Agonis dopamine untuk mencegah sindrom terlebih merangsang ovari
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled prospective study No details on randomisation Cabergoline vs no drugs Setting: Syria | |
| Participants | 272 high‐risk women undergoing ICSI with long protocol using GnRHa, E2 level on day of hCG ≥ 4000 pg/mL, ≥ 20 follicles ≥ 10 mm in diameter Quinagolide group: 136 women Control group: 136 women June 2007 to January 2010 | |
| Interventions | Quinagolide group: quinagolide (Norprolac) 150 mg/day from the day of hCG administration for 15 days (6/136 (4.41%) women developed OHSS) Control group: no drugs (126/136 (9.12%) women developed OHSS) | |
| Outcomes | OHSS symptoms assessed according to Gola's classification system, 4, 8 and 12 days after hCG administration Incidence of OHSS (quinagolide group vs control group): 6/136 vs 26/136 Live birth rate: not stated Miscarriage rate: not stated Clinical pregnancy rate: not stated, numbers reported as "similar rates" Multiple pregnancy rate: not stated Any other adverse effects of the treatment: not stated | |
| Notes | 2 different abstracts: in the Human Reproduction abstract: control group = 98 women, in the Fertility and Sterility abstract: control group = 136 women. This difference made it at risk for improper randomisation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly divided into two groups" |
| Allocation concealment (selection bias) | Unclear risk | Lack of sufficient information to permit judgement; only abstract available |
| Blinding (performance bias and detection bias) | Unclear risk | Lack of sufficient information to permit judgement; only abstract available |
| Incomplete outcome data (attrition bias) | Unclear risk | Lack of sufficient information to permit judgement; only abstract available |
| Selective reporting (reporting bias) | Unclear risk | Lack of sufficient information to permit judgement; only abstract available. No reporting on adverse effects or tolerability |
| Other bias | Unclear risk | Lack of sufficient information to permit judgement; only abstract available. 2 different abstracts with different control group size, suggesting improper randomisation |
| Methods | Parallel design, single‐centre randomised controlled trial Computer‐based randomisation Cabergoline vs placebo Setting: Spain | |
| Participants | 82 oocytes donors, high‐risk women with development of 20 to 30 follicles > 12 mm in diameter and retrieval of > 20 oocytes Exclusion criterion: coasting Cabergoline group: 41 women, only 35 women remained, because 6 women were discarded for < 20 oocytes retrieved Control group: 41 women, only 32 women remained, because 7 women were discarded for < 20 oocytes retrieved and 2 donors decided to withdraw No differences between groups in age or BMI; did not report the duration of infertility and causes of infertility | |
| Interventions | Cabergoline group: cabergoline tablet 0.5 mg/day for 8 days from the day of hCG injection Control group: placebo tablet daily for 8 days | |
| Outcomes | Moderate and severe OHSS identified by the modified classification of Golan and colleagues (Golan 1989)
Live birth rate: not stated Miscarriage rate: not stated Clinical pregnancy rate (cabergoline group vs control group): 16/41 vs 16/41 Multiple pregnancy rate: not stated Any other adverse effects of the treatment (cabergoline group vs control group): 8/41 vs 4/41 (adverse effects) | |
| Notes | Supported by Grant SAF2004‐06028 from Spanish Government | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were allocated into two groups based on a computer randomization" |
| Allocation concealment (selection bias) | Unclear risk | Lack of sufficient information to permit judgement |
| Blinding (performance bias and detection bias) | Low risk | Assessor and participants blinded |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "thirteen patients discarded for not meeting the inclusion criteria and two donors decided to withdraw" |
| Selective reporting (reporting bias) | Unclear risk | No exclusions (no live birth rated mentioned) |
| Other bias | Unclear risk | Lack of sufficient information to permit judgement |
| Methods | Parallel design, single‐centre, randomised controlled trial Computer‐based randomisation Cabergoline vs no intervention Setting: Israel | |
| Participants | 40 high‐risk women undergoing IVF/ET or IVF‐PGD, aged 18 to 40 years, serum E2 > 4000 pg/mL or the development of > 20 follicles > 12 mm in diameter Exclusion criteria: systemic disease and participating in other research studies Cabergoline group: 20 women Control group: 20 women | |
| Interventions | Cabergoline group: cabergoline tablet 0.5 mg/day for 8 days from the day of hCG injection Control group: no cabergoline | |
| Outcomes | Moderate and severe OHSS identified by the modified classification of Golan and colleagues (Golan 1989) assessed at day of ET, ET+7, ET+12
Live birth rate: not reported Miscarriage rate (cabergoline group vs control group): 0/20 vs 1/20 Clinical pregnancy rate (live heart beat) (cabergoline group vs control group): 2/20 vs 5/20 Multiple pregnancy rate (cabergoline group vs control group): 0/20 vs 1/20 Any other adverse effects of the treatment: not stated | |
| Notes | Did apply coasting to both groups in about 50% of women if serum E2 level > 5000 pg/mL | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Lack of sufficient data to permit judgement |
| Blinding (performance bias and detection bias) | Unclear risk | Neither participants nor physicians blinded, only ultrasound experts were blinded |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | No exclusions (no live birth rated mentioned) |
| Other bias | Unclear risk | Lack of sufficient data to permit judgement |
| Methods | Multicentre, prospective, randomised, double‐blind, placebo‐controlled study 3 clinics Bromocriptine vs folic acid Setting: Brazil | |
| Participants | 47 women aged < 38 years undergoing IVF with ≥ 20 follicles as assessed by transvaginal ultrasound and E2 > 3000 pg/mL on the day prior to hCG administration Exclusion criteria: hyperprolactinaemia; use of dopaminergic agents or other medications for the treatment of hyperprolactinaemia or pituitary tumours; systemic diseases, such as arterial hypertension, hypotension, orthostatic hypotension, cardiovascular disease and diabetes mellitus; polycystic ovaries Bromocriptine group: 23 women, 12/23 dropped out Folic acid group: 24 women, 7/24 dropped out | |
| Interventions | Bromocriptine group: bromocriptine 2.5 mg/day continued for 14 days Folic acid group (placebo): folic acid 2.0 mg/day continued for 14 days Capsules same appearance and form | |
| Outcomes | Incidence of OHSS (subgroups mild, moderate, severe), VEGF levels, urinary function Moderate and severe OHSS according to its OHSS criteria
Live birth rate: not stated Miscarriage rate: not stated Clinical pregnancy rate: not stated Multiple pregnancy rate: not stated Any other adverse effects of the treatment: not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated using a random number generation algorithm |
| Allocation concealment (selection bias) | Unclear risk | Lack of information to permit a judgement |
| Blinding (performance bias and detection bias) | Low risk | Double blind; medication and folic acid as a placebo in same appearance capsules |
| Incomplete outcome data (attrition bias) | High risk | High dropout numbers without dropout analysis |
| Selective reporting (reporting bias) | Unclear risk | No exclusions (no live birth rated mentioned) |
| Other bias | Unclear risk | Lack of information to permit a judgement |
| Methods | Randomised, parallel, double‐blind randomised controlled trial Quinagolide vs placebo Setting: Spain | |
| Participants | 182 women undergoing IVF and ICSI treatment and at risk of developing OHSS with ≥ 20 follicles of ≥ 10 mm on the day of hCG administration Exclusion criteria: > 30 follicles or serum E2 6000 pg/mL (or both) had cycle cancellation, previous coasting in this cycle, any clinically significant systemic disease, endocrine or metabolic abnormalities (pituitary, adrenal, pancreas, liver or kidney), history of recurrent miscarriage, undiagnosed vaginal bleeding Quinagolide 50 μg group: 51 women Quinagolide 100 μg group: 52 women Quinagolide 200 μg group: 26 women Control group: 53 women | |
| Interventions | 4 tablets for every woman (combination of placebo/quinagolide 50 μg) Quinagolide 50 μg group: quinagolide 50 μg + 3 placebo tablets once daily, continuing until the day before the serum hCG test which took place 17+2 days after oocyte retrieval Quinagolide 100 μg group: quinagolide 100 μg + 2 placebo tablets once daily, continuing until the day before the serum hCG test which took place 17+2 days after oocyte retrieval Quinagolide 200 μg group: quinagolide 200 μg + no placebo tablets once daily, continuing until the day before the serum hCG test which took place 17+2 days after oocyte retrieval Control group: 4 placebo tablets once daily, continuing until the day before the serum hCG test which took place 17+2 days after oocyte retrieval | |
| Outcomes | Moderate and severe OHSS identified by the modified classification of Golan and colleagues (Golan 1989)
Live birth rate (quinagolide 50 μg group vs quinagolide 100 μg group vs quinagolide 200 μg group vs placebo group): 23/51 vs 29/52 vs 14/26 vs 27/53 Miscarriage rate: not stated Clinical pregnancy rate (quinagolide 50 μg group vs quinagolide 100 μg group vs quinagolide 200 μg group vs placebo group): 22/51 vs 26/52 vs 11/26 vs 27/53 Multiple pregnancy rate: not stated Discontinued because of adverse events (quinagolide 50 μg group vs quinagolide 100 μg group vs quinagolide 200 μg group vs placebo group): 3/51 vs 7/52 vs 7/26 vs 0/53 Any other adverse effects of the treatment: nausea, dizziness, somnolence, diarrhoea, vomiting, lower abdominal pain, headache, abdominal distension, flatulence, upper abdominal pain, syncope | |
| Notes | Sponsored by Ferring Pharmaceuticals WHO registry reference: EUCTR2006‐000415‐15‐ES | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list prepared for each centre by a statistician not involved in the trial, and based on this the clinics were provided with individual code envelopes that were sealed to conceal the treatment group allocation |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomisation list provided to the clinics with individual code envelopes that were sealed to conceal the treatment group allocation. Block size was not disclosed |
| Blinding (performance bias and detection bias) | Low risk | Double blind (participants, staff and trial sponsor). All participants received 4 tablets (medication or placebo, or both) |
| Incomplete outcome data (attrition bias) | Low risk | Systematic OHSS evaluation performed; high‐dose arm stopped after poor tolerability of high‐dose medication |
| Selective reporting (reporting bias) | Low risk | Most of outcomes were evaluated |
| Other bias | High risk | Poor tolerability of high dose could have revealed allocated group Sponsored by Ferring Pharmaceuticals Very high‐risk women (> 30 follicles or serum E2 6000 pg/mL, or both) excluded and underwent cycle cancellation |
| Methods | Parallel, single‐centre randomised controlled trial Computer‐based randomisation Cabergoline vs no intervention Setting: Brazil | |
| Participants | 166 women undergoing IVF and ICSI treatment and at risk of developing OHSS, defined as serum E2 > 4000 pg/mL on the day of hCG administration Exclusion criteria: not stated Cabergoline group: 83 women Control group: 83 women, 3 women were withdrawn for not completing the follow‐up tests No differences between groups in age or BMI Did not report the duration of infertility and causes of infertility | |
| Interventions | All participants received routine preventive IV HA 20 g on the day of oocyte retrieval Cabergoline group: cabergoline 0.5 mg/day for 3 weeks from the day after oocyte retrieval Control group: no intervention | |
| Outcomes | Moderate and severe OHSS identified by the modified classification of Golan and colleagues (Golan 1989)
Live birth rate: not stated Miscarriage rate (cabergoline group vs control group): 1/83 vs 3/83 Clinical pregnancy rate (cabergoline group vs control group): 33/83 vs 32/83 Multiple pregnancy rate (cabergoline group vs control group): multiple pregnancies were documented in all the severe cases of OHSS in both groups (2/83 vs 1/83) Any other adverse effects of the treatment: not stated | |
| Notes | Authors reported no financial or commercial conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation |
| Allocation concealment (selection bias) | Unclear risk | Lack of sufficient information to permit judgement |
| Blinding (performance bias and detection bias) | Unclear risk | Lack of sufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Low risk | 3/200 women in control group could not complete their follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No exclusions (no live birth rated mentioned) |
| Other bias | Unclear risk | Lack of sufficient information to permit judgement |
| Methods | Single‐centre randomised controlled trial Computer‐based randomisation by independent research assistant Cabergoline vs coasting Setting: India | |
| Participants | 60 women undergoing IVF or ICSI cycles and at risk of developing OHSS, defined as the presence of preovulatory follicles ≥ 20 in both ovaries and the E2 level ≥ 2500 pg/mL Exclusion criteria: not stated Cabergoline group: 30 women Coasting group: 30 women | |
| Interventions | Cabergoline group: cabergoline 0.5 mg/day orally from the day of hCG for 8 days Coasting group: gonadotropins were withheld (while GnRHa was maintained), until the serum level of E2 started to decline in each group. 1 woman needed ascites tapped, and the remaining 29 women received 6% HES infusion | |
| Outcomes | Moderate and severe OHSS: classification not described but according to Golan (Golan 1989) criteria (from private correspondence with author)
Live birth rate: not stated Miscarriage rate (cabergoline group vs coasting group): 0/30 vs 2/30 Clinical pregnancy rate (defined as presence of gestational sac or cardiac activity 3 weeks after transfer) (cabergoline group vs coasting group): 8/30 vs 4/30 Multiple pregnancy rate (cabergoline group vs coasting group): 2/30 vs 0/30 Any other adverse effects of the treatment: cancelling of ET due to poor embryo quality (cabergoline group vs coasting group): 1/30 vs 1/30. Other adverse events not stated | |
| Notes | Received draft of full‐text article in peer review currently per private email; additional information per private correspondence with first author. 58 women received fluid of 6% HES and the remaining included woman received an ascites tap instead of HES. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of randomisation software (www.randomizer.org/) |
| Allocation concealment (selection bias) | Unclear risk | Independent research assistant allocated; concealment unclear |
| Blinding (performance bias and detection bias) | High risk | No blinding involved. The participants and clinicians were aware in which arm of the study they were |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts/loss of follow‐up in the 2 groups |
| Selective reporting (reporting bias) | Unclear risk | No exclusions (no live birth rated mentioned) |
| Other bias | High risk | OHSS identified/classification not described. 29 participants in both groups also received HES infusion, 1 participant from each group had ascites tap, unclear which participant was involved |
| Methods | Randomised trial based on blinded envelopes Cabergoline vs no intervention Setting: Russia | |
| Participants | 168 women included, but only 128 high‐risk women defined as transvaginal aspiration of ≥ 15 follicles Cabergoline group: 65 women Control group (no intervention): 63 women No significant difference between groups in somatic and obstetric anamnesis | |
| Interventions | Cabergoline group: cabergoline 0.5 mg/day from the day after oocyte retrieval for 5 days before ET day Control group: no intervention | |
| Outcomes | Moderate and severe OHSS, diagnosis OHSS not stated
Live birth rate: not stated Miscarriage rate (cabergoline group vs control group): 4/65 vs 6/63 Clinical pregnancy rate (cabergoline group vs control group): 21/65 vs 23/63 Multiple pregnancy rate: not stated Any other adverse effects of the treatment: not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Lack of information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Blinded envelopes method |
| Blinding (performance bias and detection bias) | Unclear risk | Lack of sufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No exclusions (no live birth rated mentioned) |
| Other bias | Unclear risk | Lack of sufficient information to permit judgement |
| Methods | Randomised controlled trial based on random number table Cabergoline vs albumin vs HES Setting: Iran | |
| Participants | 91 high‐risk women with E2 > 3000 pg/mL or > 20 follicles on the day of hCG administration or previous history of OHSS, or a combination Cabergoline group: 31 women Albumin group: 30 women HES group: 30 women No significant difference between groups regarding gravidity, parity, death, ectopic pregnancy, abortion and mean age | |
| Interventions | Cabergoline group: cabergoline 0.5 mg daily for 7 days after oocyte retrieval Albumin group: 2 vials (2 × 50 mL) HAs IV 30 minutes after oocyte retrieval within 4 hours HES group: 1000 mL of 6% HES IV 30 minutes after oocyte retrieval within 4 hours | |
| Outcomes | Moderate and severe OHSS identified by the classification of Golan and colleagues (Golan 1989)
Live birth rate: not stated Miscarriage rate: not stated Clinical pregnancy rate: not stated Multiple pregnancy rate: not stated Any other adverse effects of the treatment: not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Lack of sufficient information to permit judgement |
| Blinding (performance bias and detection bias) | Unclear risk | Lack of sufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No exclusions (no live birth rated mentioned) |
| Other bias | Unclear risk | Lack of sufficient information to permit judgement |
| Methods | Single‐centre, prospective randomised study ("randomly divided in two groups") Cabergoline vs no medication Setting: Tunisia | |
| Participants | 146 women undergoing IVF or ICSI and receiving GnRHa. OHSS risk defined as a plasma E2 level > 3000 pg/mL on the day of hCG administration or the development of ≥ 18 follicles > 12 mm in diameter, or both Exclusion criteria: coasting cases, aged > 40 years, history of uterine surgery, and submucosal and intramural fibromas > 5 cm Cabergoline group: 78 women Control group: 68 women | |
| Interventions | Cabergoline group: cabergoline 0.5 mg/day for 8 days starting on the day of hCG injection Control group (no intervention): no medication treatment | |
| Outcomes | Moderate and severe OHSS identified according to the criteria of Golan and colleagues (Golan 1989)
Live birth rate: not stated Miscarriage rate: only reported for women who developed OHSS (cabergoline group vs control group): 3/25 vs 6/25 Clinical pregnancy rate only reported for women who developed OHSS (cabergoline group vs control group): 20/25 vs 14/25 Multiple pregnancy rate: not stated Any other adverse effects of the treatment: not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Lack of information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Lack of information to permit judgement |
| Blinding (performance bias and detection bias) | Unclear risk | Lack of information to permit judgement |
| Incomplete outcome data (attrition bias) | High risk | No follow‐up data from the non‐OHSS women in both groups, no data on possible loss to follow‐up or dropout |
| Selective reporting (reporting bias) | High risk | Pregnancy data from the non‐OHSS women in both groups not reported |
| Other bias | High risk | Coasting cases (women at highest risk for severe OHSS) were excluded, unclear based on what criteria coasting was opted for |
| Methods | Blinded randomised controlled trial Randomisation based on computer‐generated numbers in sequentially numbered sealed envelopes Cabergoline + 6% HES vs 6% HES Setting: Spain | |
| Participants | 182 women undergoing IVF using their own oocytes and receiving GnRHa treatment and considered at risk of OHSS (all aged < 40 years). OHSS risk defined as a plasma E2 level > 3000 pg/mL on the day of hCG administration or development of 20 follicles >12 mm, or both Exclusion criteria: E2 levels > 5000 pg/mL where cycles were cancelled Cabergoline group: 88 women Control group: 94 women | |
| Interventions | Cabergoline group: slow IV infusion of 500 mL of 6% HES during follicle aspiration plus cabergoline 0.5 mg orally for 8 days starting on day of hCG administration Control group: slow IV infusion of 500 mL of 6% HES during follicle aspiration | |
| Outcomes | Moderate and severe OHSS identified by the modified classification of Golan and colleagues (Golan 1989)
Live birth rate: not stated Miscarriage rate (cabergoline + HES group vs control group): 5/88 vs 9/94 Clinical pregnancy rate (cabergoline + HES group vs control group): 43/88 vs 48/94 Multiple pregnancy rate: not stated Any other adverse effects of the treatment: not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using computer‐generated numbers |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed envelopes were used |
| Blinding (performance bias and detection bias) | Unclear risk | Both the embryologists and the gynaecologists performing oocyte aspiration, ET and post‐transfer follow‐up, were blinded to the co‐administration of cabergoline. Participants were not blinded; however, low risk of causing bias |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No exclusions (no live birth rated mentioned) |
| Other bias | Unclear risk | High‐risk cycles were cancelled (E2 > 5000 pg/mL), which might have excluded severe OHSS cases |
| Methods | Blinded randomised controlled trial Cabergoline vs prednisolone vs no intervention Setting: United Arab Emirates | |
| Participants | 200 women with polycystic ovarian syndrome undergoing IVF treatment and possibility of developing OHSS Exclusion criteria: previous oophorectomy, immune diseases that affect the permeability of blood vessels, such as systemic lupus, disseminated sclerosis and rheumatoid arthritis Cabergoline group: 75 women, 2 women lost to follow‐up Prednisolone group: 75 women, 3 women lost to follow‐up Control group (no intervention): 50 women, 2 women lost to follow‐up | |
| Interventions | Cabergoline group: cabergoline 0.5 mg tablets, 1 tablet on 2 successive days, starting from the day of hCG injection, and repeated 1 week later Prednisolone group: prednisolone 10 mg tablets twice a day to day of pregnancy test Control group: no intervention | |
| Outcomes | Moderate and severe OHSS identified by the modified classification of Golan and colleagues (Golan 1989)
Live birth rate: not stated Miscarriage rate: not stated Clinical pregnancy rate: not stated Multiple pregnancy rate: not stated Any other adverse effects of the treatment: not stated | |
| Notes | No high‐risk women identified (e.g. based on E2 or ultrasound) except that this population was young women with PCOS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Lack of information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) | Low risk | Blind to the participants |
| Incomplete outcome data (attrition bias) | Low risk | 7/200 women after randomisation could not complete their follow‐up, no reasons stated |
| Selective reporting (reporting bias) | Unclear risk | No exclusions (no live birth rated mentioned) |
| Other bias | High risk | No high‐risk women identified (e.g. based on E2 or ultrasound) except this population was young women with PCOS |
| Methods | Randomised controlled trial Computer‐based randomisation Cabergoline vs no intervention Setting: Egypt | |
| Participants | 200 women undergoing ICSI treatment and at risk of developing OHSS, defined by E2 level on day of hCG > 3500 pg/mL with ≥ 20 follicles > 12 mm diameter Cabergoline group: 100 women; 2 had empty follicles, 2 had failure of fertilisation and 1 discontinued Control group: 100 women; 3 had empty follicles and 1 had failure of fertilisation Exclusion criterion: E2 ≥ 5000 pg/mL No differences between the groups in age, BMI and causes of infertility | |
| Interventions | Cabergoline group: cabergoline tablet 0.25 mg/day for 8 days from the day of hCG injection Control group: no intervention | |
| Outcomes | Moderate and severe OHSS identified according to Golan and colleagues (Golan 1989)
Live birth rate (cabergoline group vs control group): 37/100 vs 36/100 Miscarriage rate (cabergoline group vs control group): 5/100 vs 5/100 Clinical pregnancy rate (cabergoline group vs control group): 42/100 vs 41/100 Multiple pregnancy rate: not stated Any other adverse effects of the treatment: not stated | |
| Notes | Number of women excluded for dropout (no ET because no oocytes found, no embryos yielded, etc., 1 adverse event) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation method |
| Allocation concealment (selection bias) | Unclear risk | Lack of sufficient information to permit judgement |
| Blinding (performance bias and detection bias) | Unclear risk | Lack of sufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Low risk | 9 women could not complete their follow‐up but exact reasons not stated |
| Selective reporting (reporting bias) | Low risk | Most outcomes were included |
| Other bias | Unclear risk | Lack of sufficient information to permit judgement |
| Methods | Parallel design, randomised controlled trial Block randomisation Cabergoline vs coasting Setting: Iran | |
| Participants | 60 women at risk of OHSS defined by ≥ 20 follicles in both ovaries, most being ≤ 14 mm in diameter and serum E2 level 3000 pg/mL Cabergoline group: 30 women Coasting group: 30 women Exclusion criterion: contraindication to dopamine agonists No significant differences between groups in age, BMI, menstrual cycle pattern, duration of infertility and causes of infertility | |
| Interventions | Cabergoline group: cabergoline tablet 0.5 mg/day for 7 days after hCG administration Coasting group: gonadotropin administration was ceased until serum E2 levels reached < 3000 pg/mL before hCG administration | |
| Outcomes | Moderate and severe OHSS identified by the classification of Golan and colleagues (Golan 1989)
Live birth rate: not stated Miscarriage rate: not stated Clinical pregnancy rate (cabergoline group vs coasting group): 14/30 vs 7/30 Multiple pregnancy rate: not stated Any other adverse effects of the treatment: not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table blocks according to Biostatistics in Health Systems |
| Allocation concealment (selection bias) | Unclear risk | Lack of sufficient information to permit judgement |
| Blinding (performance bias and detection bias) | Unclear risk | Lack of sufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No exclusions (no live birth rated mentioned) |
| Other bias | Unclear risk | Lack of sufficient information to permit judgement |
| Methods | Parallel, single‐centre randomised controlled trial Not blinded Computer‐based randomisation Cabergoline vs HA Setting: Iran | |
| Participants | 140 women aged 15 to 37 years Inclusion criteria: risk of developing OHSS, defined by the development of 20 to 30 follicles > 12 mm in diameter on the day of hCG administration and retrieval of > 20 oocytes, ovarian stimulation with long protocol Exclusion criteria: coasting cases, aged > 37 years, previous uterine surgery, intramural or submucosal myoma sizes > 5 cm Cabergoline group: 70 women, 1 woman lost to follow‐up Albumin group: 70 women, 1 woman lost to follow‐up No differences between groups in age, BMI, duration of infertility, type of infertility, basal FSH, LH levels and E2 levels on the day of hCG administration but there was a difference in cause of infertility. | |
| Interventions | Cabergoline group: cabergoline tablet 0.5 mg/day 7 days beginning on day of oocyte retrieval Control group: HA 20% IV infusion | |
| Outcomes | Moderate and severe OHSS identified by the modified classification of Golan and colleagues (Golan 1989)
Live birth rate: not stated Miscarriage rate (cabergoline group vs control group): 1/70 vs 3/70 Clinical pregnancy rate (cabergoline group vs control group): 20/70 vs 26/70 Multiple pregnancy rate (cabergoline group vs control group): 3/70 vs 5/70 Any other adverse effects of the treatment: not stated | |
| Notes | 1 dropout in each group. Not reported when they dropped out or if they had even started. Excluded from analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation method |
| Allocation concealment (selection bias) | Unclear risk | Lack of sufficient information to permit judgement |
| Blinding (performance bias and detection bias) | Unclear risk | Midwife open the sealed envelopes |
| Incomplete outcome data (attrition bias) | Low risk | 2/140 women lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No exclusions (no live birth rated mentioned) |
| Other bias | Unclear risk | Lack of sufficient information to permit judgement |
| Methods | Single‐centre, randomised controlled trial Blinded for sampling. No statement on blinding for allocation Randomisation not described Cabergoline vs HA Setting: Iran | |
| Participants | 95 women, every other participant sampled. > 20 oocytes during oocyte retrieval, ovary size > 10 cm, serum E2 > 2500 pg/mL, considered eligible if high risk with > 20 follicles; randomisation when confirmed > 20 follicles retrieved in both ovaries at day of hCG injection Exclusion criterion: < 20 oocytes retrieved Cabergoline group: 47 women Albumin group: 48 women | |
| Interventions | Cabergoline group: cabergoline 0.5 mg/day oral from day of hCG injection to 8 days Control group: 10 units IV HA at the start of oocyte retrieval | |
| Outcomes | Moderate and severe OHSS; identified/classification not described other than "classified according to related criteria"
Live birth rate: not stated Miscarriage rate: not stated Clinical pregnancy rate: not stated Multiple pregnancy rate: not stated Any other adverse effects of the treatment: not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The method of sampling was randomized sampling as we selected every other person. Randomization was used to allocate the patients to two groups immediately after confirmation of retrieval of >20 oocytes. but intervention started already on day 2 before retrieval (hCG administration)"! |
| Allocation concealment (selection bias) | Unclear risk | Lack of sufficient information to permit judgement |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "physician who controlled the patients was blind" |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No exclusions (no live birth rated mentioned) |
| Other bias | Unclear risk | Lack of sufficient information to permit judgement |
BMI: body mass index; E2: oestradiol; ET: embryo transfer; FSH: follicle‐stimulating hormone; GnRH: gonadotropin‐releasing hormone; GnRHa: gonadotropin‐releasing hormone agonist; HA: human albumin; hCG: human chorionic gonadotrophin; HES: hydroxyethyl starch; ICSI: intracytoplasmic sperm injection; IV: intravenous; IVF: in vitro fertilisation; LH: luteinising hormone; OHSS: ovarian hyperstimulation syndrome; PGD: preimplantation genetic diagnosis; VEGF: vascular endothelial growth factor; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not randomised, "divided into two groups according to patients convenience" | |
| Not an RCT; historic control group | |
| A pilot study, not an RCT | |
| Case report | |
| Studied co‐intervention on top of cabergoline rather than cabergoline | |
| Only women who were already developed signs of (mild) OHSS included | |
| Retrospective analysis, not an RCT | |
| Case control study, not an RCT | |
| A retrospective study, not an RCT | |
| Not an RCT | |
| Not an RCT | |
| Quasi‐randomised, odd/even participants appointed to intervention groups | |
| Case series | |
| Retrospective cohort study | |
| Quasi‐randomised (odd and even numbers) | |
| 2 differently timed cabergoline regimens, no control group | |
| Historical matched control group | |
| Not an RCT | |
| Retrospective study | |
| Evaluated 2 different cabergoline regimens on prevention of OHSS |
OHSS: ovarian hyperstimulation syndrome; RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Prospective randomised controlled trial Cabergoline vs human albumin |
| Participants | 112 high‐risk women undergoing ART Cabergoline group: 56 women Albumin group: 56 women No statistically significant differences in age, BMI, number of follicles and oocyte retrieved, and serum E2 on the day of hCG injection |
| Interventions | Cabergoline group: cabergoline tablet 0.5 mg/day until 12 days from oocytes retrieval Albumin group: 20 g IV human albumin on the day of oocyte retrieval |
| Outcomes | The OHSS frequency was significantly lower in the cabergoline group (P < 0.001). There were no significant differences in pregnancy rate, implantation and miscarriages between groups |
| Notes | Meeting abstract, no numbers mentioned, no response from authors yet |
ART: assisted reproduction technology; BMI: body mass index; E2: oestradiol; hCG: human chorionic gonadotrophin; IV: intravenous; OHSS: ovarian hyperstimulation syndrome.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Cabergoline and Coasting to Prevent OHSS; Combining Cabergoline and Coasting in Gonadotropin Releasing Hormone(GnRH)Agonist Protocol in Intracytoplasmic Sperm Injection (ICSI) to Prevent Ovarian Hyperstimulation Syndrome (OHSS): a Randomized Clinical Trial |
| Methods | RCT To randomly compare 3 study groups involving high‐risk women to 1 of 3 arms of management, either coasting for 1 to 3 days or receiving cabergoline for 8 days or coasting for 1 day plus receiving cabergoline for 8 days in women undergoing ICSI following the long luteal GnRHa protocol |
| Participants | Women undergoing ICSI Inclusion criteria:
Exclusion criteria:
|
| Interventions | Group 1: active comparator: coasting. In their ICSI cycle, participants will continue their agonist treatment while stopping the hMG injections for 1 to 3 days until drop of E2 to a safe level to prevent OHSS. Early OHSS assessed at day of ET and 7 days after this date. Late OHSS assessed 14 days after ET Group 2: active comparator: cabergoline. In their ICSI cycle, participants will take cabergoline 0.25 mg/day for 8 days from hCG triggering day to prevent OHSS. Early OHSS assessed at day of ET and 7 days after this date. Late OHSS assessed 14 days after ET Group 3: active comparator: coasting + cabergoline. In their ICSI cycle, participants will continue their agonist treatment while stopping the hMG injections for 1 day plus receiving cabergoline 0.25 mg/day for 8 days from hCG triggering day to prevent OHSS. Early OHSS assessed at day of ET and 7 days after this date. Late OHSS assessed 14 days after ET |
| Outcomes | Primary outcomes:
Secondary outcomes:
Other outcomes:
|
| Starting date | |
| Contact information | |
| Notes |
| Trial name or title | Comparative Study Between Cabergoline and Intravenous Calcium in the Prevention of Ovarian Hyperstimulation in Women with Polycystic Ovarian Disease Undergoing Intracytoplasmic Sperm Injection (ICSI) |
| Methods | |
| Participants | |
| Interventions | Cabergoline group: cabergoline (Dostinex) 0.5 mg/day oral tablets for 8 days from the day of hCG injection. Once in the trial To monitor the adherence to the medication, we ask the participant for the drug tablet return |
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Starting date | July 2013 |
| Contact information | |
| Notes |
| Trial name or title | Study of Cabergoline for Prevention of Ovarian Hyperstimulation Syndrome (OHSS) in In Vito Fertilization Cycles and Derivation of OHSS Biomarkers |
| Methods | Randomised controlled trial Endpoint classification: efficacy study Intervention model: parallel assignment Masking: double blind (participant, carer, investigator, outcomes assessor) Primary purpose: prevention |
| Participants | Inclusion criterion:
Exclusion criteria:
|
| Interventions | Cabergoline group: cabergoline 0.5 mg tablet, 1 tablet daily for 8 days Control group: placebo 1 tablet daily for 8 days |
| Outcomes | Primary outcome:
Secondary outcome:
|
| Starting date | 15 February 2012 |
| Contact information | |
| Notes | NCT01535859 |
| Trial name or title | Effect of Cabergoline on Endometrial Vascularity During Intracytoplasmic Sperm Injection |
| Methods | Allocation: non‐randomised Endpoint classification: efficacy study Intervention model: parallel assignment Masking: open label Primary purpose: diagnostic |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Cabergoline group: women AT RISK of OHSS receiving cabergoline 0.5 mg/day for 8 days from the day of oocyte pickup for prevention of hyperstimulation Control group: women AT RISK of OHSS not receiving cabergoline Control group 2: will serve as a control group and will include age‐ and BMI‐matched women NOT AT RISK of OHSS, and not receiving cabergoline |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | December 2014 |
| Contact information | |
| Notes | NCT02306564 |
| Trial name or title | Diosmin versus Cabergoline for Prevention of Ovarian Hyperstimulation Syndrome (Infertility) |
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: single group assignment Masking: single blind (participant) Primary purpose: prevention |
| Participants | 200 women at risk of OHSS during ICSI cycles will be randomly scheduled into 2 equal groups Inclusion criteria: infertile women undergoing ICSI or polycystic ovarian syndrome, aged 23 to 48 years with 1 of the following:
Exclusion criteria: none |
| Interventions | Diosmin group: diosmin 2 × 500 mg tablets every 8 hours will be given from day of hCG injection for 14 days Cabergoline group: cabergoline 1 × 0.5 mg tablet/day will be given from day of hCG injection for 8 days |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | May 2014 |
| Contact information | |
| Notes | NCT02134249 |
| Trial name or title | Cabergoline and Hydroxyethyl Starch in Ovarian Hyperstimulation Syndrome Prevention |
| Methods | Randomised open, parallel trial |
| Participants | Women aged 18 to 40 years Inclusion criterion:
Exclusion criterion:
|
| Interventions | Cabergoline group: slow infusion of 500 mL of 6% HES during follicular aspiration alone or combined with cabergoline 0.5 mg administration for 8 days, starting on the day of hCG administration Control group: slow infusion of 500 mL of 6% HES during follicular aspiration |
| Outcomes | Primary outcome: risk of OHSS Secondary outcome: pregnancy rate |
| Starting date | August 2007 |
| Contact information | None |
| Notes | NCT01530490; this is the Matorras 2013 paper |
3D: 3‐dimensional; β‐hCG: β‐human chorionic gonadotrophin; BMI: body mass index; COH: controlled ovarian hyperstimulation; E2: oestradiol; ET: embryo transfer; GnRH: gonadotropin‐releasing hormone; GnRHa: gonadotropin‐releasing hormone agonist; hCG: human chorionic gonadotrophin; HES: hydroxyethyl starch; hMG: human menopausal gonadotropin; ICSI: intracytoplasmic sperm injection; OHSS: ovarian hyperstimulation syndrome; RCT: randomised controlled trial.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Moderate or severe ovarian hyperstimulation syndrome (OHSS) Show forest plot | 8 | 1022 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.19, 0.39] |
| Analysis 1.1  Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 1 Moderate or severe ovarian hyperstimulation syndrome (OHSS). | ||||
| 1.1 Cabergoline vs placebo/no treatment | 5 | 521 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.16, 0.42] |
| 1.2 Quinagolide vs placebo | 2 | 454 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.15, 0.51] |
| 1.3 Bromocriptine vs placebo (folic acid) | 1 | 47 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.08, 1.14] |
| 2 Subgroup analysis by severity of OHSS Show forest plot | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2 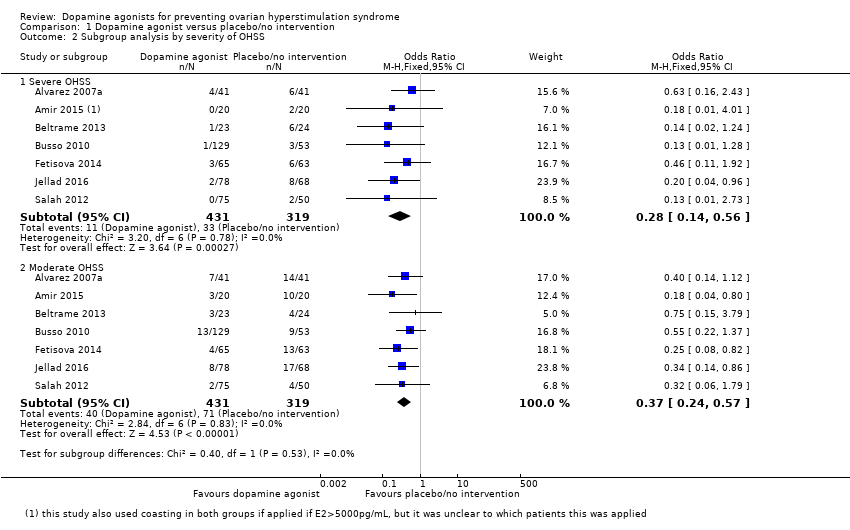 Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 2 Subgroup analysis by severity of OHSS. | ||||
| 2.1 Severe OHSS | 7 | 750 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.14, 0.56] |
| 2.2 Moderate OHSS | 7 | 750 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.24, 0.57] |
| 3 Live birth Show forest plot | 1 | 182 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.53, 1.91] |
| Analysis 1.3 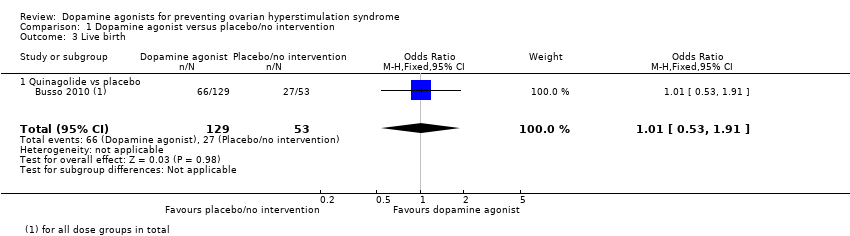 Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 3 Live birth. | ||||
| 3.1 Quinagolide vs placebo | 1 | 182 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.53, 1.91] |
| 4 Clinical pregnancy rate Show forest plot | 4 | 432 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.54, 1.22] |
| Analysis 1.4  Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 4 Clinical pregnancy rate. | ||||
| 4.1 Cabergoline vs no intervention | 3 | 250 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.48, 1.38] |
| 4.2 Quinagolide vs placebo | 1 | 182 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.43, 1.54] |
| 5 Multiple pregnancy Show forest plot | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.26] |
| Analysis 1.5  Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 5 Multiple pregnancy. | ||||
| 5.1 Cabergoline vs placebo/no treatment | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.26] |
| 6 Miscarriage Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 6 Miscarriage. | ||||
| 6.1 Cabergoline vs placebo/no treatment | 2 | 168 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.19, 2.28] |
| 7 Adverse events Show forest plot | 2 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.54 [1.49, 13.84] |
| Analysis 1.7 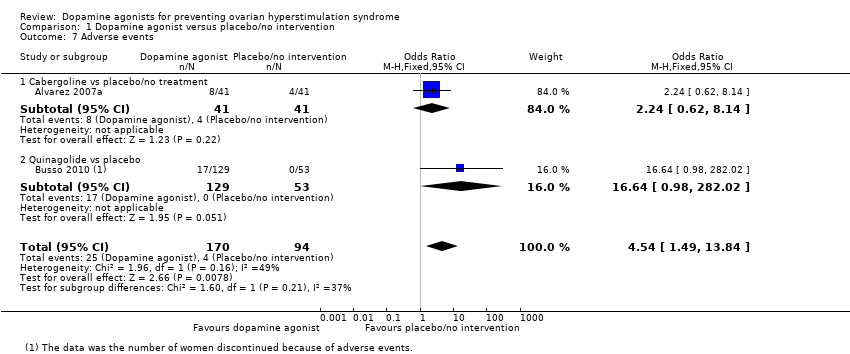 Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 7 Adverse events. | ||||
| 7.1 Cabergoline vs placebo/no treatment | 1 | 82 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.24 [0.62, 8.14] |
| 7.2 Quinagolide vs placebo | 1 | 182 | Odds Ratio (M‐H, Fixed, 95% CI) | 16.64 [0.98, 282.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Moderate or severe ovarian hyperstimulation syndrome (OHSS) Show forest plot | 3 | 548 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.31, 1.03] |
| Analysis 2.1 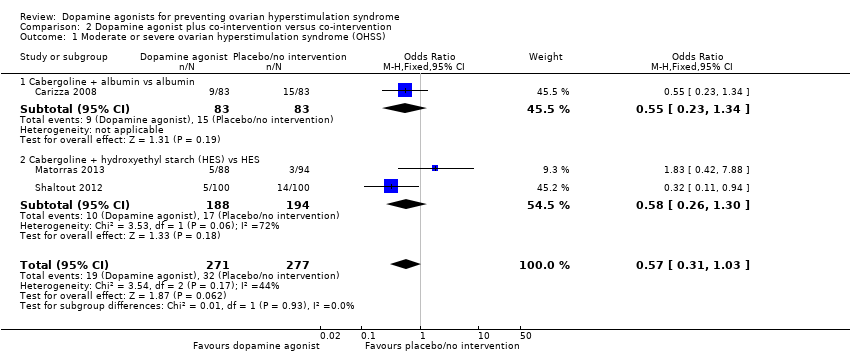 Comparison 2 Dopamine agonist plus co‐intervention versus co‐intervention, Outcome 1 Moderate or severe ovarian hyperstimulation syndrome (OHSS). | ||||
| 1.1 Cabergoline + albumin vs albumin | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.23, 1.34] |
| 1.2 Cabergoline + hydroxyethyl starch (HES) vs HES | 2 | 382 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.26, 1.30] |
| 2 Live birth Show forest plot | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.59, 1.86] |
| Analysis 2.2 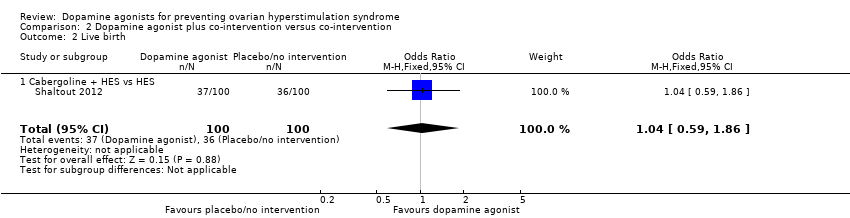 Comparison 2 Dopamine agonist plus co‐intervention versus co‐intervention, Outcome 2 Live birth. | ||||
| 2.1 Cabergoline + HES vs HES | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.59, 1.86] |
| 3 Clinical pregnancy rate Show forest plot | 3 | 548 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.71, 1.40] |
| Analysis 2.3  Comparison 2 Dopamine agonist plus co‐intervention versus co‐intervention, Outcome 3 Clinical pregnancy rate. | ||||
| 3.1 Cabergoline + albumin vs albumin | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.56, 1.96] |
| 3.2 Cabergoline + HES vs HES | 2 | 382 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.65, 1.47] |
| 4 Multiple pregnancy Show forest plot | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.18, 22.77] |
| Analysis 2.4  Comparison 2 Dopamine agonist plus co‐intervention versus co‐intervention, Outcome 4 Multiple pregnancy. | ||||
| 4.1 Cabergoline + albumin vs albumin | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.18, 22.77] |
| 5 Miscarriage Show forest plot | 3 | 548 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.30, 1.42] |
| Analysis 2.5 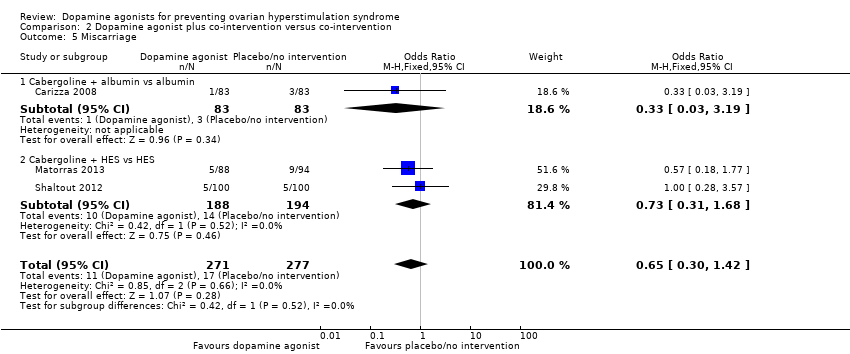 Comparison 2 Dopamine agonist plus co‐intervention versus co‐intervention, Outcome 5 Miscarriage. | ||||
| 5.1 Cabergoline + albumin vs albumin | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.19] |
| 5.2 Cabergoline + HES vs HES | 2 | 382 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.31, 1.68] |
| 6 Adverse events Show forest plot | 2 | 366 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 75.28] |
| Analysis 2.6  Comparison 2 Dopamine agonist plus co‐intervention versus co‐intervention, Outcome 6 Adverse events. | ||||
| 6.1 Cabergoline + albumin vs albumin | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Cabergoline + HES vs HES | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 75.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Moderate or severe ovarian hyperstimulation syndrome (OHSS) Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Dopamine agonist versus active interventions, Outcome 1 Moderate or severe ovarian hyperstimulation syndrome (OHSS). | ||||
| 1.1 Cabergoline vs human albumin | 3 | 296 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.12, 0.38] |
| 1.2 Cabergoline vs prednisolone | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.05, 1.33] |
| 1.3 Cabergoline vs hydroxyethyl starch (HES) | 1 | 61 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.48, 15.10] |
| 1.4 Cabergoline vs coasting | 2 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.18, 1.45] |
| 2 Clinical pregnancy rate Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 Dopamine agonist versus active interventions, Outcome 2 Clinical pregnancy rate. | ||||
| 2.1 Cabergoline vs human albumin | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.33, 1.38] |
| 2.2 Cabergoline vs coasting | 2 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.65 [1.13, 6.21] |
| 3 Multiple pregnancy Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.3 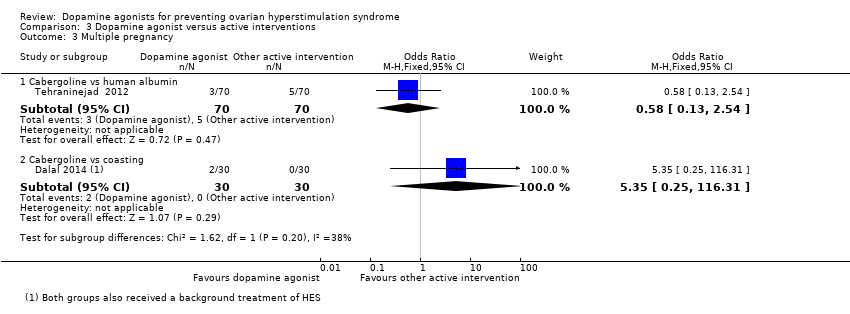 Comparison 3 Dopamine agonist versus active interventions, Outcome 3 Multiple pregnancy. | ||||
| 3.1 Cabergoline vs human albumin | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.13, 2.54] |
| 3.2 Cabergoline vs coasting | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.35 [0.25, 116.31] |
| 4 Miscarriage Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.4 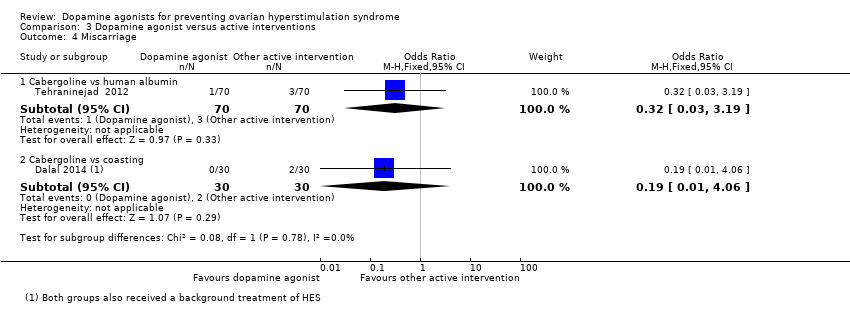 Comparison 3 Dopamine agonist versus active interventions, Outcome 4 Miscarriage. | ||||
| 4.1 Cabergoline vs human albumin | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.03, 3.19] |
| 4.2 Cabergoline vs coasting | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.06] |

Study flow diagram search August 2016.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison 1: Dopamine agonist (without co‐intervention) versus placebo/no intervention, outcome: 1.1 moderate or severe ovarian hyperstimulation syndrome.
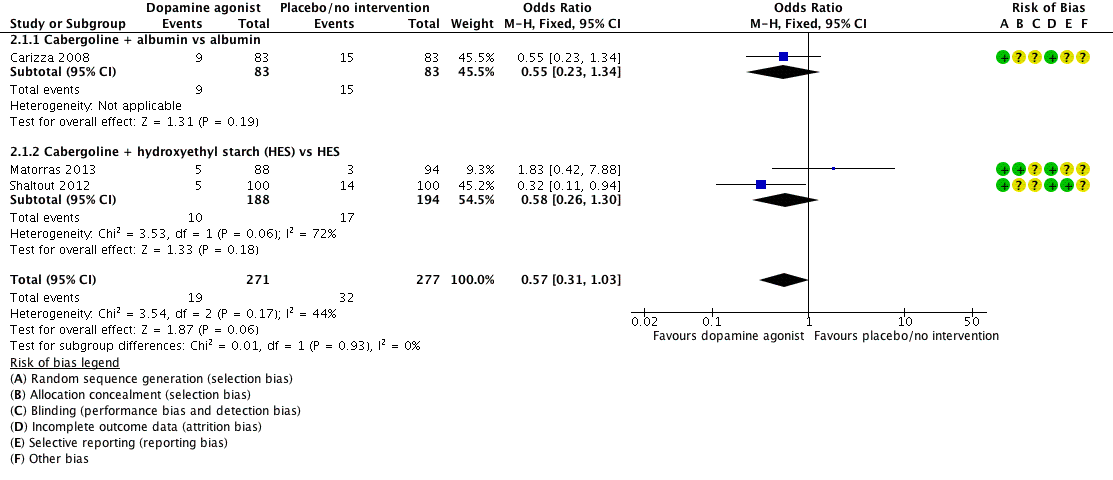
Forest plot of comparison: 2 Dopamine agonist plus co‐intervention versus co‐intervention, outcome: 2.1 Moderate or severe ovarian hyperstimulation syndrome.
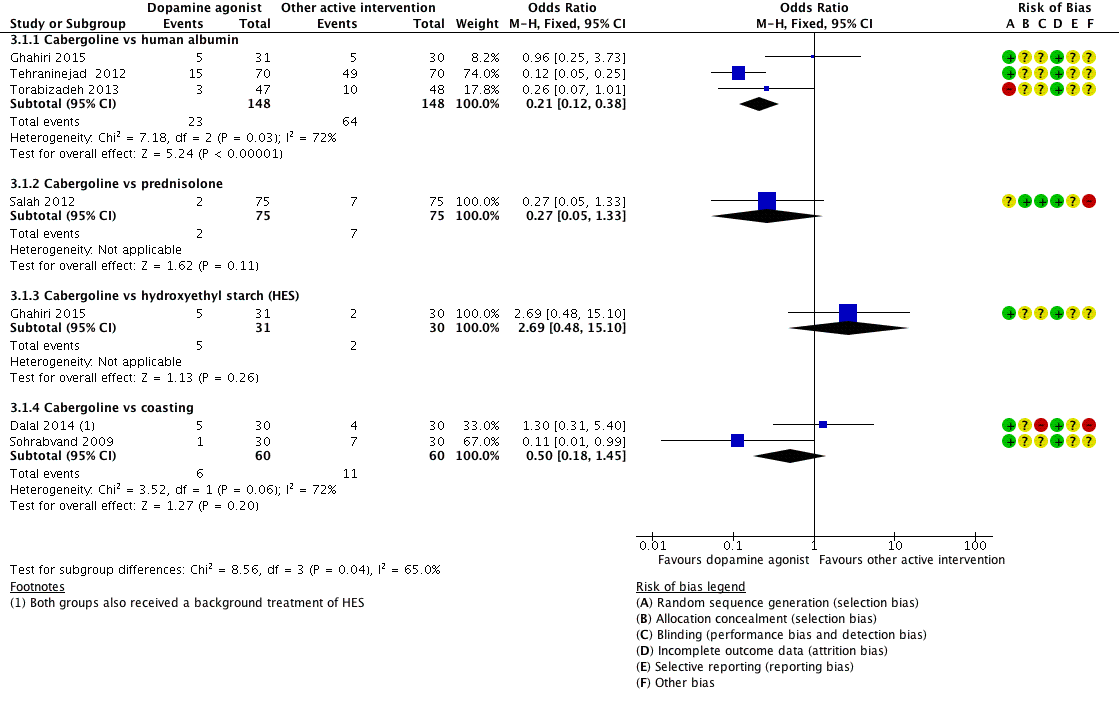
Forest plot of comparison 3: Cabergoline versus active interventions, outcome: 3.1 moderate or severe ovarian hyperstimulation syndrome.

Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 1 Moderate or severe ovarian hyperstimulation syndrome (OHSS).

Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 2 Subgroup analysis by severity of OHSS.

Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 3 Live birth.

Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 4 Clinical pregnancy rate.

Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 5 Multiple pregnancy.

Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 6 Miscarriage.
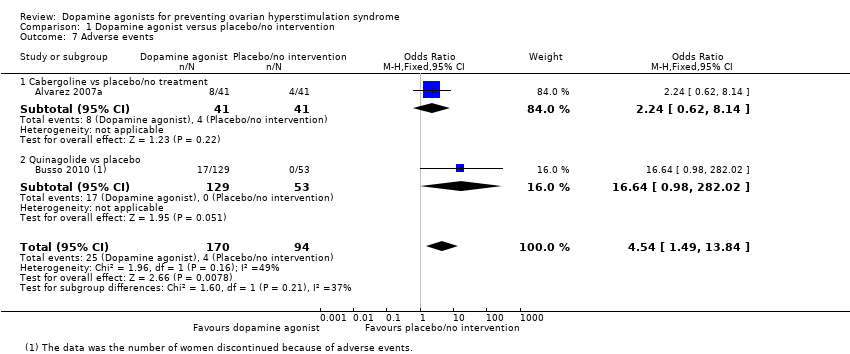
Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 7 Adverse events.
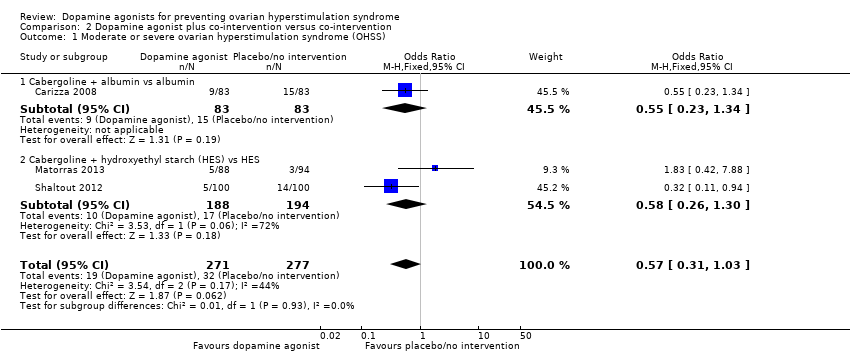
Comparison 2 Dopamine agonist plus co‐intervention versus co‐intervention, Outcome 1 Moderate or severe ovarian hyperstimulation syndrome (OHSS).
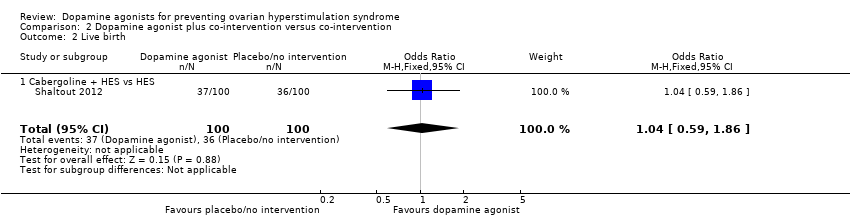
Comparison 2 Dopamine agonist plus co‐intervention versus co‐intervention, Outcome 2 Live birth.

Comparison 2 Dopamine agonist plus co‐intervention versus co‐intervention, Outcome 3 Clinical pregnancy rate.
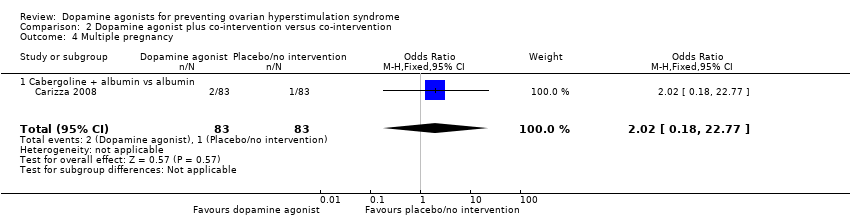
Comparison 2 Dopamine agonist plus co‐intervention versus co‐intervention, Outcome 4 Multiple pregnancy.
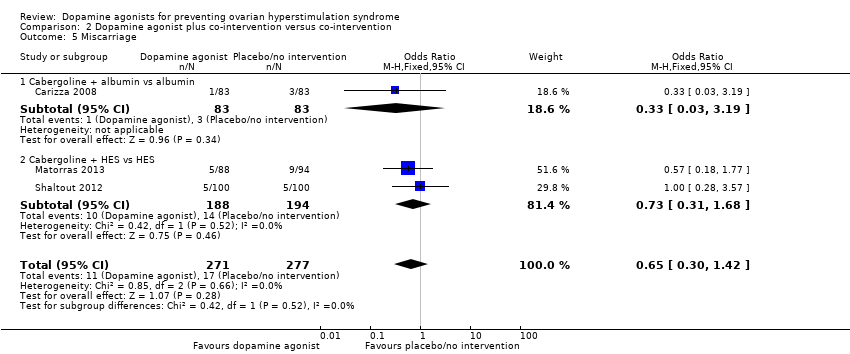
Comparison 2 Dopamine agonist plus co‐intervention versus co‐intervention, Outcome 5 Miscarriage.

Comparison 2 Dopamine agonist plus co‐intervention versus co‐intervention, Outcome 6 Adverse events.

Comparison 3 Dopamine agonist versus active interventions, Outcome 1 Moderate or severe ovarian hyperstimulation syndrome (OHSS).
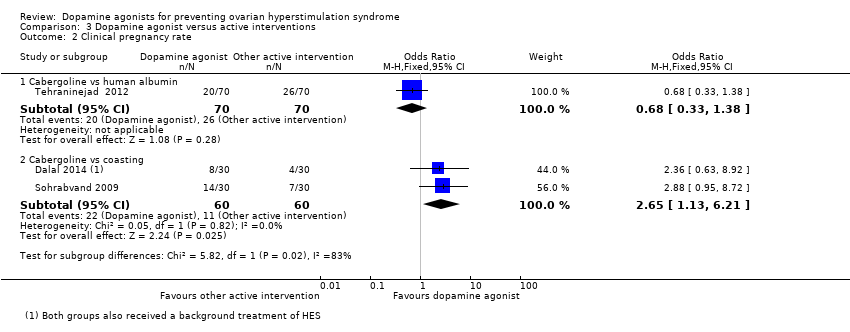
Comparison 3 Dopamine agonist versus active interventions, Outcome 2 Clinical pregnancy rate.
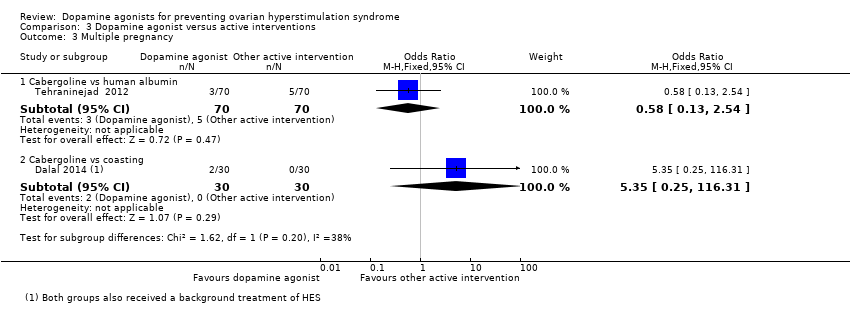
Comparison 3 Dopamine agonist versus active interventions, Outcome 3 Multiple pregnancy.

Comparison 3 Dopamine agonist versus active interventions, Outcome 4 Miscarriage.
| Dopamine agonist vs placebo/no intervention | ||||||
| Patient or population: women of reproductive age undergoing any ART therapy Settings: ART unit Intervention: dopamine agonist Comparison: placebo/no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with placebo/no intervention | Risk with dopamine agonist | |||||
| Incidence of moderate or severe OHSS | 286 per 1000 | 97 per 1000 (71 to 135) | OR 0.27 (0.19 to 0.39) | 1022 (8 studies) | ⊕⊕⊕⊝ | ‐ |
| Live birth rate | 509 per 1000 | 512 per 1000 (355 to 665) | OR 1.01 (0.53 to 1.91) | 182 | ⊕⊕⊝⊝ | |
| Clinical pregnancy rate | 401 per 1000 | 352 per 1000 (266 to 450) | OR 0.81 (0.54 to 1.22) | 432 (4 studies) | ⊕⊕⊕⊝ | |
| Multiple pregnancy | 50 per 1000 | 17 per 1000 (1 to 303) | OR 0.32 (0.01 to 8.26) | 40 | ⊕⊝⊝⊝ | |
| Miscarriage pregnancy rate | 72 per 1000 | 49 per 1000 (15 to 151) | OR 0.66 (0.19 to 2.28) | 168 (2 studies) | ⊕⊕⊝⊝ | |
| Adverse events | 43 per 1000 | 168 per 1000 (62 to 381) | OR 4.54 (1.49 to 13.84) | 264 | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ART: assisted reproductive technology; CI: confidence interval; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for serious risk of bias associated with poor reporting of study methods. 2 Downgraded one level for serious risk of imprecision: confidence interval compatible with benefit in either arm or with no difference between the groups. 3 Downgraded two levels for very serious risk of imprecision: only one event. 4 Downgraded one level for serious risk of imprecision: only 10 events. 5 Downgraded one level for serious risk of imprecision: only 29 events. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Moderate or severe ovarian hyperstimulation syndrome (OHSS) Show forest plot | 8 | 1022 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.19, 0.39] |
| 1.1 Cabergoline vs placebo/no treatment | 5 | 521 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.16, 0.42] |
| 1.2 Quinagolide vs placebo | 2 | 454 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.15, 0.51] |
| 1.3 Bromocriptine vs placebo (folic acid) | 1 | 47 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.08, 1.14] |
| 2 Subgroup analysis by severity of OHSS Show forest plot | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Severe OHSS | 7 | 750 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.14, 0.56] |
| 2.2 Moderate OHSS | 7 | 750 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.24, 0.57] |
| 3 Live birth Show forest plot | 1 | 182 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.53, 1.91] |
| 3.1 Quinagolide vs placebo | 1 | 182 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.53, 1.91] |
| 4 Clinical pregnancy rate Show forest plot | 4 | 432 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.54, 1.22] |
| 4.1 Cabergoline vs no intervention | 3 | 250 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.48, 1.38] |
| 4.2 Quinagolide vs placebo | 1 | 182 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.43, 1.54] |
| 5 Multiple pregnancy Show forest plot | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.26] |
| 5.1 Cabergoline vs placebo/no treatment | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.26] |
| 6 Miscarriage Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Cabergoline vs placebo/no treatment | 2 | 168 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.19, 2.28] |
| 7 Adverse events Show forest plot | 2 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.54 [1.49, 13.84] |
| 7.1 Cabergoline vs placebo/no treatment | 1 | 82 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.24 [0.62, 8.14] |
| 7.2 Quinagolide vs placebo | 1 | 182 | Odds Ratio (M‐H, Fixed, 95% CI) | 16.64 [0.98, 282.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Moderate or severe ovarian hyperstimulation syndrome (OHSS) Show forest plot | 3 | 548 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.31, 1.03] |
| 1.1 Cabergoline + albumin vs albumin | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.23, 1.34] |
| 1.2 Cabergoline + hydroxyethyl starch (HES) vs HES | 2 | 382 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.26, 1.30] |
| 2 Live birth Show forest plot | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.59, 1.86] |
| 2.1 Cabergoline + HES vs HES | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.59, 1.86] |
| 3 Clinical pregnancy rate Show forest plot | 3 | 548 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.71, 1.40] |
| 3.1 Cabergoline + albumin vs albumin | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.56, 1.96] |
| 3.2 Cabergoline + HES vs HES | 2 | 382 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.65, 1.47] |
| 4 Multiple pregnancy Show forest plot | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.18, 22.77] |
| 4.1 Cabergoline + albumin vs albumin | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.18, 22.77] |
| 5 Miscarriage Show forest plot | 3 | 548 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.30, 1.42] |
| 5.1 Cabergoline + albumin vs albumin | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.19] |
| 5.2 Cabergoline + HES vs HES | 2 | 382 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.31, 1.68] |
| 6 Adverse events Show forest plot | 2 | 366 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 75.28] |
| 6.1 Cabergoline + albumin vs albumin | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Cabergoline + HES vs HES | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 75.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Moderate or severe ovarian hyperstimulation syndrome (OHSS) Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Cabergoline vs human albumin | 3 | 296 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.12, 0.38] |
| 1.2 Cabergoline vs prednisolone | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.05, 1.33] |
| 1.3 Cabergoline vs hydroxyethyl starch (HES) | 1 | 61 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.48, 15.10] |
| 1.4 Cabergoline vs coasting | 2 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.18, 1.45] |
| 2 Clinical pregnancy rate Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Cabergoline vs human albumin | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.33, 1.38] |
| 2.2 Cabergoline vs coasting | 2 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.65 [1.13, 6.21] |
| 3 Multiple pregnancy Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Cabergoline vs human albumin | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.13, 2.54] |
| 3.2 Cabergoline vs coasting | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.35 [0.25, 116.31] |
| 4 Miscarriage Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Cabergoline vs human albumin | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.03, 3.19] |
| 4.2 Cabergoline vs coasting | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.06] |

