Intervenciones para el tratamiento de las úlceras de las piernas en pacientes con anemia de células falciformes
Appendices
Appendix 1. Glossary of medical terms.
| Terms | Definition | Source |
|---|---|---|
| Antithrombin III | A plasma alpha 2 glycoprotein that accounts for the major antithrombin activity of normal plasma and also inhibits several other enzymes. It is a member of the serpin superfamily. | |
| Arginine butyrate | The butyric acid salt of the amino acid arginine. Protects against lethal effect of encelphalomyocarditis virus in mice; also used to administer butyrate to stimulate globin gene expression in beta‐thalassemia and sickle cell anemia. | |
| Endothelium | A layer of epithelium that lines the heart, blood vessels (endothelium, vascular), l vessels (endothelium, lymphatic), and the serous cavities of the body. | |
| Haemoglobinopathy | A group of inherited disorders characterized by structural alterations within the haemoglobin molecule. | |
| Haemoglobin SC Disease | One of the sickle cell disorders characterized by the presence of both haemoglobin S and haemoglobin C. It is similar to, but less severe than sickle cell anaemia. | |
| Hb SS (Haemoglobin, Sickle) | An abnormal haemoglobin resulting from the substitution of valine for glutamic acid at position 6 of the beta chain of the globin moiety. The heterozygous state results in sickle cell trait, the homozygous in sickle cell anaemia. | |
| Haemoglobin SC Disease | One of the sickle cell disorders characterized by the presence of both haemoglobin S and haemoglobin C. It is similar to, but less severe than sickle cell anaemia. | |
| Isoxsuprine | A beta‐adrenergic agonist that causes direct relaxation of uterine and vascular smooth muscle. | |
| L‐carnitine | A derivative of the amino acid lysine, required for the transport of fatty acids into mitochondria for oxidation. | |
| Nitric oxide | A free radical gas produced endogenously by a variety of mammalian cells, synthesized from arginine by nitric oxide synthase. Nitric oxide is one of the endothelium‐dependent relaxing factors released by the vascular endothelium and mediates vasodilation. It also inhibits platelet aggregation, induces disaggregation of aggregated platelets, and inhibits platelet adhesion to the vascular endothelium. | |
| Oral zinc sulphate | A salt of zinc used as a supplement for treating zinc deficiency. | |
| Priapism | A prolonged painful erection that may lasts hours and is not associated with sexual activity. It is seen in patients with sickle cell anaemia, advanced malignancy, spinal trauma; and certain drug treatments. | |
| Pulmonary hypertension | Increased vascular resistance in the pulmonary circulation, usually secondary to heart diseases or lung diseases. | |
| RGD peptide matrix | Arginine‐glycine‐aspartic acid (RGD). RGD peptide matrix is designed to act as a temporary, topical synthetic extracellular matrix that substitutes for the damaged natural matrix and provides support for cell ingrowth into the ulcer site. This synthetic matrix consists of an RGD‐containing peptide complexed with sodium hyaluronate in a sterile, non‐preserved viscous gel. RGD peptide matrix (Argidene Gel®, formerly Telio‐Derm Gel®, Telios Pharmaceuticals, San Diego, CA, USA). The peptide matrix contains the arginine‐glycine‐aspartic acid amino acid sequence, by which cells in vivo become attached to extracellular matrix macromolecules via surface integrin receptors. The matrix is a sterile non‐preserved clear viscous gel, formulated in phosphate‐buffered saline and dispensed from a single‐use syringe container. The functional ingredient of RGD peptide matrix is a complex formed by the combination of a synthetic 18 amino acid peptide and sodium hyaluronate. It also contains added unconjugated sodium hyaluronate as a viscosity‐increasing agent, and therefore does not require preparation from patient samples. | |
| Solcoseryl® DuoDerm® | Solcoseryl® is a tissue metabolism activator, chemically and biologically standardized and deproteinised, non antigenic and apyrogenic haemodialized extract form the blood of healthy young calf. Solcoseryl® contains a large amount of natural low molecular substances ‐ glycolipides, nucleosides, nucleotides, amino acids, oligopeptides, irreplaceable micro elements, electrolytes. Duoderm® (Granuflex®, Convatec, Uxbridge, UK, marketed as DuoDerm® in the USA), a hydrocolloid dressing containing colloids and elastomeric and adhesive components. |
Appendix 2. LILACS search strategy 3 July 2020
| Key words |
|---|
| sickle cell disease |
((Pt ENSAYO CONTROLADO ALEATORIO OR Pt ENSAYO CLINICO CONTROLADO OR Mh ENSAYOS CONTROLADOS ALEATORIOS OR Mh DISTRIBUCIÓN ALEATORIA OR Mh METODO DOBLE CIEGO OR Mh METODO SIMPLECIEGO OR Pt ESTUDIO MULTICÉNTRICO) or ((tw ensaio or tw ensayo or tw trial) and (tw azar or tw acaso or tw placebo or tw control$ or tw aleat$ or tw random$ or (tw duplo and tw cego) or (tw doble and tw ciego) or (tw double and tw blind)) and tw clinic$)) AND NOT ((Ct ANIMALES OR Mh ANIMALES OR Ct CONEJOS OR Ct RATÓN OR MH Ratas OR MH Primates OR MH Perros OR MH Conejos OR MH Porcinos) AND NOT (Ct HUMANO AND Ct ANIMALES)) [Palabras] and sickle [Palabras del resumen] and ulcer [Palabras]
Strategy results:21
Appendix 3. Web of Science 12 March 2018
| Key words |
|---|
| sickle Search result: 83 references. |
Appendix 4. ClinicalTrials.gov 3 July 2020
"Sickle " AND "leg ulcers"
Search results: 1
Appendix 5. WHO Internation Clinical Trials Registry Platform 12 March 2018
"Sickle " AND "leg ulcers"
Search results: 7
Appendix 6. Bias definition
| Bias (Porta 2014) | Definition |
|---|---|
| Bias in the presentation of data | Error due to irregularities produced bi digit preference, incomplete data, poor techniques of measurement, technically poor laboratory procedures, or intentional attempts to mislead. |
| Selection bias | Distortions that result from procedures used to select subjects and from factors that influence participation in the study. |
| Reporting bias | Selective revealing or suppression of information. |
| Design bias | The difference between a true value and that obtained as a result of faulty design of a study. |
Appendix 7. Outcome definitions as described by the RCTs' author
| Trials | Assessment of ulcer healing | Change in ulcer size (surface area or volume) | Complete closure |
|---|---|---|---|
| "Ulcer was measured in two dimensions and photographs taken for determination of ulcer" as described by Serjeant 1970. | ‐ | ‐ | |
| It was analysed from the measured long and short dimensions of the ulcer, arbitrarily assuming and elliptical shape. | It was expressed as in real changes and percentage changes in area. | ‐ | |
| "During the weekly clinical visits, the ulcer was traced on acetate film and photographed. All ulcer areas were then calculated by computerized planimetry, using the IMAGEJ software (NIH, Bethesda, MD, USA)". | NA | Complete closure of the ulcer (to an area of 0 cm²). | |
| "The ulcer periphery was defined on the photograph, cut out, weighed, and the area of the ulcer calculated from the weight/area ratio of the photographic paper". | Default: "defined as less than 4 months satisfactorily attendance at the trial". Healed ulcers "expressed as a proportion of those ulcers likely to heal on the basis of small size (8 cm²). | ‐ | |
| "Ulcer size was measured by area (measured in square centimeters), computing an assumed ellipse from long and short axis measurements. When more than one ulcer was present, the average area calculated for that patient was used in the analysis". | Period: 12 weeks. "(1) absolute change in area (change in ulcer area over treatment period divided by number of weeks on treatment) x 12 and (2) percentage change in area (absolute change in area + area at start of treatment)". | ‐ | |
| "Changes in percent ulcer closure occurring between study commencement and endpoint". | ‐ | ‐ |
Appendix 8. Unit of randomisation versus unit of analysis
| Study | Unit of randomisation | Unit of analysis |
|---|---|---|
| participants | ulcer | |
| ulcer | ulcer | |
| participants | ulcer | |
| participants | ulcer | |
| participants | participants | |
| participants | participants |
Appendix 9. Adverse events as were reported into each trials
| Trial | Adverse events |
| There was not mention on adverse events. | |
| Solcoseryl® was well tolerated as was Eusol except for a burning sensation reported by several patients. Hydrocolloid dressing was not well tolerated, four patients expressing dislike because of the large volume of exudate which was smelly and required daily changes of dressing in the early part of the study, and two of these defaulted". | |
| "No serious adverse events were reported to be directly related to the study drug. Drug‐related adverse events related to Arginine Butyrate included headache and nausea, which were usually preventable or controlled with anti‐emetics and acetaminophen or ibuprofen therapy given prior to and during the infusions." | |
| There was not mention on adverse events. | |
| There was not mention on adverse events. | |
| "The adverse event incidence rate among the RGD peptide matrix recipients was 0.53 events per patient (17 events in 32 patients) compared with 0.70 (16 events in23 patients) in the placebo group. No adverse events were classified as either probably or definitely related to the study treatment. Three of the adverse events in the RGD peptide matrix patients (mild blistering; erythema, itching, and rash; sweating and itching) were judged to be possibly related to the study treatment compared with two such adverse events in the placebo group (swelling and impaired function of the hands; pain, tenderness, and skin discoloration)." |

Study flow diagram ‐ update Juanuary 2020

Risk of bias graph: review authors' judgements about each risk of bias domain presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias domain for each included study

Albatross plot for three trials about interventions for treating leg ulcers in people with sickle cell disease on complete leg ulcers closure
Albatross plot based on total sample size, P values and risk reduction (RR) from three trials (black points) reporting results on complete leg ulcers closure in people with sickle cell disease. Two trials (on the right side) showed positive associations (RR > 1), but neither had a P value < 0.05. One trial (on the left side) showed a negative association (RR < 1), but again did not have a P value < 0.05. This Albatross plot shows a clear heterogeneity among trials as points are spread across the width of the graph. Meta‐analysis of these small trials was not possible due to inconsistency between unit of randomisation and unit of analysis.
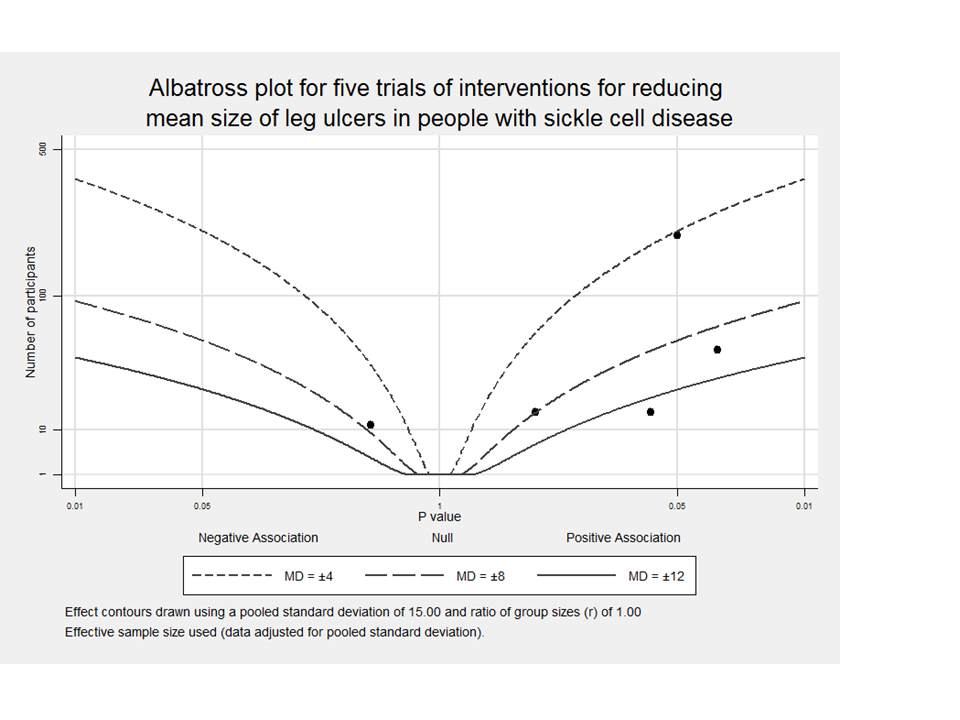
Albatross plot for five trials about interventions for treating leg ulcers in people with sickle cell disease on reducing mean size of leg ulcers
Albatross plot based on total sample size, P values and mean difference (MD) from five trials (black points) reporting results on reduction mean size of leg ulcers in people with sickle cell disease. Four trials (on the right side) showed a positive association (MD > 0). One trial (on the left side) shows a negative association (MD < 0), but with a large P value. This albatross plot shows a clear heterogeneity among trials, with no clear, consistent magnitude of effect and points spread across the graph. Meta‐analysis of these small trials was not possible due to inconsistency between unit of randomisation and unit of analysis.

Comparison 1: Isoxsuprine versus placebo, Outcome 1: Complete leg ulcer closure

Comparison 1: Isoxsuprine versus placebo, Outcome 2: Change in ulcer size

Comparison 2: Arginine butyrate plus standard care alone versus standard care alone, Outcome 1: Complete wound closure

Comparison 2: Arginine butyrate plus standard care alone versus standard care alone, Outcome 2: Change in ulcer size

Comparison 3: Propionyl‐L‐carnitine versus placebo, Outcome 1: Change in ulcer size (surface area or volume)

Comparison 4: RGD peptide matrix versus placebo, Outcome 1: Complete leg ulcer closure

Comparison 4: RGD peptide matrix versus placebo, Outcome 2: Adverse events

Comparison 5: Solcoseryl® versus antiseptic agent (Eusol), Outcome 1: Reduction in ulcer area

Comparison 6: Duoderm® versus antiseptic agent (Eusol), Outcome 1: Reduction in ulcer area
| Isoxuprine compared with placebo | ||||||
| Patient or population: individuals with sickle cell disease and a leg ulcer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Placebo | Isoxuprine | |||||
| Complete leg ulcer closure (defined as 100% epithelization or skin closure without drainage) | 182 per 1000a | 218 per 1000 | RR 1.20 | 54 ulcers (40 participants) | ⊕⊝⊝⊝ | Serjeant 1977 shows 2 inconsistencies between units of randomisation (30 or 40 participants) and unit of analysis (46 or 54 ulcers). |
| Time to ulcer closure ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1977 did not report this outcome. |
| Change in ulcer size (surface area or volume) | The mean change in ulcer size (surface area or volume) in the control groups was | The mean change in ulcer size (surface area or volume) in the intervention groups was | (40 or 30 participants) | ⊕⊝⊝⊝ | Serjeant 1977 shows 2 inconsistencies between units of randomisation (30 or 40 participants) and unit of analysis (46 or 54 ulcers). | |
| Ulcer‐free survival following treatment for SCLU (free from leg ulcer recurrence) ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1977 did not report this outcome. |
| Quality of life ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1977 did not report this outcome. |
| Amputation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1977 did not report this outcome. |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1977 did not report this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Assumed risk is based on the risks for the control group of Serjeant 1977 (18.2%). | ||||||
| Arginine butyrate plus standard care alone compared with standard care alone | ||||||
| Patient or population: individuals with sickle cell disease and a leg ulcer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Standard care alone | Arginine butyrate plus standard care alone | |||||
| Complete leg ulcer closure (defined as 100% epithelization or skin closure without drainage) | 80 per 1000a | 298 per 1000 | RR 3.72 | 23 participants 62 ulcers (1 study: McMahon 2010)b | ⊕⊝⊝⊝ | McMahon 2010 shows inconsistency between units of randomisation (23 participants) and unit of analysis (62 ulcers). |
| Time to ulcer closure ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | McMahon 2010 did not report this outcome. |
| Change in ulcer size (surface area or volume) Measured with computerized planimetry | The mean change in ulcer size (surface area or volume) in the control groups was | The mean change in ulcer size (surface area or volume) in the intervention groups was | 23 participants | ⊕⊝⊝⊝ | McMahon 2010 shows inconsistency between units of randomisation (23 participants) and unit of analysis (62 ulcers). Ulcer mean size at trial start in intervention group was two‐fold respect to control group. | |
| Ulcer‐free survival following treatment for SCLU (free from leg ulcer recurrence) ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | McMahon 2010 did not report this outcome. |
| Quality of life ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | McMahon 2010 did not report this outcome. |
| Amputation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | McMahon 2010 did not report this outcome. |
| Adverse events | See comment | See comment | Not estimable | 23 participants | See comment | McMahon 2010 reported "no serious adverse events were reported to be directly related to the study drug". |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Assumed risk is based on the risks for the control group of McMahon 2010 (8%). | ||||||
| Propionyl‐L‐carnitine compared with placebo | ||||||
| Patient or population: individuals with sickle cell disease and a leg ulcer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Placebo | Propionyl‐L‐carnitine | |||||
| Complete leg ulcer closure (defined as 100% epithelization or skin closure without drainage) ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1997 did not report this outcome. |
| Time to ulcer closure ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1997 did not report this outcome. |
| Change in ulcer size (surface area or volume) | The mean change in ulcer size (surface area or volume) in the control groups was | The mean change in ulcer size (surface area or volume) in the intervention groups was | 15 | ⊕⊝⊝⊝ | ||
| Ulcer‐free survival following treatment for SCLU (free from leg ulcer recurrence) ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1997 did not report this outcome. |
| Quality of life ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1997 did not report this outcome. |
| Amputation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1997 did not report this outcome. |
| Adverse events ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Serjeant 1997 did not report this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Trial conducted in Jamaica. | ||||||
| RGD peptide matrix compared with placebo | ||||||
| Patient or population: individuals with sickle cell disease and leg ulcer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Placebo | RGD peptide matrix | |||||
| Complete closure | 391 per 1000a | 157 per 1000 | RR 0.40 | 55 | ⊕⊝⊝⊝ | Based on number of participants. |
| Time to ulcer closure ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Wethers 1994 did not report this outcome. |
| Change in size ulcers healed ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Wethers 1994 did not report this outcome. |
| Ulcer‐free survival following treatment for SCLU (free from leg ulcer recurrence) ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Wethers 1994 did not report this outcome. |
| Quality of life ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Wethers 1994 did not report this outcome. |
| Amputation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Wethers 1994 did not report this outcome. |
| Adverse events | 696 per 1000e | 529 per 1000 | RR 0.76 | 55 | ⊕⊝⊝⊝ | Based on number of participants. Related study treatment adverse events: 1.41 (95% CI 0.27 to 7.38) based on number of participants with adverse events. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Assumed risk is based on the risks for the control group of Wethers 1994 (39.1%). | ||||||
| Solcoseryl compared with antiseptic agent | ||||||
| Patient or population: individuals with sickle cell disease and leg ulcer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Antiseptic agent | Solcoseryl | |||||
| Complete leg ulcer closure ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Time to closure ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Change in ulcer size (surface area or volume) | The mean change in ulcer size (surface area or volume) in the control groupsa was | The mean change in ulcer size (surface area or volume) in the intervention groups was | 35 ulcers in 32 participants | ⊕⊝⊝⊝ | La Grenade 1993 measured this outcome using ulcers as unit of analysis. | |
| Ulcer‐free survival following treatment for SCLU (free from leg ulcer recurrence) ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Quality of life ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Amputation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Adverse events | See comment | See comment | Not estimable | 35 ulcers in 32 participants | ⊕⊝⊝⊝ | La Grenade 1993 measured this outcome using individuals as unit of analysis. La Grenade 1993 reported "Solcoseryl® was well tolerated as was Eusol except for a burning sensation reported by several patients". |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Trial authors reported no final value from control group. | ||||||
| Duoderm®(hydroactive dressing) compared with antiseptic agent | ||||||
| Patient or population: individuals with sickle cell disease and leg ulcer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Antiseptic agent | Duoderm®(hydroactive dressing) | |||||
| Complete leg ulcer closure ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Time to closure ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Change in ulcer size (surface area or volume) | The mean change in ulcer size (surface area or volume) in the control groups was | The mean change in ulcer size (surface area or volume) in the intervention groups was | 35 ulcers in 32 participants | ⊕⊝⊝⊝ | La Grenade 1993 measured this outcome using ulcers as unit of analysis. | |
| Ulcer‐free survival following treatment for SCLU (free from leg ulcer recurrence) ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Quality of life ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Amputation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | La Grenade 1993 did not reported this outcome. |
| Adverse events | See comment | See comment | Not estimable | 35 ulcers in 32 participants | See comment | La Grenade 1993 measured this outcome using participants as unit of analysis. LLa Grenade 1993 reported "Hydrocolloid dressing was not well tolerated, four patients expressing dislike because of the large volume of exudate which was smelly and required daily changes of dressing in the early part of the study, and two of these defaulted." |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Trial authors reported no final value from control group. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Complete leg ulcer closure Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.40, 3.62] |
| 1.2 Change in ulcer size Show forest plot | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [0.00, 4.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Complete wound closure Show forest plot | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.72 [0.90, 15.35] |
| 2.2 Change in ulcer size Show forest plot | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐5.10 [‐9.65, ‐0.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Change in ulcer size (surface area or volume) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1.1 All randomised participants | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐3.90 [‐13.44, 5.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Complete leg ulcer closure Show forest plot | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.15, 1.04] |
| 4.2 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.2.1 Total | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.50, 1.17] |
| 4.2.2 Related study treatment | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.27, 7.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Reduction in ulcer area Show forest plot | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 7.10 [‐0.70, 14.90] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Reduction in ulcer area Show forest plot | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 4.12 [‐3.68, 11.92] |