鎌状赤血球病患者における下腿潰瘍を治療するための介入
アブストラクト
背景
皮膚潰瘍の発症は、鎌状赤血球病患者における罹病負担の重要な一因となっている。医療従事者には多くの治療選択肢があるが、鎌状赤血球病患者にとってどの治療法が有効であるか不明である。
目的
鎌状赤血球病患者を対象に下腿潰瘍を治療するための介入の臨床的有効性および安全性を評価すること。
検索戦略
Cochrane Cystic FibrosisおよびGenetic Disorders Group’s Haemoglobinopathies Trials Registerを検索した。
LILACS(1982年~2012年8月)、African Index Medicus(~2012年8月)、ISI Web of Knowledge(1985年~2012年8月)、Clinical Trials Search Portal of the World Health Organization(2012年8月)を検索した。特定したすべての試験の参照リストをチェックした。また、この分野で関連するランダム化試験を完了した可能性のある研究グループや個人に連絡を取った。
Cochrane Cystic Fibrosisおよび Genetic Disorders Group’s Haemoglobinopathies Trials Registerの最終検索日は2014年7月21日、Cochrane Wounds Group Trials Registerは2014年9月18日であった。
選択基準
鎌状赤血球病患者を対象とした下腿潰瘍を治療するための介入についてプラセボまたはその他の治療法と比較したランダム化比較試験。
データ収集と分析
2名の著者が独立して、選択基準を満たす試験を選択した。3名の著者が独立して、選択した試験のバイアスのリスクを評価し、データを抽出した。
主な結果
6試験が選択基準を満たした(250ヵ所の潰瘍を有する198名の参加者)。各試験では異なる介入を検証しており、本レビュー内では、全身性の薬物的介入(L‐カルニチン、酪酸アルギニン、イソクスプリン)および局所性の薬物的介入(Solcoseryl®クリーム、RGDペプチド包帯、局所抗生剤)に分類した。 3つの介入法では、潰瘍の大きさの変化について報告されている(酪酸アルギニン、RGDペプチド、L‐カルニチン)。これらのうち、RGDペプチド・マトリックスでは、対照群と比較して潰瘍の大きさが有意に減少し、平均して6.60 cm減少した(95% CI 5.51~7.69;エビデンスの質は非常に低い)。 3件の試験では、完全な回復の発生率について報告されている(イソクスプリン、酪酸アルギニン、RGDペプチド・マトリックス;エビデンスの質は低~非常に低い)。有意な効果について報告している試験はなかった。潰瘍の完全な回復までの期間、鎌状赤血球病の下腿潰瘍への治療後における無潰瘍生存率、生活の質の評価、切断の発生率について報告している試験はなかった。これらの介入の安全性に関する情報は報告されていなかった。
著者の結論
局所介入(RGDペプチド・マトリックス)によって、治療した参加者では対照群と比較して潰瘍の大きさが減少したというエビデンスがある。 有効性に関するこのエビデンスは、これらの報告に伴うバイアスのリスクが全般的に高いため、限定的である。
薬物的介入(全身性および局所性)および非薬物的介入(外科的および非外科的)という一般的な分類に基づいた結果を分析しようと試みた。しかし、アウトカムの定義における異質性およびランダム化の群と分析の群との間における不一致のため、結果を統合することができなかった。特定した試験の不足と共に、この異質性によって、メタアナリシスを行うことができなかった。
今回のコクラン・レビューでは、1つの局所的介入(RGDペプチド・マトリックス)の有効性についてある程度のエビデンスが認められた。しかし、この介入は、単一の試験報告における不十分さのため、バイアスのリスクが高いと評価された。選択したその他の試験もまた、バイアスのリスクが高いと評価された。試験結果については注意深く解釈することを推奨する。すべての介入の安全性プロファイルについて結論は出せなかった。
PICO
一般語訳
鎌状赤血球病患者における下腿潰瘍の治療
下腿潰瘍は、鎌状赤血球病患者にとって慢性の合併症である。潰瘍は、上手く治療することが難しい傾向があり、数カ月から数年かけてゆっくりと治癒が進む。潰瘍によって、生活の質に支障をきたし、不自由さが増し、仕事を休まなければならない期間が長くなり、医療システムでの治療の負担が大きくなる。そこで鎌状赤血球病患者における下腿潰瘍の治療が有効かつ安全であるかどうか検証した。
今回のコクラン・レビューでは、250カ所の潰瘍を有する198名の参加者を対象としたランダム化比較試験を6件特定した。4件のランダム化比較試験はジャマイカ、2件は米国で行われた。これらの試験では、潰瘍に直接用いる薬剤や包帯(局所性薬剤)および経口または静脈投与される薬剤(全身性薬剤)を検証した。これらの2種類では作用機序が大きく異なることから、レビューではそれらを別々に取り扱った。局所性薬剤は、Solcoseryl®クリーム、RGDペプチド包帯、局所抗生剤であった。 Solcoserylは、皮膚組織による酸素の取り込みを改善することによって、傷の治りを促すことを目的としている。局所抗生剤はまた、感染を予防するために使用される。RDGペプチド・マトリックスは、細胞増殖を促すゲルである。全身性薬剤は、L‐カルニチン、酪酸アルギニン、イソクスプリンであった。酪酸アルギニンは静脈内投与すると、傷の治りを促すと考えられており、L‐カルニチンは経口投与すると、組織の低酸素状態を改善すると考えられており、イソクスプリンは塩酸イソクスプリンとして経口投与すると、傷への血流を増加させると考えられている。
局所的介入(RDGペプチド・マトリックス)の1つの治療法によって、治療した参加者では対照と比較して潰瘍の大きさが減少した。しかし、この試験報告に伴う不十分さによるバイアスのリスクが高いため、この効果については注意深く解釈する必要がある。
鎌状赤血球病および慢性下腿潰瘍の患者を治療するための介入の使用に関するエビデンスは強固なものではなかった。このレビューで選択したすべてのランダム化臨床試験では、バイアスのリスクが高かった。このシステマティック・レビューから、鎌状赤血球病患者の下腿潰瘍の治癒を改善するための介入の有益性や有害性を評価するには、十分にデザインされた質の高いランダム化試験が必要であることが示された。
Authors' conclusions
Summary of findings
| Isoxuprine compared to placebo for leg ulcer in people with sickle cell disease | ||||||
| Patient or population: patients with leg ulcer in people with sickle cell disease | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo | Isoxuprine | |||||
| Incidence of complete closure | See comment | See comment | Not estimable | 54 ulcers | ⊕⊝⊝⊝ | This trial shows inconsistency between units of randomisation (30 participants) and unit of analysis (54 ulcers). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Sequence generation, allocation concealment, blinding: unclear. Incomplete outcome data and selective report. CI: confidence interval | ||||||
| arginine butyrate plus standard local care compared to standard local care for sickle cell in people with sickle cell disease | ||||||
| Patient or population: sickle cell in people with sickle cell disease | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| standard local care | arginine butyrate plus standard local care | |||||
| Complete healing | See comment | See comment | Not estimable | 23 participants 62 ulcers | ⊕⊝⊝⊝ | This trial shows inconsistency between units of randomisation (23 participants) and unit of analysis (62 ulcers). |
| Change in ulcer size | See comment | See comment | Not estimable | (1 study; McMahon 2010) | ⊕⊝⊝⊝ | This trial shows inconsistency between units of randomisation (23 participants) and unit of analysis (62 ulcers). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Unclear allocation concealment CI: confidence interval | ||||||
| L‐carnitine compared to placebo for leg ulcer in people with sickle cell disease | ||||||
| Patient or population: leg ulcer in people with sickle cell disease | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo | L‐carnitine | |||||
| Change in ulcer size | See comment | See comment | Not estimable | 15 | ⊕⊝⊝⊝ | Mean difference: ‐3.90 (95% CI ‐13.44 to 5.64). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Random sequence generation: unclear. CI: confidence interval | ||||||
| RGD peptide matrix compared to placebo for leg ulcer in people with sickle cell disease | ||||||
| Patient or population: patients with leg ulcer in people with sickle cell disease | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo | RGD peptide matrix | |||||
| Complete closure | See comment | See comment | Not estimable | 55 | ⊕⊝⊝⊝ | Risk ratio: 0.40 (95% CI 0.15 to 1.04). |
| Change in size ulcers healed | See comment | See comment | Not estimable | 55 | ⊕⊝⊝⊝ | Mean difference: 6.60 (95% CI 5.51 to 7.69). |
| Total adverse events | See comment | See comment | Not estimable | 55 | ⊕⊝⊝⊝ | Risk ratio: 0.76 (95 CI 0.50 to 1.17). |
| Related study treatment adverse events | See comment | See comment | Not estimable | 33 | ⊕⊝⊝⊝ | Risk Ratio: 1.41 (95% CI 0.27 to 7.38). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Sequence generation and allocation concealment: unclear CI: confidence interval | ||||||
Background
See Appendix 1 for a glossary of medical terms.
Description of the condition
Sickle cell disease (SCD) is an inherited disease and the most common haemoglobinopathy worldwide (Ballas 2010; Orkin 2010; Rees 2010). Recently, the clinical history and others aspects of SCD have been reviewed (Mousa 2010; Prabhakar 2010; Serjeant 2010). It is common among people with sub‐Saharan African, Indian, Middle Eastern or Mediterranean ancestry (Creary 2007; Modell 2008). Sickle cell disease is a major public health problem (Modell 2008) with an estimated 70% of sufferers living in Africa (Makani 2007). Screening programmes for detecting SCD are ongoing worldwide (e.g. Bardakdjian‐Michau 2009; Daudt 2007; Henthorn 2004; Mañú 2009; Gulbis 2006; Tshilolo 2008). The term SCD includes sickle cell anaemia (Hb SS), haemoglobin S combined with haemoglobin C (Hb SC), haemoglobin S associated with ß thalassemia (Sß0 Thal and Sß+ Thal) and other less prevalent double heterozygous conditions which cause clinical disease (Steinberg 2009; Weatherall 2006). Haemoglobin S combined with normal haemoglobin (A) is known as the sickle cell trait (AS), which is generally asymptomatic and is not part of this review.
One chronic complication of SCD is the sickle cell leg ulcer (SCLU) (Figure 1) (Knox‐Macaulay 1983; Minniti 2010; Ramalho 1985). The frequency of skin ulceration makes it an important contributor to the morbidity burden faced by people with SCD, and resistance to therapy makes chronicity an important feature of the condition. Although there is no universally accepted leg ulcer duration that defines the condition as chronic, a research definition from Jamaica has defined a chronic leg ulcer as "an active ulcer recorded at least twice over a minimum period of three months" (Alexander 2004; Serjeant 2005). A SCLU can be a physically disabling complication, with potentially negative psychological and social consequences. It has been considered a marker of disease severity by some authors (Alleyne 1977; Cumming 2008; Eckman 1996; Halabi ‐Tawil 2008). The rationale behind this severity statement is based on an increasing risk of priapism and pulmonary hypertension in those individuals with SCLU (Halabi ‐Tawil 2008; Serarslan 2009). People with homozygous SCD and a leg ulcer have a higher prevalence of pulmonary hypertension compared to people with homozygous SCD without a leg ulcer (Serarslan 2009). These vasculopathies have a common denominator: a chronic intravascular hyper‐haemolysis which generate a lower level of nitric oxide (Akinsheye 2010; Taylor 2008). Others have used multivariate statistical modelling to explore possible clinical phenotypes in people with homozygous SCD and have suggested a 'leg ulcer phenotype' (Alexander 2004).

Individuals with Hb SS are more likely to experience a leg ulcer than those with other genotypes (Ankra‐Badu 1992; Koshy 1989). Geographically, the reported prevalence of this complication varies. In Nigeria, the prevalence of SCLU ranges between 7.5% for people with HbSS and 1.5% for people with HbSC (Akinyanju 1979; Durosinmi 1991). According to the 'Cooperative Study of Sickle Cell Disease' in the USA, leg ulcers affected 2.5% of people with SCD, with a higher rate of between 4% and 5% among those with HbSS (Koshy 1989). In Jamaica, a lifetime prevalence of any leg ulceration was reported to be between 70% and 80% (Serjeant 2005). More recently a prevalence of ulcers lasting six months or more was reported as 29.5% and the cumulative incidence as 16.7% (Cumming 2008). These prevalence rate variations are partly due to differing patient age distributions and analysis methodologies. It is generally agreed that leg ulcers are most commonly reported in adolescence and early adulthood in individuals with SCD (Serjeant 2005).
The SCLUs generally occur in areas with less subcutaneous fat, with thin skin, and with decreased blood flow (Trent 2004). The commonest sites are the medial and lateral malleoli (ankles), often becoming circumferential if not controlled early; the medial malleolus is more commonly involved than the lateral malleolus (Serjeant 2005; Trent 2004) (Figure 1). Less common sites are the anterior tibial area, dorsum of the foot, and achilles tendon area (Trent 2004).
The pathogenesis of the SCLU is complex (Aslan 2007; Hagar 2008; Kato 2007; Kato 2009; Mack 2006; Morris 2008; Paladino 2007; Serjeant 2005; Trent 2004; Wood 2008). One or more of the following mechanisms could play a role in the development of leg ulcers in people with SCD:
-
decreased nitric oxide: the haemolysis (breakdown) of the sickle red blood cells releases haemoglobin into the blood stream which consumes nitric oxide (a powerful vasodilator agent) to the blood, perhaps leading to impaired endothelial function (Mack 2006);
-
infectious process: the role of bacterial infection or colonization, or both, is illustrated by the growth of organisms such as Staphylococcus aureus, Pseudomonas aeruginosa and group A streptococci from ulcer swabs, signs of local inflammation and regional lymphadenopathy (MacFarlane 1986; Mohan 2000; Sehgal 1992);
-
venous incompetence: low oxygen tension in the venous system, inevitable turbulence around venous valves and high white blood cell and platelet counts promote endothelial adhesion and chronic ischemia (Chalchal 2001; Clare 2002; Cumming 2008; Mohan 2000; Serjeant 2005);
-
blood hypercoagulability: an acquired antithrombin III deficiency that has been described in individuals with SCLUs, along with evidence of fibrinolysis (D‐dimer fragment, and fibrinogen or fibrin degradation products) (Cacciola 1990a);
-
defective immunity: the complement system contributes to the immune system's defence against infection, and an inability to fix this system has been proposed as a risk factor for developing this chronic complication (Morgan 1981);
-
genetic risk factors: there is a relationship between genes of the TGF‐beta/BMP superfamily and endothelial function and nitric oxide biology which may explain the genesis of the SCLU (Nolan 2006; Ofosu 1987; Steinberg 2009).
-
postural vasoconstriction: by using the laser Doppler flowmeter, it had been demonstrated that individuals with SCLU have a low red cell flux at the ulcer or scar site (Mohan 1997).
In brief, the increased susceptibility to leg ulcers in people with SCD is due to a chronic ischemia and defective immunity. Chronic ischemia may be explained by a blood hypercoagulability, venous incompetence, or postural vasoconstriction, with these mechanisms conditioned by a low nitric oxide that may itself be genetically influenced.
Description of the intervention
There are a wide range of possible treatments for leg ulcers in people with SCD, and many of these treatments have been considered as interventions among people with leg or foot ulcers resulting from other pathologies, e.g. a silver‐based wound dressing and topical agents for treating diabetic foot ulcers (Bergin 2006; O'Meara 2010), the debridement of diabetic foot ulcers (Edwards 2002), oral zinc for arterial and venous leg ulcers (Wilkinson 1998) and compression for venous leg ulcers (O'Meara 2009). Conventional care would generally include occlusive dressing (Palfreyman 2006; Vermeulen 2004; Wasiak 2008) and debridement and cleansing (Moore 2005; Nelson 2000; Smith 2011). It is unknown if ulcers in people with SCD might experience differential benefits of these common treatments compared to ulcers from other pathologies.
Many possible treatments exist as adjuncts to conventional treatment and we have classified these into two major treatment groups: pharmaceutical interventions; and non‐pharmaceutical interventions.
-
Pharmaceutical interventions
-
Systemic pharmaceutical interventions:
-
vascular drugs (such as pentoxifylline (blood viscosity‐reducing agent) (Frost 1990), isoxsuprine hydrochloride (β‐adrenergic receptor stimulant) (Serjeant 1977), xanthinol nicotinate (vasodilator) (Afifi 1979));
-
antioxidant agents (such as L‐carnitine) (Harrel 1990; Serjeant 1997);
-
recombinant agents and related (such as recombinant human erythropoietin (erythropoiesis‐stimulating agent which increases the haemoglobin levels) (al‐Momen 1991), antithrombin III concentrate (potent coagulation inhibitor) (Cacciola 1989));
-
growth factors: such as Bosentan (a receptor endothelin receptor blocker) (Lionnet 2008).
-
minerals (oral zinc sulphate) (Serjeant 1970).
-
pharmacologic stimulation of HbF synthesis agents (such as arginine butyrate) (Sher 1994);
-
-
Topical pharmaceutical interventions:
-
antibiotics and antiseptics (such as topical antibiotic (Baum 1987), collagen dressing (Reindorf 1989), natural honey (Okany 2004));
-
growth factors and related (such as topical granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) (Alikhan 2004; Mery 2004; Pieters 1995), RGD peptide matrix, Solcoseryl® (a tissue stimulating agent) (La Grenade 2003));
-
steroids (such as cortisone ‐ a potent anti‐inflammatory) (Rice 1953);
-
miscellaneous (such as topical opioids) (Ballas 2002).
-
-
-
Non‐pharmaceutical interventions
-
Non‐pharmaceutical surgical interventions:
-
reconstructive surgery (free flap transfer) (Spence 1985);
-
cell therapy (e.g. human skin equivalent (Gordon 2003); allogeneic keratinocytes (Amini‐Adle 2007));
-
-
Non‐pharmaceutical and non‐surgical:
-
laser therapy (e.g. InGaP (670 nm) laser) (Lucena 2007);
-
miscellaneous (e.g. hyperbaric oxygen) (Espinosa 1992).
-
-
These interventions might occur alone or in combination. Recently, for example, a combination of treatment approaches (antibacterial agent, zinc oxide, bandages and debridement) have been proposed for improving the care of people with SCLUs (Schleucher 2007).
Why it is important to do this review
Even with rigorous conventional care, leg ulcers tend to be indolent and intractable, healing slowly over months or years (Ballas 2002; Minniti 2010). In the USA, the average duration of a SCLU has been reported to exceed three years (Wethers 1994) with recurrence rates ranging from 25% to 52% (Koshy 1989). The decreased quality of life, increased disability, absence from work and high utilization of health care resources can severely affect the lives of people with SCLUs (Cumming 2008; Halabi ‐Tawil 2008). This is an update of a previously published review (Martí‐Carvajal 2012).
In this review, we assessed the clinical effectiveness and safety of interventions for treating leg ulcers in people with SCD.
Objectives
To determine whether any clinical interventions (used either alone or in combination) are effective and safe when treating leg ulcers in people with SCD.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
People with all types of SCD who have been diagnosed with a leg ulcer and treated in a hospital or community setting, or both.
Types of interventions
Single or combination treatment regimen (with each treatment classified as pharmaceutical or non‐pharmaceutical as detailed below) compared to either conventional care or another treatment regimen for leg ulcers in people with SCD.
1. Pharmaceutical interventions
Systemic interventions
-
vascular drugs (such as pentoxifylline (blood viscosity‐reducing agent), isoxsuprine hydrochloride (β‐adrenergic receptor stimulant), xanthinol nicotinate (vasodilator));
-
antioxidant agents (such as L‐carnitine);
-
recombinant agents and related (such as recombinant human erythropoietin (erythropoiesis‐stimulating agent which increases the haemoglobin levels), antithrombin III concentrate (potent coagulation inhibitor));
-
growth factors: such as bosentan (a receptor endothelin receptor blocker);
-
pharmacologic stimulation of HbF synthesis agents (such as arginine butyrate);
-
oral zinc sulphate.
Topical interventions
-
antibiotics and antiseptics (such as topical antibiotic, collagen dressing, natural honey);
-
growth factors and related (such as topical granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), RGD peptide matrix, Solcoseryl® (a tissue stimulating agent));
-
steroids (such as cortisone (a potent anti‐inflammatory));
-
dressing;
-
debriding agents;
-
compression;
-
miscellaneous (such as topical opioids).
2. Non‐pharmaceutical interventions
-
reconstructive surgery (free flap transfer);
-
cell therapy (e.g. human skin equivalent; allogeneic keratinocytes);
-
laser therapy (e.g. InGaP (670 nm) laser);
-
miscellaneous (e.g. hyperbaric oxygen).
Types of outcome measures
Primary outcomes
-
Incidence of complete closure (defined as 100% epithelization or skin closure without drainage)
-
Time to ulcer closure
-
Change in ulcer size (surface area or volume)
Secondary outcomes
-
Ulcer‐free survival following treatment for SCLUs (free from leg ulcer recurrence)
-
Quality of life measures (based on any item from a validated scale, e.g. SF‐36, EuroQoL, WHOQOL‐BREF (Asnani 2009))
-
Incidence of amputation
-
Adverse events
-
any adverse event defined as "any untoward medical occurrence that may present during treatment with a pharmaceutical product but which does not necessarily have a causal relationship with this treatment" (Nebeker 2004).
-
adverse drug reactions defined as "a response to a drug which is noxious and uninitiated and which occurs at doses normally used in man for prophylaxis, diagnosis, or therapy of disease, or for the modification of physiologic functions" (Nebeker 2004).
-
Search methods for identification of studies
We searched for trials, irrespective of publication status (trials may be unpublished or published as an article, an abstract, or a letter), language or country. No limit was applied with respect to the period of follow‐up.
Electronic searches
We identified relevant trials from the Cystic Fibrosis and Genetic Disorders (CFGD) Group's Haemoglobinopathies Trials Register using the terms: (sickle cell OR (haemoglobinopathies AND general)) AND leg ulcers.
Date of the last search of the Cochrane Cystic Fibrosis and Genetic Disorders Group Haemoglobinopathies Trials Register: 21 July 2014.
The CFGD Group's Haemoglobinopathies Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of The Cochrane Library) and quarterly searches of MEDLINE. Unpublished work is identified by searching the abstract books of five major conferences: the European Haematology Association conference; the American Society of Hematology conference; the British Society for Haematology Annual Scientific Meeting; the Caribbean Health Research Council Meetings; and the National Sickle Cell Disease Program Annual Meeting. For full details of all searching activities for the register, please see the relevant section of the Cystic Fibrosis and Genetic Disorders Group Module.
We also searched the specialised register of the Cochrane Wounds Group (Cochrane Wounds Group). Date of the last search of the specialised register of the Cochrane Wounds Group: 18 September 2014.
We also searched the following resources using the keywords: (sickle cell OR (haemoglobinopathies AND general)) AND leg ulcers (to August 2012).
Searching other resources
We also checked the reference lists of all the trials identified by the above methods. We contacted a number of key researchers (by email), asking whether they knew of studies assessing treatments for SCLUs in people with SCD.
Data collection and analysis
Data collection and analysis procedures are described below, and follow documented Cochrane Collaboration methodologies (Higgins 2011a).
Selection of studies
Two authors independently selected studies for inclusion, and extracted data. A third author was always included when two authors disagreed, with all disagreements resolved by group discussion.
Data extraction and management
We extracted the following groups of data:
-
demographics (age, sex, country);
-
characteristic of the ulcer (anatomic site, size, number of ulcers, presence of infection, how long the patient has had the ulcer);
-
sickle cell genotype (SS, SC);
-
phenotypic expression (e.g. total haemoglobin, fetal haemoglobin).
One review author, Jennifer M Knight‐Madden, interviewed Dr Graham R. Serjeant, an author on four of the included RCTs (Baum 1987; La Grenade 1993; Serjeant 1977; Serjeant 1997) to clarify aspects of these trials that were unclear from the published trial reports.
Although potential interventions were broadly classed as pharmaceutical and non‐pharmaceutical, we identified only pharmaceutical interventions and present results under two major groupings (systemic and topical). Given the clinical diversity of the included interventions, we do not regard it as appropriate to combine data for meta‐analysis at this time.
For future updates, If we are able to find RCTs reporting these data, we plan to report our endpoints at between three and six months, and greater than six months: work from the Jamaican sickle cell cohort suggest that most leg ulcers take a minimum of between three and six months to heal (Serjeant 2005).
Assessment of risk of bias in included studies
We followed the domain‐based evaluation for risk of bias in included RCTs (Higgins 2011a). All review authors independently assessed the risk of bias of the trials according to the Cochrane Handbook for Systemtic Reviews of Interventions (Higgins 2011a). The disagreements were resolved through discussion.
We assessed the following domains as low, unclear, or high risk of bias:
-
generation of allocation sequence;
-
allocation concealment;
-
blinding (of participants, personnel and outcome assessors);
-
incomplete outcome data;
-
selective reporting;
-
free of other bias.
1. Generation of allocation sequence (checking for possible selection bias)
We described, for each included trial, whether the method used to generate the allocation sequence was reported in sufficient detail to allow an assessment of whether it produced comparable groups. We assessed the method as having one of the following risks of bias:
-
low risk (any truly random process, e.g. random number table; computer random number generator);
-
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk (if the trial was described as randomised, but the method used for the allocation sequence generation was not described).
2. Allocation concealment (checking for possible selection bias)
We described, for each included trial, whether the method used to conceal the allocation sequence was reported in sufficient detail and determined whether group allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as having one of the following risks of bias:
-
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk (if the trial was described as randomised, but the method used to conceal the allocation was not described).
3. Blinding or masking (checking for possible performance bias)
We described, for each included trial, the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We judged trials at low risk of bias if they were blinded, or if we judged that the lack of blinding could not have affected the results. We aimed to assess blinding separately for different outcomes or classes of outcomes. We assessed the methods as having one of the following risks of bias:
-
low, high or unclear risk for participants;
-
low, high or unclear risk for personnel;
-
low, high or unclear risk for outcome assessors.
4. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We assessed the methods as having one of the following risks of bias:
-
low risk (the numbers and reasons for dropouts and withdrawals in all intervention groups were described or if it was specified that there were no dropouts or withdrawals);
-
high risk (the number or reasons for dropouts and withdrawals were not described).
-
unclear risk (the report gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated);
We further examined the percentages of overall dropouts in each trial and per randomisation arm and aimed to evaluate whether an intention‐to‐treat analysis had been performed or could be performed from the published information.
5. Selective reporting bias (reporting bias due to selective outcome reporting)
We described, for each included trial, how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as having one of the following risks of bias:
-
low risk (any one of the following: the study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way or the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon);
-
high risk (any one of the following: not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub scales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study);
-
unclear risk (insufficient information to permit judgement of ‘low risk’ or ‘high risk’).
6. Free of other bias (bias due to problems not covered elsewhere in the table)
We described, for each included study, any important concerns we have about other possible sources of bias and made assessments as follows:
-
low risk of bias (the trial appears to be free of other components that could put it at risk of bias);
-
high risk of bias (there are other factors in the trial that could put it at risk of bias;
-
unclear risk of bias (the trial may or may not be free of other components that could put it at risk of bias); e.g. ascertainment bias, bias in the presentation of data, design bias (Porta 2008). Please refer to the appendices for further details (Appendix 2).
7. Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook for Systemtic Reviews of Interventions (Higgins 2011a). In reference to the six domains above, we assessed the likely magnitude and direction of any bias and whether we considered it was likely to impact on the review findings.
Trials that achieved a 'low risk' assessment for adequate generation of allocation sequence, allocation concealment, blinding, handling of incomplete outcome data, and no selective outcome reporting, and that were without other risks of bias, were considered to be at an overall low risk of bias, while trials that were assessed as either 'high risk' or 'unclear risk' on the majority of domains were considered to be at a high risk of bias. We aimed to explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
For binary outcome measures (incidence of complete closure, adverse events), we calculated the risk ratio (RR) with 95% confidence intervals (CIs) for each outcome. For continuous outcomes (change in ulcer size measured using surface area or volume), we calculated the mean difference with 95% CIs.
For the future update, if the search strategy finds new RCTs, we will plan to assess of treatment effect as follows:
-
for binary outcome measures (incidence of complete closure, incidence of amputation, adverse events, and adverse drug reaction), we plan to calculate the risk ratio (RR) with 95% confidence intervals (CIs) for each outcome.
-
for continuous outcomes (quality of life), we will use the standardized mean difference with 95% CIs, since different scales may be used to measure quality of life. Change in ulcer size (surface area or volume) will be measured using a pooled estimate of treatment effect by calculating the mean difference with 95% CIs. If statistical information is missing (such as standard deviations), we will try to extract them from other relevant information in the paper, such as P values and CIs
-
for time‐to‐event outcome (time to ulcer closure and ulcer‐free survival following treatment for SCLU), we plan to calculate the hazard ratios (HR) with 95% CIs for each outcome.
Unit of analysis issues
This review has treated the participant as the unit of analysis. This is important as the meta‐analytic techniques used assume independence between measurements, and more than one treated ulcer per participant would not be statistically independent. A result of ignoring this unit‐of‐analysis issue could be overly optimistic confidence intervals. We found six RCTs and of these, four used the ulcer as their unit of analysis (La Grenade 1993; Baum 1987; McMahon 2010; Serjeant 1977). For future updates, and if the review authors receive extra requested information from study authors, we plan to incorporate multiple ulcers per participant into our quantitative analyses using 'approximate analyses of cluster‐randomised trials of meta‐analysis', according to theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Due to problems with assuring a successful treatment 'washout' period, we do not consider cross‐over trials an appropriate study design for assessing wound healing, and we have not included cross‐over trials in this systematic review.
Dealing with missing data
For all included studies, we recorded the levels of participant attrition. Attrition rates were not stated in four of the six trial reports, and for these trials we approached the lead author for additional information on levels of missing data (Baum 1987; La Grenade 1993; Serjeant 1977; Serjeant 1997). This has allowed an intention‐to‐treat analysis for all included studies. For future updates, if necessary, we will seek full reports from authors where studies have been published in abstract form, presented at meetings or reported to the co‐authors. Where information is missing or unclear, we will contact the primary investigator. In order to allow an intention‐to‐treat analysis, we grouped data by allocated treatment groups, irrespective of later exclusion (regardless of cause) or loss to follow‐up.
Assessment of heterogeneity
For future updates, when more trials are included, we plan to test for heterogeneity between studies using a standard chi‐squared test and I2 statistic (Higgins 2003). The chi‐squared test is a statistical test for heterogeneity, whereas I2 assesses the impact of heterogeneity on the meta‐analysis. We will use the following I2 ranges to interpret heterogeneity:
-
0% to 40%: might not be important;
-
30% to 60%: may represent moderate heterogeneity;
-
50% to 90%: may represent substantial heterogeneity;
-
75% to 100%: considerable heterogeneity.
In the presence of substantial heterogeneity we plan to explore this heterogeneity by pre‐specified subgroup analysis. We will also evaluate the extend of heterogeneity by visual inspection of the forest plot (Higgins 2011).
Assessment of reporting biases
Comprehensive searches were done by two authors to minimize publication and reporting biases. We compared the 'Methods' section of the full published paper to the 'Results' section to ensure that all outcomes which were measured, were reported. For future updates, If we are able to include 10 RCTs or more in a single meta‐analysis, we will assess whether the review is subject to possible publication bias by using a funnel plot to graphically illustrate variability between trials. If asymmetry is detected, we will explore causes other than publication bias.
Data synthesis
No meta‐analysis was undertaken in this review. For future updates, any meta‐analyses will be performed separately for each intervention group (pharmaceutical systemic, pharmaceutical topical, non‐pharmaceutical surgical, non‐pharmaceutical non‐surgical). If the eligible trials are sufficiently homogenous, we will summarize their findings using a fixed‐effect model. However, if we find statistical heterogeneity using the I2 statistic (I2 > 50%) we will use a random‐effects model (Higgins 2003; Higgins 2011). Clinical variability (variability in types of participants, interventions, or outcomes) may prevent us from pooling trials.
Summary of findings tables
We used the GRADE proposals to assess the quality of the body of evidence associated with the following outcomes: complete closure; change in ulcer size; and safety (total adverse events and related study treatment adverse events) (Guyatt 2011). We constructed summary of findings tables (SoF) using the GRADEPro software (summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4) (GRADEPro 2014). The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers within‐study risk of bias (methodological quality), the directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias (Balshem 2011; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g; Guyatt 2011h; Guyatt 2012).
We would have used (and will apply these for future updates, if possible) the principles of the GRADE system to assess the quality of the body of evidence associated with others outcomes of this review which were not assessed by the included RCTs in this review: time to ulcer closure; ulcer‐free survival following for SCLUs (free from leg ulcer recurrence); quality of life; and incidence of amputation.
Subgroup analysis and investigation of heterogeneity
For future updates, if we find clinical heterogeneity, we plan to conduct subgroup analyses as follows:
-
SS type versus SC type (Appendix 1);
-
follow‐up duration.
These subgroup analyses will be only conducted for primary outcomes.
Sensitivity analysis
No sensitivity analyses were undertaken in this review. If sufficient data had been available, we would have used the following procedures (and will apply these for future updates, if possible). We would have compared RCTs with high versus low methodological quality (studies classified as having a 'low risk of bias' versus those identified as having a 'high risk of bias') and RCTs that performed intention‐to treat versus per‐protocol analyses (Higgins 2011).
For future updates, we will also evaluate the risk of attrition bias, as estimated by the percentage of participants lost. Trials with a total attrition of more than 30% or where differences between the groups exceed 10%, or both, would be excluded from meta‐analysis but included in the review (Higgins 2011a).
Results
Description of studies
Results of the search
We identified 88 references using our search strategy (see Figure 2 for search result details). Six RCTs met our inclusion criteria (Baum 1987; La Grenade 1993; McMahon 2010; Serjeant 1977; Serjeant 1997; Wethers 1994). These RCTs were published between 1977 and 2010, four in Jamaica (Baum 1987; La Grenade 1993; Serjeant 1977; Serjeant 1997) and two in The United States of America (McMahon 2010; Wethers 1994). There were 250 ulcers studied among 198 participants (trial sample sizes ranged from 15 to 55 participants). A detailed description of the included trials is provided in the Characteristics of included studies table.

Flowchart of last search of the Group's Trials Register: 25 May 2012.
Included studies
The six included RCTs report the following baseline characteristics (Baum 1987; La Grenade 1993; McMahon 2010; Serjeant 1977; Serjeant 1997; Wethers 1994).
Clinical characteristics
1. Genotype of SCD
Two RCTs only included patients with HbSS (Baum 1987; La Grenade 1993). Three RCTs included patients with HbSS, HbSC, HbSS‐O (Arab), HbSβ thalassaemia, HbSC Harlem, HbS (Serjeant 1977; Serjeant 1997; Wethers 1994). One trial reported 92.3% (24 out of 26) of the participants with HbSS (McMahon 2010).
2. Leg ulceration duration, leg ulcer diameter on entry to the trial and number of ulcer per patient
Two RCTs mentioned leg ulceration duration at baseline (Baum 1987; Wethers 1994). Five RCTs mentioned leg ulcer diameter on trial entry (Baum 1987; La Grenade 1993; McMahon 2010; Serjeant 1997; Wethers 1994). One trial excluded patients with large leg ulcers, but did not define the limits of inclusion (Baum 1987). Two RCTs reported baseline ulcer size using median area (Baum 1987; La Grenade 1993). Two RCTs reported baseline ulcer size using mean area (McMahon 2010; Wethers 1994). Three RCTs reported the number of ulcers per patient (Baum 1987; McMahon 2010; Serjeant 1977). SeeCharacteristics of included studies for details.
Intervention characteristics
1. Type of intervention
Each trial investigated a different intervention to treat SCLUs. Baum assessed topical antibiotics (Baum 1987), La Grenade assessed Solcoseryl® and DuoDerm as two arms of a three‐arm trial (La Grenade 1993), McMahon assessed arginine butyrate (McMahon 2010), Serjeant assessed isoxsuprine (Serjeant 1977) and propionyl‐L‐carnitine (Serjeant 1997), and Wether assessed RGD peptide matrix (Wethers 1994).
2. Administration route of intervention
Two RCTs used an oral administration (Serjeant 1977;Serjeant 1997). Three RCTs used topical interventions (Baum 1987; La Grenade 1993; Wethers 1994). One RCT used an intravenous intervention (McMahon 2010).
Outcome characteristics
Three RCTs explicitly stated their primary outcomes (Baum 1987; La Grenade 1993; Wethers 1994). The primary outcomes were pain (Baum 1987), healing rate (La Grenade 1993) and changes in per cent ulcer closure (Wethers 1994). According to Dr Graham Serjeant, interviewed by one author of this review (JKM), 'ulcer area‐change' was the primary outcome assessed in four trials that he was involved in (Baum 1987; La Grenade 1993; Serjeant 1977; Serjeant 1997). No RCTs explicitly described their secondary outcomes. These outcome measurements or definitions, as they were shown in each RCT, are listed within an appendix (Appendix 3).
Methodology characteristics
1. Units of randomisation and of analysis
Five RCTs declared participants as the unit of randomisation (Baum 1987; McMahon 2010; Serjeant 1977; Serjeant 1997; Wethers 1994). One RCT chose leg ulcers as the unit of randomisation (La Grenade 1993).
However, from the published papers, it was clear that two RCTs used participants as the unit of analysis (Serjeant 1997; Wethers 1994) and that four reported ulcers as the unit of analysis (La Grenade 1993; McMahon 2010; Serjeant 1977; Baum 1987). See the appendices for a summary of the unit of randomisation and the unit of analysis (Appendix 4). For those trials with ulcer as the unit of analysis, data have been narratively reported. We have contacted trial authors requesting further information that would allow us to accommodate the increased correlation that comes from multiple ulcers 'clustered' within an individual into our meta‐analysis. If received, we would use this information in a future update of the review.
2. Number of comparison groups
Five RCTs were conducted comparing two groups (Baum 1987; McMahon 2010; Serjeant 1977; Serjeant 1997; Wethers 1994). One RCT was conducted comparing three groups (La Grenade 1993).
3. A priori sample size estimation
One RCT was conducted with an a priori sample size estimation (Serjeant 1997). Five RCTs did not report information on sample size calculation a priori (Baum 1987; La Grenade 1993; McMahon 2010; Serjeant 1977; Wethers 1994). Dr Graham Serjeant (04 February 2011) has confirmed that four of the trials were not conducted using sample size a priori (Baum 1987; La Grenade 1993; Serjeant 1977; Serjeant 1997).
4. Follow‐up period
The follow‐up period ranged between two and six months: eight weeks (Baum 1987); ten weeks (Wethers 1994); twelve weeks (La Grenade 1993; McMahon 2010; Serjeant 1997); and six months (Serjeant 1977).
5. Description of the inclusion and exclusion criteria
One RCT did not describe the inclusion criteria (Serjeant 1977) and three RCTs did not describe the exclusion criteria (La Grenade 1993; Serjeant 1977; Serjeant 1997).
6. Multiple trials reports
The search strategy identified three included RCTs with multiple preliminary reports (Baum 1987; McMahon 2010; Wethers 1994).
Excluded studies
Eight studies were excluded (Afifi 1979; Cacciola 1990b; Lucena 2007; Neves 2010; Okany 2004; Paggiaro 2010; Serjeant 1970; Sawyer 1979). Overall, the majority of these studies were non‐RCT or case reports. See the Characteristics of excluded studies table for a detailed account of the reasons for exclusion.
Risk of bias in included studies
None of the included RCTs were graded as having an overall low risk of bias. The risk of bias of all domains listed above for all included trials are summarised in Figure 3 and Figure 4.
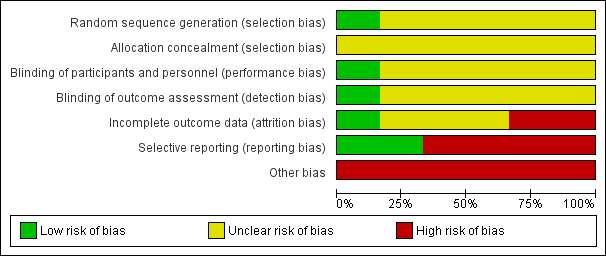
Risk of bias graph: review authors' judgements about each risk of bias domain presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias domain for each included study
Allocation
One RCT used an adequate method for sequence generation (graded as low risk) (McMahon 2010). We assessed five RCTs as having an unclear risk of bias in relation to the method used for generating the allocation sequence (Baum 1987; La Grenade 1993; Serjeant 1977; Serjeant 1997; Wethers 1994). No information was reported on allocation concealment by any of the included trials (Baum 1987; La Grenade 1993; McMahon 2010; Serjeant 1977; Serjeant 1997; Wethers 1994). See Characteristics of included studies for details.
Blinding
We assessed participant and personnel blinding separately. Three RCTs were described within the published papers as blinded trials (Serjeant 1977; Serjeant 1997; Wethers 1994). We judged one trial to have an adequate blinding process (low risk) (Wethers 1994). No information was reported about this domain in the remaining five trials (unclear risk) (Baum 1987; La Grenade 1993; McMahon 2010; Serjeant 1977; Serjeant 1997). SeeCharacteristics of included studies for details.
Incomplete outcome data
One RCT was assessed as having adequately reported outcome data (low risk) (Wethers 1994). Three RCTs were assessed as having an unclear risk in this domain (Baum 1987; McMahon 2010; Serjeant 1997), and two as having a high risk of bias in this domain (La Grenade 1993; Serjeant 1977). SeeCharacteristics of included studies for details.
Selective reporting
Two RCTs show adequate reporting (graded as low risk) (McMahon 2010; Wethers 1994). The remaining four RCTs were assessed as having a high risk of bias for this domain (Baum 1987; La Grenade 1993; Serjeant 1977; Serjeant 1997). SeeCharacteristics of included studies for details.
Other potential sources of bias
All of the included RCTs show some type of bias (ascertainment bias, bias in the presentation of data, design bias). SeeCharacteristics of included studies for details. SeeAppendix 2 for bias definitions.
Effects of interventions
See: Summary of findings for the main comparison Isoxuprine compared to placebo for leg ulcer in people with sickle cell disease; Summary of findings 2 Arginine butyrate plus standard local care compared to standard local care for sickle cell in people with sickle cell disease; Summary of findings 3 L‐carnitine compared to placebo for leg ulcer in people with sickle cell disease; Summary of findings 4 RGD peptide matrix compared to placebo for leg ulcer in people with sickle cell disease
Six RCTs were conducted for treating leg ulcers in people with SCD (Baum 1987; La Grenade 1993; McMahon 2010; Serjeant 1977; Serjeant 1997; Wethers 1994). The results are based on five RCTs (La Grenade 1993; McMahon 2010; Serjeant 1977; Serjeant 1997; Wethers 1994) as Baum did not report on any of the outcomes pre‐defined in our protocol (Baum 1987). Seesummary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4. Results were based on 168 participants with 210 ulcers. All RCTs assessed pharmaceutical interventions. Due to the differences in the modes of action of the pharmaceutical interventions used in the trials, we regarded it as inappropriate to combine the data available. Please note, as referred to above, for those trials where ulcers were the unit of analysis (instead of the participant), data have been narratively reported and not included in the meta‐analysis (La Grenade 1993; McMahon 2010; Serjeant 1977).
Primary outcomes
1. Pharmaceutical interventions
Systemic interventions
Three trials investigated systemic interventions (McMahon 2010; Serjeant 1977; Serjeant 1997).
Primary outcomes
1. Incidence of complete closure (defined as 100% epithelization or skin closure without drainage)
-
Vascular drugs
Isoxsuprine
One trial (involving 30 patients as the unit of randomisation and 46 ulcers as the unit of analysis) compared isoxsuprine with placebo (Serjeant 1977). This trial reported on: healed ulcers (23.9% (11 out of 46: 7 in the isoxsuprine group versus 4 in the control group); improved ulcers (36.9% (17 out of 46: 8 in the isoxsuprine group versus 9 in the control group); no changes in the ulcers (15.2% (7 out of 46: 5 in the isoxsuprine group versus 2 in the control group); and deteriorated ulcers (23.9% (11 out of 46: 8 in the isoxsuprine group versus 3 in the control group) (Serjeant 1977).
-
Pharmacologic stimulation of HbF synthesis agents
Arginine butyrate
One trial (involving 23 patients as the unit of randomisation and 62 ulcers as the unit of analysis) assessed arginine butyrate plus standard local care versus standard local care. This trial reported a complete closure incidence of 30% (11 out of 37) in the arginine butyrate group compared with 8% (2 out of 25) in the control group at 12 weeks of trial (P = 0.056) (McMahon 2010).
2. Time to ulcer closure
This outcome was not reported on in any of the RCTs.
3. Change in ulcer size (surface area or volume)
-
Vascular drugs
Isoxsuprine
One trial of 54 ulcers assessing this drug reported that a mean overall change in six‐month period was ‐1.4 cm2/ulcer in the treatment group and +0.7 cm2 in the controls (Serjeant 1977).
-
Antioxidant agents
L‐carnitine
One trial (Serjeant 1997) of 15 participants reported this outcome over the course of the trial with a non‐statistically significant change in ulcer size noted in the L‐carnitine group compared with those non‐receiving L‐carnitine, MD: ‐3.90 cm2, 95% CI ‐13.44 to 5.64 (P = 0.42). (Analysis 1.1).
-
Pharmacologic stimulation of HbF synthesis agents
Arginine butyrate
One trial reported that the mean decrease in ulcer area was nearly four times greater for the treatment arm in the 10 cm2 to 40 cm2 category (53% versus 12% closure) and nearly twice as many ulcers in the treatment arm healed in the larger than 40 cm2 group (43% versus 23% in the control arm) (McMahon 2010).
Secondary outcomes
1. Ulcer‐free survival following treatment for SCLU (free from leg ulcer recurrence)
This outcome was not reported on in any of the RCTs.
2. Quality of life measures (based on any item from a validated scale, e.g. SF‐36, EuroQoL, WHOQOL‐BREF)
This outcome was not reported on in any of the RCTs.
3. Incidence of amputation
This outcome was not reported on in any of the RCTs.
4. Adverse events
-
Pharmacologic stimulation of HbF synthesis agents
Arginine butyrate (McMahon 2010).
One trial reported that no serious adverse events were reported to be directly related to the study drug (McMahon 2010). Prior to arginine butyrate treatment on this study, port‐a‐cath infections did occur in two participants who had ports for their standard sickle cell care.
Topical pharmaceutical interventions
Three trials investigated topical interventions (Baum 1987; La Grenade 1993; Wethers 1994). The results are based on two RCTs (La Grenade 1993; Wethers 1994)
Primary outcomes
1. Incidence of complete closure (defined as 100% epithelization or skin closure without drainage)
-
Growth factors and related
One trial of 55 participants and 14 events reported this outcome over the course of the trial with a non‐statistically significant increase in complete closure noted in the RGD peptide matrix group compared with the placebo group, RR 0.40, 95% CI 0.15 to 1.04 (P = 0.06) (Wethers 1994) (Analysis 2.1).
2. Time to ulcer closure
This outcome was not reported on in any of the RCTs.
3. Change in ulcer size
-
Growth factors and related
One trial reported this outcome over the course of the trial with a statistically significant change in ulcer size noted in the RGD peptide matrix group compared with the placebo group, MD 6.60 cm2 (95% CI 5.51 to 7.69) (P <0.001) (Wethers 1994) (Analysis 2.2).
A further trial of 32 participants and 49 ulcers reported this outcome over the course of the trial with a non‐statistically significant change in ulcer size noted in the Solcoseryl® and hydrocolloid dressing compared with those non‐receiving Solcoseryl® and hydrocolloid dressing (La Grenade 1993). It was reported that "The mean difference in reduction of ulcer area between Solcoseryl® and controls was 7.1 cm2 (95% IC ‐0.7 to 14.9), and between hydrocolloid dressing and controls was 4.2 cm2 (95% CI ‐3.6 to 12.0). The mean difference in relative ulcer size between Solcoseryl® and controls was 28.3 cm2 (95% CI ‐1.9 to 58.5), and between hydrocolloid dressing and controls was ‐1.3 cm2 (95% CI ‐31.5 to 28.9)".
Secondary outcomes
1. Ulcer‐free survival following treatment for SCLU (free from leg ulcer recurrence)
This outcome was not reported on in any of the RCTs.
2. Quality of life measures (based on any item from a validated scale, e.g. SF‐36, EuroQoL, WHOQOL‐BREF)
This outcome was not reported on in any of the RCTs.
3. Incidence of amputation
This outcome was not reported on in any of the RCTs.
4. Adverse events
-
Growth factors and related
One trial of 55 participants and 33 events reported the total occurrence of adverse events over the course of the trial with a non‐statistically significant increase of this outcome noted in the RGD peptide matrix group compared with the placebo group, RR 0.76 (95% CI 0.50 to 1.17) (P = 0.21) (Wethers 1994) (Analysis 2.3). Wethers (for 33 participants and 5 events) also reported the treatment‐related adverse event over the course of the trial, which showed a non‐statistically significant increase of this outcome in the RGD peptide matrix group compared with the placebo group,RR 1.41 (95% CI 0.27 to 7.38) (P = 0.68) (Wethers 1994) (Analysis 2.3).
One trial assessing Solcoseryl® and hydrocolloid dressing reported that "Solcoseryl® was well tolerated as was Eusol except for a burning sensation reported by several patients (La Grenade 1993). Hydrocolloid dressing was not well tolerated, four patients expressing dislike because of the large volume of exudate which was smelly and required daily changes of dressing in the early part of the study, and two of these defaulted".
Discussion
Summary of main results
This review of interventions for treating leg ulcers in people with SCD included six studies (198 participants with 250 ulcers) which were grouped as systemic pharmaceutical interventions (L‐carnitine (Serjeant 1997), arginine butyrate (McMahon 2010), isoxsuprine hydrochloride (Serjeant 1977)) and topical pharmaceutical interventions (Solcoseryl® cream (La Grenade 1993), RGD peptide dressing (Wethers 1994), and topical antibiotics (Baum 1987). One trial was included but not reported any pre‐specified outcome in this Cochrane review (Baum 1987). Of these, RGD peptide dressing was effective in reducing the size of treated ulcers compared with placebo (Wethers 1994). The evidence for the use of interventions to treat people with sickle cell disease is not strong. Although there is some encouraging evidence for the use of RGD peptide matrix to reduce ulcer size, given the inadequacies with the associated trial reports, we currently feel that further confirmatory work is needed. Trials had a high risk of bias and failed to show beneficial effects. Please refer to the following tables for details (summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4).
Overall completeness and applicability of evidence
This review provides inconclusive evidence on the assessed interventions due to both heterogeneity between trials, and inadequate information provided by trial reports (Hopewell 2010). During this review, we have identified the following issues, which we feel are particularly relevant to consider as further work is planned. Generally, heterogeneity between trials prevented the pooling of results, with the main areas of variation between trials being differences in outcome definition, and inconsistency between unit of randomisation and unit of analysis (Appendix 4). In this regard, it has been recently suggested that trials adopt an agreed set of core outcomes for each medical condition (Clarke 2007). This approach could help to reduce the impact of outcome reporting bias (Kirkham 2010). In particular, a lack of reported inclusion and exclusion criteria and a lack of reported outcome definitions hampered the ability to compare trials (Appendix 3).
Quality of the evidence
The main source of bias in the included studies was the lack of detail in describing the generation of the randomisation sequences or the allocation concealment (Baum 1987; La Grenade 1993; Serjeant 1977; Serjeant 1997; Wethers 1994). We interviewed an author who was on four of the six included studies, who provided information on outstanding details. Trials also lacked detail about their blinding processes. The review authors' assessment of the risk of bias of the included studies has been described previously and a summary can be found in Figure 3 and Figure 4. The studies were classified as having a high risk of bias. Uncertainty remains about possible harms from the interventions, due to a lack of information presented on safety data.
Potential biases in the review process
In the process of performing a systematic review, there is a group of biases called significance‐chasing biases (Ioannidis 2010). These includes publication bias, selective outcome reporting bias, selective analysis reporting bias, and fabrication bias (Ioannidis 2010). Publication bias represents a major threat to the validity of systematic reviews, particularly in reviews such as this one that include small trials. However, we believe that this Cochrane review has a low risk of publication bias due to the thorough trial search process. We contacted the main author of four included RCTs. Selective outcome reporting bias operates through suppression of information on specific outcomes and has similarities to study publication bias, in that ‘negative’ results remain unpublished (Ioannidis 2010). This Cochrane review found that four out of the six included RCTs have high risk of selective outcome reporting (Baum 1987; La Grenade 1993; Serjeant 1977; Serjeant 1997).

Flowchart of last search of the Group's Trials Register: 25 May 2012.

Risk of bias graph: review authors' judgements about each risk of bias domain presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias domain for each included study
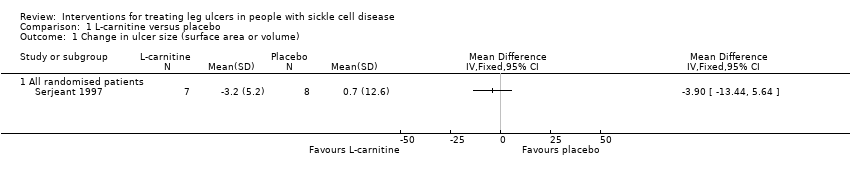
Comparison 1 L‐carnitine versus placebo, Outcome 1 Change in ulcer size (surface area or volume).

Comparison 2 RGD peptide matrix versus placebo, Outcome 1 Incidence of complete closure.

Comparison 2 RGD peptide matrix versus placebo, Outcome 2 Change in size of ulcers healed.

Comparison 2 RGD peptide matrix versus placebo, Outcome 3 Adverse events.
| Isoxuprine compared to placebo for leg ulcer in people with sickle cell disease | ||||||
| Patient or population: patients with leg ulcer in people with sickle cell disease | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo | Isoxuprine | |||||
| Incidence of complete closure | See comment | See comment | Not estimable | 54 ulcers | ⊕⊝⊝⊝ | This trial shows inconsistency between units of randomisation (30 participants) and unit of analysis (54 ulcers). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Sequence generation, allocation concealment, blinding: unclear. Incomplete outcome data and selective report. CI: confidence interval | ||||||
| arginine butyrate plus standard local care compared to standard local care for sickle cell in people with sickle cell disease | ||||||
| Patient or population: sickle cell in people with sickle cell disease | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| standard local care | arginine butyrate plus standard local care | |||||
| Complete healing | See comment | See comment | Not estimable | 23 participants 62 ulcers | ⊕⊝⊝⊝ | This trial shows inconsistency between units of randomisation (23 participants) and unit of analysis (62 ulcers). |
| Change in ulcer size | See comment | See comment | Not estimable | (1 study; McMahon 2010) | ⊕⊝⊝⊝ | This trial shows inconsistency between units of randomisation (23 participants) and unit of analysis (62 ulcers). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Unclear allocation concealment CI: confidence interval | ||||||
| L‐carnitine compared to placebo for leg ulcer in people with sickle cell disease | ||||||
| Patient or population: leg ulcer in people with sickle cell disease | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo | L‐carnitine | |||||
| Change in ulcer size | See comment | See comment | Not estimable | 15 | ⊕⊝⊝⊝ | Mean difference: ‐3.90 (95% CI ‐13.44 to 5.64). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Random sequence generation: unclear. CI: confidence interval | ||||||
| RGD peptide matrix compared to placebo for leg ulcer in people with sickle cell disease | ||||||
| Patient or population: patients with leg ulcer in people with sickle cell disease | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo | RGD peptide matrix | |||||
| Complete closure | See comment | See comment | Not estimable | 55 | ⊕⊝⊝⊝ | Risk ratio: 0.40 (95% CI 0.15 to 1.04). |
| Change in size ulcers healed | See comment | See comment | Not estimable | 55 | ⊕⊝⊝⊝ | Mean difference: 6.60 (95% CI 5.51 to 7.69). |
| Total adverse events | See comment | See comment | Not estimable | 55 | ⊕⊝⊝⊝ | Risk ratio: 0.76 (95 CI 0.50 to 1.17). |
| Related study treatment adverse events | See comment | See comment | Not estimable | 33 | ⊕⊝⊝⊝ | Risk Ratio: 1.41 (95% CI 0.27 to 7.38). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Sequence generation and allocation concealment: unclear CI: confidence interval | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in ulcer size (surface area or volume) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 All randomised patients | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of complete closure Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Change in size of ulcers healed Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Total | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Related study treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |


