Agonistas dopaminérgicos para la prevención del síndrome de hiperestimulación ovárica
Resumen
Antecedentes
El síndrome de hiperestimulación ovárica (SHEO) es una complicación potencialmente grave de la estimulación ovárica en la tecnología de reproducción asistida (TRA). Se caracteriza por el agrandamiento de los ovarios y un desplazamiento agudo de líquido del espacio intravascular al tercer espacio, lo que provoca distención abdominal, un aumento del riesgo de tromboembolia venosa y una disminución de la perfusión orgánica. La mayoría de los casos son leves, pero en el 3% al 8% de los ciclos de fecundación in vitro (FIV) ocurren formas moderadas o graves de SHEO. Los agonistas dopaminérgicos se introdujeron como una intervención de prevención secundaria para el SHEO en las mujeres con alto riesgo de presentarlo que se someten a tratamiento con TRA.
Objetivos
Evaluar la efectividad y la seguridad de los agonistas dopaminérgicos en la prevención del SHEO en mujeres con alto riesgo de presentarlo sometidas a tratamiento con TRA.
Métodos de búsqueda
Se realizaron búsquedas en las siguientes bases de datos desde el inicio hasta el 4 de mayo de 2020: Registro especializado del Grupo Cochrane de Ginecología y fertilidad (Cochrane Gynaecology and Fertility Group), CENTRAL, MEDLINE, Embase, CINAHL y PsycINFO para encontrar ensayos controlados aleatorizados (ECA) que evaluaran el efecto de los agonistas dopaminérgicos sobre las tasas de SHEO. También se realizaron búsquedas manuales en las listas de referencias y en la literatura gris.
Criterios de selección
Se consideraron para inclusión los ECA que compararon los agonistas dopaminérgicos con placebo/ninguna intervención u otra intervención para la prevención del SHEO en la TRA. Los desenlaces principales fueron la incidencia del SHEO moderado o grave y la tasa de nacidos vivos. Los desenlaces secundarios fueron las tasas de embarazo clínico, embarazo múltiple, aborto espontáneo y eventos adversos.
Obtención y análisis de los datos
Dos autores de la revisión examinaron de forma independiente los títulos, los resúmenes y los textos completos de las publicaciones, seleccionaron los estudios, extrajeron los datos y evaluaron el riesgo de sesgo. Los desacuerdos se resolvieron mediante consenso. Los resultados agrupados se informaron como odds ratios (OR) e intervalo de confianza (IC) del 95% con el método de Mantel‐Haenszel. Se utilizó el método GRADE para evaluar la calidad general de la evidencia.
Resultados principales
La búsqueda identificó seis nuevos ECA, lo que dio como resultado que en esta revisión actualizada se incluyeran 22 ECA con 3171 mujeres con alto riesgo de SHEO. Los agonistas dopaminérgicos fueron la cabergolina, la quinagolida y la bromocriptina.
Agonistas dopaminérgicos versus placebo o ninguna intervención
Los agonistas dopaminérgicos probablemente redujeron el riesgo de SHEO moderado o grave en comparación con placebo/ninguna intervención (OR 0,32; IC del 95%: 0,23 a 0,44; diez estudios, 1202 participantes; evidencia de calidad moderada). Lo anterior indica que, si se supone que el riesgo de presentar SHEO moderado o grave tras la administración de placebo/ninguna intervención es del 27%, el riesgo tras la administración de agonistas dopaminérgicos estaría entre el 8% y el 14%. No hay certeza cerca del efecto de los agonistas dopaminérgicos sobre las tasas de nacidos vivos (OR 0,96; IC del 95%: 0,60 a 1,55; tres estudios, 362 participantes; evidencia de calidad baja). Tampoco se sabe con certeza el efecto de los agonistas dopaminérgicos sobre el embarazo clínico, el embarazo múltiple, el aborto espontáneo o los eventos adversos (evidencia de calidad muy baja a baja).
Agonistas dopaminérgicos más cointervención versus cointervención
El agonista dopaminérgico más una cointervención (hidroxietilalmidón, albúmina humana o el aplazamiento de la estimulación ovárica o "coasting") podría disminuir el riesgo de SHEO moderado o grave en comparación con la cointervención (OR 0,48; IC del 95%: 0,28 a 0,84; cuatro estudios, 748 participantes; evidencia de calidad baja). Los agonistas dopaminérgicos podrían mejorar las tasas de nacidos vivos (OR 1,21; IC del 95%: 0,81 a 1,80; dos estudios, 400 participantes; evidencia de calidad baja. Los agonistas dopaminérgicos podrían mejorar las tasas de embarazo clínico y de aborto espontáneo, pero no se sabe si mejoran las tasas de embarazo múltiple o los eventos adversos (evidencia de calidad muy baja a baja).
Agonistas dopaminérgicos versus otras intervenciones activas
No hay certeza de que la cabergolina mejore el riesgo de SHEO moderado o grave en comparación con la albúmina humana (OR 0,21; IC del 95%: 0,12 a 0,38; tres estudios, 296 participantes; evidencia de calidad muy baja), la prednisolona (OR 0,27; IC del 95%: 0,05 a 1,33; un estudio; 150 participantes; evidencia de calidad muy baja), el hidroxietilalmidón (OR 2,69; IC del 95%: 0,48 a 15,10; un estudio, 61 participantes; evidencia de calidad muy baja), el aplazamiento o coasting (OR 0,42; IC del 95%: 0,18 a 0,95; tres estudios, 320 participantes; evidencia de calidad muy baja), la infusión de calcio (OR 1,83; IC del 95%: 0,88 a 3,81; I² = 81%; dos estudios, 400 participantes; evidencia de calidad muy baja) o la diosmina (OR 2,85; IC del 95%: 1,35 a 6,00; un estudio, 200 participantes; evidencia de calidad muy baja). No hay certeza del efecto de los agonistas dopaminérgicos sobre las tasas de nacidos vivos (OR 1,08; IC del 95%: 0,73 a 1,59; dos estudios, 430 participantes; evidencia de calidad baja). No hay certeza del efecto de los agonistas dopaminérgicos sobre el embarazo clínico, el embarazo múltiple o el aborto espontáneo (evidencia de calidad baja a moderada). No se informaron eventos adversos.
Conclusiones de los autores
Los agonistas dopaminérgicos probablemente reducen la incidencia del SHEO moderado o grave en comparación con el placebo o ninguna intervención, aunque no se sabe con certeza el efecto sobre los eventos adversos ni los desenlaces del embarazo (nacidos vivos, embarazo clínico, aborto espontáneo). Los agonistas dopaminérgicos más una cointervención podrían disminuir las tasas de SHEO moderado o grave en comparación con la cointervención solamente, pero no hay certeza de que los agonistas dopaminérgicos afecten los desenlaces del embarazo. En comparación con otras intervenciones activas, no hay certeza de los efectos de los agonistas dopaminérgicos sobre el SHEO moderado o grave ni los desenlaces del embarazo.
PICO
Resumen en términos sencillos
¿Los agonistas de la dopamina pueden prevenir el síndrome de hiperestimulación ovárica en mujeres sometidas a tratamiento de fertilidad con FIV o ICSI?
¿Por qué se ha elaborado esta revisión Cochrane?
Se quería determinar si los agonistas de la dopamina son efectivos y seguros para prevenir el síndrome de hiperestimulación ovárica (SHEO) en mujeres con alto riesgo de presentarlo (p.ej., mujeres con ovarios poliquísticos o un número elevado de óvulos después de la estimulación ovárica) sometidas a fecundación in vitro (FIV) o a inyección intracitoplasmática de espermatozoides (ICSI). ¿Qué eficacia tienen estos medicamentos en comparación con otros tipos de medicamentos o con la interrupción de la estimulación ovárica durante unos días (aplazamiento o coasting)?
Antecedentes
La FIV (los óvulos y los espermatozoides se mezclan en un laboratorio y el embrión resultante se introduce en el útero) o la ICSI (un procedimiento de FIV en el que se inyecta un solo espermatozoide directamente en un óvulo en un laboratorio y el embrión resultante se introduce en el útero) son tratamientos para la infertilidad. Para ello se estimulan los ovarios (órganos reproductores femeninos) para que produzcan más óvulos mediante la administración de un medicamento hormonal a las mujeres. El SHEO es una complicación de la estimulación de los ovarios en el tratamiento de FIV o ICSI, en la que se desarrollan demasiados óvulos, los ovarios se inflaman y el líquido se filtra a otras partes del cuerpo, lo que provoca inflamación del estómago, coágulos de sangre y una reducción de la sangre y el oxígeno en órganos importantes. En la mayoría de los casos, la afección es leve y se resuelve sin tratamiento, pero algunas mujeres desarrollan una forma moderada o grave de SHEO que requiere hospitalización. No hay cura para el SHEO, salvo esperar a que ceda y controlar los síntomas hasta que desaparezcan.
Los agonistas de la dopamina son un medicamento que podría evitar la pérdida de líquido de los vasos sanguíneos a otras partes del cuerpo, lo que constituye un problema importante en el SHEO.
Se han indicado varios tratamientos para prevenir el SHEO. Por ejemplo, el aplazamiento o los medicamentos que mantienen el líquido en los vasos sanguíneos (agonistas de la dopamina, albúmina humana, hidroxietilalmidón, calcio o diosmina) o que favorecen la función de los órganos (prednisolona).
Qué se encontró
Se encontraron 22 ensayos controlados aleatorizados (un tipo de estudio que ofrece la evidencia más fiable sobre los efectos de un tratamiento) que incluían a 3171 mujeres con alto riesgo de SHEO y que evaluaron tres agonistas de la dopamina (cabergolina, bromocriptina y quinagolida). Seis estudios son nuevos en esta actualización. Los desenlaces principales fueron el número de nuevos casos (incidencia) de SHEO moderado a grave y la tasa de nacidos vivos. La evidencia está actualizada hasta el 4 de mayo de 2020.
Resultados clave
Agonistas de la dopamina frente a placebo/ningún tratamiento
Los agonistas de la dopamina parecen reducir la incidencia del SHEO moderado o grave en las mujeres con alto riesgo de presentarlo, en comparación con el placebo (un tratamiento simulado) o ningún tratamiento. Esto indica que de cada 100 mujeres que se someten a FIV o ICSI, 27 mujeres que reciben placebo o ningún tratamiento tendrán SHEO moderado o grave, en comparación con entre ocho y 14 mujeres que reciben agonistas de la dopamina. Los agonistas de la dopamina podrían mejorar los desenlaces del embarazo, pero todavía no se sabe si pueden aumentar los efectos secundarios leves, como las molestias estomacales, la sensación de malestar o los mareos. No está claro el efecto de los agonistas de la dopamina sobre los desenlaces del embarazo, ya que se han informado datos escasos.
Agonista de la dopamina más otro tratamiento frente a otro tratamiento
El uso de agonistas de la dopamina combinados con otro tratamiento activo podría reducir el riesgo de presentar SHEO moderado o grave en comparación con utilizar otro tratamiento activo solo. Esto significa que de 100 mujeres que reciben otro tratamiento activo solo para el SHEO, 11 mujeres tendrán SHEO moderado o grave en comparación con entre tres y nueve mujeres que utilicen agonistas de la dopamina más otro tratamiento activo. Todavía no se sabe si la dopamina combinada con otro tratamiento mejora los desenlaces del embarazo ni los efectos secundarios.
Agonista de la dopamina frente a otro tratamiento
No se sabe si el agonista de la dopamina cabergolina disminuye las tasas de SHEO en comparación con otros tratamientos activos (p.ej., hidroxietilalmidón, prednisolona, infusión de calcio o aplazamiento). No se sabe si la cabergolina mejora los desenlaces del embarazo en comparación con otras intervenciones. No hubo efectos secundarios en el único estudio de esta comparación.
Calidad de la evidencia
La calidad de la evidencia varió de muy baja a moderada. Las limitaciones incluyeron el informe deficiente de los métodos de estudio y la imprecisión (muy pocos episodios, muy pocos estudios incluidos) en algunas comparaciones.
Authors' conclusions
Summary of findings
| Dopamine agonist vs placebo/no intervention | ||||||
| Patient or population: women of reproductive age undergoing any ART therapy Settings: ART unit Intervention: dopamine agonist Comparison: placebo/no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with placebo/no intervention | Risk with dopamine agonist | |||||
| Incidence of moderate or severe OHSS | 268 per 1000 | 105 per 1000 | OR 0.32 | 1202 | ⊕⊕⊕⊝ | — |
| Live birth rate | 324 per 1000 | 315 per 1000 | OR 0.96 | 362 | ⊕⊕⊝⊝ | — |
| Clinical pregnancy rate | 307 per 1000 | 289 per 1000 | OR 0.92 | 530 | ⊕⊕⊝⊝ | — |
| Multiple pregnancy rate | 50 per 1000 | 17 per 1000 (1 to 303) | OR 0.32 (0.01 to 8.26) | 40 | ⊕⊝⊝⊝ | — |
| Miscarriage rate | 72 per 1000 | 49 per 1000 (15 to 151) | OR 0.66 (0.19 to 2.28) | 168 (2 RCTs) | ⊕⊕⊝⊝ | — |
| Any other adverse events | 43 per 1000 | 168 per 1000 (62 to 381) | OR 4.54 (1.49 to 13.84) | 264 | ⊕⊝⊝⊝ | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ART: assisted reproductive technology; CI: confidence interval; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for risk of bias associated with poor reporting of study methods. | ||||||
| Dopamine agonist plus co‐intervention vs co‐intervention | ||||||
| Patient or population: women of reproductive age undergoing any ART therapy Settings: ART unit Intervention: dopamine agonist plus co‐intervention Comparison: co‐intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with co‐intervention only | Risk with dopamine agonist plus co‐intervention | |||||
| Incidence of moderate or severe OHSS | 109 per 1000 | 55 per 1000 (33 to 93) | OR 0.48 (0.28 to 0.84) | 748 (4 RCTs) | ⊕⊕⊝⊝ | — |
| Live birth rate | 380 per 1000 | 426 per 1000 (332 to 525) | OR 1.21 (0.81 to 1.80) | 400 (2 studies) | ⊕⊕⊝⊝ | — |
| Clinical pregnancy rate | 443 per 1000 | 469 per 1000 (398 to 542) | OR 1.11 (0.83 to 1.49) | 748 (4 studies) | ⊕⊕⊝⊝ | — |
| Multiple pregnancy rate | 12 per 1000 | 24 per 1000 (2 to 217) | OR 2.02 (0.18 to 22.77) | 166 (1 study) | ⊕⊝⊝⊝ | — |
| Miscarriage rate | 61 per 1000 | 41 per 1000 (19 to 85) | OR 0.65 (0.30 to 1.42) | 548 (3 studies) | ⊕⊕⊝⊝ | — |
| Any other adverse events | 0 per 1000 | 0 per 1000 (0 to 0) | OR 3.03 (0.12 to 75.28) | 366 (2 studies) | ⊕⊝⊝⊝ | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ART: assisted reproductive technology; CI: confidence interval; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for serious risk of bias associated with poor reporting of study methods. | ||||||
| Dopamine agonist vs other active intervention | |||||||
| Patient or population: women of reproductive age undergoing any ART therapy Settings: ART unit Intervention: dopamine agonist Comparison: other active intervention | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | ||
|---|---|---|---|---|---|---|---|
| Risk with other active intervention | Risk with dopamine agonist | ||||||
| Incidence of moderate or severe OHSS | Cabergoline vs human albumin | 432 per 1000 | 138 per 1000 (84 to 225) | OR 0.21 (0.12 to 0.38) | 296 | ⊕⊝⊝⊝ | — |
| Cabergoline vs prednisolone | 93 per 1000 | 27 per 1000 (5 to 120) | OR 0.27 (0.05 to 1.33) | 150 | ⊕⊝⊝⊝ | — | |
| Cabergoline vs hydroxyethyl starch | 67 per 1000 | 161 per 1000 (33 to 519) | OR 2.69 (0.48 to 15.10) | 61 | ⊕⊝⊝⊝ | — | |
| Cabergoline vs coasting | 125 per 1000 | 57 per 1000 (25 to 119) | OR 0.42 (0.18 to 0.95) | 320 | ⊕⊝⊝⊝ | — | |
| Cabergoline vs calcium infusion | 60 per 1000 | 105 per 1000 (53 to 196) | OR 1.83 (0.88 to 3.81) | 400 | ⊕⊝⊝⊝ | — | |
| Cabergoline vs diosmin | 120 per 1000 | 280 per 1000 (155 to 450) | OR 2.85 (1.35 to 6.00) | 200 | ⊕⊝⊝⊝ | — | |
| Live birth rate | Cabergoline vs coasting or calcium infusion | 395 per 1000 | 414 per 1000 (323 to 510) | OR 1.08 (0.73 to 1.59) | 430 | ⊕⊕⊝⊝ | — |
| Clinical pregnancy rate | Cabergoline vs human albumin, coasting, calcium infusion, or diosmin | 432 per 1000 | 442 per 1000 (381 to 503) | OR 1.04 (0.81 to 1.33) | 1060 | ⊕⊕⊕⊝ | — |
| Multiple pregnancy rate | Cabergoline vs human albumin, coasting, or diosmin | 130 per 1000 | 115 per 1000 (66 to 192) | OR 0.87 (0.47 to 1.59) | 400 | ⊕⊕⊝⊝ | — |
| Miscarriage rate | Cabergoline vs human albumin, coasting, calcium infusion, or diosmin | 79 per 1000 | 54 per 1000 (29 to 97) | OR 0.66 (0.35 to 1.25) | 630 | ⊕⊕⊝⊝ | — |
| Any other adverse events | Cabergoline vs calcium infusion | 0 per 1000 | 0 per 1000 (0 to 0) | Not estimable | 170 (1 RCT) | Not estimable | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ART: assisted reproductive technology; CI: confidence interval; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio; RCT: randomised controlled trial. | |||||||
| GRADE Working Group grades of evidence | |||||||
| aDowngraded one level for risk of bias associated with poor reporting of study methods. | |||||||
Background
Description of the condition
Ovarian hyperstimulation syndrome (OHSS) is a complication of assisted reproduction technology (ART) treatment. It can occur following exposure of the ovaries of susceptible women to human chorionic gonadotrophin (hCG) or luteinising hormone (LH) during controlled ovarian stimulation with follicle‐stimulating hormone (FSH). Women at risk of OHSS are generally young and have polycystic ovary syndrome (PCOS) (Costello 2012). OHSS is characterised by enlarged ovaries and an acute fluid shift from the intravascular space to the third space (mainly to the abdominal or thoracic cavity), which may result in an accumulation of fluid in the peritoneal cavity and pleura, an elevation of haematocrit, and a decrease in organ perfusion (Aboulghar 2003; Soares 2008; Vloeberghs 2009). Its symptoms range from abdominal bloating and a feeling of fullness to shortness of breath (Vloeberghs 2009). OHSS was classified as mild, moderate or severe by Golan and colleagues (Golan 1989), modified from Rabau and colleagues (Rabau 1967) by incorporating ultrasonographic measurement of the stimulated ovaries. Despite measures adopted by physicians to prevent these sequelae, mild OHSS may affect up to 33% of in vitro fertilisation (IVF) cycles. Moderate or severe OHSS arises in 3% to 8% of IVF cycles (RCOG 2006). Young women with low body mass index and polycystic ovaries are at particular risk of OHSS and the only way to avoid the condition for women with fallopian tube compromise, or whose partner has impaired semen parameters, is to undergo in vitro oocyte maturation, which is an approach that is not available in most centres (Walls 2015).
The pathophysiology of OHSS is not yet completely elucidated. Increased vascular permeability causing the loss of fluid into the third space (abdominal and pleural cavity) is the central feature of clinically significant OHSS, which triggers events that result in the associated symptoms (such as abdominal pain and distension) (Ata 2009). Most cases of OHSS have been associated with the use of hCG to trigger oocyte maturation prior to oocyte retrieval, however, it is recognised that hCG has no direct effect on the vascular system (Gómez 2002). Vasoactive substances are released by the ovaries in response to hCG administration. It is almost certain that vascular endothelial growth factor (VEGF) is a key substance that induces vascular hyperpermeability, leading to a shift of fluids from the intravascular system to the third space (Busso 2009; Soares 2008). Higher production of VEGF from the many follicles during stimulation by ovarian steroids and hCG appears to be the specific key process leading to the development of OHSS in women at high risk of OHSS.
Description of the intervention
Severe OHSS is a potentially life‐threatening condition that occurs in women undergoing ART cycles. Several measures have been introduced to prevent OHSS (Prakash 2009). These include cycle cancellation or 'coasting' (D'Angelo 2017; Delvigne 2002), use of intravenous fluids (Youssef 2010; Youssef 2016), cryopreservation of embryos rather than immediate fresh embryo transfer (D'Angelo 2007), and the use of progesterone as luteal phase support rather than hCG (van der Linden 2015). More recent treatments include 'minimal stimulation IVF' (using a combination of medications to gently stimulate the ovaries), in vitro maturation of oocytes (letting oocytes mature in vitro) (Walls 2012), the use of 'natural cycle' IVF (collecting and fertilising one egg released during the normal monthly cycle and without the use of fertility drugs) (Edwards 2007), the use of metformin in women with PCOS (Tso 2014), the use of gonadotropin‐releasing hormone (GnRH) antagonist, as opposed to GnRH agonist for ovarian downregulation (a prerequisite to assist in the timing of oocyte retrieval), adjusting stimulation protocols (Al‐Inany 2011), and the use of an agonist trigger prior to oocyte retrieval in an antagonist cycle (Casper 2015). Despite their availability, there is no consensus on what would be the most favourable strategy to prevent OHSS, and none of these strategies have led to the eradication of OHSS (Aboulghar 2009). Research suggests that the use of dopamine agonists may be a promising strategy for the prevention and treatment of OHSS (Busso 2009; Castelo‐Branco 2009).
How the intervention might work
With a better understanding of the pathophysiology of OHSS and recognition of the important role of VEGF in the development of OHSS, a series of blockers, such as SU5416 (a potent and selective inhibitor of the vascular endothelial growth factor receptor (VEGFR)), were introduced to reverse the hCG action on vascular permeability by targeting VEGFR‐2 expressed on human ovaries (Gómez 2002). However, these anti‐angiogenic drugs could not be used clinically to prevent or treat OHSS due to their adverse effect profile (such as thromboembolism) (Glade‐Bender 2003; Kuenen 2003), and the possibility of affecting embryo implantation (Pauli 2005; Rockwell 2002). Another approach is to consider the use of a dopamine agonist, which shows similar effects to anti‐angiogenic drugs on vascular permeability and appears not to exert adverse effects (Castelo‐Branco 2009; Soares 2012). Moreover, dopamine agonists have been used for many years in other fields of medicine, for example to treat elevated serum prolactin levels. However, since the dopamine agonist cabergoline has been associated with fibrotic valvular heart disease when used chronically, other types of dopamine agonists are now being examined for use in OHSS. Possible advantages are the different pharmacokinetic profiles (e.g. shorter half‐life of the drugs (about 17 hours for quinagolide versus about 65 hours for cabergoline)) thereby reducing exposure of embryos to possible teratogenic effects (Busso 2010), and in case of bromocriptine, lower costs and longer experience in use during pregnancy (Beltrame 2013).
Research findings in animal models of OHSS, as well as in humans, have shown that cabergoline can prevent the increase in vascular permeability (Gómez 2006). Several clinical trials have also evaluated the clinical value of cabergoline and showed that prophylactic use of cabergoline was associated with a decrease in the severity of OHSS (Manno 2005). Therefore, dopamine agonists may provide a new, specific, and non‐toxic approach to the prevention and treatment of OHSS (Alvarez 2007a; Knoepfelmacher 2006).
Why it is important to do this review
Though short‐term use of dopamine agonists for preventing OHSS represents no significant risk for women, long‐term data on its effectiveness and safety requires corroboration. An increased incidence of cardiac valve regurgitation is suggested when women took cabergoline or pergolide for treating Parkinson's disease or hyperprolactinaemia (Budayr 2020; Kars 2008; Martin 2009; Schade 2007; Trifiro 2012; Zanettini 2007). Clinical studies have increasingly suggested that cabergoline can be safely administered in ART for preventing OHSS without influencing pregnancy outcomes. Moreover, the role of other dopamine agonists (e.g. quinagolide and bromocriptine) for preventing OHSS remain uncertain due to lack of robust evidence for their efficacy and safety. This review aimed to summarise the available evidence from randomised controlled trials (RCTs) to determine whether dopamine agonists can reduce the incidence of moderate or severe OHSS in women at high risk of OHSS undergoing ART and identify any safety concerns.
Objectives
To assess the effectiveness and safety of dopamine agonists in preventing OHSS in women at high risk of developing OHSS when undergoing ART treatment.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished RCTs investigating the effectiveness and safety of dopamine agonists compared with placebo/no intervention or another intervention. We handled conference abstracts in the same way as full publications. We excluded quasi‐randomised trials and, in the case of cross‐over trials, included only pre‐crossover data.
Types of participants
Women of reproductive age at high risk of OHSS (as defined by the studies) and undergoing any ART therapy.
Types of interventions
Trials were eligible for inclusion when they evaluated any dose of dopamine agonist alone or as an add‐on therapy versus placebo, no intervention, or other active treatments.
Types of outcome measures
Both primary and secondary outcome measures were defined for this review.
Primary outcomes
-
Incidence of moderate or severe OHSS (as determined by study authors) per woman randomised.
-
Live birth rate (as a result of an embryo transferred in a fresh cycle using fertilised oocytes from the same menstrual cycle) defined as a live infant born after 20 weeks' gestation per woman randomised.
Secondary outcomes
-
Clinical pregnancy rate (as a result of an embryo transferred in a fresh cycle using fertilised oocytes from the same menstrual cycle) per woman randomised.
-
Multiple pregnancy rate (as a result of an embryo transferred in a fresh cycle using fertilised oocytes from the same menstrual cycle) per woman randomised.
-
Miscarriage rate (following an embryo transferred in a fresh cycle using fertilised oocytes from the same menstrual cycle) per woman randomised.
-
Any other adverse events of the treatment per woman randomised.
Search methods for identification of studies
See: Cochrane Gynaecology and Fertility (CGF) (formerly Menstrual Disorders and Subfertility Group, MDSG) guidance for writing all sections of systematic reviews (CGF).
We searched for published and unpublished articles in any language, that described or might have described RCTs of dopamine agonists (and more specifically cabergoline, quinagolide, or bromocriptine) for preventing OHSS, in consultation with the Cochrane Gynaecology and Fertility Information Specialist.
Electronic searches
We searched:
-
the Cochrane Gynaecology and Fertility Group's Specialised Register using key terms on a Procite platform (searched 4 May 2020; Appendix 1). This register also contains unpublished trial abstracts;
-
the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO), Web platform (searched 4 May 2020; Appendix 2; CENTRAL included the ongoing trials from clinicaltrials.gov and the World Health Organization International Clinical Trials Registry Platform);
-
MEDLINE, Ovid (searched from 1946 to 4 May 2020; Appendix 3);
-
Embase, Ovid (searched from 1980 to 4 May 2020; Appendix 4);
-
PsycINFO, Ovid (searched from 1806 to 4 May 2020; Appendix 5);
-
CINAHL, EBSCO (searched from 1961 to 4 May 2020; Appendix 6).
We also searched the Epistemonikos database, which contains systematic reviews that can be useful for reference checking for trials (www.epistemonikos.org/en).
We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials, which appears in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 4; Higgins 2019).
We combined the Embase searches with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN; (https://www.sign.ac.uk/what‐we‐do/methodology/search‐filters/).
Searching other resources
We searched the citation lists of relevant publications and included studies, review articles, and abstracts of conferences, and asked manufacturers, experts, and specialists in the field for any trials that they were aware of.
We conducted handsearching in the appropriate journals of gynaecology and reproductive medicine; the conference proceedings (for abstracts) of the European Society for Human Reproduction and Embryology (ESHRE) and the American Society for Reproductive Medicine (ASRM), as well as related textbooks.
We searched for conference abstracts on the Web of Science (wokinfo.com/).
Data collection and analysis
Selection of studies
Two review authors (SM and HT) independently reviewed the titles and abstracts of the trials, in accordance with the search protocol. We reviewed full‐text articles and considered them for inclusion. If the published study was judged to contain insufficient information, we contacted trial authors. Two review authors (SM and HT) independently critically appraised the trials against the inclusion criteria. We resolved any disagreements by consensus or referral to a third review author (RH).
Data extraction and management
Two review authors (SM and HT) independently extracted data using a piloted data extraction form (Appendix 7). We compared the two sets of extracted data and resolved discrepancies by discussion. The data extraction forms included methodological quality. We included this information in the review and presented it in the Characteristics of included studies and Characteristics of excluded studies tables following the guidance of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
Assessment of risk of bias in included studies
Two review authors (SM and HT) independently critically assessed risk of bias in all included studies, including the following domains: sequence generation; allocation concealment; blinding of participants, personnel, and outcome assessors; incomplete outcome data; selective outcome reporting, and other bias (described in Cochrane's tool for assessing risk of bias) (Higgins 2011). We judged each domain at low risk of bias, high risk of bias, or unclear risk of bias for either a lack of information or uncertainty regarding the potential for bias, with any disagreements resolved by consensus or by a discussion with a third review author (RH).
Measures of treatment effect
We anticipated that all data would be dichotomous. We used the numbers of events in the control and intervention groups of each study to calculate odds ratios (OR) with 95% confidence intervals (CI).
Unit of analysis issues
The primary analysis unit was per woman randomised.
Dealing with missing data
Our meta‐analysis used an intention‐to‐treat (ITT) approach, meaning that we included all women randomised in the analysis, in the groups to which they were randomised. In case of missing data, we contacted the trial authors by e‐mail. We assumed that events did not occur in the women for whom data were unobtainable.
Assessment of heterogeneity
We carried out a test for statistical heterogeneity for each meta‐analysis and assessed heterogeneity using the I²statistic. This quantifies inconsistency, describing the impact of heterogeneity on the meta‐analysis and measuring the degree of inconsistency across studies. We considered an I²statistic less than 25% as low‐level heterogeneity, 25% to 50% as moderate‐level heterogeneity, and higher than 50% as high‐level heterogeneity (Higgins 2019).
Assessment of reporting biases
We planned to use a funnel plot to assess the potential for reporting bias where 10 or more trials per comparison reported data.
Data synthesis
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We pooled data where appropriate, using the Mantel‐Haenszel method. We used a fixed‐effect model as we did not anticipate finding large amounts of heterogeneity. We combined data to calculate pooled ORs and 95% CIs in the following.
-
Dopamine agonist versus placebo/no intervention, subgrouped by type of dopamine agonist (cabergoline versus quinagolide versus bromocriptine) and severity of OHSS.
-
Dopamine agonist plus co‐intervention versus co‐intervention, subgrouped by type of dopamine agonist (if available) and type of co‐intervention.
-
Dopamine agonist versus other active interventions, subgrouped by type of dopamine agonist (if available) and type of other active intervention.
We performed statistical analyses using Review Manager 5 (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
We considered the following subgroup analyses to assess any differences in effect within these subgroups:
-
type of dopamine agonist;
-
type of co‐intervention;
-
type of other active interventions;
-
severity of OHSS (severe OHSS versus moderate OHSS).
Sensitivity analysis
We performed sensitivity analyses for the primary outcome of moderate or severe OHSS to test whether the review conclusion would be different. We conducted a sensitivity analysis by changing the underlying model to random effects to determine any difference resulting from the choice of the fixed‐effect model. We also considered excluding studies with high risk of bias for any domain.
Summary of findings and assessment of the certainty of the evidence
We generated a 'Summary of findings' table using GRADEpro and Cochrane methods (GRADEpro GDT 2015; Higgins 2011). This table evaluated the overall quality of the body of evidence for the main review comparison (dopamine agonists versus placebo or no intervention) for the main review outcomes (i.e. incidence of moderate or severe OHSS, live birth rate, multiple pregnancy rate, clinical pregnancy rate, miscarriage rate, and any other adverse effect). We also developed 'Summary of findings' tables for the comparisons of dopamine agonist plus co‐intervention versus co‐intervention and dopamine agonist versus other active intervention. According to GRADE criteria, we assessed the following factors that might decrease the quality level of a body of evidence: study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness, and publication bias. We incorporated judgements about evidence quality (high, moderate, low, and very low) into reporting of results for each outcome. Two review authors (HT and SM) independently conducted evidence grading, and resolved disagreements by consensus.
Results
Description of studies
We included all RCTs in ART reporting on dopamine agonists for the prevention of OHSS.
Results of the search
This updated search was performed up to May 2020. In this 2021 updated review, we included six additional trials (Bassiouny 2018; Elnory 2018; El‐Shaer 2019; Kilic 2015; Saad 2017; Singh 2017). This resulted in 22 included trials (Alhalabi 2011; Alvarez 2007a; Amir 2015; Bassiouny 2018; Beltrame 2013; Busso 2010; Carizza 2008; Dalal 2014; Elnory 2018; El‐Shaer 2019; Fetisova 2014; Ghahiri 2015; Jellad 2017; Kilic 2015; Matorras 2013; Saad 2017; Salah 2012; Shaltout 2012; Singh 2017; Sohrabvand 2009; Tehraninejad 2012; Torabizadeh 2013). See Figure 1 for the PRISMA flow chart.
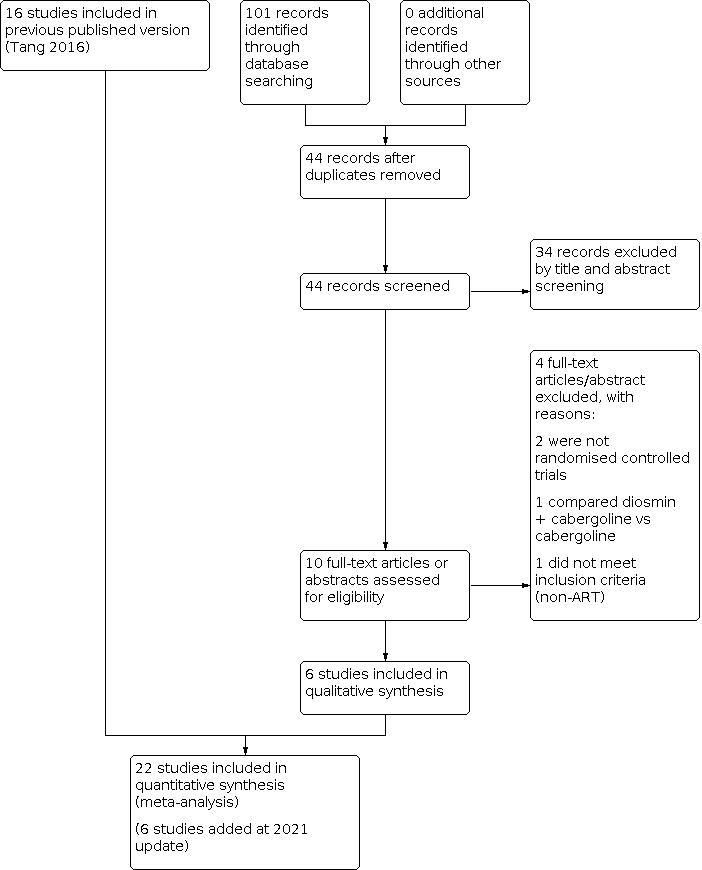
Study flow diagram search May 2020. ART: assisted reproduction technology.
We excluded 23 studies, of which three were excluded in the 2020 update (Saad 2019; Seyam 2018; Zahran 2018).
There are currently five ongoing studies, which will be checked in the future update (El Khattan 2015; Hendricks 2015; IRCT2016071428930N1; Kamel 2015; Khaled 2014). One meeting abstract is awaiting classification (Ahmadi 2010).
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; and Characteristics of ongoing studies tables.
Included studies
We included 22 studies (Alhalabi 2011; Alvarez 2007a; Amir 2015; Bassiouny 2018; Beltrame 2013; Busso 2010; Carizza 2008; Dalal 2014; Elnory 2018; El‐Shaer 2019; Fetisova 2014; Ghahiri 2015; Jellad 2017; Kilic 2015; Matorras 2013; Saad 2017; Salah 2012; Shaltout 2012; Singh 2017; Sohrabvand 2009; Tehraninejad 2012; Torabizadeh 2013) (see Characteristics of included studies table). We contacted some trial authors for more detailed information (Dalal 2014; Fetisova 2014; Ghahiri 2015; Jellad 2017; Salah 2012; Shaltout 2012; Sohrabvand 2009; Tehraninejad 2012).
Participants
Twenty‐two studies enrolled 3171 women at high risk of OHSS undergoing IVF or ICSI. One of these studies included only oocyte donors (Alvarez 2007a).
The studies were performed in 11 different countries: five studies in Egypt (Bassiouny 2018; Elnory 2018; El‐Shaer 2019; Saad 2017; Shaltout 2012); four studies in Iran (Ghahiri 2015; Sohrabvand 2009; Tehraninejad 2012; Torabizadeh 2013); three in Spain (Alvarez 2007a; Busso 2010; Matorras 2013); two in Brazil (Beltrame 2013; Carizza 2008); two in India (Dalal 2014; Singh 2017); and one each from Syria (Alhalabi 2011), Israel (Amir 2015), United Arab Emirates (Salah 2012), Russia (Fetisova 2014), Tunisia (Jellad 2017), and Turkey (Kilic 2015).
One study included women with PCOS only (Salah 2012), without additional risk factors for OHSS (such as a minimum oestradiol (E2 or number of follicles/oocytes retrieved), whereas other studies either excluded women with PCOS (Beltrame 2013), or included women with and without PCOS (Alhalabi 2011; Alvarez 2007a; Amir 2015; Bassiouny 2018; Busso 2010; Carizza 2008; Elnory 2018; Fetisova 2014; Ghahiri 2015; Jellad 2017; Kilic 2015; Matorras 2013; Saad 2017; Shaltout 2012; Singh 2017; Sohrabvand 2009; Tehraninejad 2012; Torabizadeh 2013).
The definition of 'high risk of OHSS' varied widely between studies; some used a minimum number of follicles of a certain diameter (18 or more over 12 mm at day of hCG (El‐Shaer 2019; Jellad 2017); 20 or more over 12 mm at day of hCG (Alhalabi 2011; Amir 2015; Kilic 2015; Matorras 2013; Shaltout 2012), with or without a minimum E2 level at day of hCG (greater than 2500 pg/mL (Dalal 2014 (mentioned only number of 20 or more follicles without mentioning size of follicles); Torabizadeh 2013); greater than 3000 pg/mL (Elnory 2018; Ghahiri 2015; Jellad 2017; Kilic 2015; Matorras 2013; Saad 2017; Sohrabvand 2009); greater than 3500 pg/mL (Bassiouny 2018; Shaltout 2012); greater than 4000 pg/mL (Alhalabi 2011; Amir 2015; Carizza 2008)). Five studies also incorporated the retrieval of 20 or more oocytes as a criterion (Alvarez 2007a; Ghahiri 2015; Sohrabvand 2009; Tehraninejad 2012; Torabizadeh 2013), whereas one study used transvaginal aspiration of 15 or more follicles (Fetisova 2014). Three studies also considered women with previous history of OHSS as high risk (Elnory 2018; Ghahiri 2015; Saad 2017). Two studies also included women with polycystic ovaries (i.e. more than 24 antral follicles at baseline ultrasound examination) (Elnory 2018; Saad 2017). One study included women with 13 or more follicles greater than 11 mm (Singh 2017). One study included only oocyte donors who consequently did not proceed to have an embryo transferred (Alvarez 2007a). Most studies selected women aged between 18 and 40 years.
Some studies excluded women with very high E2 levels (greater than 5000 pg/mL (Kilic 2015; Matorras 2013; Shaltout 2012); greater than 6000 pg/mL (Busso 2010; Singh 2017)), because of their very high risk of developing OHSS, and assigned those women to cycle cancellation. One study excluded coasting cases, without stating when a woman was eligible for coasting (Jellad 2017). One study cancelled cycles after randomisation and cryopreserved all embryos when early OHSS was detected on embryo transfer day (Bassiouny 2018).
Interventions
Comparisons with cabergoline
Seven studies involving 701 women compared cabergoline with placebo or no intervention (Alvarez 2007a; Amir 2015; Fetisova 2014; Jellad 2017; Kilic 2015; Salah 2012; Singh 2017). Amir 2015 also used coasting in almost half of the women in both the intervention and control group. We tried to contact the authors to retrieve more information about which women received coasting and whether these women developed OHSS, but received no reply. Other studies excluded women who were received coasting.
Four studies gave oral cabergoline 0.5 mg daily for eight days from the day of hCG injection (Alvarez 2007a; Amir 2015; Jellad 2017; Kilic 2015), one study gave oral cabergoline 0.5 mg daily from the day after oocyte retrieval for five days before embryo transfer day (Fetisova 2014), and one study gave oral cabergoline 0.5 mg on two successive days, starting from the day of hCG injection and repeated one week later (Salah 2012). Salah 2012 also had a third treatment arm of oral prednisolone 10 mg daily from the day of hCG injection to the day of the pregnancy test (Salah 2012).
Two studies involving 382 women compared cabergoline plus hydroxyethyl starch (HES) versus HES alone (500 mL of HES by intravenous infusion during follicle aspiration plus oral cabergoline 0.5 mg daily for eight days starting on the day of hCG administration for Matorras 2013; 500 mL of HES by intravenous infusion on day of follicle aspiration and oral cabergoline 0.25 mg daily for eight days starting on the day of hCG administration for Shaltout 2012).
Two studies involving 235 women compared oral cabergoline 0.5 mg daily with human albumin (albumin 20 g 20% on day of oocyte retrieval and cabergoline for seven days beginning on the day of oocyte retrieval in Tehraninejad 2012; albumin 10 units 20% on day of oocyte retrieval and cabergoline for eight days beginning on the day of hCG injection in Torabizadeh 2013).
One study with 91 women involved three arms (oral cabergoline 0.5 mg daily for seven days after oocyte retrieval versus albumin (100 mL intravenous 30 minutes after retrieval within four hours) versus 6% HES 1000 mL intravenous 30 minutes after oocyte retrieval within four hours) (Ghahiri 2015).
One study involving 166 women compared cabergoline 0.5 mg daily for three weeks beginning the day after oocyte retrieval plus albumin 20 g on day of oocyte retrieval versus albumin 20 g alone (Carizza 2008).
Two studies involving 120 women compared cabergoline 0.5 mg daily for seven or eight days after hCG administration versus coasting with gonadotropin administration withheld until serum E2 level was below 3000 pg/mL or serum E2 level started to decline before hCG administration) (Dalal 2014; Sohrabvand 2009). However, Dalal 2014 also gave 6% HES to 58 women and the remaining included woman received an ascites tap instead of HES.
One study involving 300 women compared cabergoline plus coasting (stopping receiving human menopausal gonadotrophin (hMG) for one day while continuing agonist injections and cabergoline 0.25 mg/day for eight days from hCG administration) versus cabergoline (0.25 mg/day for eight days from hCG administration) versus coasting (stopping receiving hMG for one day while continuing agonist injections) (Bassiouny 2018).
Two studies involving 400 women compared oral cabergoline 0.5 mg daily for seven days starting at day of ovum pick‐up with calcium infusion (10 mL of calcium gluconate 10%, in 200 mL 0.9% saline solution given intravenously on the day of ovum pick‐up and days one, two, and three after day of ovum pick‐up over 30 minutes) (Elnory 2018; El‐Shaer 2019).
One study involving 200 women compared oral cabergoline 0.5 mg daily for eight days starting at day of hCG injection with oral diosmin 1000 mg/eight hours for two weeks starting at day of hCG injection (Saad 2017).
Comparisons with quinagolide
Two studies involving 454 women compared quinagolide versus placebo (quinagolide 150 µg daily for 15 days beginning on the day of hCG administration for Alhalabi 2011; three subgroups with doses of quinagolide 50 μg daily, 100 μg daily, and 200 µg daily from the day of hCG administration until the day of serum hCG test (which was 17 days, standard deviation 2 days, after oocyte retrieval) for Busso 2010).
Comparisons with bromocriptine
One trial involving 47 women compared bromocriptine 2.5 mg daily versus folic acid 2.0 mg daily (as a placebo), both for 14 days, beginning the day of hCG administration (Beltrame 2013).
Outcomes
All 22 included studies reported the incidence of severe or moderate OHSS but only six studies reported live birth rate (Bassiouny 2018; Busso 2010; Elnory 2018; Kilic 2015; Shaltout 2012; Singh 2017). Fifteen studies reported clinical pregnancy rate (Alvarez 2007a; Amir 2015; Bassiouny 2018; Busso 2010; Carizza 2008; Dalal 2014; Elnory 2018; Fetisova 2014; Kilic 2015; Matorras 2013; Saad 2017; Shaltout 2012; Singh 2017; Sohrabvand 2009; Tehraninejad 2012). Torabizadeh 2013 only reported pregnancy rates of the women who developed moderate or severe OHSS (no significant difference between groups) and Alhalabi 2011 and El‐Shaer 2019 only mentioned that pregnancy rates were 'equal' between groups, without providing data on this outcome. Nine studies reported miscarriage rate (Amir 2015; Busso 2010; Carizza 2008; Dalal 2014; Fetisova 2014; Matorras 2013; Saad 2017; Shaltout 2012; Tehraninejad 2012), five studies reported multiple pregnancy rate (Amir 2015; Carizza 2008; Dalal 2014; Saad 2017; Tehraninejad 2012), and five studies reported any other adverse events of the treatment (Alvarez 2007a; Busso 2010; Carizza 2008; El‐Shaer 2019; Shaltout 2012).
Excluded studies
We excluded 23 studies after examining full‐text reports and obtaining clarifications from original authors. The reasons for exclusion are explained in the Characteristics of excluded studies table.
Studies awaiting classification
We classified one meeting abstract as awaiting classification due to lack of information for assessment despite attempts to contact the authors (Ahmadi 2010).
Ongoing studies
From the trial registries, five ongoing or recently finished trials had potential to be included in this review but were not published yet as abstracts or full‐text papers (El Khattan 2015; Hendricks 2015; IRCT2016071428930N1; Kamel 2015; Khaled 2014).
Risk of bias in included studies
We assessed the risk of bias for each study using the Cochrane 'Risk of bias' tool (Higgins 2011). The 'Risk of bias' graph and 'Risk of bias' summary were presented in Figure 2 and Figure 3. We contacted the original authors by e‐mail to clarify any information on methodological quality and study characteristics that were unclear (see 'Risk of bias' table in the Characteristics of included studies table).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Generation of random sequence
Fifteen trials clearly reported the generation of random sequence and were judged at low risk: fourteen trials used computer‐generated randomisation (Alvarez 2007a; Amir 2015; Beltrame 2013; Busso 2010; Carizza 2008; Dalal 2014; Elnory 2018; Ghahiri 2015; Kilic 2015; Matorras 2013; Shaltout 2012; Singh 2017; Sohrabvand 2009; Tehraninejad 2012), and one trial reported using QuickCalcs to perform a block random to assign the participants into three groups (Bassiouny 2018). Six trials were assessed as unclear due to lack of information to judge the randomisation process (Alhalabi 2011; El‐Shaer 2019; Fetisova 2014; Jellad 2017; Saad 2017; Salah 2012). One trial mentioned that randomised sampling was also performed by selecting "every other person," before actual randomisation took place, which we judged as high risk of bias (Torabizadeh 2013).
Allocation concealment
Six trials clearly reported the method of allocation concealment and were assessed at low risk of bias: five trials reported they allocated with sealed or closed envelopes (Bassiouny 2018; Busso 2010; Fetisova 2014; Matorras 2013; Salah 2012), and one study mentioned that the treatment allocation schedule was stored by an infertility consultant (Elnory 2018). The other 16 trials were judged unclear due to a lack of detailed allocation information (Alhalabi 2011; Alvarez 2007a; Amir 2015; Beltrame 2013; Carizza 2008; Dalal 2014; El‐Shaer 2019; Ghahiri 2015; Jellad 2017; Kilic 2015; Saad 2017; Shaltout 2012; Singh 2017; Sohrabvand 2009; Tehraninejad 2012; Torabizadeh 2013).
Blinding
Performance bias
Performance bias was high in five studies due to lack of blinding of the participants or clinicians (Amir 2015; Bassiouny 2018; Dalal 2014; Elnory 2018; Tehraninejad 2012). Five studies were at low risk of performance bias (Alvarez 2007a; Beltrame 2013; Busso 2010; Matorras 2013; Salah 2012), and 12 studies were judged as unclear due to lack of information to perform judgement (Alhalabi 2011; Carizza 2008; El‐Shaer 2019; Fetisova 2014; Ghahiri 2015; Jellad 2017; Kilic 2015; Saad 2017; Shaltout 2012; Singh 2017; Sohrabvand 2009; Torabizadeh 2013).
Detection bias
Three studies were at low risk of bias because they reported that the outcome assessor was blinded (Alvarez 2007a; Amir 2015; Matorras 2013); and three studies were at high risk of bias, as blinding was not performed (Bassiouny 2018; Dalal 2014; Elnory 2018). The other 16 studies were judged at unclear risk of bias, as information was inadequately reported for this domain (Alhalabi 2011; Beltrame 2013; Busso 2010; Carizza 2008; El‐Shaer 2019; Fetisova 2014; Ghahiri 2015; Jellad 2017; Kilic 2015; Saad 2017; Salah 2012; Shaltout 2012; Singh 2017; Sohrabvand 2009; Tehraninejad 2012; Torabizadeh 2013).
Incomplete outcome data
Sixteen trials were at low risk of attrition bias (Alvarez 2007a; Amir 2015; Busso 2010; Carizza 2008; Dalal 2014; Elnory 2018; Fetisova 2014; Ghahiri 2015; Kilic 2015; Matorras 2013; Saad 2017; Salah 2012; Shaltout 2012; Sohrabvand 2009; Tehraninejad 2012; Torabizadeh 2013). Eleven studies reported the information on dropouts and described the exact reasons (Alvarez 2007a; Bassiouny 2018; Busso 2010; Dalal 2014; Elnory 2018; Ghahiri 2015; Kilic 2015; Saad 2017; Shaltout 2012; Singh 2017; Tehraninejad 2012). Two studies only stated that women withdrew from the study, without exact reasons (Carizza 2008; Salah 2012), but only a small proportion of women (less than 5%) were lost to follow‐up, which does not have a clinically relevant impact on observed effect size, and hence we rated the studies at low risk of bias (Amir 2015; Fetisova 2014; Matorras 2013; Sohrabvand 2009; Torabizadeh 2013). Four trials were at high risk of bias (Bassiouny 2018; Beltrame 2013; Jellad 2017; Singh 2017): in two trials, women withdrew due to E2 greater than 6000 pg/mL (Singh 2017) or OHSS (Bassiouny 2018), which affected the cases of OHSS reported. Beltrame 2013 had high dropout (40%) without mentioning reasons for dropout; Jellad 2017 only reported on the subgroups of women within each arm of the study that actually went on to develop OHSS while data from the non‐OHSS participants were lacking. Two trials were at unclear due to lack of information to be judged (Alhalabi 2011; El‐Shaer 2019).
Selective reporting
Six trials were at low risk of reporting bias because they reported the primary outcome of live birth rate (Bassiouny 2018; Busso 2010; Elnory 2018; Kilic 2015; Shaltout 2012; Singh 2017)).
One trial was judged as high risk of bias due to the fact that pregnancy and miscarriage rates were reported only for the women per arm that actually developed OHSS (Jellad 2017). The remaining 15 studies were at unclear risk of bias (Alhalabi 2011; Alvarez 2007a; Amir 2015; Beltrame 2013; Carizza 2008; Dalal 2014; El‐Shaer 2019; Fetisova 2014; Ghahiri 2015; Matorras 2013; Saad 2017; Salah 2012; Sohrabvand 2009; Tehraninejad 2012; Torabizadeh 2013).
Other potential sources of bias
Three trials were at high risk of other bias (Busso 2010; Dalal 2014; Salah 2012): one trial included young women with PCOS without other high‐risk factors identified (e.g. based on E2 or ultrasound) (Salah 2012). Dalal 2014 reported that 29 participants in both groups also received HES infusion, and one participant from each group also had ascites drained but it was unclear who exactly received these extra interventions.
Effects of interventions
See: Summary of findings 1 Dopamine agonist versus placebo/no intervention; Summary of findings 2 Dopamine agonist plus co‐intervention versus co‐intervention; Summary of findings 3 Dopamine agonist versus other active intervention
1. Dopamine agonist versus placebo/no intervention
1.1. Primary outcomes
1.1.1. Incidence of moderate or severe ovarian hyperstimulation syndrome
Ten studies reported the incidence of moderate or severe OHSS (Alhalabi 2011; Alvarez 2007a; Amir 2015; Beltrame 2013; Busso 2010; Fetisova 2014; Jellad 2017; Kilic 2015; Salah 2012; Singh 2017). Dopamine agonists were probably associated with a lower risk of moderate or severe OHSS than placebo/no intervention (OR 0.32, 95% CI 0.23 to 0.44; 10 studies, 1202 participants; I² = 13%; moderate‐quality evidence; Analysis 1.1; Figure 4). This suggests that if the risk of moderate or severe OHSS following placebo/no intervention is assumed to be 27%, the risk following dopamine agonists would be between 8% and 14%.

Forest plot of comparison 1: Dopamine agonist (without co‐intervention) versus placebo/no intervention, outcome: 1.1 moderate or severe ovarian hyperstimulation syndrome.
Subgroup analysis
We performed a subgroup analysis by type of dopamine agonist, which showed no evidence of a difference among these three types of dopamine agonist (P = 0.85). When compared with placebo/no intervention, cabergoline (OR 0.34, 95% CI 0.23 to 0.51; I² = 31%; 7 studies, 701 participants), and quinagolide (OR 0.28, 95% CI 0.15 to 0.51; I² = 30%; 2 studies, 454 participants) were associated with a lower risk of moderate or severe OHSS (Analysis 1.1; Figure 4). However, there was probably little or no difference between bromocriptine and placebo (OR 0.29, 95% CI 0.08 to 1.14; 1 study, 47 participants) (Analysis 1.1; Figure 4). Also, for the subgroup analysis by severity of OHSS, there was no difference between severe OHSS and moderate OHSS (P = 0.77). Dopamine agonists probably improve the risk of both severe OHSS (OR 0.27, 95% CI 0.14 to 0.51; I² = 0%; 9 studies, 930 participants) and moderate OHSS (OR 0.46, 95% CI 0.31 to 0.68; I² = 2%; 9 studies, 930 participants) when compared to placebo or no intervention (Analysis 1.2).
Sensitivity analysis
We conducted a prespecified sensitivity analysis by excluding four studies with high risk of bias from Analysis 1.1, the lower incidence of moderate or severe OHSS with dopamine agonists compared with placebo/no intervention remained unchanged (OR 0.28, 95% CI 0.17 to 0.46; I² = 0%; 10 studies, 552 participants). Also, use of a random‐effects model did not affect the results.
1.1.2. Live birth rate
Three trials reported data on live birth rate (Busso 2010; Kilic 2015; Singh 2017). We are uncertain of the effect of dopamine agonists on live birth rate compared with placebo/no intervention (OR 0.96, 95% CI 0.60 to 1.55; I² = 0%; 3 studies, 362 participants; low‐quality evidence; Analysis 1.3). This suggests that if the chance of live birth following placebo/no intervention is assumed to be 32%, the risk following dopamine agonists would be between 22% and 43%. In the subgroup analysis by type of dopamine agonist, the test for subgroup differences showed we are uncertain of an effect of cabergoline compared to placebo/no intervention (OR 0.91, 95% CI 0.44 to 1.87; I² = 0%; 2 studies, 180 participants) and the effect of quinagolide compared to placebo/no intervention (OR 1.01, 95% CI 0.53 to 1.91; 1 study, 182 participants), with a P value of 0.83.
1.2. Secondary outcomes
1.2.1. Clinical pregnancy rate
Five trials reported clinical pregnancy rate (Amir 2015; Busso 2010; Fetisova 2014; Kilic 2015; Singh 2017). We are uncertain of the effect of dopamine agonist when compared to placebo/no intervention (OR 0.92, 95% CI 0.63 to 1.37; I² = 0%; 5 studies, 530 participants; low‐quality evidence; Analysis 1.4). This suggests that if the chance of clinical pregnancy following placebo/no intervention is assumed to be 31%, the risk following dopamine agonists would be between 22% and 38%. We are uncertain of the effect of between cabergoline compared to placebo/no intervention (OR 1.00, 95% CI 0.61 to 1.64; I² = 0%; 4 studies, 348 participants), and between quinagolide compared to placebo (OR 0.81, 95% CI 0.43 to 1.54; 1 study, 182 participants).
1.2.2. Multiple pregnancy rate
One study reported multiple pregnancy rate (Amir 2015). We are uncertain whether dopamine agonist improves multiple pregnancy rate compared with placebo/no intervention (OR 0.32, 95% CI 0.01 to 8.26; 1 study, 40 participants; very low‐quality evidence; Analysis 1.5). This suggests that if the chance of multiple pregnancy following placebo/no intervention is assumed to be 5%, the risk following dopamine agonists would be between 1% and 30%.
1.2.3. Miscarriage rate
Two studies reported miscarriage rate (Amir 2015; Fetisova 2014). We are uncertain of the effect of dopamine agonist on miscarriage rate compared with placebo/no intervention (OR 0.66, 95% CI 0.19 to 2.28; I² = 0%; 2 studies, 168 participants; low‐quality evidence; Analysis 1.6). This suggests that if the risk of miscarriage following placebo/no intervention is assumed to be 7%, the risk following dopamine agonists would be between 2% and 15%.
1.2.4. Any other adverse events of the treatment
Two trials reported adverse events (Alvarez 2007a; Busso 2010). We are uncertain whether dopamine agonists increased risk of adverse events (OR 4.54, 95% CI 1.49 to 13.84; I² = 49%; 2 studies, 264 participants; very low‐quality evidence; Analysis 1.7). This suggests that if the risk of any other adverse events following placebo/no intervention is assumed to be 4%, the risk following dopamine agonists would be between 6% and 38%. Subgroup analysis by type of dopamine agonist showed no difference among dopamine agonists (P = 0.21).
We are uncertain whether cabergoline increases adverse effects compared to placebo/no intervention (OR 2.24, 95% CI 0.62 to 8.14; 1 study; 82 participants; Analysis 1.7). One trial reported that 17 women in the quinagolide group discontinued because of adverse events and no women in the placebo group (OR 16.64, 95% CI 0.98 to 282.02; 1 study; 182 participants) (Analysis 1.7).
2. Dopamine agonist plus co‐intervention versus co‐intervention
Four studies compared dopamine agonist plus co‐intervention versus co‐intervention (Bassiouny 2018; Carizza 2008; Matorras 2013; Shaltout 2012). All four studies used cabergoline. The co‐interventions were HES (Matorras 2013; Shaltout 2012), albumin (Carizza 2008), and coasting (Bassiouny 2018).
2.1. Primary outcomes
2.1.1. Incidence of severe or moderate ovarian hyperstimulation syndrome
Four studies reported the incidence of moderate or severe OHSS (Bassiouny 2018; Carizza 2008; Matorras 2013; Shaltout 2012). Dopamine agonists plus co‐intervention may decrease the risk of moderate or severe OHSS compared with co‐intervention alone (OR 0.48, 95% CI 0.28 to 0.84; I² = 40%; 4 studies, 748 participants; low‐quality evidence; Analysis 2.1; Figure 5). This suggests that if the risk of moderate or severe OHSS following placebo/no intervention is assumed to be 11%, the risk following dopamine agonists would be between 3% and 9%. Subgroup analysis by type of co‐intervention did not alter this conclusion. We were uncertain of the effects between the following: cabergoline plus albumin group versus albumin group (OR 0.55, 95% CI 0.23 to 1.34; 1 study, 166 participants), cabergoline plus HES group versus HES group (OR 0.58, 95% CI 0.26 to 1.30; I² = 72%; 2 studies, 382 participants), or between cabergoline plus coasting group versus coasting group (OR 0.21, 95% CI 0.04 to 0.98; 1 study, 200 participants); all were low‐quality evidence (Analysis 2.1; Figure 5).

Forest plot of comparison: 2 Dopamine agonist plus co‐intervention versus co‐intervention, outcome: 2.1 Moderate or severe ovarian hyperstimulation syndrome.
Our sensitivity analysis by excluding one study (Bassiouny 2018) with high risk of bias (OR 0.57, 95% CI 0.31 to 1.03; I² = 44%; 3 studies, 548 participants) or changing analysis model (OR 0.50, 95% CI 0.23 to 1.08; I² = 40%; 4 studies, 748 participants) did not change these results.
2.1.2. Live birth rate
Two trials reported data on live birth rate (Bassiouny 2018; Shaltout 2012). We are uncertain of the effect of dopamine agonist plus co‐intervention on live birth rate compared with co‐intervention alone (OR 1.21, 95% CI 0.81 to 1.80; I² = 0%; 2 studies, 400 participants; low‐quality evidence). This suggests that if the chance of live birth following placebo/no intervention is assumed to be 38%, the risk following dopamine agonists would be between 33% and 53%. Subgroup analysis by type of co‐intervention showed no difference between subgroups (P = 0.49). We are uncertain whether cabergoline plus HES improved live birth compared to HES alone (OR 1.04, 95% CI 0.59 to 1.86; 1 study, 200 participants) or of cabergoline plus coasting compared to coasting alone (OR 1.38, 95% CI 0.79 to 2.42; 1 study, 200 participants) (Analysis 2.2).
2.2. Secondary outcomes
2.2.1. Clinical pregnancy rate
Four trials reported the clinical pregnancy rate (Bassiouny 2018; Carizza 2008; Matorras 2013; Shaltout 2012). We are uncertain of the effect of dopamine agonist plus co‐intervention versus co‐intervention alone (OR 1.11, 95% CI 0.83 to 1.49; I² = 0%; 4 studies, 748 participants; low‐quality evidence; Analysis 2.3). This suggests that if the chance of clinical pregnancy following placebo/no intervention is assumed to be 44%, the risk following dopamine agonists would be between 40% and 54%. In the subgroup analysis, we found no difference between subgroups (P = 0.47). We are uncertain whether cabergoline plus albumin improved clinical pregnancy rate compared to albumin (OR 1.05, 95% CI 0.56 to 1.96; 1 study, 166 participants), cabergoline plus HES compared to HES (OR 0.98, 95% CI 0.65 to 1.47; I² = 0%; 2 studies, 382 participants), or cabergoline plus coasting compared to coasting (OR 1.49, 95% CI 0.86 to 2.61; 1 study, 200 participants; Analysis 2.3).
2.2.2. Multiple pregnancy rate
One study reported multiple pregnancy rate (Carizza 2008). We are uncertain of the effect of cabergoline plus albumin on multiple pregnancy rate compared with albumin (OR 2.02, 95% CI 0.18 to 22.77; 1 study, 166 participants; very low‐quality evidence; Analysis 2.4). This suggests that if the chance of multiple pregnancy following placebo/no intervention is assumed to be 1%, the risk following dopamine agonists would be between 0.2% and 22%.
2.2.3. Miscarriage rate
Three studies reported miscarriage rate (Carizza 2008; Matorras 2013; Shaltout 2012). We are uncertain of the effect of dopamine agonist plus co‐intervention on miscarriage rates compared with co‐intervention (OR 0.65, 95% CI 0.30 to 1.42; I² = 0%; 3 studies, 548 participants; low‐quality evidence; Analysis 2.5). This suggests that if the risk of miscarriage following placebo/no intervention is assumed to be 6%, the risk following dopamine agonists would be between 2% and 9%. We found no difference between subgroups (P = 0.52) and the results showed that we are uncertain whether cabergoline plus albumin compared to albumin (OR 0.33, 95% CI 0.03 to 3.19; 1 study, 166 participants), or cabergoline plus HES compared to HES (OR 0.73, 95% CI 0.31 to 1.68; I² = 0%; 2 studies, 382 participants) improved miscarriage rate (Analysis 2.5).
2.2.4. Any other adverse events of the treatment
Two trials reported adverse events (Carizza 2008; Shaltout 2012). We are uncertain whether dopamine agonist plus co‐intervention increases risk of adverse events (OR 3.03, 95% CI 0.12 to 75.28; I² = 0%; 2 studies, 366 participants; very low‐quality evidence; Analysis 2.6. One trial with 166 participants detected no adverse events (Carizza 2008).
3. Dopamine agonist versus other active intervention
3.1. Primary outcomes
3.1.1. Incidence of moderate or severe ovarian hyperstimulation syndrome
Ten studies reported the incidence of moderate or severe OHSS when comparing dopamine agonist with several other active interventions (Bassiouny 2018; Dalal 2014; Elnory 2018; El‐Shaer 2019; Ghahiri 2015; Saad 2017; Salah 2012; Sohrabvand 2009; Tehraninejad 2012; Torabizadeh 2013). There was significant heterogeneity between subgroups, therefore, we reported the results of each subgroup only (Figure 6).

Funnel plot of comparison: 3 Dopamine agonist versus other active interventions, outcome: 3.1 Incidence of moderate or severe ovarian hyperstimulation syndrome (OHSS).
3.1.1.1. Cabergoline versus human albumin
Three studies reported the incidence of moderate or severe OHSS (Ghahiri 2015; Tehraninejad 2012; Torabizadeh 2013). We are uncertain whether cabergoline decreases the incidence of severe or moderate OHSS compared with human albumin (OR 0.21, 95% CI 0.12 to 0.38; I² = 72%; 3 studies, 296 participants; very low‐quality evidence; Analysis 3.1; Figure 7). This suggests that if the risk of moderate or severe OHSS following human albumin is assumed to be 43%, the risk following dopamine agonists would be between 8% and 23%.

Forest plot of comparison 3: Cabergoline versus active interventions, outcome: 3.1 moderate or severe ovarian hyperstimulation syndrome.
3.1.1.2. Cabergoline versus prednisolone
One study reported the incidence of moderate or severe OHSS (Salah 2012). We are uncertain of the effect of cabergoline on risk of moderate or severe OHSS compared with prednisolone (OR 0.27, 95% CI 0.05 to 1.33; 1 study, 150 participants; very low‐quality evidence; Analysis 3.1; Figure 7). This suggests that if the risk of moderate or severe OHSS following prednisolone is assumed to be 9%, the risk following dopamine agonists would be between 0.5% and 12%.
3.1.1.3. Cabergoline versus hydroxyethyl starch
One study reported the incidence of moderate or severe OHSS (Ghahiri 2015). We are uncertain of the effect of cabergoline on risk of moderate or severe OHSS compared with HES (OR 2.69, 95% CI 0.48 to 15.10; 1 study, 61 participants; very low‐quality evidence; Analysis 3.1; Figure 7). This suggests that if the risk of moderate or severe OHSS following HES is assumed to be 7%, the risk following dopamine agonists would be between 3% and 52%.
3.1.1.4. Cabergoline versus coasting
Three studies reported the incidence of moderate or severe OHSS (Bassiouny 2018; Dalal 2014; Sohrabvand 2009). We are uncertain whether cabergoline decreases the risk of moderate or severe OHSS compared with coasting (OR 0.42, 95% CI 0.18 to 0.95; I² = 50%; 3 studies, 320 participants; very low‐quality evidence; Analysis 3.1; Figure 7). This suggests that if the risk of moderate or severe OHSS following coasting is assumed to be 13%, the risk following dopamine agonists would be between 3% and 12%.
3.1.1.5. Cabergoline versus calcium infusion
Two studies reported the incidence of moderate or severe OHSS (Elnory 2018; El‐Shaer 2019). We are uncertain of the effect of cabergoline on risk of moderate or severe OHSS when compared with calcium infusion (OR 1.83, 95% CI 0.88 to 3.81; I² = 81%; 2 studies, 400 participants; very low‐quality evidence; Analysis 3.1; Figure 7). This suggests that if the risk of moderate or severe OHSS following calcium infusion is assumed to be 6%, the risk following dopamine agonists would be between 5% and 20%.
3.1.1.6. Cabergoline versus diosmin
One study reported the incidence of moderate or severe OHSS (Saad 2017). We are uncertain of the effect of cabergoline on risk of moderate or severe OHSS compared with diosmin (OR 2.85, 95% CI 1.35 to 6.00; 1 study, 200 participants; very low‐quality evidence; Analysis 3.1; Figure 7). This suggests that if the risk of moderate or severe OHSS following diosmin is assumed to be 12%, the risk following dopamine agonists would be between 16% and 45%.
3.1.2. Live birth rate
Two studies reported the data on live birth rate (Bassiouny 2018; Elnory 2018). We are uncertain whether dopamine agonist improves live birth rate (OR 1.08, 95% CI 0.73 to 1.59; I² = 0%; 2 studies, 430 participants; low‐quality evidence) (Analysis 3.2). This suggests that if the chance of live birth following other active intervention is assumed to be 40%, the risk following dopamine agonist would be between 32% and 51%. Both trials included cabergoline. Our subgroup analysis by type of other active intervention showed that we are uncertain whether cabergoline improves live birth rate when compared to coasting (OR 1.04, 95% CI 0.59 to 1.83; 1 study, 200 participants) and when compared to calcium infusion (OR 1.11, 95% CI 0.66 to 1.89; 1 study, 230 participants) (Analysis 3.2).
3.2. Secondary outcomes
3.2.1. Clinical pregnancy rate
Seven studies reported clinical pregnancy rate (Bassiouny 2018; Dalal 2014; Elnory 2018; El‐Shaer 2019; Saad 2017; Sohrabvand 2009; Tehraninejad 2012). The pooled results showed probably little or no difference in clinical pregnancy rate between dopamine agonists compared with other active interventions (OR 1.04, 95% CI 0.81 to 1.33; I² = 11%; 7 studies, 1060 participants; moderate‐quality evidence). This suggests that if the chance of clinical pregnancy following other active intervention is assumed to be 43%, the risk following dopamine agonist would be between 38% and 50%. All trials evaluated the dopamine agonist cabergoline. Subgroup analysis by type of other active intervention showed that we were uncertain whether cabergoline improves clinical pregnancy rates when compared to human albumin (OR 0.68, 95% CI 0.33 to 1.38; 1 study, 140 participants), coasting (OR 1.46, 95% CI 0.92 to 2.32; I² = 28%; 3 studies, 320 participants), calcium infusion (OR 1.00, 95% CI 0.67 to 1.49; I² = 0%; 2 studies, 400 participants), or diosmin (OR 0.89, 95% CI 0.51 to 1.55; I² = 0%; 1 study, 200 participants) (Analysis 3.3).
3.2.2. Multiple pregnancy rate
Three studies reported multiple pregnancy rate (Dalal 2014; Saad 2017; Tehraninejad 2012). We are uncertain of the effect of dopamine agonist on multiple pregnancy rate (OR 0.87, 95% CI 0.47 to 1.59; I² = 0%; 3 studies, 400 participants; low‐quality evidence; Analysis 3.4). Subgroup analysis by type of other active intervention showed that we were uncertain of the effect of cabergoline on multiple pregnancy rate when compared to human albumin (OR 0.58, 95% CI 0.13 to 2.54; 1 study, 140 participants), coasting (OR 5.35, 95% CI 0.25 to 116.31; 1 study, 60 participants), or diosmin (OR 0.83, 95% CI 0.41 to 1.67; 1 study, 200 participants) (Analysis 3.4).
3.2.3. Miscarriage rate
Four studies reported the miscarriage rate (Dalal 2014; Elnory 2018; Saad 2017; Tehraninejad 2012). We are uncertain of the effect of dopamine agonist on miscarriage rate (OR 0.66, 95% CI 0.35 to 1.25; I² = 0%; 4 studies, 630 participants; low‐quality evidence; Analysis 3.5). Furthermore, in our subgroup analysis by type of other active intervention, we were uncertain of the effect of cabergoline on miscarriage rate when compared to human albumin (OR 0.32, 95% CI 0.03 to 3.19; 1 study, 140 participants), coasting (OR 0.19, 95% CI 0.01 to 4.06; 1 study, 60 participants), calcium infusion (OR 0.63, 95% CI 0.27 to 1.48; 1 study, 230 participants), or diosmin (OR 1.21, 95% CI 0.36 to 4.11; 1 study, 200 participants) (Analysis 3.5).
3.2.4. Any other adverse effects of the treatment
One study reported that there were no adverse events when comparing cabergoline versus calcium infusion (Analysis 3.6) (El‐Shaer 2019).
Discussion
Summary of main results
This systematic review evaluated the effectiveness and safety of dopamine agonists for preventing OHSS in women at high risk of OHSS during ART treatment and performed meta‐analyses. Ten trials compared dopamine agonist with placebo or no intervention, four trials compared dopamine agonist in combination with co‐intervention with co‐intervention and 10 trials compared dopamine agonists with other active interventions (one trial compared DA with two other interventions). Overall, when compared with placebo or no intervention, dopamine agonists had a lower risk of developing moderate or severe OHSS without influencing pregnancy outcomes such as live birth rate for those women who proceeded to have a fresh embryo transfer, clinical pregnancy rate, multiple pregnancy rate, and miscarriage rate. However, data on the live birth rate were scarce or incomplete in the included trials.
There was an increased risk of adverse events, which occurred rarely and were mild, associated with dopamine agonists particularly when using quinagolide. Cabergoline was associated with a lower risk of moderate or severe OHSS, without influencing pregnancy outcomes when compared with placebo or no intervention. Quinagolide appeared to reduce the risk of moderate or severe OHSS, but might increase the incidence of adverse events, although the reported events were mainly very mild gastrointestinal and central nervous system symptoms, especially compared to the risks of severe OHSS. With the limited data available, bromocriptine did not influence the incidence of moderate or severe OHSS.
Dopamine agonist plus co‐intervention may reduce the risk of moderate or severe OHSS compared to co‐intervention alone. We are uncertain of the effect of dopamine agonist plus co‐intervention and co‐intervention in other outcomes of interest.
When compared with other active interventions, we reported OHSS data in subgroups per type of intervention, due to large heterogeneity across subgroups. When compared with human albumin and coasting, cabergoline might reduce the incidence of moderate or severe OHSS, but dopamine agonists might increase that risk compared to diosmin. We are uncertain of an effect on OHSS rates when comparing cabergoline to other active interventions such as prednisolone or HES or calcium gluconate infusion. Also, we were uncertain of any effect on pregnancy outcomes and adverse events when comparing cabergoline versus other active interventions.
The quality of the evidence for the comparison of dopamine agonist with placebo/no intervention was moderate but for the other comparators the evidence was low or very low. The main limitations causing these quality judgements were poor reporting of study methods (mostly lack of details on randomisation and blinding), heterogeneity across trials, and risk of imprecision (low number of events or small sample sizes).
Overall completeness and applicability of evidence
Compared with the previous published version of this review (Tang 2016), we included six additional trials. In total, this updated Cochrane Review included 22 trials involving 3171 women at high risk of OHSS. The study populations varied among trials regarding the definition of 'women at high risk' of OHSS. This may influence the incidence of OHSS and limits the applicability of study results in practice. However, as some trials even excluded the truly 'high risk of OHSS' women from participating, we do not know whether this effect of dopamine agonists could also be seen when these women were not excluded. Most of the trials defined moderate or severe OHSS according to Golan's classification (Golan 1989), but five trials used other definitions (undefined, or following the criteria defined by Mathur and colleagues (Mathur 2007) or Humaidan and colleagues (Humaidan 2010). This may induce bias when pooling the data of the various studies. Only a few studies reported pregnancy outcomes such as live birth. The influence of dopamine agonists on pregnancy outcomes requires further study; however, many units will practice an embryo 'freeze‐all' approach for women at risk of OHSS and, therefore, data for pregnancy outcomes may not be forthcoming. Most of the trials evaluated the dopamine agonist cabergoline, whereas two trials evaluated quinagolide and one trial evaluated bromocriptine. In addition, our evidence was applicable in low‐ to middle‐income countries as most trials were performed in these countries. Finally, due to the lack of studies comparing a dopamine agonist with another dopamine agonist, we were unable to determine which dopamine agonist is most effective in preventing OHSS.
Quality of the evidence
The methodological quality of the 22 included trials varied. Fifteen trials used correct random sequence generation, and only five trials had a low risk of bias in the domain of allocation concealment. Four trials were either single or double blind. One trial was at high risk of bias due to a high percentage of dropouts without reported reasons (Beltrame 2013). All trials reported the outcomes of OHSS, but only six studies provided the primary outcome of 'live birth rate.' See Figure 2 and Figure 3 for the 'Risk of bias' assessments of the included studies.
Moreover, the overall body of evidence for primary outcomes between dopamine agonist and placebo or no intervention was moderate. The main reasons for downgrading the quality of the evidence were: poor reporting of study methods (e.g. 36% of RCTs did not report the methods of allocation concealment or blinding) and risk of imprecision (e.g. low number of events). See summary of findings Table 1; summary of findings Table 2; and summary of findings Table 3 for more details.
Potential biases in the review process
We tried to identify all eligible trials by conducting a systematic review of the literature without restrictions of publication type or language. Moreover, we contacted the authors of trials for more information about any unpublished data. For the missing participants who did not report the outcome, we assumed that the events did not occur.
Agreements and disagreements with other studies or reviews
Our results are in agreement with most of the systematic reviews and meta‐analyses on dopamine agonists for the prevention for OHSS (Baumgarten 2013; Guo 2016; Kalampokas 2013; Kasum 2014; Leitao 2014; Youssef 2010). The first systematic review published in 2010 included only four RCTs with 570 women, and showed that cabergoline might reduce the incidence of OHSS. However, it found no evidence of a reduction in severe OHSS (Youssef 2010), which is consistent with our previous Cochrane Review (Tang 2016). This might be caused by a small sample size or low event rate of severe OHSS. In 2014, another systematic review included eight trials involving 858 women and showed that cabergoline could reduce the risk of moderate or severe OHSS, as well as severe OHSS (Leitao 2014). In 2016, one systematic review and network meta‐analysis of 31 RCTs involving 7181 women showed that cabergoline was superior to placebo or human albumin, or glucocorticoid in decreasing OHSS incidence, and there was no evidence of a difference between cabergoline and other active interventions (e.g. aspirin, HES, calcium infusion or metformin). However, until 2016, few systematic reviews included types of dopamine agonist other than cabergoline. One systematic review showed that a dopamine agonist appeared to be effective for the prevention of OHSS (Baumgarten 2013). Moreover, there was no evidence of adverse effects on pregnancy outcomes (Baumgarten 2013; Leitao 2014; Youssef 2010). Compared with previous systematic reviews, our review includes more trials and women, and can, therefore, draw a more robust conclusion that the use of dopamine agonists could reduce the incidence of moderate or severe OHSS.
In future OHSS trials, it will probably be considered unethical to withhold women who are at risk of OHSS from having all their embryos frozen for replacement in a subsequent cycle, as current embryo survival rates after freezing are generally excellent and the transfer of a frozen embryo in an unstimulated cycle avoids the risk of OHSS in that cycle.
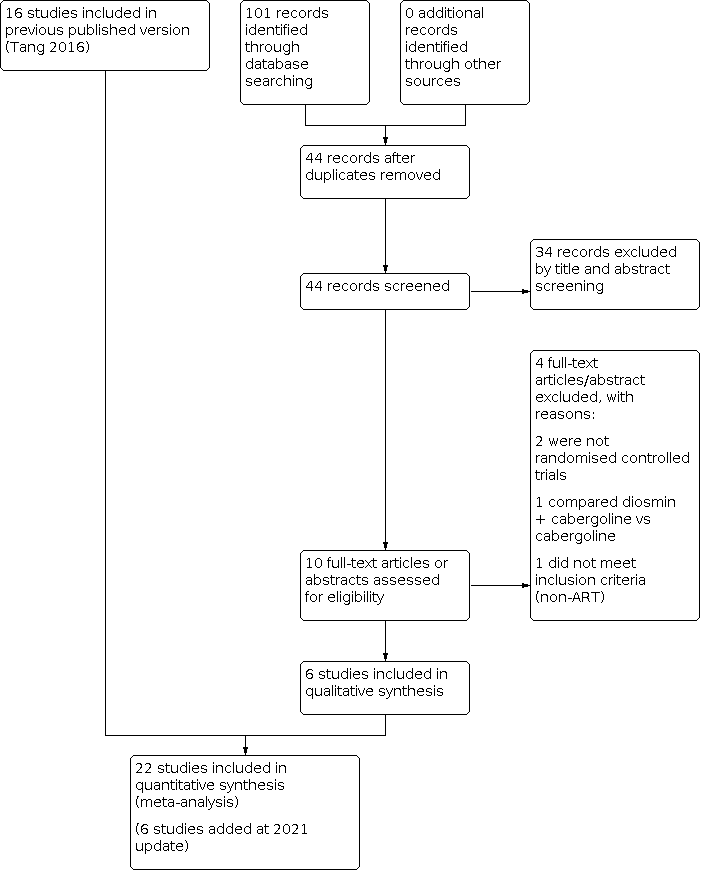
Study flow diagram search May 2020. ART: assisted reproduction technology.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison 1: Dopamine agonist (without co‐intervention) versus placebo/no intervention, outcome: 1.1 moderate or severe ovarian hyperstimulation syndrome.

Forest plot of comparison: 2 Dopamine agonist plus co‐intervention versus co‐intervention, outcome: 2.1 Moderate or severe ovarian hyperstimulation syndrome.

Funnel plot of comparison: 3 Dopamine agonist versus other active interventions, outcome: 3.1 Incidence of moderate or severe ovarian hyperstimulation syndrome (OHSS).

Forest plot of comparison 3: Cabergoline versus active interventions, outcome: 3.1 moderate or severe ovarian hyperstimulation syndrome.

Comparison 1: Dopamine agonist versus placebo/no intervention, Outcome 1: Incidence of moderate or severe ovarian hyperstimulation syndrome (OHSS)

Comparison 1: Dopamine agonist versus placebo/no intervention, Outcome 2: Subgroup analysis by severity of OHSS

Comparison 1: Dopamine agonist versus placebo/no intervention, Outcome 3: Live birth rate

Comparison 1: Dopamine agonist versus placebo/no intervention, Outcome 4: Clinical pregnancy rate

Comparison 1: Dopamine agonist versus placebo/no intervention, Outcome 5: Multiple pregnancy rate

Comparison 1: Dopamine agonist versus placebo/no intervention, Outcome 6: Miscarriage rate

Comparison 1: Dopamine agonist versus placebo/no intervention, Outcome 7: Any other adverse events

Comparison 2: Dopamine agonist plus co‐intervention (DA+co‐int) versus co‐intervention (co‐int), Outcome 1: Incidence of moderate or severe ovarian hyperstimulation syndrome (OHSS)

Comparison 2: Dopamine agonist plus co‐intervention (DA+co‐int) versus co‐intervention (co‐int), Outcome 2: Live birth rate

Comparison 2: Dopamine agonist plus co‐intervention (DA+co‐int) versus co‐intervention (co‐int), Outcome 3: Clinical pregnancy rate

Comparison 2: Dopamine agonist plus co‐intervention (DA+co‐int) versus co‐intervention (co‐int), Outcome 4: Multiple pregnancy rate

Comparison 2: Dopamine agonist plus co‐intervention (DA+co‐int) versus co‐intervention (co‐int), Outcome 5: Miscarriage rate

Comparison 2: Dopamine agonist plus co‐intervention (DA+co‐int) versus co‐intervention (co‐int), Outcome 6: Any other adverse events

Comparison 3: Dopamine agonist versus other active interventions, Outcome 1: Incidence of moderate or severe ovarian hyperstimulation syndrome (OHSS)

Comparison 3: Dopamine agonist versus other active interventions, Outcome 2: Live birth rate

Comparison 3: Dopamine agonist versus other active interventions, Outcome 3: Clinical pregnancy rate

Comparison 3: Dopamine agonist versus other active interventions, Outcome 4: Multiple pregnancy rate

Comparison 3: Dopamine agonist versus other active interventions, Outcome 5: Miscarriage rate

Comparison 3: Dopamine agonist versus other active interventions, Outcome 6: Any other adverse events
| Dopamine agonist vs placebo/no intervention | ||||||
| Patient or population: women of reproductive age undergoing any ART therapy Settings: ART unit Intervention: dopamine agonist Comparison: placebo/no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with placebo/no intervention | Risk with dopamine agonist | |||||
| Incidence of moderate or severe OHSS | 268 per 1000 | 105 per 1000 | OR 0.32 | 1202 | ⊕⊕⊕⊝ | — |
| Live birth rate | 324 per 1000 | 315 per 1000 | OR 0.96 | 362 | ⊕⊕⊝⊝ | — |
| Clinical pregnancy rate | 307 per 1000 | 289 per 1000 | OR 0.92 | 530 | ⊕⊕⊝⊝ | — |
| Multiple pregnancy rate | 50 per 1000 | 17 per 1000 (1 to 303) | OR 0.32 (0.01 to 8.26) | 40 | ⊕⊝⊝⊝ | — |
| Miscarriage rate | 72 per 1000 | 49 per 1000 (15 to 151) | OR 0.66 (0.19 to 2.28) | 168 (2 RCTs) | ⊕⊕⊝⊝ | — |
| Any other adverse events | 43 per 1000 | 168 per 1000 (62 to 381) | OR 4.54 (1.49 to 13.84) | 264 | ⊕⊝⊝⊝ | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ART: assisted reproductive technology; CI: confidence interval; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for risk of bias associated with poor reporting of study methods. | ||||||
| Dopamine agonist plus co‐intervention vs co‐intervention | ||||||
| Patient or population: women of reproductive age undergoing any ART therapy Settings: ART unit Intervention: dopamine agonist plus co‐intervention Comparison: co‐intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with co‐intervention only | Risk with dopamine agonist plus co‐intervention | |||||
| Incidence of moderate or severe OHSS | 109 per 1000 | 55 per 1000 (33 to 93) | OR 0.48 (0.28 to 0.84) | 748 (4 RCTs) | ⊕⊕⊝⊝ | — |
| Live birth rate | 380 per 1000 | 426 per 1000 (332 to 525) | OR 1.21 (0.81 to 1.80) | 400 (2 studies) | ⊕⊕⊝⊝ | — |
| Clinical pregnancy rate | 443 per 1000 | 469 per 1000 (398 to 542) | OR 1.11 (0.83 to 1.49) | 748 (4 studies) | ⊕⊕⊝⊝ | — |
| Multiple pregnancy rate | 12 per 1000 | 24 per 1000 (2 to 217) | OR 2.02 (0.18 to 22.77) | 166 (1 study) | ⊕⊝⊝⊝ | — |
| Miscarriage rate | 61 per 1000 | 41 per 1000 (19 to 85) | OR 0.65 (0.30 to 1.42) | 548 (3 studies) | ⊕⊕⊝⊝ | — |
| Any other adverse events | 0 per 1000 | 0 per 1000 (0 to 0) | OR 3.03 (0.12 to 75.28) | 366 (2 studies) | ⊕⊝⊝⊝ | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ART: assisted reproductive technology; CI: confidence interval; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for serious risk of bias associated with poor reporting of study methods. | ||||||
| Dopamine agonist vs other active intervention | |||||||
| Patient or population: women of reproductive age undergoing any ART therapy Settings: ART unit Intervention: dopamine agonist Comparison: other active intervention | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | ||
|---|---|---|---|---|---|---|---|
| Risk with other active intervention | Risk with dopamine agonist | ||||||
| Incidence of moderate or severe OHSS | Cabergoline vs human albumin | 432 per 1000 | 138 per 1000 (84 to 225) | OR 0.21 (0.12 to 0.38) | 296 | ⊕⊝⊝⊝ | — |
| Cabergoline vs prednisolone | 93 per 1000 | 27 per 1000 (5 to 120) | OR 0.27 (0.05 to 1.33) | 150 | ⊕⊝⊝⊝ | — | |
| Cabergoline vs hydroxyethyl starch | 67 per 1000 | 161 per 1000 (33 to 519) | OR 2.69 (0.48 to 15.10) | 61 | ⊕⊝⊝⊝ | — | |
| Cabergoline vs coasting | 125 per 1000 | 57 per 1000 (25 to 119) | OR 0.42 (0.18 to 0.95) | 320 | ⊕⊝⊝⊝ | — | |
| Cabergoline vs calcium infusion | 60 per 1000 | 105 per 1000 (53 to 196) | OR 1.83 (0.88 to 3.81) | 400 | ⊕⊝⊝⊝ | — | |
| Cabergoline vs diosmin | 120 per 1000 | 280 per 1000 (155 to 450) | OR 2.85 (1.35 to 6.00) | 200 | ⊕⊝⊝⊝ | — | |
| Live birth rate | Cabergoline vs coasting or calcium infusion | 395 per 1000 | 414 per 1000 (323 to 510) | OR 1.08 (0.73 to 1.59) | 430 | ⊕⊕⊝⊝ | — |
| Clinical pregnancy rate | Cabergoline vs human albumin, coasting, calcium infusion, or diosmin | 432 per 1000 | 442 per 1000 (381 to 503) | OR 1.04 (0.81 to 1.33) | 1060 | ⊕⊕⊕⊝ | — |
| Multiple pregnancy rate | Cabergoline vs human albumin, coasting, or diosmin | 130 per 1000 | 115 per 1000 (66 to 192) | OR 0.87 (0.47 to 1.59) | 400 | ⊕⊕⊝⊝ | — |
| Miscarriage rate | Cabergoline vs human albumin, coasting, calcium infusion, or diosmin | 79 per 1000 | 54 per 1000 (29 to 97) | OR 0.66 (0.35 to 1.25) | 630 | ⊕⊕⊝⊝ | — |
| Any other adverse events | Cabergoline vs calcium infusion | 0 per 1000 | 0 per 1000 (0 to 0) | Not estimable | 170 (1 RCT) | Not estimable | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ART: assisted reproductive technology; CI: confidence interval; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio; RCT: randomised controlled trial. | |||||||
| GRADE Working Group grades of evidence | |||||||
| aDowngraded one level for risk of bias associated with poor reporting of study methods. | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Incidence of moderate or severe ovarian hyperstimulation syndrome (OHSS) Show forest plot | 10 | 1202 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.23, 0.44] |
| 1.1.1 Cabergoline vs placebo/no treatment | 7 | 701 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.23, 0.51] |
| 1.1.2 Quinagolide vs placebo | 2 | 454 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.15, 0.51] |
| 1.1.3 Bromocriptine vs placebo (folic acid) | 1 | 47 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.08, 1.14] |
| 1.2 Subgroup analysis by severity of OHSS Show forest plot | 9 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Severe OHSS | 9 | 930 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.14, 0.51] |
| 1.2.2 Moderate OHSS | 9 | 930 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.31, 0.68] |
| 1.3 Live birth rate Show forest plot | 3 | 362 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.60, 1.55] |
| 1.3.1 Cabergoline vs placebo/no treatment | 2 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.44, 1.87] |
| 1.3.2 Quinagolide vs placebo/no treatment | 1 | 182 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.53, 1.91] |
| 1.4 Clinical pregnancy rate Show forest plot | 5 | 530 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.63, 1.37] |
| 1.4.1 Cabergoline vs placebo/no intervention | 4 | 348 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.61, 1.64] |
| 1.4.2 Quinagolide vs placebo/no treatment | 1 | 182 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.43, 1.54] |
| 1.5 Multiple pregnancy rate Show forest plot | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.26] |
| 1.5.1 Cabergoline vs placebo/no treatment | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.26] |
| 1.6 Miscarriage rate Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.6.1 Cabergoline vs placebo/no treatment | 2 | 168 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.19, 2.28] |
| 1.7 Any other adverse events Show forest plot | 2 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.54 [1.49, 13.84] |
| 1.7.1 Cabergoline vs placebo/no treatment | 1 | 82 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.24 [0.62, 8.14] |
| 1.7.2 Quinagolide vs placebo | 1 | 182 | Odds Ratio (M‐H, Fixed, 95% CI) | 16.64 [0.98, 282.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Incidence of moderate or severe ovarian hyperstimulation syndrome (OHSS) Show forest plot | 4 | 748 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.28, 0.84] |
| 2.1.1 Cabergoline + albumin vs albumin | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.23, 1.34] |
| 2.1.2 Cabergoline + hydroxyethyl starch (HES) vs HES | 2 | 382 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.26, 1.30] |
| 2.1.3 Cabergoline + coasting vs coasting | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.04, 0.98] |
| 2.2 Live birth rate Show forest plot | 2 | 400 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.81, 1.80] |
| 2.2.1 Cabergoline + HES vs HES | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.59, 1.86] |
| 2.2.2 Cabergoline + coasting vs coasting | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.79, 2.42] |
| 2.3 Clinical pregnancy rate Show forest plot | 4 | 748 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.83, 1.49] |
| 2.3.1 Cabergoline + albumin vs albumin | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.56, 1.96] |
| 2.3.2 Cabergoline + HES vs HES | 2 | 382 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.65, 1.47] |
| 2.3.3 Cabergoline + coasting vs coasting | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.86, 2.61] |
| 2.4 Multiple pregnancy rate Show forest plot | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.18, 22.77] |
| 2.4.1 Cabergoline + albumin vs albumin | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.18, 22.77] |
| 2.5 Miscarriage rate Show forest plot | 3 | 548 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.30, 1.42] |
| 2.5.1 Cabergoline + albumin vs albumin | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.19] |
| 2.5.2 Cabergoline + HES vs HES | 2 | 382 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.31, 1.68] |
| 2.6 Any other adverse events Show forest plot | 2 | 366 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 75.28] |
| 2.6.1 Cabergoline + albumin vs albumin | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.6.2 Cabergoline + HES vs HES | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 75.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Incidence of moderate or severe ovarian hyperstimulation syndrome (OHSS) Show forest plot | 10 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1.1 Cabergoline vs human albumin | 3 | 296 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.12, 0.38] |
| 3.1.2 Cabergoline vs prednisolone | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.05, 1.33] |
| 3.1.3 Cabergoline vs hydroxyethyl starch (HES) | 1 | 61 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.48, 15.10] |
| 3.1.4 Cabergoline vs coasting | 3 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.18, 0.95] |
| 3.1.5 Cabergoline vs calcium infusion | 2 | 400 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.88, 3.81] |
| 3.1.6 Cabergoline vs diosmin | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.85 [1.35, 6.00] |
| 3.2 Live birth rate Show forest plot | 2 | 430 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.73, 1.59] |
| 3.2.1 Cabergoline vs coasting | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.59, 1.83] |
| 3.2.2 Cabergoline vs calcium infusion | 1 | 230 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.66, 1.89] |
| 3.3 Clinical pregnancy rate Show forest plot | 7 | 1060 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.81, 1.33] |
| 3.3.1 Cabergoline vs human albumin | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.33, 1.38] |
| 3.3.2 Cabergoline vs coasting | 3 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.92, 2.32] |
| 3.3.3 Cabergoline vs calcium infusion | 2 | 400 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.67, 1.49] |
| 3.3.4 Cabergoline vs diosmin | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.51, 1.55] |
| 3.4 Multiple pregnancy rate Show forest plot | 3 | 400 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.47, 1.59] |
| 3.4.1 Cabergoline vs human albumin | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.13, 2.54] |
| 3.4.2 Cabergoline vs coasting | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.35 [0.25, 116.31] |
| 3.4.3 Cabergoline vs diosmin | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.41, 1.67] |
| 3.5 Miscarriage rate Show forest plot | 4 | 630 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.35, 1.25] |
| 3.5.1 Cabergoline vs human albumin | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.03, 3.19] |
| 3.5.2 Cabergoline vs coasting | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.06] |
| 3.5.3 Cabergoline vs calcium infusion | 1 | 230 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.27, 1.48] |
| 3.5.4 Cabergoline vs diosmin | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.36, 4.11] |
| 3.6 Any other adverse events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.6.1 Cabergoline vs calcium infusion | 1 | 170 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |

