 15 August 2016), see Appendix 8;
-
The OpenSIGLE database, for European grey literature, Web platform (from inception up to 15 August 2016); opensigle.inist.fr/).
-
We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials, which appears in the Cochrane Handbook for Systematic Reviews of Interventions (Section 6.4.11) (Higgins 2011).
We combined the Embase searches with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/mehodology/filters.html#random).
Searching other resources
We searched the citation lists of relevant publications and included studies, review articles and abstracts of conferences, and asked manufacturers, experts and specialists in the field for any trials that they were aware of.
We conducted handsearching in the appropriate journals of gynaecology and reproductive medicine; the conference proceedings (for abstracts) of the European Society for Human Reproduction and Embryology (ESHRE) and the American Society for Reproductive Medicine (ASRM), as well as related textbooks.
We searched for conference abstracts on the Web of Science (wokinfo.com/).
Data collection and analysis
Selection of studies
Two review authors (SM and HT) independently reviewed the titles and abstracts of the trials, in accordance with the search protocol. We review full‐text articles and considered them for inclusion. If the published study was judged to contain insufficient information, we contacted trial authors. Two review authors (SM and HT) independently critically appraised the trials against the inclusion criteria. We resolved any disagreements by consensus or referral to a third review author (RH).
Data extraction and management
Two review authors (SM and HT) independently extracted data using a piloted data extraction form (Appendix 10). We compared the two sets of extracted data and resolved discrepancies by discussion. The data extraction forms included methodological quality and allocation scores. We included this information in the review and presented it in the Characteristics of included studies and Characteristics of excluded studies tables following the guidance of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of risk of bias in included studies
Two review authors (SM and HT) independently critically assessed risk of bias in all studies included in this review, including the following domains: sequence generation; allocation concealment; blinding of participants, personnel and outcome assessors; incomplete outcome data; and selective outcome reporting (described in Cochrane's tool for assessing risk of bias) (Higgins 2011). We judged each domain as being at low risk of bias, high risk of bias or unclear risk of bias for either a lack of information or uncertainty regarding the potential for bias, with any disagreements resolved by consensus or by discussion with a third author (RH).
Measures of treatment effect
We anticipated that all data would be dichotomous. We used the numbers of events in the control and intervention groups of each study to calculate odds ratios (OR) with 95% confidence intervals (CI).
Unit of analysis issues
The primary analysis unit was per woman randomised.
Dealing with missing data
Our meta‐analysis used an intention‐to‐treat (ITT) approach, meaning that we included all women randomised in the analysis, in the groups to which they were randomised. In case of missing data, we contacted the trial authors by email. We assumed that events did not occur in the women for whom data were unobtainable. The imputation undertaken was subjected to sensitivity analysis.
Assessment of heterogeneity
We carried out a test for statistical heterogeneity for each meta‐analysis and assessed heterogeneity by the I2 statistic. This quantifies inconsistency, describing the impact of heterogeneity on the meta‐analysis and measuring the degree of inconsistency across studies. We considered an I2 statistic less than 25% as low level heterogeneity, 25% to 50% as moderate level heterogeneity and higher than 50% as high level heterogeneity (Higgins 2011).
Assessment of reporting biases
We planned to use a funnel plot to assess the potential for reporting bias where 10 or more trials per comparison reported data.
Data synthesis
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We pooled data where appropriate, using the Mantel‐Haenszel method. Before pooling data from more than one primary study, we considered heterogeneity. If heterogeneity was low or moderate, we used a fixed‐effect model, otherwise we used a random‐effects model, with further investigation (subgroup analysis) to explore the possible causes of the heterogeneity. We combined data to calculate pooled ORs and 95% CIs.
We stratified the primary analysis by type of intervention (cabergoline, quinagolide or bromocriptine)
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses where there were sufficient data (at least five studies per comparison in the analysis). We performed analyses to determine effects within the following subgroups:
-
severity of OHSS (severe OHSS versus moderate OHSS);
-
dose of dopamine agonist (high dose versus low dose).
Sensitivity analysis
We planned a sensitivity analysis for the primary review outcomes by excluding the studies with high risk of bias for any domain. In addition, we tested the effect by using a random‐effects model and evaluated the impact of bias from assumptions made about missing data.
Overall quality of the body of evidence: 'Summary of findings' table
We generated a 'Summary of findings' table using GRADEpro software (GRADEpro GDT 2015). This table evaluated the overall quality of the body of evidence for the main review comparison (dopamine agonists versus placebo or no intervention) for the main review outcomes (i.e. incidence of moderate or severe OHSS, live birth rate, multiple pregnancy rate, clinical pregnancy rate miscarriage rate and any other adverse effect), using GRADE criteria. We assessed the following factors that might decrease the quality level of a body of evidence: study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness and publication bias. We incorporated judgements about evidence quality (high, moderate, low and very low) into reporting of results for each outcome. Two review authors independently conducted evidence grading, and resolved disagreements by consensus.
Results
Description of studies
Results of the search
As the scope of this review was broadened for the update and we added new key terms, the literature searches were first run without a date restriction, up to 15 September 2015; an updated date‐restricted search was performed from September 2015 up to 15 August 2016. After excluding duplicate abstracts, we retrieved 212 citations using the search strategy). After independent evaluation by two review authors, we excluded 171 articles (non‐RCT, quasi‐RCT, animal experiment). Two review authors (SM and HT) independently reviewed the remaining 41 articles for possible inclusion. Finally, we included 14 new RCTs for meta‐analysis in this update, and categorised one study as 'awaiting classification' (Ahmadi 2010) (Figure 1).

Study flow diagram search August 2016.
See the inclusion and exclusion criteria for the studies in the Characteristics of included studies; Characteristics of excluded studies; and Characteristics of studies awaiting classification tables.
Included studies
In total, we included 16 studies (Alhalabi 2011; Alvarez 2007a; Amir 2015; Beltrame 2013; Busso 2010; Carizza 2008; Dalal 2014; Fetisova 2014; Ghahiri 2015; Jellad 2016; Matorras 2013; Salah 2012; Shaltout 2012; Sohrabvand 2009; Tehraninejad 2012; Torabizadeh 2013) (see Characteristics of included studies table). We contacted some trial authors for more detailed information (Dalal 2014; Fetisova 2014; Ghahiri 2015; Jellad 2016; Salah 2012; Shaltout 2012; Sohrabvand 2009; Tehraninejad 2012). In addition, we classified one meeting abstract as 'awaiting classification' due to lack of information for assessment despite attempts to contact the authors (Ahmadi 2010). From the trial registries, six ongoing or recently finished trials had potential to be included in this review, but were not published yet as abstracts or full‐text papers (Bassiouny 2015; El Khattan 2015; Hendricks 2015; Kamel 2015; Khaled 2014; NCT01530490). We attempted to contact the authors to inquire about the trials' status (e.g. recruiting phase, analysis phase, finished but unpublished or publication pending), but only one trial author replied (Bassiouny 2015), who confirmed that the trial was in the analysis phase. See Characteristics of studies awaiting classification and Characteristics of ongoing studies table.
Participants
The 16 studies enrolled 2091 high‐risk women. One study included only oocyte donors (Alvarez 2007a).
The studies were performed in ten different countries: four studies came from Iran (Ghahiri 2015; Sohrabvand 2009; Tehraninejad 2012; Torabizadeh 2013); three from Spain (Alvarez 2007a; Busso 2010; Matorras 2013);two from Brazil ( Beltrame 2013; Carizza 2008); and one each from Syria (Alhalabi 2011), Israel (Amir 2015), United Arab Emirates (Salah 2012), Russia (Fetisova 2014), Egypt (Shaltout 2012), Tunisia (Jellad 2016), and India (Dalal 2014).
One study included women with PCOS only (Salah 2012), without additional risk factors for OHSS (such as a minimum oestradiol (E2 or number of follicles/oocytes retrieved), whereas other studies either excluded women with PCOS (Beltrame 2013), or included women with and without PCOS (Alhalabi 2011; Alvarez 2007a; Amir 2015; Busso 2010; Carizza 2008; Fetisova 2014; Ghahiri 2015; Jellad 2016; Matorras 2013; Shaltout 2012; Sohrabvand 2009; Tehraninejad 2012; Torabizadeh 2013).
Most studies selected women aged under 37 years or under 40 years, but Salah 2012 selected women aged 25 to 35 years at high risk for OHSS. The definition of 'high risk of OHSS' varied widely between studies; some used a minimum number of follicles of a certain diameter (18 or more over 12 mm at day of hCG (Jellad 2016); 20 or more over 12 mm at day of hCG (Alhalabi 2011; Amir 2015; Matorras 2013; Shaltout 2012), with or without a minimum E2 level at day of hCG (greater than 2500 pg/mL (Torabizadeh 2013 and Dalal 2014 (the latter mentioned only number of 20 or more follicles without mentioning size of follicles)); greater than 3000 pg/mL (Ghahiri 2015; Jellad 2016; Matorras 2013; Sohrabvand 2009); greater than 3500 pg/mL (Shaltout 2012); greater than 4000 pg/mL (Alhalabi 2011; Amir 2015; Carizza 2008)). Five studies also incorporated the retrieval of 20 or more oocytes as a criterion (Alvarez 2007a; Ghahiri 2015; Sohrabvand 2009; Tehraninejad 2012; Torabizadeh 2013), whereas one study used transvaginal aspiration of 15 or more follicles (Fetisova 2014). One study also considered women with previous history of OHSS as high risk (Ghahiri 2015). One study included only oocyte donors who consequently did not proceed to have an embryo transferred (Alvarez 2007a).
Some studies excluded women with very high E2 levels (greater than 5000 pg/mL (Matorras 2013; Shaltout 2012); greater than 6000 pg/mL (Busso 2010)) because of their very high risk to develop OHSS, and assigned those women to cycle cancellation. One study excluded coasting cases, without stating when a woman was eligible for coasting (Jellad 2016).
Interventions
Comparisons with cabergoline
Five studies involving 521 women compared cabergoline in the treatment group with placebo or no intervention in the control group (Alvarez 2007a; Amir 2015; Fetisova 2014; Jellad 2016; Salah 2012). Amir 2015 also used coasting in almost half of the women in both the intervention and control group. We tried to contact the authors to retrieve more information about which women received coasting and whether these women developed OHSS, but received no reply. Other studies excluded women that were received coasting.
Three studies gave oral cabergoline 0.5 mg daily for eight days from the day of hCG injection (Alvarez 2007a; Amir 2015; Jellad 2016), one study gave oral cabergoline 0.5 mg daily from the day after oocyte retrieval for five days before embryo transfer day (Fetisova 2014), and one study gave oral cabergoline 0.5 mg on two successive days, starting from the day of hCG injection and repeated one week later (Salah 2012). The Salah 2012 study also had a third treatment arm of oral prednisolone 10 mg daily from the day of hCG injection to the day of the pregnancy test (Salah 2012).
Two studies involving 382 women compared cabergoline plus hydroxyethyl starch (HES) versus HES alone (500 mL of HES by intravenous infusion during follicle aspiration plus oral cabergoline 0.5 mg daily for eight days starting on the day of hCG administration for Matorras 2013; 500 mL of HES by intravenous infusion on day of follicle aspiration and oral cabergoline 0.25 mg daily by mouth for eight days starting on the day of hCG administration for Shaltout 2012).
Two studies involving 235 women compared oral cabergoline 0.5 mg daily with human albumin (albumin 20 g 20% on day of oocyte retrieval and cabergoline for seven days beginning on the day of oocyte retrieval in Tehraninejad 2012; albumin 10 units 20% on day of oocyte retrieval and cabergoline for eight days beginning on the day of hCG injection in Torabizadeh 2013).
One study with 91 women involved three arms (oral cabergoline 0.5 mg daily for seven days after oocyte retrieval versus albumin (100 mL intravenous 30 minutes after retrieval within four hours) versus 6% HES 1000 mL intravenous 30 minutes after oocyte retrieval within four hours) (Ghahiri 2015).
One study involving 166 women compared cabergoline 0.5 mg daily for three weeks beginning the day after oocyte retrieval plus albumin 20 g on day of oocyte retrieval versus albumin 20 g alone (Carizza 2008).
Two studies involving 120 women compared cabergoline with coasting (cabergoline group received cabergoline 0.5 mg daily for seven or eight days after hCG administration and coasting group had gonadotropin administration withheld until serum E2 level was below 3000 pg/mL or serum E2 level started to decline before hCG administration) (Dalal 2014; Sohrabvand 2009). However, a fluid of 6% HES was also given to 58 women in the study by Dalal 2014, and the remaining included woman received an ascites tap instead of HES.
Comparisons with quinagolide
Two studies involving 454 women compared quinagolide versus placebo (quinagolide 150 µg daily for 15 days beginning on the day of hCG administration for Alhalabi 2011; three subgroups with doses of quinagolide 50 μg daily, 100 μg daily and 200 µg daily from the day of hCG administration until the day of serum hCG test (which was 17 ± 2 days after oocyte retrieval) for Busso 2010).
Comparisons with bromocriptine
One trial involving 47 women compared bromocriptine 2.5 mg daily versus folic acid 2.0 mg daily (as a placebo), both for 14 days, beginning the day of hCG administration (Beltrame 2013).
Outcomes
All 16 included studies reported the incidence of severe or moderate OHSS but only two studies reported on live birth rate (Busso 2010; Shaltout 2012). Ten studies reported the clinical pregnancy rate (Alvarez 2007a; Amir 2015; Busso 2010; Carizza 2008; Dalal 2014; Fetisova 2014; Matorras 2013; Shaltout 2012; Sohrabvand 2009; Tehraninejad 2012). Torabizadeh 2013 only reported pregnancy rates of the women who developed moderate or severe OHSS (no significant difference between groups) and Alhalabi 2011 only mentioned that pregnancy rates were 'equal' between groups, without providing data on this outcome. Eight studies reported miscarriage rate (Amir 2015; Busso 2010; Carizza 2008; Dalal 2014; Fetisova 2014; Matorras 2013; Shaltout 2012; Tehraninejad 2012), four studies reported multiple pregnancy rate (Amir 2015; Carizza 2008; Dalal 2014; Tehraninejad 2012), and four studies reported any other adverse events of the treatment (Alvarez 2007a; Busso 2010; Carizza 2008; Shaltout 2012).
Excluded studies
We excluded 26 studies in the 2015 and 2016 searches together. The reasons for exclusion are explained in the Characteristics of excluded studies table.
Risk of bias in included studies
Figure 2 and Figure 3 summarise the risk of bias. We contacted the original authors by e‐mail to clarify any information on methodological quality and study characteristics that were unclear (see 'Risk of bias' table in the Characteristics of included studies table).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
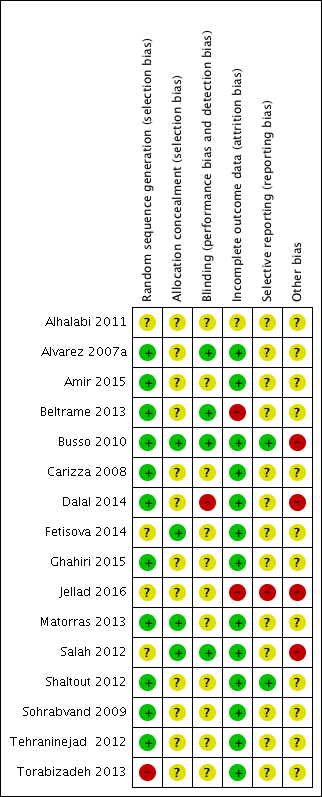
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Sequence generation (selection bias)
Eleven trials used computer‐generated randomisation (Alvarez 2007a; Amir 2015; Beltrame 2013; Busso 2010; Carizza 2008; Dalal 2014; Ghahiri 2015; Matorras 2013; Shaltout 2012; Sohrabvand 2009; Tehraninejad 2012). The randomisation process of three trials remained unclear from the publications (Alhalabi 2011; Fetisova 2014; Salah 2012). One trial mentioned that the already randomised participants were subsequently also included 'every other person', which we judged as high risk of bias (Torabizadeh 2013).
Allocation
Of the 16 included trials, four trials reported they allocated with sealed or closed envelopes (Busso 2010; Fetisova 2014; Matorras 2013; Salah 2012). The other 12 trials were unclear due to lack of detailed allocation information (Alhalabi 2011; Alvarez 2007a; Amir 2015; Beltrame 2013; Carizza 2008; Dalal 2014; Ghahiri 2015; Jellad 2016; Shaltout 2012; Sohrabvand 2009; Tehraninejad 2012; Torabizadeh 2013).
Blinding
Three studies were blinded to both assessors and participants (Alvarez 2007a; Beltrame 2013; Busso 2010), and one study was only blinded to participants (Salah 2012), while in five other studies used no blinding (Carizza 2008; Dalal 2014; Ghahiri 2015; Shaltout 2012; Tehraninejad 2012). One study blinded neither the women nor the lead physicians but did blind the ultrasound reporters (Amir 2015). Two studies blinded the lead physicians, but not the participants (Matorras 2013; Torabizadeh 2013). Seven studies reported no or limited information on blinding (Alhalabi 2011; Carizza 2008; Fetisova 2014; Jellad 2016; Shaltout 2012; Sohrabvand 2009; Tehraninejad 2012). For objective outcomes (e.g. pregnancy outcomes or live birth rate), blinding is not as important as for subjective outcomes, so we rated the unblinded studies as unclear rather than high risk of bias.
Incomplete outcome data
Six studies reported the information on dropouts and described the exact reasons (Alvarez 2007a; Busso 2010; Dalal 2014; Ghahiri 2015; Shaltout 2012; Tehraninejad 2012). Two other studies only stated that women withdrew from the study, without exact reasons (Carizza 2008; Salah 2012). However, only a small proportion of women (less than 5%) were lost to follow‐up, which does not have a clinically relevant impact on observed effect size, and hence we rated the studies at low risk of bias (Amir 2015; Fetisova 2014; Matorras 2013; Sohrabvand 2009; Torabizadeh 2013). The study of Beltrame 2013 had a high dropout number (40%) without mentioning reasons for dropout, and was therefore at high risk of bias. Jellad 2016 only reported on the subgroups of women within each arm of the study that actually went on to develop OHSS. Data from the non‐OHSS participants were lacking.
Selective reporting
Only two studies reported on the primary outcome of live birth rate (Busso 2010; Shaltout 2012). Fourteen studies reported on the primary outcome of incidence of moderate or severe OHSS.
Ten studies fully reported pregnancy rates (Alvarez 2007a; Amir 2015; Busso 2010; Carizza 2008; Dalal 2014; Fetisova 2014; Matorras 2013; Shaltout 2012; Sohrabvand 2009; Tehraninejad 2012), and two studies mentioned pregnancy rates without complete data (Alhalabi 2011; Torabizadeh 2013). Four studies reported multiple pregnancy (Amir 2015; Carizza 2008; Dalal 2014; Tehraninejad 2012). Eight studies reported miscarriage rate (Amir 2015; Busso 2010; Carizza 2008; Dalal 2014; Fetisova 2014; Matorras 2013; Shaltout 2012; Tehraninejad 2012). Jellad 2016 only reported pregnancy and miscarriage rates of the women in each arm that actually developed OHSS. Four studies reported adverse events (Alvarez 2007a; Busso 2010; Carizza 2008; Shaltout 2012).
Because of limited (fewer than 10) trials included per comparison, we were unable to make this assessment for the primary outcomes in this version of the review. In future updates of the review, where 10 or more trials are included, we will use a visual inspection of the funnel plot to look at reporting biases.
Other potential sources of bias
One study was sponsored by Ferring Pharmaceuticals (Busso 2010). One trial included young women with PCOS without other high risk factors identified (e.g. based on E2 or ultrasound) (Salah 2012).
Effects of interventions
See: Summary of findings for the main comparison Dopamine agonist versus placebo/no intervention
1 Dopamine agonist versus placebo/no intervention
Primary outcomes
1.1 Incidence of moderate or severe ovarian hyperstimulation syndrome per woman randomised
Eight studies reported the incidence of moderate or severe OHSS (Alhalabi 2011; Alvarez 2007a; Amir 2015; Beltrame 2013; Busso 2010; Fetisova 2014; Jellad 2016; Salah 2012). Dopamine agonists were associated with a lower risk of moderate or severe OHSS as compared with placebo/no intervention (OR 0.27, 95% CI 0.19 to 0.39; 1022 participants; 8 studies; I2 = 0%; moderate quality evidence) (Analysis 1.1; Figure 4). This suggests that if 28.6% of women taking placebo or no intervention experience moderate or severe OHSS, between 7.1% and 13.5% of women taking dopamine agonists will do so. When compared with placebo/no intervention, cabergoline (OR 0.26, 95% CI 0.16 to 0.42; 521 participants; 5 studies I2 = 0%), and quinagolide (OR 0.28, 95% CI 0.15 to 0.51; 454 participants; 2 studies; I2 = 30%) were associated with a lower risk of moderate or severe OHSS (Analysis 1.1; Figure 4). However, there was no evidence of a difference between bromocriptine and placebo (OR 0.29, 95% CI 0.08 to 1.14; 47 participants; 1 study) (Analysis 1.1; Figure 4).

Forest plot of comparison 1: Dopamine agonist (without co‐intervention) versus placebo/no intervention, outcome: 1.1 moderate or severe ovarian hyperstimulation syndrome.
Subgroup analyses
1.1.1 severity of ovarian hyperstimulation syndrome The effect estimates were similar in the two subgroups and the test for subgroup differences showed no evidence of a difference between them (test for subgroup differences: Chi2 = 0.40, degrees of freedom (df) = 1 (P = 0.53), I2 = 0%) (Analysis 1.2).
1.1.2 Dose of dopamine agonist All studies with cabergoline used a dose of 0.5 mg daily so subgroup analysis for dose was not possible.
1.2 Live birth rate per woman randomised
One study reported data on live birth rate (Busso 2010). There was no evidence of a difference between dopamine agonist (only including quinagolide) and placebo/no intervention (OR 1.01, 95% CI 0.53 to 1.91; 182 participants; 1 study; low quality evidence) (Analysis 1.3). This suggests that if 51% of women taking placebo or no intervention experience live birth, between 36% and 67% of women taking dopamine agonists will do so. In addition, there was no evidence of a difference between quinagolide and placebo/no intervention in the subgroup analysis for dose.
Secondary outcomes
1.3 Clinical pregnancy rate
Four trials reported the clinical pregnancy rate (Alvarez 2007a; Amir 2015; Busso 2010; Fetisova 2014). There was no evidence of a difference between dopamine agonist and placebo/no intervention (OR 0.81, 95% CI 0.54 to 1.22; 432 participants; 4 studies; I2 = 0%; moderate quality evidence) (Analysis 1.4). This suggests that if 40% of women taking placebo or no intervention experience clinical pregnancy, between 27% and 45% of women taking dopamine agonists will do so. There was no evidence of a difference between cabergoline and placebo/no intervention (OR 0.81, 95% CI 0.48 to 1.38; 250 participants; 3 studies; I2 = 0%), and between quinagolide and placebo (OR 0.81, 95% CI 0.43 to 1.54; 182 participants; 1 study).
1.4 Multiple pregnancy rate
Only one study reported multiple pregnancy rate (Amir 2015), and there was no evidence of difference between cabergoline and placebo (OR 0.32, 95% CI 0.01 to 8.26; 40 participants; 1 study; very low quality evidence) (Analysis 1.5). This suggests that if 5% of women taking placebo or no intervention experience multiple pregnancy, between 1% and 30% of women taking dopamine agonists will do so.
1.5 Miscarriage rate
Two studies reported miscarriage rate (Amir 2015; Fetisova 2014). There was no conclusive evidence of a difference between dopamine agonist and placebo/no intervention (OR 0.66, 95% CI 0.19 to 2.28; 168 participants; 2 studies; I2 = 0%; low quality evidence) (Analysis 1.6). This suggests that if 7% of women taking placebo or no intervention experience a miscarriage, between 2% and 15% of women taking dopamine agonists will do so.
1.6 Any other adverse events of the treatment
Two trials reported adverse events (Alvarez 2007a; Busso 2010). Dopamine agonists were associated with an increased risk of adverse events (OR 4.54, 95% CI 1.49 to 13.84; 264 participants; 2 studies; I2 = 49%; very low quality evidence) (Analysis 1.7). This suggests that if 4% of women taking placebo or no intervention experience adverse events, between 6% and 38% of women taking dopamine agonists will do so. However, there was no conclusive evidence of a difference between cabergoline and placebo/no intervention (OR 2.24, 95% CI 0.62 to 8.14; 82 participants; 1 study) (Analysis 1.7). One trial reported that 17 women in the quinagolide group discontinued because of adverse events and no women in the placebo group (OR 16.64, 95% CI 0.98 to 282.02; 182 participants; 1 study) (Analysis 1.7) (Busso 2010).
2 Dopamine agonist plus co‐intervention versus co‐intervention
Three studies compared dopamine agonist plus co‐intervention versus co‐intervention. The co‐interventions were HES (two RCTs) and albumin (one RCT).
Primary outcomes
2.1 Incidence of severe or moderate ovarian hyperstimulation syndrome per woman randomised
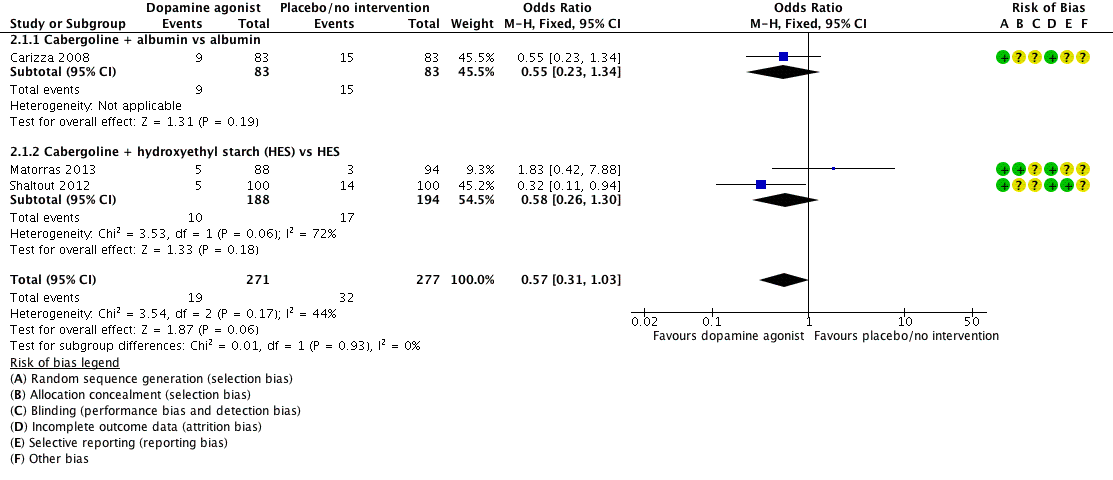
Three studies reported the incidence of moderate or severe OHSS (Carizza 2008; Matorras 2013; Shaltout 2012). Dopamine agonists plus co‐intervention were not significantly associated with a lower risk of moderate or severe OHSS as compared with co‐intervention alone (OR 0.57, 95% CI 0.31 to 1.03; 548 participants; 3 studies; I2 = 44%) (Analysis 2.1; Figure 5). There was no evidence of a difference between the cabergoline plus albumin group and the albumin group (OR 0.55, 95% CI 0.23 to 1.34; 166 participants; 1 study), or between the cabergoline plus HES group versus the HES group (OR 0.58, 95% CI 0.26 to 1.30; 382 participants; 2 studies; I2 = 72%) (Analysis 2.1; Figure 5). As we included only three studies, we did not perform a subgroup analysis.

Forest plot of comparison: 2 Dopamine agonist plus co‐intervention versus co‐intervention, outcome: 2.1 Moderate or severe ovarian hyperstimulation syndrome.
2.2 Live birth rate per woman randomised
One study reported data on live birth rate (Shaltout 2012). There was no evidence of a difference between cabergoline plus HES and HES (OR 1.04, 95% CI 0.59 to 1.86; 200 participants; 1 study) (Analysis 2.2).
Secondary outcomes
2.3 Clinical pregnancy rate
Three trials reported the clinical pregnancy rate (Carizza 2008; Matorras 2013; Shaltout 2012). There was no evidence of a difference between dopamine agonist plus co‐intervention and co‐intervention alone (OR 1.00, 95% CI 0.71 to 1.40; 548 participants; 3 studies; I2 = 0%) (Analysis 2.3). There was no evidence of a difference between cabergoline plus albumin and albumin (OR 1.05, 95% CI 0.56 to 1.96; 166 participants; a study), and between cabergoline plus HES and HES (OR 0.98, 95% CI 0.65 to 1.47; 382 participants; 2 studies; I2 = 0%) (Analysis 2.3).
2.4 Multiple pregnancy rate
Only one study reported multiple pregnancy rate (Carizza 2008). There was no evidence of a difference between cabergoline plus albumin and albumin (OR 2.02, 95% CI 0.18 to 22.77; 166 participants; 1 study) (Analysis 2.4).
2.5 Miscarriage rate
Three studies reported miscarriage rate (Carizza 2008; Matorras 2013; Shaltout 2012). There was no conclusive evidence of a difference between dopamine agonist plus co‐intervention and co‐intervention (OR 0.65, 95% CI 0.30 to 1.42; 548 participants; 3 studies; I2 = 0%) (Analysis 2.5). There was no evidence of a difference between cabergoline plus albumin and albumin (OR 0.33, 95% CI 0.03 to 3.19; 166 participants; 1 study), and between cabergoline plus HES and HES (OR 0.73, 95% CI 0.31 to 1.68; 382 participants; 2 studies; I2 = 0%) (Analysis 2.5).
2.6 Any other adverse events of the treatment
Two trials reported adverse events (Carizza 2008; Shaltout 2012). Dopamine agonists plus co‐intervention were associated with an increased risk of adverse events (OR 3.03, 95% CI 0.12 to 75.28; 366 participants; 2 studies; I2 = 0%) (Analysis 2.6). However, there was no inclusive evidence of a difference between cabergoline plus HES and HES (OR 3.03, 95% CI 0.12 to 75.28; 200 participants; 1 study). One trial detected no adverse events (Carizza 2008).
3 Dopamine agonist versus other active intervention
3.1 Cabergoline versus human albumin
Primary outcomes
3.1.1 Incidence of severe or moderate ovarian hyperstimulation syndrome per woman randomised
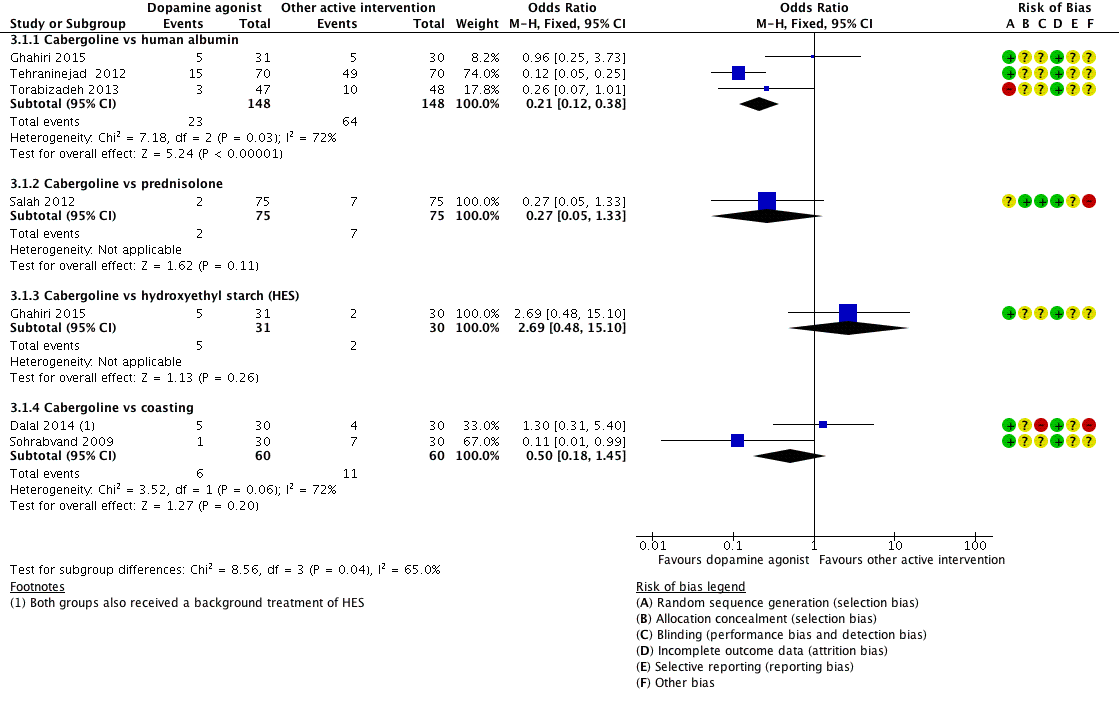
Three studies reported the incidence of moderate or severe OHSS for the comparison of cabergoline versus human albumin (Ghahiri 2015; Tehraninejad 2012; Torabizadeh 2013). Cabergoline was associated with a lower incidence of severe or moderate OHSS than human albumin (OR 0.21, 95% CI 0.12 to 0.38; 296 participants; 3 studies; I2 = 72%) (Analysis 3.1; Figure 6).
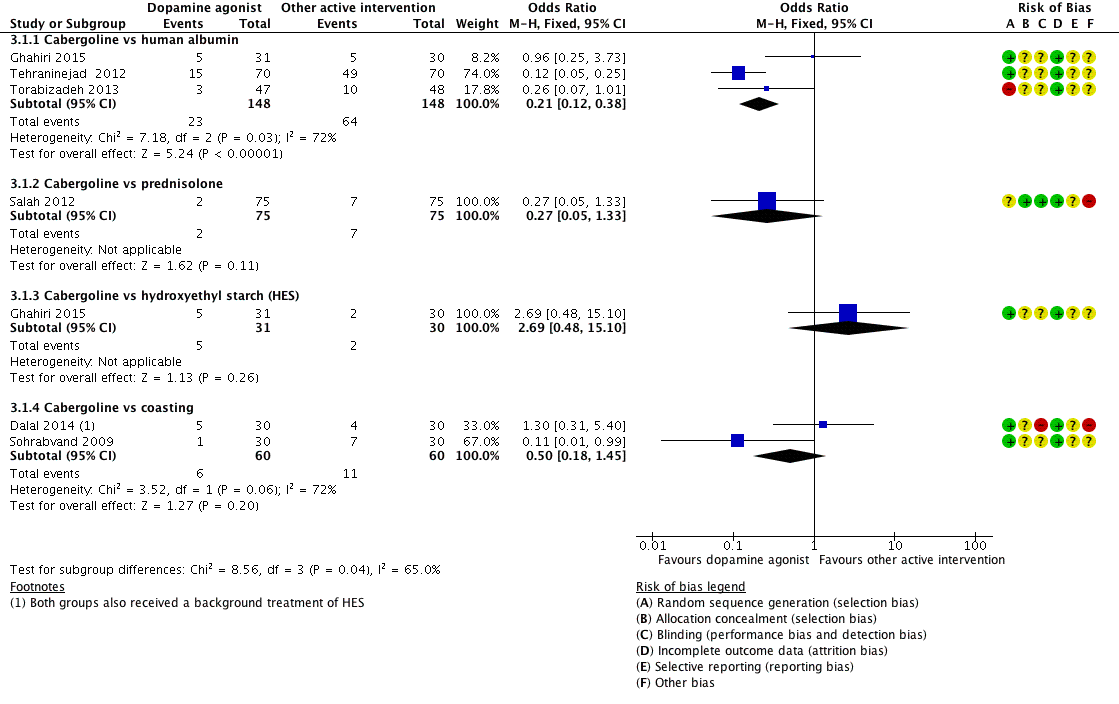
Forest plot of comparison 3: Cabergoline versus active interventions, outcome: 3.1 moderate or severe ovarian hyperstimulation syndrome.
3.1.2 Live birth rate per woman randomised
We found no trials comparing cabergoline versus human albumin on live birth rate.
Secondary outcomes
3.1.3 Clinical pregnancy rate
There was no evidence of any difference between the cabergoline and human albumin groups in the study of Tehraninejad 2012 (OR 0.68, 95% CI 0.33 to 1.38; 140 participants; 1 study) (Analysis 3.2). The study of Torabizadeh 2013 only reported pregnancy rates in women who developed moderate or severe OHSS.
3.1.4 Multiple pregnancy rate
There was no evidence of a difference between the cabergoline and human albumin groups (OR 0.58, 95% CI 0.13 to 2.54; 140 participants; 1 study) (Analysis 3.3).
3.1.5 Miscarriage rate
There was no evidence of any difference between the cabergoline and human albumin groups (OR 0.32, 95% CI 0.03 to 3.19; 140 participants; 1 study) (Analysis 3.4).
3.1.6 Any other adverse effects of the treatment
There trials reported no data comparing cabergoline versus prednisolone on any other adverse effects.
3.2 Cabergoline versus prednisolone
3.2.1 Incidence of severe or moderate ovarian hyperstimulation syndrome per woman randomised
Only one trial reported on the comparison of cabergoline versus prednisolone in the incidence of severe or moderate OHSS (Salah 2012). There was no evidence of a difference between the groups (OR 0.27, 95% CI 0.05 to 1.33; 150 participants; 1 study) (Analysis 3.1; Figure 6).
3.2.2 Live birth rate per woman randomised
We found no trials comparing cabergoline versus prednisolone on live birth rate.
Secondary outcomes
3.2.3 Clinical pregnancy rate
We found no trials comparing cabergoline versus prednisolone on clinical pregnancy rate.
3.2.4 Multiple pregnancy rate
We found no trials comparing cabergoline versus prednisolone on multiple pregnancy rate.
3.2.5 Miscarriage rate
We found no trials comparing cabergoline versus prednisolone on miscarriage rate.
3.2.6 Any other adverse effects of the treatment
We found no trials comparing cabergoline versus prednisolone on adverse effects.
3.3 Cabergoline versus hydroxyethyl starch
Primary outcomes
3.3.1 Incidence of severe or moderate ovarian hyperstimulation syndrome per woman randomised
There was no evidence of a difference between the cabergoline and HES group in incidence of severe or moderate OHSS (OR 2.69, 95% CI 0.48 to 15.10; 61 participants; 1 study) (Analysis 3.1; Figure 6).
3.3.2 Live birth rate per woman randomised
We found no trials comparing cabergoline versus HES on live birth rate.
Secondary outcomes
3.3.3 Clinical pregnancy rate
We found no trials comparing cabergoline versus HES on clinical pregnancy rate.
3.3.4 Multiple pregnancy rate
We found no trials comparing cabergoline versus HES on multiple pregnancy rate.
3.3.5 Miscarriage rate
We found no trials comparing cabergoline versus HES on miscarriage rate.
3.3.6 Any other adverse effects of the treatment
We found no trials comparing cabergoline versus HES on adverse effects.
3.4 Cabergoline versus coasting
Primary outcomes
3.4.1 Incidence of severe or moderate ovarian hyperstimulation syndrome per woman randomised
Two trials provided data on the incidence of severe or moderate OHSS (Dalal 2014; Sohrabvand 2009). There was no evidence of a difference between the groups (OR 0.50, 95% CI 0.18 to 1.45; 120 participants; 2 studies; I2 = 72%) (Analysis 3.1; Figure 6).
3.4.2 Live birth rate per woman randomised
We found no trials comparing cabergoline versus coasting on live birth rate.
Secondary outcomes
3.4.3 Clinical pregnancy rate
There was a higher clinical pregnancy rate with cabergoline compared with coasting clinical pregnancy rate (OR 2.65, 95% CI 1.13 to 6.21; 120 participants; 2 studies; I2 = 0%) (Analysis 3.2).
3.4.4 Multiple pregnancy rate
There was no evidence of a difference between the cabergoline and coasting on multiple pregnancy rate (OR 5.35, 95% CI 0.25 to 116.31; 60 participants; 1 study) (Analysis 3.3).
3.4.5 Miscarriage rate
There was no evidence of a difference between the cabergoline and coasting on miscarriage rate (OR 0.19, 95% CI 0.01 to 4.06; 60 participants; 1 study) (Analysis 3.4).
Publication bias
A funnel plot was not necessary as we included fewer than 10 trials in the analyses. This will be assessed in future updates if there are 10 or more trials.
Sensitivity analysis
We conducted a prespecified sensitivity analysis. When we excluded four studies with high risk of bias from Analysis 1.1 (Beltrame 2013; Busso 2010; Jellad 2016; Salah 2012), the lower incidence of moderate or severe OHSS with dopamine agonists compared with placebo/no intervention remained unchanged (OR 0.25, 95% CI 0.15 to 0.41; 522 participants; 4 studies; I2 = 0%). The results were similar for moderate or severe OHSS between cabergoline and human albumin (OR 0.20, 95% CI 0.11 to 0.38; 201 participants; 2 studies; I2 = 86%) when we excluded Torabizadeh 2013 from Analysis 3.1. However, cabergoline became associated with a lower risk of moderate or severe OHSS than coasting (OR 0.11. 95% CI 0.01 to 0.99; 60 participants; 1 study) when Dalal 2014 was excluded from Analysis 3.1. In addition, use of a random‐effects model or the assumptions made about missing data did not affect the results.
Discussion
Summary of main results
This systematic review evaluated the effectiveness and safety of dopamine agonists for preventing OHSS in high‐risk women during ART treatment and performed a meta‐analysis. Eight trials compared dopamine agonist with placebo or no intervention, three trials compared dopamine agonist in combination with co‐intervention with co‐intervention and five trials compared dopamine agonists with other active interventions. Overall, when compared with placebo or no intervention, dopamine agonists had a lower risk of developing moderate or severe OHSS without influencing pregnancy outcomes such as live birth rate for those women who proceeded to have a fresh embryo transfer, clinical pregnancy rate, multiple pregnancy rate and miscarriage rate. However, data on the live birth rate were scarce in the included trials. In general in OHSS trials, it will be considered unethical to withhold women who are at risk of OHSS of having all their embryos frozen for replacement in a subsequent cycle, as current embryo survival rates after freezing are generally excellent and the transfer of a frozen embryo in an unstimulated cycle avoids the risk of OHSS in that cycle.
There was an increased risk of adverse events, which occurred rarely, associated with dopamine agonists particularly when using quinagolide. The use of cabergoline was associated with a lower risk of moderate or severe OHSS, without influencing pregnancy outcomes when compared with placebo or no intervention. Quinagolide appeared to reduce the risk of moderate or severe OHSS, but might increase the incidence of adverse events. With the limited data available, bromocriptine did not influence the incidence of moderate or severe OHSS. There was no evidence of a difference between dopamine agonist plus co‐intervention and co‐intervention in the outcomes of interest. For dopamine agonists compared with other active interventions, we found only a comparison with cabergoline. Compared with human albumin, cabergoline might reduce the incidence of moderate or severe OHSS, but there was no evidence of a difference for comparisons between cabergoline and prednisolone, HES or coasting. Cabergoline was associated with a higher clinical pregnancy rate than coasting. In other respects, there was no evidence of a difference between cabergoline and other active interventions with respect to the other studied outcomes.
The quality of the evidence for the comparison of dopamine agonist with placebo/no intervention was generally moderate; the main limitations were poor reporting of study methods (mostly lack of details on randomisation and blinding), heterogeneity across trials and risk of imprecision (low events or small sample sizes).
Overall completeness and applicability of evidence
Compared with previous review (Tang 2012), we included 14 additional trials. In total, this updated Cochrane Review included 16 trials involving 2091 high‐risk women. The study populations varied among these trials regarding the definition of 'high‐risk' of OHSS. This may influence the incidence of OHSS and limits the applicability of study results in practice. However, as some trials even excluded the truly high‐risk women from participating, it can be postulated that the effect of dopamine agonists could be even larger when these women would have been included. Most of the trials defined moderate or severe OHSS according to Golan's classification (Golan 1989), but three trials used other definitions, which may induce bias when pooling the data of the various studies. Only a few studies reported pregnancy outcomes such as live birth. The influence of dopamine agonists on pregnancy outcomes requires further study; however, many units will practice an embryo 'freeze‐all' approach for women at risk of OHSS and therefore data for pregnancy outcomes may not be forthcoming. Most of the trials evaluated the dopamine agonist cabergoline, whereas two trials evaluated quinagolide and one trial evaluated bromocriptine. In addition, our evidence was applicable in low‐ to middle‐income countries as most trials were performed in these countries. Finally, due to the lack of studies comparing a dopamine agonist with another dopamine agonist, we are unable to determine which dopamine agonist is most effective in preventing OHSS.
Quality of the evidence
The methodological quality of the 16 included trials varied. Eleven trials used correct random sequence generation, and only four trials had a low risk of bias in the domain of allocation concealment. Four trials were either single or double blind. One trial was at high risk of bias due to a high percentage of dropouts without reported reasons (Beltrame 2013). All trials reported the outcomes of OHSS, but only two studies provided the primary outcome of 'live birth rate'. See Figure 2 and Figure 3 for the 'Risk of bias' assessments of the included studies.
Moreover, the overall body of evidence for primary outcomes between dopamine agonist and placebo or no intervention was moderate. The main reasons for downgrading the quality of the evidence were: poor reporting of study methods (e.g. 25% of RCTs did not report the methods of allocation concealment or blinding) and risk of imprecision (e.g. low events). See summary of findings Table for the main comparison for more details.
Potential biases in the review process
We tried to identify all eligible trials by conducting a systematic review of the literature without restrictions of publication type or language. Moreover, we contacted the authors of trials for more information about any unpublished data. In addition, we made assumptions about missing data, but they seemed to be robust in the sensitivity analysis.
Agreements and disagreements with other studies or reviews
Our results are in agreement with most of the systematic reviews and meta‐analyses on dopamine agonists for the prevention for OHSS (Baumgarten 2013; Guo 2016; Kalampokas 2013; Kasum 2014; Leitao 2014; Youssef 2010). The first systematic review published in 2010 included only four RCTs with 570 women, and showed that cabergoline might reduce the incidence of OHSS. However, it did not show evidence of a reduction in severe OHSS (Youssef 2010), which is consistent with our previous Cochrane Review (Tang 2012). This might be caused by a small sample size or low event rate of severe OHSS. In 2014, another systematic review included eight trials involving 858 women and showed that cabergoline could reduce the risk of moderate or severe OHSS, as well as severe OHSS (Leitao 2014). In 2016, one systematic review and network meta‐analysis of 31 RCTs involving 7181 women showed that cabergoline was superior to placebo or human albumin, or glucocorticoid in decreasing OHSS incidence, and there was no evidence of any difference between cabergoline and other active interventions (e.g. aspirin, HES, calcium infusion or metformin). However, until 2016, few systematic reviews included types of dopamine agonist other than cabergoline. One systematic review showed that a dopamine agonist appeared to be effective for the prevention of OHSS (Baumgarten 2013). Moreover, no evidence of adverse effects on pregnancy outcomes was detected (Baumgarten 2013; Leitao 2014; Youssef 2010). Compared with previous systematic reviews, our review includes more trials and women, and can therefore draw a more robust conclusion that the use of dopamine agonists could reduce the incidence of moderate or severe OHSS.

Study flow diagram search August 2016.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison 1: Dopamine agonist (without co‐intervention) versus placebo/no intervention, outcome: 1.1 moderate or severe ovarian hyperstimulation syndrome.
Forest plot of comparison: 2 Dopamine agonist plus co‐intervention versus co‐intervention, outcome: 2.1 Moderate or severe ovarian hyperstimulation syndrome.
Forest plot of comparison 3: Cabergoline versus active interventions, outcome: 3.1 moderate or severe ovarian hyperstimulation syndrome.

Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 1 Moderate or severe ovarian hyperstimulation syndrome (OHSS).

Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 2 Subgroup analysis by severity of OHSS.

Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 3 Live birth.

Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 4 Clinical pregnancy rate.

Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 5 Multiple pregnancy.

Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 6 Miscarriage.
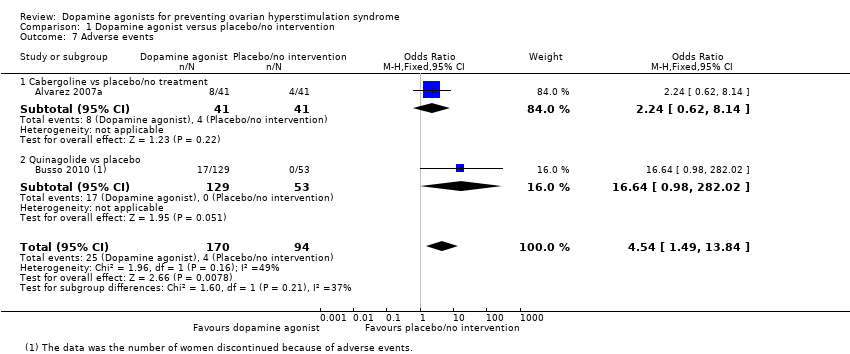
Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 7 Adverse events.
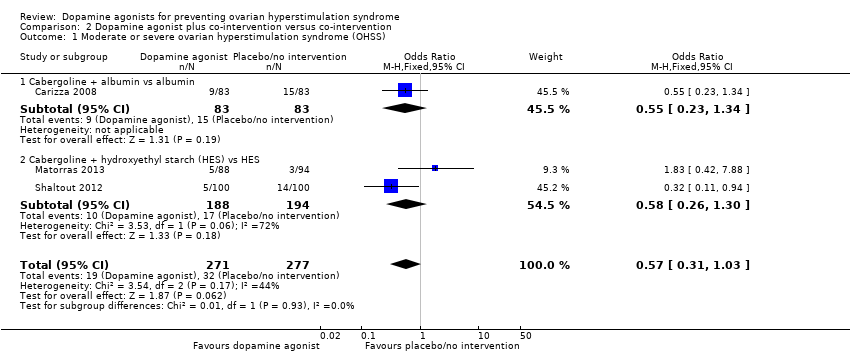
Comparison 2 Dopamine agonist plus co‐intervention versus co‐intervention, Outcome 1 Moderate or severe ovarian hyperstimulation syndrome (OHSS).
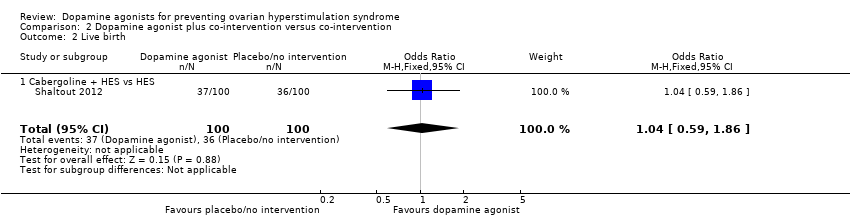
Comparison 2 Dopamine agonist plus co‐intervention versus co‐intervention, Outcome 2 Live birth.

Comparison 2 Dopamine agonist plus co‐intervention versus co‐intervention, Outcome 3 Clinical pregnancy rate.
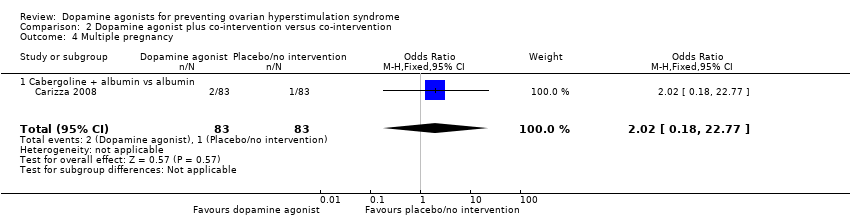
Comparison 2 Dopamine agonist plus co‐intervention versus co‐intervention, Outcome 4 Multiple pregnancy.
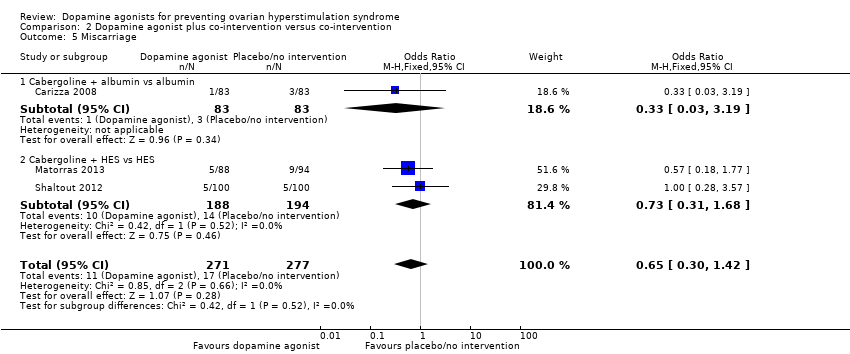
Comparison 2 Dopamine agonist plus co‐intervention versus co‐intervention, Outcome 5 Miscarriage.

Comparison 2 Dopamine agonist plus co‐intervention versus co‐intervention, Outcome 6 Adverse events.

Comparison 3 Dopamine agonist versus active interventions, Outcome 1 Moderate or severe ovarian hyperstimulation syndrome (OHSS).
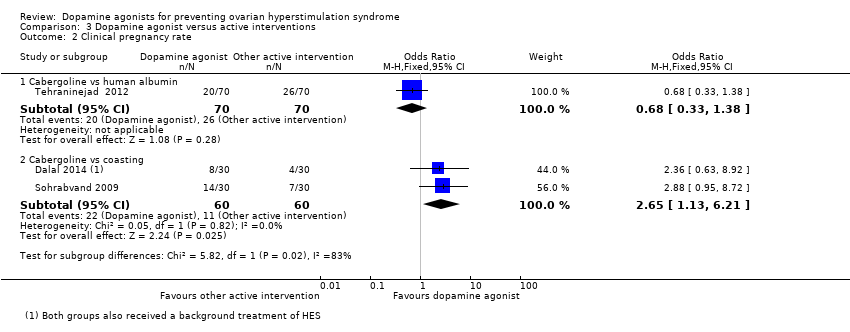
Comparison 3 Dopamine agonist versus active interventions, Outcome 2 Clinical pregnancy rate.
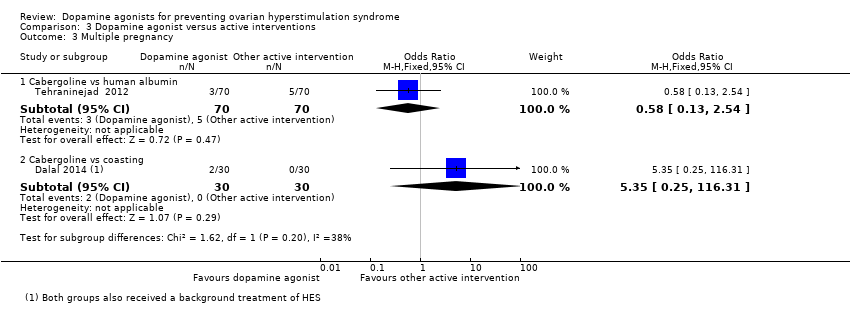
Comparison 3 Dopamine agonist versus active interventions, Outcome 3 Multiple pregnancy.

Comparison 3 Dopamine agonist versus active interventions, Outcome 4 Miscarriage.
| Dopamine agonist vs placebo/no intervention | ||||||
| Patient or population: women of reproductive age undergoing any ART therapy Settings: ART unit Intervention: dopamine agonist Comparison: placebo/no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with placebo/no intervention | Risk with dopamine agonist | |||||
| Incidence of moderate or severe OHSS | 286 per 1000 | 97 per 1000 (71 to 135) | OR 0.27 (0.19 to 0.39) | 1022 (8 studies) | ⊕⊕⊕⊝ | ‐ |
| Live birth rate | 509 per 1000 | 512 per 1000 (355 to 665) | OR 1.01 (0.53 to 1.91) | 182 | ⊕⊕⊝⊝ | |
| Clinical pregnancy rate | 401 per 1000 | 352 per 1000 (266 to 450) | OR 0.81 (0.54 to 1.22) | 432 (4 studies) | ⊕⊕⊕⊝ | |
| Multiple pregnancy | 50 per 1000 | 17 per 1000 (1 to 303) | OR 0.32 (0.01 to 8.26) | 40 | ⊕⊝⊝⊝ | |
| Miscarriage pregnancy rate | 72 per 1000 | 49 per 1000 (15 to 151) | OR 0.66 (0.19 to 2.28) | 168 (2 studies) | ⊕⊕⊝⊝ | |
| Adverse events | 43 per 1000 | 168 per 1000 (62 to 381) | OR 4.54 (1.49 to 13.84) | 264 | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ART: assisted reproductive technology; CI: confidence interval; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for serious risk of bias associated with poor reporting of study methods. 2 Downgraded one level for serious risk of imprecision: confidence interval compatible with benefit in either arm or with no difference between the groups. 3 Downgraded two levels for very serious risk of imprecision: only one event. 4 Downgraded one level for serious risk of imprecision: only 10 events. 5 Downgraded one level for serious risk of imprecision: only 29 events. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Moderate or severe ovarian hyperstimulation syndrome (OHSS) Show forest plot | 8 | 1022 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.19, 0.39] |
| 1.1 Cabergoline vs placebo/no treatment | 5 | 521 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.16, 0.42] |
| 1.2 Quinagolide vs placebo | 2 | 454 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.15, 0.51] |
| 1.3 Bromocriptine vs placebo (folic acid) | 1 | 47 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.08, 1.14] |
| 2 Subgroup analysis by severity of OHSS Show forest plot | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Severe OHSS | 7 | 750 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.14, 0.56] |
| 2.2 Moderate OHSS | 7 | 750 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.24, 0.57] |
| 3 Live birth Show forest plot | 1 | 182 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.53, 1.91] |
| 3.1 Quinagolide vs placebo | 1 | 182 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.53, 1.91] |
| 4 Clinical pregnancy rate Show forest plot | 4 | 432 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.54, 1.22] |
| 4.1 Cabergoline vs no intervention | 3 | 250 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.48, 1.38] |
| 4.2 Quinagolide vs placebo | 1 | 182 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.43, 1.54] |
| 5 Multiple pregnancy Show forest plot | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.26] |
| 5.1 Cabergoline vs placebo/no treatment | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.26] |
| 6 Miscarriage Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Cabergoline vs placebo/no treatment | 2 | 168 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.19, 2.28] |
| 7 Adverse events Show forest plot | 2 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.54 [1.49, 13.84] |
| 7.1 Cabergoline vs placebo/no treatment | 1 | 82 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.24 [0.62, 8.14] |
| 7.2 Quinagolide vs placebo | 1 | 182 | Odds Ratio (M‐H, Fixed, 95% CI) | 16.64 [0.98, 282.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Moderate or severe ovarian hyperstimulation syndrome (OHSS) Show forest plot | 3 | 548 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.31, 1.03] |
| 1.1 Cabergoline + albumin vs albumin | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.23, 1.34] |
| 1.2 Cabergoline + hydroxyethyl starch (HES) vs HES | 2 | 382 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.26, 1.30] |
| 2 Live birth Show forest plot | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.59, 1.86] |
| 2.1 Cabergoline + HES vs HES | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.59, 1.86] |
| 3 Clinical pregnancy rate Show forest plot | 3 | 548 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.71, 1.40] |
| 3.1 Cabergoline + albumin vs albumin | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.56, 1.96] |
| 3.2 Cabergoline + HES vs HES | 2 | 382 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.65, 1.47] |
| 4 Multiple pregnancy Show forest plot | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.18, 22.77] |
| 4.1 Cabergoline + albumin vs albumin | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.18, 22.77] |
| 5 Miscarriage Show forest plot | 3 | 548 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.30, 1.42] |
| 5.1 Cabergoline + albumin vs albumin | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.19] |
| 5.2 Cabergoline + HES vs HES | 2 | 382 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.31, 1.68] |
| 6 Adverse events Show forest plot | 2 | 366 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 75.28] |
| 6.1 Cabergoline + albumin vs albumin | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Cabergoline + HES vs HES | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 75.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Moderate or severe ovarian hyperstimulation syndrome (OHSS) Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Cabergoline vs human albumin | 3 | 296 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.12, 0.38] |
| 1.2 Cabergoline vs prednisolone | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.05, 1.33] |
| 1.3 Cabergoline vs hydroxyethyl starch (HES) | 1 | 61 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.48, 15.10] |
| 1.4 Cabergoline vs coasting | 2 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.18, 1.45] |
| 2 Clinical pregnancy rate Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Cabergoline vs human albumin | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.33, 1.38] |
| 2.2 Cabergoline vs coasting | 2 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.65 [1.13, 6.21] |
| 3 Multiple pregnancy Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Cabergoline vs human albumin | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.13, 2.54] |
| 3.2 Cabergoline vs coasting | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.35 [0.25, 116.31] |
| 4 Miscarriage Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Cabergoline vs human albumin | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.03, 3.19] |
| 4.2 Cabergoline vs coasting | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.06] |

