Intervenciones para el tratamiento de las úlceras de las piernas en pacientes con anemia de células falciformes
Resumen
Antecedentes
La frecuencia de ulceración de la piel contribuye de forma importante a la carga de morbilidad de los pacientes con anemia de células falciformes. El profesional sanitario dispone de muchas opciones de tratamiento, aunque se desconoce qué tratamientos se han evaluado con respecto a la efectividad en pacientes con anemia de células falciformes. Esta es una actualización de una revisión Cochrane publicada anteriormente.
Objetivos
Evaluar la efectividad clínica y los efectos perjudiciales de las intervenciones para el tratamiento de las úlceras de las piernas en pacientes con anemia de células falciformes.
Métodos de búsqueda
Se realizaron búsquedas en el Registro de ensayos de hemoglobinopatías del Grupo Cochrane de Fibrosis quística y enfermedades genéticas (Cochrane Cystic Fibrosis and Genetic Disorders Group).
Se hicieron búsquedas en LILACS (1982 hasta enero de 2020), ISI Web of Knowledge (1985 hasta enero de 2020) y en el Clinical Trials Search Portal de la Organización Mundial de la Salud (enero de 2020). Se comprobaron las listas de referencias de todos los ensayos identificados. Se estableció contacto con grupos o individuos que hubieran completado ensayos aleatorizados relevantes en esta área.
Fecha de la búsqueda más reciente en el Registro de ensayos de hemoglobinopatías del Grupo Cochrane de Fibrosis quística y enfermedades genéticas: 13 de enero de 2020; fecha de la última búsqueda en el registro de ensayos del Grupo Cochrane de Heridas: 17 de febrero de 2017.
Criterios de selección
Ensayos controlados aleatorizados de intervenciones para el tratamiento de las úlceras de las piernas en pacientes con anemia de células falciformes en comparación con placebo o un tratamiento alternativo.
Obtención y análisis de los datos
Dos autores de la revisión evaluaron de forma independiente los estudios para inclusión. Los tres autores de la revisión, de forma independiente, evaluaron el riesgo de sesgo de los estudios incluidos y extrajeron los datos. Para evaluar la calidad general de la evidencia se utilizaron los criterios GRADE.
Resultados principales
Seis estudios cumplieron los criterios de inclusión (198 participantes con 250 úlceras). Cada ensayo investigó una intervención diferente que en esta revisión se agruparon en intervenciones farmacéuticas sistémicas (L‐carnitina, butirato de arginina, isoxsuprina) e intervenciones farmacéuticas tópicas (crema Solcoseryl®, apósito de matriz peptídica de arginina‐glicina‐ácido aspártico (RGD), antibióticos tópicos). En la revisión no se incluyeron ensayos sobre intervenciones farmacéuticas. Todos los ensayos tuvieron un riesgo general de sesgo poco claro o alto y cuatro recibieron patrocinio de empresas farmacéuticas. No fue posible agrupar los resultados debido a la heterogeneidad en las definiciones de los desenlaces y a la incoherencia entre las unidades de asignación al azar y las de análisis.
Tres intervenciones informaron sobre el cambio en el tamaño de la úlcera (butirato de arginina, apósito de matriz peptídica RGD, L‐carnitina). De estas, sólo el butirato de arginina mostró una reducción del tamaño de la úlcera en comparación con un grupo de control, reducción media ‐5,10 cm² (IC del 95%: ‐9,65 a ‐0,55), pero no se sabe si esto reduce el tamaño de la úlcera en comparación con la atención estándar sola, ya que la certeza de la evidencia se ha considerado muy baja. Tres ensayos informaron sobre el cierre completo de la úlcera de la pierna (isoxsuprina, butirato de arginina, matriz peptídica RGD; evidencia de calidad muy baja). Ninguno informó un beneficio clínico. Ningún ensayo informó sobre el tiempo transcurrido hasta la cicatrización completa de la úlcera; la supervivencia sin úlceras después del tratamiento de las úlceras de las piernas en pacientes con anemia de células falciformes, medidas de calidad de vida, la incidencia de amputación o los efectos perjudiciales.
Conclusiones de los autores
Dada la muy baja calidad de la evidencia identificada en esta revisión Cochrane actualizada, no se sabe con certeza si alguna de las intervenciones farmacéuticas evaluadas reduce el tamaño de la úlcera o provoca el cierre de la úlcera de la pierna en participantes tratados comparados con controles. Sin embargo, esta intervención se consideró con alto riesgo de sesgo debido a las deficiencias en el informe del único ensayo. Otros estudios incluidos también se consideraron con riesgo de sesgo alto o poco claro. El perfil de efectos perjudiciales de todas las intervenciones continúa sin ser concluyente.
PICO
Resumen en términos sencillos
Tratamientos para las úlceras de las piernas en pacientes con anemia de células falciformes
Pregunta de la revisión
Se revisó la evidencia para la efectividad clínica y los efectos perjudiciales de las intervenciones para el tratamiento de las úlceras de las piernas en pacientes con anemia de células falciformes. Esta es una actualización de una revisión Cochrane publicada anteriormente.
Antecedentes
Las úlceras de las piernas son una complicación crónica en los pacientes que presentan anemia de células falciformes. Las úlceras tienden a ser difíciles de tratar de forma exitosa y cicatrizan lentamente durante meses o años. Pueden perturbar gravemente la calidad de vida, aumentar la discapacidad, requerir ausencia prolongada del lugar de trabajo e implican una carga alta de atención en los sistemas de asistencia sanitaria. Se analizó si los tratamientos para las úlceras de las piernas en los pacientes con anemia de células falciformes fueron efectivos y seguros.
Fecha de la búsqueda
La evidencia está actualizada hasta el 13 de enero de 2020.
Características de los estudios
En esta actualización de revisión Cochrane se han incluido seis ensayos controlados aleatorizados con 198 participantes con 250 úlceras. Cuatro de los ensayos controlados aleatorizados se realizaron en Jamaica y dos en los EE.UU. Estos ensayos incluyeron fármacos o apósitos aplicados directamente a la úlcera (fármacos tópicos) y fármacos administrados por vía oral o por vía intravenosa (fármacos sistémicos). Debido a que los mecanismos de acción de estos dos grupos son muy diferentes, se trataron por separado en toda la revisión. Los agentes tópicos incluyeron crema Solcoseryl®, apósito de matriz peptídica RGD y antibióticos tópicos. Solcoseryl tiene como objetivo mejorar la administración de oxígeno al tejido cutáneo y promover así la cicatrización de la herida. Los antibióticos tópicos también se utilizan para prevenir la infección. La matriz peptídica RGD es un gel que promueve el crecimiento de las células. Las intervenciones sistémicas incluyeron el butirato de arginina, la L‐carnitina y la isoxsuprina. El butirato de arginina por vía intravenosa acelera la cicatrización de la herida, la L‐carnitina por vía oral mejora la hipoxia tisular y se considera que la isoxsuprina por vía oral como clorhidrato de isoxsuprina dilata los vasos sanguíneos, lo que aumenta el flujo sanguíneo a la herida afectada.
Resultados clave
Dada la muy baja calidad de la evidencia, no se sabe con certeza si alguna de las intervenciones farmacéuticas evaluadas reduce el tamaño de la úlcera o provoca el cierre de la úlcera de la pierna. Ninguno de los ensayos incluidos informó sobre otros desenlaces de interés: el tiempo transcurrido hasta la cicatrización completa de la úlcera, la supervivencia sin úlceras después del tratamiento de las úlceras de las piernas en pacientes con anemia de células falciformes, las medidas de calidad de vida, la incidencia de amputación o los efectos perjudiciales.
Calidad de la evidencia
Existe muy poca evidencia sobre el uso de intervenciones para tratar a los pacientes con anemia de células falciformes y ulceración crónica de las piernas. Todos los ensayos clínicos aleatorizados que se incluyeron en esta revisión se asociaron con un riesgo de sesgo alto o poco claro, lo que proporcionó evidencia de calidad muy baja. Esta revisión sistemática ha mostrado la necesidad de ensayos aleatorizados bien diseñados y de alta calidad para evaluar los efectos beneficiosos y perjudiciales de las intervenciones para mejorar la cicatrización de las úlceras de las piernas en los pacientes con anemia de células falciformes.
Authors' conclusions
Summary of findings
| Isoxuprine compared with placebo | ||||||
| Patient or population: individuals with sickle cell disease and a leg ulcer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Placebo | Isoxuprine | |||||
| Complete leg ulcer closure (defined as 100% epithelization or skin closure without drainage) | 182 per 1000a | 218 per 1000 | RR 1.20 | 54 ulcers (40 participants) | ⊕⊝⊝⊝ | Serjeant 1977 shows 2 inconsistencies between units of randomisation (30 or 40 participants) and unit of analysis (46 or 54 ulcers). |
| Time to ulcer closure ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1977 did not report this outcome. |
| Change in ulcer size (surface area or volume) | The mean change in ulcer size (surface area or volume) in the control groups was | The mean change in ulcer size (surface area or volume) in the intervention groups was | (40 or 30 participants) | ⊕⊝⊝⊝ | Serjeant 1977 shows 2 inconsistencies between units of randomisation (30 or 40 participants) and unit of analysis (46 or 54 ulcers). | |
| Ulcer‐free survival following treatment for SCLU (free from leg ulcer recurrence) ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1977 did not report this outcome. |
| Quality of life ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1977 did not report this outcome. |
| Amputation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1977 did not report this outcome. |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1977 did not report this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Assumed risk is based on the risks for the control group of Serjeant 1977 (18.2%). | ||||||
| Arginine butyrate plus standard care alone compared with standard care alone | ||||||
| Patient or population: individuals with sickle cell disease and a leg ulcer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Standard care alone | Arginine butyrate plus standard care alone | |||||
| Complete leg ulcer closure (defined as 100% epithelization or skin closure without drainage) | 80 per 1000a | 298 per 1000 | RR 3.72 | 23 participants 62 ulcers (1 study: McMahon 2010)b | ⊕⊝⊝⊝ | McMahon 2010 shows inconsistency between units of randomisation (23 participants) and unit of analysis (62 ulcers). |
| Time to ulcer closure ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | McMahon 2010 did not report this outcome. |
| Change in ulcer size (surface area or volume) Measured with computerized planimetry | The mean change in ulcer size (surface area or volume) in the control groups was | The mean change in ulcer size (surface area or volume) in the intervention groups was | 23 participants | ⊕⊝⊝⊝ | McMahon 2010 shows inconsistency between units of randomisation (23 participants) and unit of analysis (62 ulcers). Ulcer mean size at trial start in intervention group was two‐fold respect to control group. | |
| Ulcer‐free survival following treatment for SCLU (free from leg ulcer recurrence) ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | McMahon 2010 did not report this outcome. |
| Quality of life ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | McMahon 2010 did not report this outcome. |
| Amputation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | McMahon 2010 did not report this outcome. |
| Adverse events | See comment | See comment | Not estimable | 23 participants | See comment | McMahon 2010 reported "no serious adverse events were reported to be directly related to the study drug". |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Assumed risk is based on the risks for the control group of McMahon 2010 (8%). | ||||||
| Propionyl‐L‐carnitine compared with placebo | ||||||
| Patient or population: individuals with sickle cell disease and a leg ulcer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Placebo | Propionyl‐L‐carnitine | |||||
| Complete leg ulcer closure (defined as 100% epithelization or skin closure without drainage) ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1997 did not report this outcome. |
| Time to ulcer closure ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1997 did not report this outcome. |
| Change in ulcer size (surface area or volume) | The mean change in ulcer size (surface area or volume) in the control groups was | The mean change in ulcer size (surface area or volume) in the intervention groups was | 15 | ⊕⊝⊝⊝ | ||
| Ulcer‐free survival following treatment for SCLU (free from leg ulcer recurrence) ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1997 did not report this outcome. |
| Quality of life ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1997 did not report this outcome. |
| Amputation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1997 did not report this outcome. |
| Adverse events ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1997 did not report this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Trial conducted in Jamaica. | ||||||
| RGD peptide matrix compared with placebo | ||||||
| Patient or population: individuals with sickle cell disease and leg ulcer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Placebo | RGD peptide matrix | |||||
| Complete closure | 391 per 1000a | 157 per 1000 | RR 0.40 | 55 | ⊕⊝⊝⊝ | Based on number of participants. |
| Time to ulcer closure ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Wethers 1994 did not report this outcome. |
| Change in size ulcers healed ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Wethers 1994 did not report this outcome. |
| Ulcer‐free survival following treatment for SCLU (free from leg ulcer recurrence) ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Wethers 1994 did not report this outcome. |
| Quality of life ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Wethers 1994 did not report this outcome. |
| Amputation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Wethers 1994 did not report this outcome. |
| Adverse events | 696 per 1000e | 529 per 1000 | RR 0.76 | 55 | ⊕⊝⊝⊝ | Based on number of participants. Related study treatment adverse events: 1.41 (95% CI 0.27 to 7.38) based on number of participants with adverse events. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Assumed risk is based on the risks for the control group of Wethers 1994 (39.1%). | ||||||
| Solcoseryl compared with antiseptic agent | ||||||
| Patient or population: individuals with sickle cell disease and leg ulcer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Antiseptic agent | Solcoseryl | |||||
| Complete leg ulcer closure ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Time to closure ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Change in ulcer size (surface area or volume) | The mean change in ulcer size (surface area or volume) in the control groupsa was | The mean change in ulcer size (surface area or volume) in the intervention groups was | 35 ulcers in 32 participants | ⊕⊝⊝⊝ | La Grenade 1993 measured this outcome using ulcers as unit of analysis. | |
| Ulcer‐free survival following treatment for SCLU (free from leg ulcer recurrence) ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Quality of life ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Amputation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Adverse events | See comment | See comment | Not estimable | 35 ulcers in 32 participants | ⊕⊝⊝⊝ | La Grenade 1993 measured this outcome using individuals as unit of analysis. La Grenade 1993 reported "Solcoseryl® was well tolerated as was Eusol except for a burning sensation reported by several patients". |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Trial authors reported no final value from control group. | ||||||
| Duoderm®(hydroactive dressing) compared with antiseptic agent | ||||||
| Patient or population: individuals with sickle cell disease and leg ulcer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Antiseptic agent | Duoderm®(hydroactive dressing) | |||||
| Complete leg ulcer closure ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Time to closure ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Change in ulcer size (surface area or volume) | The mean change in ulcer size (surface area or volume) in the control groups was | The mean change in ulcer size (surface area or volume) in the intervention groups was | 35 ulcers in 32 participants | ⊕⊝⊝⊝ | La Grenade 1993 measured this outcome using ulcers as unit of analysis. | |
| Ulcer‐free survival following treatment for SCLU (free from leg ulcer recurrence) ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Quality of life ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Amputation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Adverse events | See comment | See comment | Not estimable | 35 ulcers in 32 participants | See comment | La Grenade 1993 measured this outcome using participants as unit of analysis. LLa Grenade 1993 reported "Hydrocolloid dressing was not well tolerated, four patients expressing dislike because of the large volume of exudate which was smelly and required daily changes of dressing in the early part of the study, and two of these defaulted." |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Trial authors reported no final value from control group. | ||||||
Background
See Appendix 1 for a glossary of medical terms.
Description of the condition
Sickle cell disease (SCD) is an inherited disease and the most common haemoglobinopathy worldwide (Ballas 2020; Barnett 2017; Lettre 2016). Recently, the clinical aspects of SCD have been reviewed (Piel 2017; Sundd 2019; Ware 2017). It is common among people with sub‐Saharan African, Indian, Middle Eastern or Mediterranean ancestry (Lettre 2016). Sickle cell disease is a major public health problem (Modell 2008) with an estimated 70% of sufferers living in Africa (Makani 2007). Screening programmes for detecting SCD are ongoing worldwide (e.g. Bardakdjian‐Michau 2009; Daudt 2007; Henthorn 2004; Mañú 2009; Gulbis 2006; Tshilolo 2008). The term SCD includes sickle cell anaemia (Hb SS), haemoglobin S combined with haemoglobin C (Hb SC), haemoglobin S associated with β thalassemia (Sβ0 Thal and Sβ+ Thal) and other less prevalent double heterozygous conditions which cause clinical disease (Steinberg 2009; Weatherall 2006). Haemoglobin S combined with normal haemoglobin (A) is known as the sickle cell trait (AS), which is generally asymptomatic and is not part of this review.
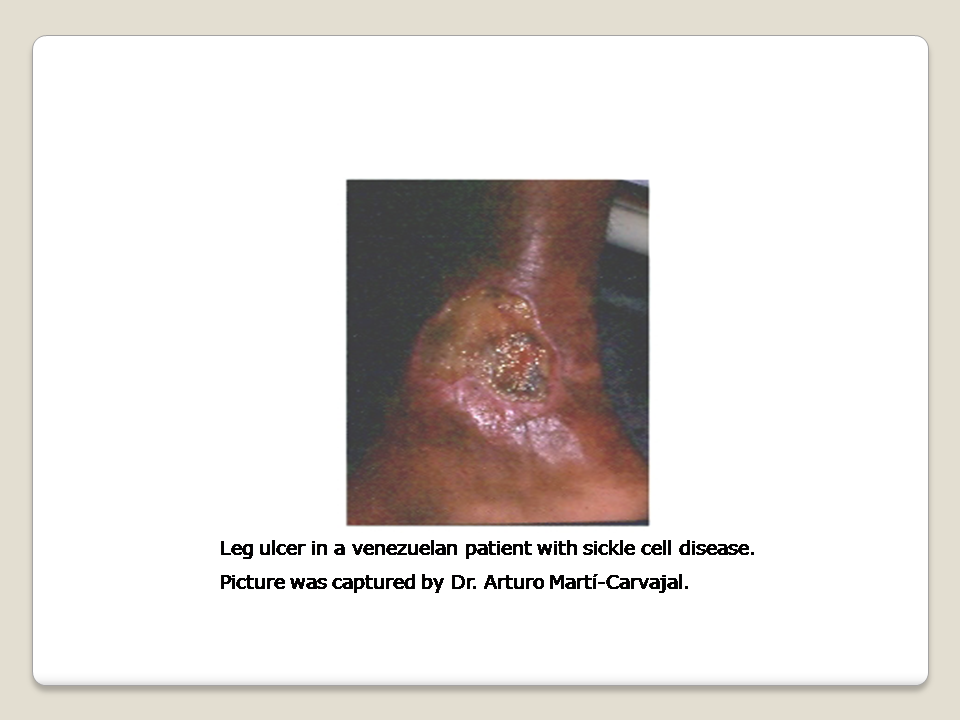
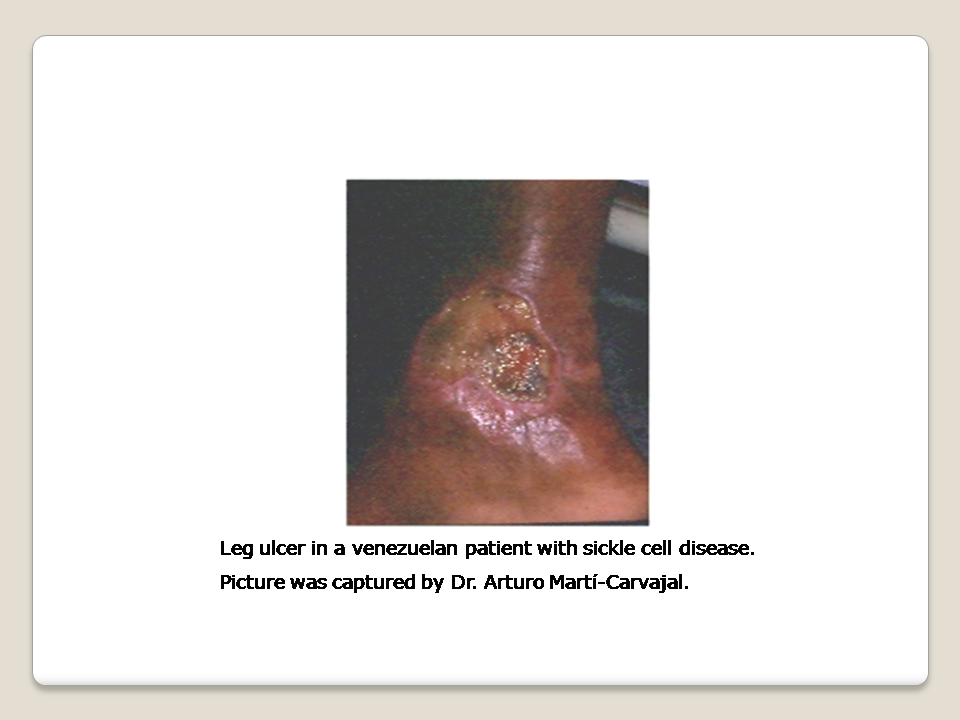
One chronic complication of SCD is the sickle cell leg ulcer (SCLU) (Figure 1) (AlDallal 2019; Delaney 2013; Minniti 2010; Mouba 2014; Ndiaye 2016), and it is a component of named "Mortal quintet of sickle cell diseases" (Helvaci 2015). The frequency of skin ulceration makes it an important contributor to the morbidity burden faced by people with SCD, and resistance to therapy makes chronicity an important feature of the condition. Although there is no universally accepted leg ulcer duration that defines the condition as chronic, a research definition from Jamaica has defined a chronic leg ulcer as "an active ulcer recorded at least twice over a minimum period of three months" (Alexander 2004; Serjeant 2005). A SCLU can be a physically disabling complication, with potentially negative psychological and social consequences. It has been considered a marker of disease severity by some authors (Alleyne 1977; Cumming 2008; Eckman 1996; Halabi ‐Tawil 2008). The rationale behind this severity statement is based on an increasing risk of priapism and pulmonary hypertension in those individuals with SCLU (Halabi ‐Tawil 2008; Serarslan 2009). People with homozygous SCD and a leg ulcer have a higher prevalence of pulmonary hypertension compared to people with homozygous SCD without a leg ulcer (Serarslan 2009). These vasculopathies have a common denominator: a chronic intravascular hyper‐haemolysis which generate a lower level of nitric oxide (Akinsheye 2010; Steinberg 2014; Taylor 2008). Others have used multivariate statistical modelling to explore possible clinical phenotypes in people with homozygous SCD and have suggested a 'leg ulcer phenotype' (Alexander 2004).
Individuals with Hb SS are more likely to experience a leg ulcer than those with other genotypes (Ankra‐Badu 1992; Koshy 1989). Geographically, the reported prevalence of this complication varies. In Nigeria, the prevalence of SCLU ranges between 7.5% for people with HbSS and 1.5% for people with HbSC (Akinyanju 1979; Durosinmi 1991). According to the 'Cooperative Study of Sickle Cell Disease' in the USA, leg ulcers affected 2.5% of people with SCD, with a higher rate of between 4% and 5% among those with HbSS (Koshy 1989). In Jamaica, a lifetime prevalence of any leg ulceration was reported to be between 70% and 80% (Serjeant 2005). More recently a prevalence of ulcers lasting six months or more was reported as 29.5% and the cumulative incidence as 16.7% (Cumming 2008). These prevalence rate variations are partly due to differing patient age distributions and analysis methodologies. It is generally agreed that leg ulcers are most commonly reported in adolescence and early adulthood in individuals with SCD (Serjeant 2005). Leg ulcer area of less than 8 cm² and of less than nine weeks' duration are associated with an increasing chance for complete leg ulcer healing at week 24 rather than the clinical or biological variables of SCD per se (Senet 2017).
The SCLUs generally occur in areas with less subcutaneous fat, with thin skin, and with decreased blood flow (Trent 2004). The commonest sites are the medial and lateral malleoli (ankles), often becoming circumferential if not controlled early; the medial malleolus is more commonly involved than the lateral malleolus (Serjeant 2005; Trent 2004) (Figure 1). Less common sites are the anterior tibial area, dorsum of the foot, and achilles tendon area (Trent 2004).
The pathogenesis of the SCLU is complex (Aslan 2007; Hagar 2008; Kato 2007; Kato 2009; Mack 2006; Morris 2008; Paladino 2007; Serjeant 2005; Trent 2004; Wood 2008). One or more of the following mechanisms could play a role in the development of leg ulcers in people with SCD:
-
decreased nitric oxide: the haemolysis (breakdown) of the sickle red blood cells releases haemoglobin into the blood stream which consumes nitric oxide (a powerful vasodilator agent) to the blood, perhaps leading to impaired endothelial function (Mack 2006);
-
infectious process: the role of bacterial infection or colonization, or both, is illustrated by the growth of organisms such as Staphylococcus aureus, Pseudomonas aeruginosa and group A streptococci from ulcer swabs, signs of local inflammation and regional lymphadenopathy (MacFarlane 1986; Mohan 2000; Sehgal 1992);
-
venous incompetence: low oxygen tension in the venous system, inevitable turbulence around venous valves and high white blood cell and platelet counts promote endothelial adhesion and chronic ischaemia (Chalchal 2001; Clare 2002; Cumming 2008; Mohan 2000; Serjeant 2005);
-
blood hypercoagulability: an acquired antithrombin III deficiency that has been described in individuals with SCLUs, along with evidence of fibrinolysis (D‐dimer fragment, and fibrinogen or fibrin degradation products) (Cacciola 1990a);
-
defective immunity: the complement system contributes to the immune system's defence against infection, and an inability to fix this system has been proposed as a risk factor for developing this chronic complication (Morgan 1981);
-
genetic risk factors: there is a relationship between genes of the TGF‐beta/BMP superfamily and endothelial function and nitric oxide biology which may explain the genesis of the SCLU (Nolan 2006; Ofosu 1987; Steinberg 2009).
-
postural vasoconstriction: by using the laser Doppler flowmeter, it had been demonstrated that individuals with SCLU have a low red cell flux at the ulcer or scar site (Mohan 1997).
In brief, the increased susceptibility to leg ulcers in people with SCD is due to a chronic ischaemia and defective immunity. Chronic ischaemia may be explained by a blood hypercoagulability, venous incompetence, or postural vasoconstriction, with these mechanisms conditioned by a low nitric oxide that may itself be genetically influenced. Recently, it has been reported novel genes associated with leg ulcers in sickle cell anemia (de Carvalho‐Siqueira 2019).
Description of the intervention
There are a wide range of possible treatments for leg ulcers in people with SCD, and many of these treatments have been considered as interventions among people with leg or foot ulcers resulting from other pathologies, e.g. a silver‐based wound dressing and topical agents for treating diabetic foot ulcers (Bergin 2006; Monfort 2020; O'Meara 2010), the debridement of diabetic foot ulcers (Edwards 2002), oral zinc for arterial and venous leg ulcers (Wilkinson 1998) and compression for venous leg ulcers (O'Meara 2009). Conventional care would generally include occlusive dressing (Palfreyman 2006; Vermeulen 2004; Wasiak 2008) and debridement and cleansing (Moore 2005; Nelson 2000; Smith 2011). It is unknown if ulcers in people with SCD might experience differential benefits of these common treatments compared to ulcers from other pathologies.
Many possible treatments exist as adjuncts to conventional treatment and we have classified these into two major treatment groups: pharmaceutical interventions; and non‐pharmaceutical interventions.
-
Pharmaceutical interventions
-
Systemic pharmaceutical interventions:
-
vascular drugs (such as pentoxifylline (blood viscosity‐reducing agent) (Frost 1990), isoxsuprine hydrochloride (β‐adrenergic receptor stimulant) (Serjeant 1977), xanthinol nicotinate (vasodilator) (Afifi 1979));
-
antioxidant agents (such as L‐carnitine) (Harrel 1990; Serjeant 1997);
-
recombinant agents and related (such as recombinant human erythropoietin (erythropoiesis‐stimulating agent which increases the haemoglobin levels) (al‐Momen 1991), antithrombin III concentrate (potent coagulation inhibitor) (Cacciola 1989));
-
growth factors: such as Bosentan (a receptor endothelin receptor blocker) (Lionnet 2008).
-
minerals (oral zinc sulphate) (Serjeant 1970).
-
pharmacologic stimulation of HbF synthesis agents (such as arginine butyrate) (Sher 1994);
-
-
Topical pharmaceutical interventions:
-
antibiotics and antiseptics (such as topical antibiotic (Baum 1987), collagen dressing (Reindorf 1989), natural honey (Okany 2004));
-
growth factors and related (such as topical granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) (Alikhan 2004; Mery 2004; Pieters 1995), RGD peptide matrix, Solcoseryl® (a tissue stimulating agent) (La Grenade 2003));
-
steroids (such as cortisone ‐ a potent anti‐inflammatory) (Rice 1953);
-
sodium nitrite (Minniti 2014);
-
miscellaneous (such as topical opioids) (Ballas 2002).
-
-
-
Non‐pharmaceutical interventions
-
Non‐pharmaceutical surgical interventions:
-
reconstructive surgery (free flap transfer) (Spence 1985);
-
cell therapy (e.g. human skin equivalent (Gordon 2003); allogeneic keratinocytes (Amini‐Adle 2007));
-
Stem cell therapy with bone marrow mononuclear cells (Meneses 2016);
-
-
Non‐pharmaceutical and non‐surgical:
-
laser therapy (e.g. InGaP (670 nm) laser) (Lucena 2007);
-
miscellaneous (e.g. hyperbaric oxygen) (Espinosa 1992).
-
-
How the intervention might work
The interventions described above might occur alone or in combination. Recently, for example, a combination of treatment approaches (antibacterial agent, zinc oxide, bandages and debridement) have been proposed for improving the care of people with SCLUs (Schleucher 2007).
Why it is important to do this review
Even with rigorous conventional care, leg ulcers tend to be indolent and intractable, healing slowly over months or years (Ballas 2002; Minniti 2010). In the USA, the average duration of a SCLU has been reported to exceed three years (Wethers 1994) with recurrence rates ranging from 25% to 52% (Koshy 1989). The decreased quality of life, social isolation, increased disability, absence from work and high utilization of health care resources can severely affect the lives of people with SCLUs (Coleman 2016; Cumming 2008; Halabi ‐Tawil 2008; Umeh 2017). This is an update of a previously published Cochrane Review (Martí‐Carvajal 2012; Martí‐Carvajal 2014).
In this updated review, we assessed the clinical effectiveness and harms of interventions for treating leg ulcers in people with SCD.
Objectives
To determine whether any clinical interventions (used either alone or in combination) are effective and harms when treating leg ulcers in people with SCD.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
People with all types of SCD who have been diagnosed with a leg ulcer and treated in a hospital or community setting, or both.
Types of interventions
Single or combination treatment regimen (with each treatment classified as pharmaceutical or non‐pharmaceutical as detailed below) compared to either conventional care or another treatment regimen for leg ulcers in people with SCD.
1. Pharmaceutical interventions
Systemic interventions
-
vascular drugs (such as pentoxifylline (blood viscosity‐reducing agent), isoxsuprine hydrochloride (β‐adrenergic receptor stimulant), xanthinol nicotinate (vasodilator));
-
antioxidant agents (such as L‐carnitine);
-
recombinant agents and related (such as recombinant human erythropoietin (erythropoiesis‐stimulating agent which increases the haemoglobin levels), antithrombin III concentrate (potent coagulation inhibitor));
-
growth factors: such as bosentan (a receptor endothelin receptor blocker);
-
pharmacologic stimulation of HbF synthesis agents (such as arginine butyrate);
-
oral zinc sulphate.
Topical interventions
-
antibiotics and antiseptics (such as topical antibiotic, collagen dressing, natural honey);
-
growth factors and related (such as topical granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), RGD peptide matrix, Solcoseryl® (a tissue stimulating agent));
-
steroids (such as cortisone (a potent anti‐inflammatory));
-
dressing;
-
debriding agents;
-
compression;
-
sodium nitrite;
-
miscellaneous (such as topical opioids).
2. Non‐pharmaceutical interventions
-
reconstructive surgery (free flap transfer);
-
cell therapy (e.g. human skin equivalent; allogeneic keratinocytes);
-
stem cell therapy with bone marrow mononuclear cells;
-
laser therapy (e.g. InGaP (670 nm) laser);
-
miscellaneous (e.g. hyperbaric oxygen).
Types of outcome measures
We assessed the following outcome measures.
Primary outcomes
-
Complete leg ulcer closure (defined as 100% epithelization or skin closure without drainage)
-
Time to ulcer closure
-
Change in ulcer size (surface area or volume)
Secondary outcomes
-
Ulcer‐free survival following treatment for SCLUs (free from leg ulcer recurrence)
-
Quality of life measures (based on any item from a validated scale, e.g. SF‐36, EuroQoL, WHOQOL‐BREF (Asnani 2009))
-
Amputation
-
Adverse events
-
any adverse event defined as "any untoward medical occurrence that may present during treatment with a pharmaceutical product but which does not necessarily have a causal relationship with this treatment" (Nebeker 2004).
-
adverse drug reactions defined as "a response to a drug which is noxious and uninitiated and which occurs at doses normally used in man for prophylaxis, diagnosis, or therapy of disease, or for the modification of physiologic functions" (Nebeker 2004).
-
Search methods for identification of studies
We searched for trials, irrespective of publication status (trials may be unpublished or published as an article, an abstract, or a letter), language or country. No limit was applied with respect to the period of follow‐up.
Electronic searches
We identified relevant trials from the Cystic Fibrosis and Genetic Disorders (CFGD) Group's Haemoglobinopathies Trials Register using the terms: (sickle cell OR (haemoglobinopathies AND general)) AND leg ulcers.
Date of the last search of the Cochrane Cystic Fibrosis and Genetic Disorders Group Haemoglobinopathies Trials Register: 13 January 2020.
The Haemoglobinopathies Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library) and weekly searches of MEDLINE. Unpublished work is identified by searching the abstract books of five major conferences: the European Haematology Association conference; the American Society of Hematology conference; the British Society for Haematology Annual Scientific Meeting; the Caribbean Public Health Agency Annual Scientific Meeting (formerly the Caribbean Health Research Council Meeting); and the National Sickle Cell Disease Program Annual Meeting. For full details of all searching activities for the register, please see the relevant section of the Cochrane Cystic Fibrosis and Genetic Disorders Group's website.
We also searched the specialised register of the Cochrane Wounds Group (Cochrane Wounds Group). Date of the last search of the specialised register of the Cochrane Wounds Group: 17 February 2017.
For the previous version of this review we also searched the following resources using the keywords: (sickle cell OR (haemoglobinopathies AND general)) AND leg ulcers (10 October 2016).
-
Latin American and Caribbean Health Sciences Information System (LILACS) (from 1982) (Appendix 2);
-
ISI Web of Knowledge (from 1985) (Appendix 3);
-
Clinical Trials Search Portal of the World Health Organization (Appendix 5);
For the 2020 update, we were unable to find information in African Index Medicus as currently the website is not functioning correctly (8 May 2020).
For this update, we were unable to find information in Clinical Trials Search Portal of the World Health Organization: "Due to heavy traffic generated by the COVID‐19 outbreak, the ICTRP Search Portal is not accessible from outside WHO temporarily. Please subscribe to the ICTRP list server if you wish to be notified when the search portal is working again. Information on how to subscribe can be found on the same page below." (8 May 2020).
Searching other resources
We also checked the reference lists of all the trials identified by the above methods. We contacted a number of key researchers (by email), asking whether they knew of studies assessing treatments for SCLUs in people with SCD.
Data collection and analysis
Data collection and analysis procedures are described below, and follow documented Cochrane methodologies (Higgins 2011a).
Selection of studies
Two authors independently selected studies for inclusion, and extracted data. A third author was always included when two authors disagreed, with all disagreements resolved by group discussion.
Data extraction and management
We extracted the following groups of data:
-
demographics (age, sex, country);
-
characteristic of the ulcer (anatomic site, size, number of ulcers, presence of infection, how long the individual has had the ulcer);
-
sickle cell genotype (SS, SC);
-
phenotypic expression (e.g. total haemoglobin, fetal haemoglobin).
One review author, Jennifer M Knight‐Madden, interviewed Dr Graham R. Serjeant, an author on four of the included RCTs (Baum 1987; La Grenade 1993; Serjeant 1977; Serjeant 1997) to clarify aspects of these trials that were unclear from the published trial reports.
Although potential interventions were broadly classed as pharmaceutical and non‐pharmaceutical, we identified only pharmaceutical interventions and present results under two major groupings (systemic and topical). Given the clinical diversity of the included interventions, we do not regard it as appropriate to combine data for meta‐analysis at this time.
For future updates, If we are able to find RCTs reporting these data, we plan to report our endpoints at between three and six months, and greater than six months: work from the Jamaican sickle cell cohort suggest that most leg ulcers take a minimum of between three and six months to heal (Serjeant 2005).
Assessment of risk of bias in included studies
We followed the domain‐based evaluation for risk of bias in included RCTs (Higgins 2011a). All review authors independently assessed the risk of bias of the trials according to the CochraneHandbook for Systemtic Reviews of Interventions (Higgins 2011a). The disagreements were resolved through discussion.
We assessed the following domains as low, unclear, or high risk of bias:
-
generation of allocation sequence;
-
allocation concealment;
-
blinding (of participants, personnel and outcome assessors);
-
incomplete outcome data;
-
selective reporting;
-
free of other bias.
1. Generation of allocation sequence (checking for possible selection bias)
We described, for each included trial, whether the method used to generate the allocation sequence was reported in sufficient detail to allow an assessment of whether it produced comparable groups. We assessed the method as having one of the following risks of bias:
-
low risk (any truly random process, e.g. random number table; computer random number generator);
-
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk (if the trial was described as randomised, but the method used for the allocation sequence generation was not described).
2. Allocation concealment (checking for possible selection bias)
We described, for each included trial, whether the method used to conceal the allocation sequence was reported in sufficient detail and determined whether group allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as having one of the following risks of bias:
-
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk (if the trial was described as randomised, but the method used to conceal the allocation was not described).
3. Blinding or masking (checking for possible performance bias)
We described, for each included trial, the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We judged trials at low risk of bias if they were blinded, or if we judged that the lack of blinding could not have affected the results. We aimed to assess blinding separately for different outcomes or classes of outcomes. We assessed the methods as having one of the following risks of bias:
-
low, high or unclear risk for participants;
-
low, high or unclear risk for personnel;
-
low, high or unclear risk for outcome assessors.
4. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We assessed the methods as having one of the following risks of bias:
-
low risk (the numbers and reasons for dropouts and withdrawals in all intervention groups were described or if it was specified that there were no dropouts or withdrawals);
-
high risk (the number or reasons for dropouts and withdrawals were not described).
-
unclear risk (the report gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated);
We further examined the percentages of overall dropouts in each trial and per randomisation arm and aimed to evaluate whether an intention‐to‐treat analysis had been performed or could be performed from the published information.
5. Selective reporting bias (reporting bias due to selective outcome reporting)
We described, for each included trial, how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as having one of the following risks of bias:
-
low risk (any one of the following: the study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way or the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon);
-
high risk (any one of the following: not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub scales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study);
-
unclear risk (insufficient information to permit judgement of ‘low risk’ or ‘high risk’).
6. Free of other bias (bias due to problems not covered elsewhere in the table)
We described, for each included study, any important concerns we have about other possible sources of bias and made assessments as follows:
-
low risk of bias (the trial appears to be free of other components that could put it at risk of bias);
-
high risk of bias (there are other factors in the trial that could put it at risk of bias;
-
unclear risk of bias (the trial may or may not be free of other components that could put it at risk of bias); e.g. ascertainment bias, bias in the presentation of data, design bias (Porta 2014). Please refer to the appendices for further details (Appendix 6).
7. Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the CochraneHandbook for Systemtic Reviews of Interventions (Higgins 2011a). In reference to the six domains above, we assessed the likely magnitude and direction of any bias and whether we considered it was likely to impact on the review findings.
Trials that achieved a 'low risk' assessment for adequate generation of allocation sequence, allocation concealment, blinding, handling of incomplete outcome data, and no selective outcome reporting, and that were without other risks of bias, were considered to be at an overall low risk of bias, while trials that were assessed as either 'high risk' or 'unclear risk' on the majority of domains were considered to be at a high risk of bias. We aimed to explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
For binary outcome measures (incidence of complete closure, adverse events), we calculated the risk ratio (RR) with 95% confidence intervals (CIs) for each outcome. For continuous outcomes (change in ulcer size measured using surface area or volume), we calculated the mean difference (MD) with 95% CIs.
For the future update, if the search strategy finds new RCTs, we will plan to assess treatment effect as follows:
-
for binary outcome measures (incidence of complete closure, incidence of amputation, adverse events, and adverse drug reaction), we plan to calculate the risk ratio (RR) with 95% CIs for each outcome.
-
for continuous outcomes (quality of life), we will use the standardized MD with 95% CIs, since different scales may be used to measure quality of life. Change in ulcer size (surface area or volume) will be measured using a pooled estimate of treatment effect by calculating the mean difference with 95% CIs. If statistical information is missing (such as standard deviations (SDs)), we will try to extract them from other relevant information in the paper, such as P values and CIs
-
for time‐to‐event outcome (time to ulcer closure and ulcer‐free survival following treatment for SCLU), we plan to calculate the hazard ratios (HR) with 95% CIs for each outcome.
Unit of analysis issues
Within this review we aimed to treat the participant as the unit of analysis. This is important as the meta‐analytic techniques used assume independence between measurements, and more than one treated ulcer per participant would not be statistically independent. A result of ignoring this unit of analysis issue could be overly optimistic CIs. However, of the six included trials, two trials report on the number of participants (Serjeant 1997; Wethers 1994) while the remaining four used the ulcer as their unit of analysis (Baum 1987; La Grenade 1993; McMahon 2010; Serjeant 1977). For future updates, and if the review authors receive extra requested information from study authors, we plan to incorporate multiple ulcers per participant into our quantitative analyses using 'approximate analyses of cluster‐randomised trials of meta‐analysis', according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Due to problems with assuring a successful treatment 'washout' period, we do not consider cross‐over trials an appropriate study design for assessing wound healing, and we have not included cross‐over trials in this systematic review.
Dealing with missing data
For all included studies, we recorded the levels of participant attrition. Attrition rates were not stated in four of the six trial reports, and for these trials we approached the lead author for additional information on levels of missing data (Baum 1987; La Grenade 1993; Serjeant 1977; Serjeant 1997). This has allowed an intention‐to‐treat analysis for all included studies. For future updates, if necessary, we will seek full reports from authors where studies have been published in abstract form, presented at meetings or reported to the co‐authors. Where information is missing or unclear, we will contact the primary investigator. In order to allow an intention‐to‐treat analysis, we grouped data by allocated treatment groups, irrespective of later exclusion (regardless of cause) or loss to follow‐up.
Assessment of heterogeneity
For future updates, when more trials are included, we plan to test for heterogeneity between studies using a standard chi‐squared test and I² statistic (Higgins 2003). The Chi² test is a statistical test for heterogeneity, whereas I² assesses the impact of heterogeneity on the meta‐analysis. We will use the following I² ranges to interpret heterogeneity:
-
0% to 40%: might not be important;
-
30% to 60%: may represent moderate heterogeneity;
-
50% to 90%: may represent substantial heterogeneity;
-
75% to 100%: considerable heterogeneity.
In the presence of substantial heterogeneity we plan to explore this heterogeneity by pre‐specified subgroup analysis. We will also evaluate the extend of heterogeneity by visual inspection of the forest plot (Higgins 2019).
We showed an albatross plot as a visual display and presentation of data to be transparent reporting in this Cochrane review with lack of meta‐analysis (Harrison 2017; McKenzie 2019).
Assessment of reporting biases
Comprehensive searches were done by two authors to minimize publication and reporting biases. We compared the 'Methods' section of the full published paper to the 'Results' section to ensure that all outcomes which were measured, were reported. For future updates, If we are able to include 10 RCTs or more in a single meta‐analysis, we will assess whether the review is subject to possible publication bias by using a funnel plot to graphically illustrate variability between trials. If asymmetry is detected, we will explore causes other than publication bias.
Data synthesis
No meta‐analysis was undertaken in this review. For future updates, any meta‐analyses will be performed separately for each intervention group (pharmaceutical systemic, pharmaceutical topical, non‐pharmaceutical surgical, non‐pharmaceutical non‐surgical). If the eligible trials are sufficiently homogenous, we will summarize their findings using a fixed‐effect model. However, if we find statistical heterogeneity using the I² statistic (I² > 50%) we will use a random‐effects model (Higgins 2003; Higgins 2019). Clinical variability (variability in types of participants, interventions, or outcomes) may prevent us from pooling trials.
Threshold for clinical relevance
Due to P values, the ‘gold standard’ of statistical validity, are not as reliable as many scientists assume (Nuzzo 2014), we estimated the threshold for clinical relevance with the Bayes factor (Jakobsen 2014) which has been pointed out as a complementary statistical evidence to P values (Lin 2015). Bayes factor is a likelihood ratio indicate the relative strength of evidence for two theories (Dienes 2014; Goodman 1999; Goodman 2005). The Bayes factor is a comparison of how well two hypotheses (the null hypothesis ‐H0‐ and the alternative hypothesis ‐H1‐) predict the data (Goodman 1999). The Bayes factor provides a continuous measure of evidence for H1 over H0. When the Bayes factor is 1 (evidence is insensitive, the data is equally well predicted by both models and the evidence does not favour either model over the other (1 means the data are equally well predicted by H1 as H0, so it should not be interpreted as favouring H0; rather the evidence does not point either way). As the Bayes factor increase above 1 (towards infinity) the evidence favours H1 over H0. As the Bayes factor decreases below 1 (towards 0) the evidence favours H0 over H1 (Dienes 2014). We used Dienes' Calculator for estimating Bayes factor.
Subgroup analysis and investigation of heterogeneity
For future updates, if we identify any clinical heterogeneity, we plan to conduct subgroup analyses as follows:
-
SS type versus SC type (Appendix 1);
-
follow‐up duration.
These subgroup analyses will be only conducted for primary outcomes.
Sensitivity analysis
No sensitivity analyses were undertaken in this review. If sufficient data had been available, we would have used the following procedures (and will apply these for future updates, if possible). We would have compared RCTs with high versus low methodological quality (studies classified as having a 'low risk of bias' versus those identified as having a 'high risk of bias') and RCTs that performed intention‐to treat versus per protocol analyses (Higgins 2019).
For future updates, we will also evaluate the risk of attrition bias, as estimated by the percentage of participants lost. Trials with a total attrition of more than 30% or where differences between the groups exceed 10%, or both, would be excluded from meta‐analysis but included in the review (Higgins 2011a).
Summary of findings and assessment of the certainty of the evidence
Summary of findings tables
We used the GRADE proposals to assess the quality of the body of evidence (six comparisons) associated with the following outcomes: complete leg ulcer closure; time to ulcer closure; change in ulcer size; ulcer‐free survival following for SCLUs (free from leg ulcer recurrence); quality of life; and incidence of amputation; and safety (total adverse events and related study treatment adverse events) (Schünemann 2019). We constructed summary of findings tables (SoF) using the GRADEPro software (GRADEPro 2014). The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers within‐study risk of bias (methodological quality), the directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias (Schünemann 2019).
See summary of findings Table 1; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4; summary of findings Table 5; summary of findings Table 6.
Results
Description of studies
Results of the search
In the original version of the review, we identified 88 references using our search strategy. For the 2020 update, we identified a further 158 references (see Figure 2 for search result details) and one of these trials (two references) has been added to 'Studies awaiting classification' (You 2019). Therefore, there remain six trials included in this updated Cochrane Review (Baum 1987; La Grenade 1993; McMahon 2010; Serjeant 1977; Serjeant 1997; Wethers 1994). These RCTs were published between 1977 and 2010, four in Jamaica (Baum 1987; La Grenade 1993; Serjeant 1977; Serjeant 1997), and two in the USA (McMahon 2010; Wethers 1994). There were 250 ulcers studied among 198 participants (trial sample sizes ranged from 15 to 55 participants). A detailed description of the included trials is provided in the Characteristics of included studies table.
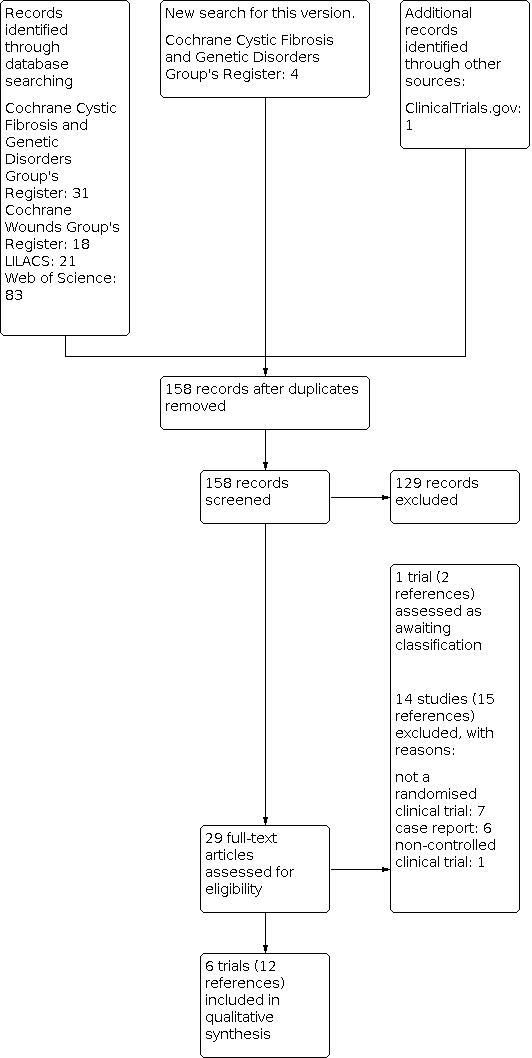
Study flow diagram ‐ update Juanuary 2020
Included studies
The six included RCTs report the following baseline characteristics (Baum 1987; La Grenade 1993; McMahon 2010; Serjeant 1977; Serjeant 1997; Wethers 1994).
Clinical characteristics
1. Genotype of SCD
Two RCTs only included people with HbSS (Baum 1987; La Grenade 1993). Three RCTs included people with HbSS, HbSC, HbSS‐O (Arab), HbSβ thalassaemia, HbSC Harlem, HbS (Serjeant 1977; Serjeant 1997; Wethers 1994). One trial reported 92.3% (24 out of 26) of the participants with HbSS (McMahon 2010).
2. Leg ulceration duration, leg ulcer diameter on entry to the trial and number of ulcer per participant
Two RCTs mentioned leg ulceration duration at baseline (Baum 1987; Wethers 1994). Five RCTs mentioned leg ulcer diameter on trial entry (Baum 1987; La Grenade 1993; McMahon 2010; Serjeant 1997; Wethers 1994).
One trial excluded people with large leg ulcers, but did not define the limits of inclusion (Baum 1987). Two RCTs reported baseline ulcer size using median area (Baum 1987; La Grenade 1993). Two RCTs reported baseline ulcer size using mean area (McMahon 2010; Wethers 1994). Three RCTs reported the number of ulcers per participant (Baum 1987; McMahon 2010; Serjeant 1977). SeeCharacteristics of included studies for details. McMahon 2010 included also one participant with SCD suffering diabetes which had large ulcer (> 300 cm²).
Intervention characteristics
1. Type of intervention
Each trial investigated a different intervention to treat SCLUs. Baum assessed topical antibiotics (Baum 1987), La Grenade assessed Solcoseryl® and DuoDerm® as two arms of a three‐arm trial (La Grenade 1993), McMahon assessed arginine butyrate (McMahon 2010), Serjeant assessed isoxsuprine and propionyl‐L‐carnitine (Serjeant 1977), and Wethers assessed RGD peptide matrix (Wethers 1994)..
2. Administration route of intervention
Two RCTs used an oral administration (Serjeant 1977Serjeant 1997). ThreeRCTs used topical interventions (Baum 1987; La Grenade 1993; Wethers 1994). One RCT used an intravenous intervention (McMahon 2010).
Outcome characteristics
Three RCTs explicitly stated their primary outcomes (Baum 1987; La Grenade 1993; Wethers 1994). The primary outcomes were pain (Baum 1987), healing rate (La Grenade 1993) and changes in per cent ulcer closure (Wethers 1994). According to Dr Graham Serjeant, interviewed by one author of this review (JKM), 'ulcer area‐change' was the primary outcome assessed in four trials that he was involved in (Baum 1987; La Grenade 1993; Serjeant 1977; Serjeant 1997). The other RCTs did not explicitly describe their secondary outcomes. These outcome measurements or definitions, as they were shown in each RCT, are listed within an appendix (Appendix 7).
Methodology characteristics
1. Location of the trials
Four trials were conducted in Jamaica by the same team (Baum 1987; La Grenade 1993; Serjeant 1977; Serjeant 1997), and two trials were conducted in the USA (McMahon 2010; Wethers 1994).
2. Units of randomisation and of analysis
Five RCTs declared participants as the unit of randomisation (Baum 1987; McMahon 2010; Serjeant 1977; Serjeant 1997; Wethers 1994). One RCT chose leg ulcers as the unit of randomisation (La Grenade 1993).
However, from the published papers, it was clear that two RCTs used participants as the unit of analysis (Serjeant 1997; Wethers 1994) and four reported ulcers as the unit of analysis (La Grenade 1993; McMahon 2010; Serjeant 1977; Baum 1987). See the appendices for a summary of the unit of randomisation and the unit of analysis (Appendix 8). For those trials with ulcer as the unit of analysis, data have been narratively reported. We have contacted trial authors requesting further information that would allow us to accommodate the increased correlation that comes from multiple ulcers 'clustered' within an individual into our meta‐analysis. If received, we would use this information in a future update of the review.
3. Number of comparison groups
Five RCTs were conducted comparing two groups (Baum 1987; McMahon 2010; Serjeant 1977; Serjeant 1997; Wethers 1994). One RCT was conducted comparing three groups (La Grenade 1993).
4. A priori sample size estimation
One RCT was conducted with an a priori sample size estimation (Serjeant 1997). Four RCTs did not report information on a priori sample size calculation (Baum 1987; La Grenade 1993; McMahon 2010; Serjeant 1977; Wethers 1994). Dr Graham Serjeant (04 February 2011) has confirmed that four of the trials were not conducted using a priori sample size (Baum 1987; La Grenade 1993; Serjeant 1977; Serjeant 1997).
5. Follow‐up period
The follow‐up period ranged between two and six months: eight weeks (Baum 1987); ten weeks (Wethers 1994); 12 weeks (La Grenade 1993; McMahon 2010; Serjeant 1997); and six months (Serjeant 1977).
6. Description of the inclusion and exclusion criteria
One RCT did not describe the inclusion criteria (Serjeant 1977) and three RCTs did not describe the exclusion criteria (La Grenade 1993; Serjeant 1977; Serjeant 1997).
7. Multiple trials reports
The search strategy identified three included RCTs with multiple preliminary reports (Baum 1987; McMahon 2010; Wethers 1994).
8. Drug company sponsorship
Drug company sponsored five trials (Baum 1987; La Grenade 1993; Serjeant 1977; Serjeant 1997; Wethers 1994). The financing is unknown in one trial (McMahon 2010).
Excluded studies
Fourteen studies were excluded (Afifi 1979; Bonini‐Domingos 2012; Cacciola 1990b; Connor 2018; Lucena 2007; Massenburg 2016; Meneses 2016; Minniti 2014; Neves 2010; Okany 2004; Paggiaro 2010; Sawyer 1979; Serjeant 1970; Valentino 2017). Overall, the majority of these studies were non‐RCT or case reports. See the Characteristics of excluded studies table for a detailed account of the reasons for exclusion.
Studies awaiting classification
This Cochrane Review includes two studies awaiting classification (Friedrisch 2016; You 2019). Regarding the Friedrisch trial, we contacted to the main author of the published abstract, however, we have not yet received any reply. The You trial is reported on a trials registry and as an abstract only and we await the publication of the full trial report in order to fully assess this and access relevant outcome data, See the Characteristics of studies awaiting classification table (You 2019).
Ongoing studies
This Cochrane Systematic review identified one ongoing trial (NCT04058197). It is assessing the clinical benefits and harms of deferoxamine intradermal compared with placebo. See the Characteristics of ongoing studies table for additional information.
Risk of bias in included studies
None of the included RCTs were graded as having an overall low risk of bias. The risk of bias of all domains listed above for all included trials are summarised in Figure 3 and Figure 4. SeeCharacteristics of included studies for details.

Risk of bias graph: review authors' judgements about each risk of bias domain presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias domain for each included study
Allocation
Random sequence generation
One RCT used an adequate method for sequence generation (graded as low risk) (McMahon 2010). We assessed the remaining five RCTs as having an unclear risk of bias in relation to the method used for generating the allocation sequence (Baum 1987; La Grenade 1993; Serjeant 1977; Serjeant 1997; Wethers 1994).
Allocation concealment
No information was reported on allocation concealment by any of the included trials (Baum 1987; La Grenade 1993; McMahon 2010; Serjeant 1977; Serjeant 1997; Wethers 1994). See Characteristics of included studies for details.
Blinding
We assessed participant and personnel blinding separately. Three RCTs were described within the published papers as blinded trials (Serjeant 1977; Serjeant 1997; Wethers 1994). We judged one trial to have an adequate blinding process (low risk) (Wethers 1994). No information was reported about this domain in the remaining five trials (unclear risk) (Baum 1987; La Grenade 1993; McMahon 2010; Serjeant 1977; Serjeant 1997).
Incomplete outcome data
One RCT was assessed as having adequately reported outcome data (low risk) (Wethers 1994). Three RCTs were assessed as having an unclear risk in this domain (Baum 1987; McMahon 2010; Serjeant 1997), and two as having a high risk of bias in this domain (La Grenade 1993; Serjeant 1977).
Selective reporting
Two RCTs show adequate reporting (graded as low risk) (McMahon 2010; Wethers 1994). The remaining four RCTs were assessed as having a high risk of bias for this domain (Baum 1987; La Grenade 1993; Serjeant 1977; Serjeant 1997).
Other potential sources of bias
All of the included RCTs show some type of bias (ascertainment bias, bias in the presentation of data, design bias). SeeCharacteristics of included studies for details. SeeAppendix 6 for bias definitions.
Effects of interventions
See: Summary of findings 1 Isoxsuprine compared with placebo for leg ulcers in people with sickle cell disease; Summary of findings 2 Arginine butyrate plus standard care alone compared with standard care alone for leg ulcers in people with sickle cell disease; Summary of findings 3 Propionyl‐L‐carnitine compared with placebo for leg ulcers in people with sickle cell disease; Summary of findings 4 RGD peptide matrix compared with placebo for leg ulcers in people with sickle cell disease; Summary of findings 5 Solcoseryl compared with antiseptic agent for leg ulcers in people with sickle cell disease; Summary of findings 6 Duoderm® (hydroactive dressing) compared with antiseptic agent for leg ulcers in people with sickle cell disease
The quality of the evidence has been graded for those outcomes included in the summary of findings tables. For the definitions of these gradings, please refer to the summary of findings tables (summary of findings Table 1; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4; summary of findings Table 5; summary of findings Table 6).
The included trials all assessed pharmaceutical interventions; no trials assessed non‐pharmaceutical interventions.
Six RCTs were conducted for treating leg ulcers in people with SCD (Baum 1987; La Grenade 1993; McMahon 2010; Serjeant 1977; Serjeant 1997; Wethers 1994). All RCTs assessed pharmaceutical interventions. The results are based on five RCTs (La Grenade 1993; McMahon 2010; Serjeant 1977; Serjeant 1997; Wethers 1994) as Baum 1987 did not report on any of the outcomes pre‐defined in our protocol .
Results were based on 168 participants with 210 ulcers (La Grenade 1993; McMahon 2010; Serjeant 1977; Serjeant 1997; Wethers 1994)..
Due to the differences in the modes of action of the pharmaceutical interventions used in the trials and differences among the unit of randomisation and analysis, we regarded it as inappropriate to combine the data available. As referred above, for those trials where ulcers were the unit of analysis (instead of the participant), data have been narratively reported (La Grenade 1993; McMahon 2010; Serjeant 1977).
1. Pharmaceutical interventions
Systemic interventions
Three trials investigated systemic interventions (McMahon 2010; Serjeant 1977; Serjeant 1997).
Primary outcomes
1. Complete leg ulcer closure (defined as 100% epithelization or skin closure without drainage)
-
Vascular drugs
Isoxsuprine versus placebo
Serjeant 1977 compared isoxsuprine with placebo and found inconclusive evidence regarding complete leg ulcer closure, RR 1.20 (95% CI 0.40 to 3.62) (participants = 40/ ulcers = 54, very low quality of evidence) (Analysis 1.1). Bayes factor was 1.98 which means that there is a likelihood of 1.98 times for isoxsuprine than placebo for reaching a complete leg ulcer closure, i.e. it favours to alternative hypothesis. See summary of findings Table 1.
-
Pharmacologic stimulation of HbF synthesis agents
Arginine butyrate plus standard care versus standard care alone
McMahon found inconclusive evidence in terms of complete leg ulcer closure comparing arginine butyrate versus standard care alone, RR 3.72 (95% CI 0.90 to 15.35) (23 participants as the unit of randomisation and 62 ulcers as the unit of analysis; very low quality evidence) (McMahon 2010) (Analysis 2.1). Bayes factor was 4.47 which means that there is a likelihood of 4.47 times for arginine butyrate than placebo for reaching a complete leg ulcer closure, i.e. it favours to alternative hypothesis. See summary of findings Table 3.
2. Time to ulcer closure
This outcome was not reported on in any of the RCTs.
3. Change in ulcer size (surface area or volume)
-
Vascular drugs
Isoxsuprine versus placebo
Serjeant 1977 of 54 ulcers in 40 participants comparing isoxsuprine with placebo reported inconclusive evidence in six‐month period, MD 2.10 cm² (95% CI 0.00 to 4.20) (participants = 40, very low quality of evidence). Bayes factor was 4.15 which means that there is a likelihood of 4.15 times for isoxsuprine than placebo for reaching a change in ulcer size, i.e. it favours to alternative hypothesis. See summary of findings Table 1.
-
Pharmacologic stimulation of HbF synthesis agents
Arginine butyrate plus standard care versus standard care alone
One trial found reduction in ulcer size favouring to participants allocated to arginine butyrate rather than those receiving standard care alone, MD ‐5.10 cm² (95% CI ‐9.65 to ‐0.55) (participants = 23; very low‐quality evidence) (McMahon 2010) (Analysis 2.2). See summary of findings Table 3. However, the two groups were not comparable at the start of the trial. Mean baseline ulcer area (cm²) in arginine butyrate plus standard care was 50.6 versus 25.7 for standard care alone.
-
Antioxidant agents
Propionyl‐L‐carnitine
Comparison between propionyl‐L‐carnitine and placebo found uncertain effect regarding change in ulcer size at 12‐weeks, MD ‐3.90 (95% CI ‐13.44 to 5.64) (participants = 15, very low‐quality evidence) (Serjeant 1997) (Analysis 3.1). Bayes factor was 0.0 which means that there is a likelihood of zero times for propionyl‐L‐carnitine than placebo for reaching a change in ulcer size, i.e. it favours to null hypothesis. See summary of findings Table 3.
Secondary outcomes
1. Ulcer‐free survival following treatment for SCLU (free from leg ulcer recurrence)
This outcome was not reported on any of the RCTs.
2. Quality of life measures (based on any item from a validated scale, e.g. SF‐36, EuroQoL, WHOQOL‐BREF)
This outcome was not reported on any of the RCTs.
3. Amputation
This outcome was not reported on any of the RCTs.
4. Adverse events
-
Vascular drugs
Isoxsuprine versus placebo
Serjeant did not report any data or information on adverse events (Serjeant 1977) (Appendix 9).
-
Pharmacologic stimulation of HbF synthesis agents
Arginine butyrate (McMahon 2010).
One trial reported that no serious adverse events were reported to be directly related to the study drug (McMahon 2010). Prior to arginine butyrate treatment on this study, port‐a‐cath infections did occur in two participants who had ports for their standard sickle cell care (Appendix 9).
-
Antioxidant agents
Propionyl‐L‐carnitine
Serjeant did not report any data or information on adverse events (Serjeant 1997) (Appendix 9).
Topical pharmaceutical interventions
Three trials investigated topical interventions (Baum 1987; La Grenade 1993; Wethers 1994). The results are based on two RCTs (La Grenade 1993; Wethers 1994); Baum did not report on any of the outcomes pre‐defined in our protocol (Baum 1987).
Primary outcomes
1. Complete leg ulcer closure (defined as 100% epithelization or skin closure without drainage)
-
Growth factors and related
Wethers found no conclusive evidence comparing RGD peptide matrix versus placebo in terms of complete leg ulcer closure (5/32 (15.6%) versus 9/23 (39.1%), RR 0.40 (95% CI 0.15 to 1.04) (participants = 55, very low quality evidence) (Wethers 1994) (Analysis 4.1). Bayes factor was 1.17 which means that there is a likelihood of 1.17 times for RGD peptide matrix than placebo for reaching a complete leg ulcer closure, i.e. it should be interpreted as no differences. See summary of findings Table 4.
2. Time to ulcer closure
This outcome was not reported on any of the RCTs.
3. Change in ulcer size
-
Growth factors and related
One trial of 32 participants and 49 ulcers (unit of randomisation and analysis) reported this outcome. Solcoseryl® compared with antiseptic agent (Eusol) showed no difference regarding change in ulcer size, MD 7.10 cm² (95% CI ‐0.70 to 14.90); ulcers = 35; very low quality evidence). Factor Bayes was 3.18 which means here is a likelihood of 3.18 times for Solcoseryl® than control for reaching a reduction of ulcer size i.e. higher likelihood of true for supporting evidence came from alternative hypothesis. The comparison of hydrocolloid dressing (Duoderm® ) with antiseptic agent (Eusol) found inconclusive evidence in terms change in ulcer size, MD 4.12 (95% CI ‐3.68 to 11.92) (ulcers = 35; very low quality evidence). Factor Bayes was 1.04 which means the data are equally well predicted by hydrocolloid dressing (Duoderm® ) as control, so it should not be interpreted as favouring control; rather the evidence does not point either way (La Grenade 1993). See Analysis 5.1 and Analysis 6.1, respectively. See summary of findings Table 5and summary of findings Table 6, respectively.
Secondary outcomes
1. Ulcer‐free survival following treatment for SCLU (free from leg ulcer recurrence)
This outcome was not reported on any of the RCTs.
2. Quality of life measures (based on any item from a validated scale, e.g. SF‐36, EuroQoL, WHOQOL‐BREF)
This outcome was not reported on any of the RCTs.
3. Incidence of amputation
This outcome was not reported on any of the RCTs.
4. Adverse events
-
Growth factors and related
One trial found inconclusive evidence comparing RGD peptide matrix group and the placebo group regarding total occurrence of adverse events (17/32 (53.1%) versus 16/23(69.6%); RR 0.76 (95% CI 0.50 to 1.17) (very low quality of evidence) (Wethers 1994) (Analysis 4.2). In terms of treatment‐related adverse event Wethers 1994 reported also uncertain evidence (3/17 (17.64%) versus 2/16 (12.5%); RR 1.41 (95% CI 0.27 to 7.38) (participants = 55, very low quality of evidence) (Analysis 4.2). See summary of findings Table 4,
One trial assessing Solcoseryl® and hydrocolloid dressing reported that "Solcoseryl® was well tolerated as was Eusol except for a burning sensation reported by several patients" (La Grenade 1993). Hydrocolloid dressing was not well tolerated, four patients expressing dislike because of the large volume of exudate which was smelly and required daily changes of dressing in the early part of the study, and two of these defaulted" (La Grenade 1993). See summary of findings Table 5and summary of findings Table 6, respectively.
Discussion
Summary of main results
This updated review of interventions for treating leg ulcers in people with SCD included seven trials (198 participants with 250 ulcers) which were grouped as systemic pharmaceutical interventions (L‐carnitine (Serjeant 1997), arginine butyrate (McMahon 2010), isoxsuprine hydrochloride (Serjeant 1977)) and topical pharmaceutical interventions (Solcoseryl® cream (La Grenade 1993), arginine‐glycine‐aspartic acid (RGD) peptide dressing (Wethers 1994), ), and topical antibiotics (Baum 1987)). One trial was included but not reported any pre‐specified outcome in this Cochrane Review (Baum 1987). Furthermore, it did not report the number of ulcers. In Jamaica, a team of researchers conducted four trials (Baum 1987; La Grenade 1993; Serjeant 1977; Serjeant 1997). Two trials were conducted in USA by different research groups (McMahon 2010; Wethers 1994). From these interventions, arginine butyrate seems to have benefit in reducing ulcer size respect to control (McMahon 2010). But, effect size is very small with a very low quality of evidence. Harm profile remains uncertain for all interventions. The evidence for the use of interventions to treat people with SCD is very low. Trials were underpowered and had a high risk of bias. Drug companies sponsored five trials (Baum 1987; La Grenade 1993; Serjeant 1977; Serjeant 1997; Wethers 1994). Please refer to the following tables for details (summary of findings Table 1; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4; summary of findings Table 5; summary of findings Table 6).
Overall completeness and applicability of evidence
This updated review provides inconclusive evidence on the assessed interventions due to both heterogeneity between trials, and inadequate information provided by trial reports (Hopewell 2010). During this review, we have identified the following issues, which we feel are particularly relevant to consider as further work is planned. Generally, heterogeneity between trials prevented the pooling of results, with the main areas of variation between trials being differences in outcome definition, and inconsistency between unit of randomisation and unit of analysis (Appendix 8). In this regard, it has been recently suggested that trials adopt an agreed set of core outcomes for each medical condition (Clarke 2007). This approach could help to reduce the impact of outcome reporting bias (Kirkham 2018). In particular, a lack of reported inclusion and exclusion criteria and a lack of reported outcome definitions hampered the ability to compare trials (Appendix 7). This updated review shows albatross plots, which are showing the results from trials whose effect sizes could not be pooled due to inconsistency between their units of randomisation and analysis (Harrison 2017; McKenzie 2019). Albatross plots show obvious heterogeneity across the trials (Figure 5; Figure 6).

Albatross plot for three trials about interventions for treating leg ulcers in people with sickle cell disease on complete leg ulcers closure
Albatross plot based on total sample size, P values and risk reduction (RR) from three trials (black points) reporting results on complete leg ulcers closure in people with sickle cell disease. Two trials (on the right side) showed positive associations (RR > 1), but neither had a P value < 0.05. One trial (on the left side) showed a negative association (RR < 1), but again did not have a P value < 0.05. This Albatross plot shows a clear heterogeneity among trials as points are spread across the width of the graph. Meta‐analysis of these small trials was not possible due to inconsistency between unit of randomisation and unit of analysis.

Albatross plot for five trials about interventions for treating leg ulcers in people with sickle cell disease on reducing mean size of leg ulcers
Albatross plot based on total sample size, P values and mean difference (MD) from five trials (black points) reporting results on reduction mean size of leg ulcers in people with sickle cell disease. Four trials (on the right side) showed a positive association (MD > 0). One trial (on the left side) shows a negative association (MD < 0), but with a large P value. This albatross plot shows a clear heterogeneity among trials, with no clear, consistent magnitude of effect and points spread across the graph. Meta‐analysis of these small trials was not possible due to inconsistency between unit of randomisation and unit of analysis.
The only intervention that seems to show some small benefits was arginine butyrate (McMahon 2010). However, that trial has a major pitfall which was an imbalance of mean area of ulcer between arginine butyrate (50.6 cm²) and control group (25.7cm²) at the start of the trial. Obviously, at the trial end, it seems to have a larger size of effect due to intervention (28.3 cm²) than in the control group (23.2 cm²). It has been pointed out that large initial values may have largest differences (Harrel 2017). Furthermore, this trial was not powered for any outcome, and showed inconsistency among unit of randomisation (participants) and unit of analysis (ulcer). It is a reason to explain fails to translate into benefits for patients (Heneghan 2017).
This updated Cochrane Review found that a team of researchers conducted four included trials (Baum 1987; La Grenade 1993; Serjeant 1977; Serjeant 1997). There are studies pointing out that the single‐centre trials overrates the treatment effect compared with multicenter trials (Bafeta 2012; Dechartres 2011; Inaba 2009; Unverzagt 2013).
Another finding (data not shown) was the dichotomization of continuous outcome performed in some trials to show potential benefit effect of the interventions. This issue has been rejected due to potentials misleading results (Mabikwa 2017; MacCallum 2002; Royston 2006; Senn 2005).
The fact that this updated review did not find strong differences between any experimental drug and placebo groups should not be interpreted as meaning that medication study and placebo have the same efficacy (Alderson 2004). The first possible explanation is failure to achieve an appropriate sample size (Peduzzi 1996). Five included trials reported no information about a priori sample size estimation (Baum 1987; La Grenade 1993; McMahon 2010; Serjeant 1977; Wethers 1994). Therefore, it represents a threat to statistical conclusion validity which is due to a low statistical power (Matthay 2020). It is defined as "an insufficiently powered experiment may incorrectly conclude that there is no relationship between treatment and outcome" (Matthay 2020). Furthermore, trials with small sample size are associated with inconsistency, heterogeneity, incoherence for clinical decision‐making and with lack of non‐comparability, i.e. confusion bias (Martí‐Carvajal 2018). Simulation studies have shown that sample sizes well over 1000 participants are needed before simple randomisation can bring what we expect: a fair and even distribution of prognostic factors among participants (Martí‐Carvajal 2018; Nguyen 2017).
Thus, overall completeness and applicability of evidence is poor due to potential spurious findings.
Quality of the evidence
The main source of bias in the included studies was the lack of detail in describing the generation of the randomisation sequences or the allocation concealment (Baum 1987; La Grenade 1993; Serjeant 1977; Serjeant 1997; Wethers 1994). We interviewed an author who was on four of the six included studies, who provided information on outstanding details. Trials also lacked detail about their blinding processes. The review authors' assessment of the risk of bias of the included studies has been described previously and a summary can be found in Figure 3 and Figure 4. The trials were classified as having a high risk of bias. Uncertainty remains about possible harms from the interventions, due to a lack of information presented on harms data. Furthermore, trials had smallness of sample size which yielded wide confidence intervals (Altman 2004; Stratford 2010; Castellini 2018). See summary of findings Table 1; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4; summary of findings Table 5; summary of findings Table 6 for details.
Potential biases in the review process
In the process of performing a systematic review, there is a group of biases called significance‐chasing biases (Ioannidis 2010). These includes publication bias, selective outcome reporting bias, selective analysis reporting bias, and fabrication bias (Ioannidis 2010). Publication bias represents a major threat to the validity of systematic reviews, particularly in reviews such as this one that includes small trials. However, we could not perform a funnel plot to explore the risk of bias because we included less than ten studies. To minimize publication bias we contacted the main author of four included RCTs. Selective outcome reporting bias operates through suppression of information on specific outcomes and has similarities to study publication bias, in that ‘negative’ results remain unpublished (Ioannidis 2010). This updated Cochrane review found that four out of the six included RCTs have high risk of selective outcome reporting (Baum 1987; La Grenade 1993; Serjeant 1977; Serjeant 1997).
Agreements and disagreements with other studies or reviews
It has been reported that even with rigorous conventional care, leg ulcers tend to be indolent and intractable, healing slowly over months or years (Ballas 2002; Minniti 2010).

Study flow diagram ‐ update Juanuary 2020

Risk of bias graph: review authors' judgements about each risk of bias domain presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias domain for each included study

Albatross plot for three trials about interventions for treating leg ulcers in people with sickle cell disease on complete leg ulcers closure
Albatross plot based on total sample size, P values and risk reduction (RR) from three trials (black points) reporting results on complete leg ulcers closure in people with sickle cell disease. Two trials (on the right side) showed positive associations (RR > 1), but neither had a P value < 0.05. One trial (on the left side) showed a negative association (RR < 1), but again did not have a P value < 0.05. This Albatross plot shows a clear heterogeneity among trials as points are spread across the width of the graph. Meta‐analysis of these small trials was not possible due to inconsistency between unit of randomisation and unit of analysis.
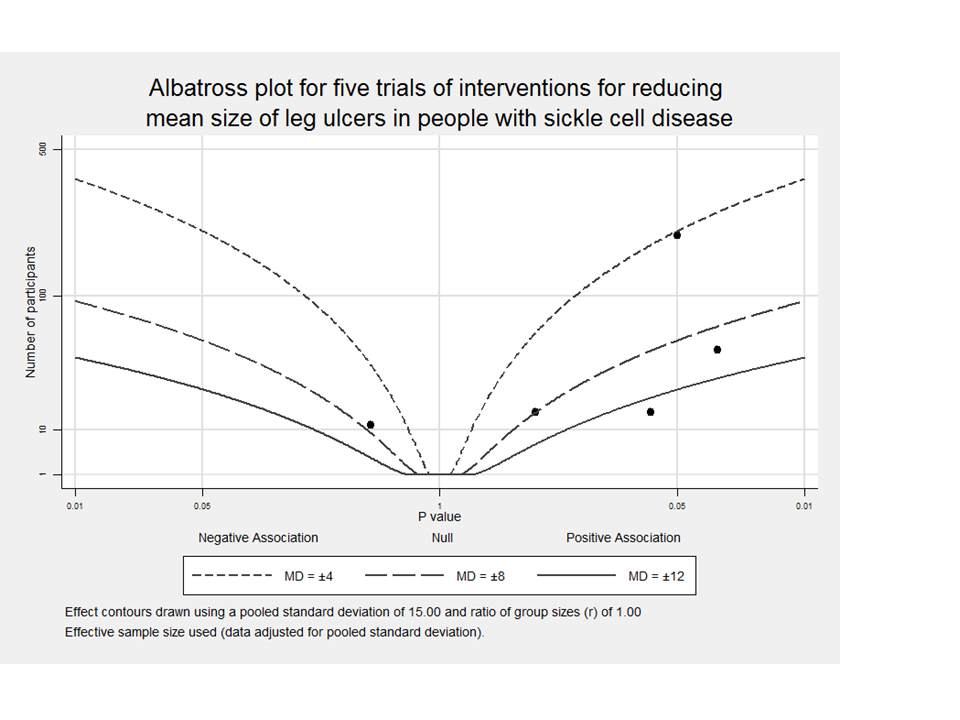
Albatross plot for five trials about interventions for treating leg ulcers in people with sickle cell disease on reducing mean size of leg ulcers
Albatross plot based on total sample size, P values and mean difference (MD) from five trials (black points) reporting results on reduction mean size of leg ulcers in people with sickle cell disease. Four trials (on the right side) showed a positive association (MD > 0). One trial (on the left side) shows a negative association (MD < 0), but with a large P value. This albatross plot shows a clear heterogeneity among trials, with no clear, consistent magnitude of effect and points spread across the graph. Meta‐analysis of these small trials was not possible due to inconsistency between unit of randomisation and unit of analysis.

Comparison 1: Isoxsuprine versus placebo, Outcome 1: Complete leg ulcer closure

Comparison 1: Isoxsuprine versus placebo, Outcome 2: Change in ulcer size

Comparison 2: Arginine butyrate plus standard care alone versus standard care alone, Outcome 1: Complete wound closure

Comparison 2: Arginine butyrate plus standard care alone versus standard care alone, Outcome 2: Change in ulcer size

Comparison 3: Propionyl‐L‐carnitine versus placebo, Outcome 1: Change in ulcer size (surface area or volume)

Comparison 4: RGD peptide matrix versus placebo, Outcome 1: Complete leg ulcer closure

Comparison 4: RGD peptide matrix versus placebo, Outcome 2: Adverse events

Comparison 5: Solcoseryl® versus antiseptic agent (Eusol), Outcome 1: Reduction in ulcer area

Comparison 6: Duoderm® versus antiseptic agent (Eusol), Outcome 1: Reduction in ulcer area
| Isoxuprine compared with placebo | ||||||
| Patient or population: individuals with sickle cell disease and a leg ulcer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Placebo | Isoxuprine | |||||
| Complete leg ulcer closure (defined as 100% epithelization or skin closure without drainage) | 182 per 1000a | 218 per 1000 | RR 1.20 | 54 ulcers (40 participants) | ⊕⊝⊝⊝ | Serjeant 1977 shows 2 inconsistencies between units of randomisation (30 or 40 participants) and unit of analysis (46 or 54 ulcers). |
| Time to ulcer closure ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1977 did not report this outcome. |
| Change in ulcer size (surface area or volume) | The mean change in ulcer size (surface area or volume) in the control groups was | The mean change in ulcer size (surface area or volume) in the intervention groups was | (40 or 30 participants) | ⊕⊝⊝⊝ | Serjeant 1977 shows 2 inconsistencies between units of randomisation (30 or 40 participants) and unit of analysis (46 or 54 ulcers). | |
| Ulcer‐free survival following treatment for SCLU (free from leg ulcer recurrence) ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1977 did not report this outcome. |
| Quality of life ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1977 did not report this outcome. |
| Amputation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1977 did not report this outcome. |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1977 did not report this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Assumed risk is based on the risks for the control group of Serjeant 1977 (18.2%). | ||||||
| Arginine butyrate plus standard care alone compared with standard care alone | ||||||
| Patient or population: individuals with sickle cell disease and a leg ulcer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Standard care alone | Arginine butyrate plus standard care alone | |||||
| Complete leg ulcer closure (defined as 100% epithelization or skin closure without drainage) | 80 per 1000a | 298 per 1000 | RR 3.72 | 23 participants 62 ulcers (1 study: McMahon 2010)b | ⊕⊝⊝⊝ | McMahon 2010 shows inconsistency between units of randomisation (23 participants) and unit of analysis (62 ulcers). |
| Time to ulcer closure ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | McMahon 2010 did not report this outcome. |
| Change in ulcer size (surface area or volume) Measured with computerized planimetry | The mean change in ulcer size (surface area or volume) in the control groups was | The mean change in ulcer size (surface area or volume) in the intervention groups was | 23 participants | ⊕⊝⊝⊝ | McMahon 2010 shows inconsistency between units of randomisation (23 participants) and unit of analysis (62 ulcers). Ulcer mean size at trial start in intervention group was two‐fold respect to control group. | |
| Ulcer‐free survival following treatment for SCLU (free from leg ulcer recurrence) ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | McMahon 2010 did not report this outcome. |
| Quality of life ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | McMahon 2010 did not report this outcome. |
| Amputation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | McMahon 2010 did not report this outcome. |
| Adverse events | See comment | See comment | Not estimable | 23 participants | See comment | McMahon 2010 reported "no serious adverse events were reported to be directly related to the study drug". |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Assumed risk is based on the risks for the control group of McMahon 2010 (8%). | ||||||
| Propionyl‐L‐carnitine compared with placebo | ||||||
| Patient or population: individuals with sickle cell disease and a leg ulcer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Placebo | Propionyl‐L‐carnitine | |||||
| Complete leg ulcer closure (defined as 100% epithelization or skin closure without drainage) ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1997 did not report this outcome. |
| Time to ulcer closure ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1997 did not report this outcome. |
| Change in ulcer size (surface area or volume) | The mean change in ulcer size (surface area or volume) in the control groups was | The mean change in ulcer size (surface area or volume) in the intervention groups was | 15 | ⊕⊝⊝⊝ | ||
| Ulcer‐free survival following treatment for SCLU (free from leg ulcer recurrence) ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1997 did not report this outcome. |
| Quality of life ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1997 did not report this outcome. |
| Amputation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1997 did not report this outcome. |
| Adverse events ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1997 did not report this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Trial conducted in Jamaica. | ||||||
| RGD peptide matrix compared with placebo | ||||||
| Patient or population: individuals with sickle cell disease and leg ulcer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Placebo | RGD peptide matrix | |||||
| Complete closure | 391 per 1000a | 157 per 1000 | RR 0.40 | 55 | ⊕⊝⊝⊝ | Based on number of participants. |
| Time to ulcer closure ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Wethers 1994 did not report this outcome. |
| Change in size ulcers healed ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Wethers 1994 did not report this outcome. |
| Ulcer‐free survival following treatment for SCLU (free from leg ulcer recurrence) ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Wethers 1994 did not report this outcome. |
| Quality of life ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Wethers 1994 did not report this outcome. |
| Amputation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Wethers 1994 did not report this outcome. |
| Adverse events | 696 per 1000e | 529 per 1000 | RR 0.76 | 55 | ⊕⊝⊝⊝ | Based on number of participants. Related study treatment adverse events: 1.41 (95% CI 0.27 to 7.38) based on number of participants with adverse events. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Assumed risk is based on the risks for the control group of Wethers 1994 (39.1%). | ||||||
| Solcoseryl compared with antiseptic agent | ||||||
| Patient or population: individuals with sickle cell disease and leg ulcer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Antiseptic agent | Solcoseryl | |||||
| Complete leg ulcer closure ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Time to closure ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Change in ulcer size (surface area or volume) | The mean change in ulcer size (surface area or volume) in the control groupsa was | The mean change in ulcer size (surface area or volume) in the intervention groups was | 35 ulcers in 32 participants | ⊕⊝⊝⊝ | La Grenade 1993 measured this outcome using ulcers as unit of analysis. | |
| Ulcer‐free survival following treatment for SCLU (free from leg ulcer recurrence) ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Quality of life ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Amputation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Adverse events | See comment | See comment | Not estimable | 35 ulcers in 32 participants | ⊕⊝⊝⊝ | La Grenade 1993 measured this outcome using individuals as unit of analysis. La Grenade 1993 reported "Solcoseryl® was well tolerated as was Eusol except for a burning sensation reported by several patients". |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Trial authors reported no final value from control group. | ||||||
| Duoderm®(hydroactive dressing) compared with antiseptic agent | ||||||
| Patient or population: individuals with sickle cell disease and leg ulcer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Antiseptic agent | Duoderm®(hydroactive dressing) | |||||
| Complete leg ulcer closure ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Time to closure ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Change in ulcer size (surface area or volume) | The mean change in ulcer size (surface area or volume) in the control groups was | The mean change in ulcer size (surface area or volume) in the intervention groups was | 35 ulcers in 32 participants | ⊕⊝⊝⊝ | La Grenade 1993 measured this outcome using ulcers as unit of analysis. | |
| Ulcer‐free survival following treatment for SCLU (free from leg ulcer recurrence) ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Quality of life ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Amputation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Adverse events | See comment | See comment | Not estimable | 35 ulcers in 32 participants | See comment | La Grenade 1993 measured this outcome using participants as unit of analysis. LLa Grenade 1993 reported "Hydrocolloid dressing was not well tolerated, four patients expressing dislike because of the large volume of exudate which was smelly and required daily changes of dressing in the early part of the study, and two of these defaulted." |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Trial authors reported no final value from control group. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Complete leg ulcer closure Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.40, 3.62] |
| 1.2 Change in ulcer size Show forest plot | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [0.00, 4.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Complete wound closure Show forest plot | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.72 [0.90, 15.35] |
| 2.2 Change in ulcer size Show forest plot | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐5.10 [‐9.65, ‐0.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Change in ulcer size (surface area or volume) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1.1 All randomised participants | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐3.90 [‐13.44, 5.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Complete leg ulcer closure Show forest plot | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.15, 1.04] |
| 4.2 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.2.1 Total | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.50, 1.17] |
| 4.2.2 Related study treatment | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.27, 7.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Reduction in ulcer area Show forest plot | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 7.10 [‐0.70, 14.90] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Reduction in ulcer area Show forest plot | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 4.12 [‐3.68, 11.92] |