Terapias psicológicas versus fármacos antidepresivos, solos y en combinación para la depresión en niños y adolescentes
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Duration: 12 weeks Follow‐up assessment points: Post‐intervention, 28 weeks Funded by: NHS Health Technology Assessment (HTA) Programme, Central Manchester and Manchester Children’s University Hospitals | |
| Participants | N = 208 Adolescents only (11 to 17 years) Depression diagnoses included: DSM‐IV; criteria for major or probable major depression (four symptoms with psychosocial impairment). Participants also had to obtain a score of 7 or more on the Health of the Nation Outcome scales for children and adolescents (HoNOSCA; Gowers 1999) Baseline risk of suicide: Measured using the suicidality items from the Kiddie Schedule for Affective Disorders and Schizophrenia Present and Lifetime Version (K‐SADS‐PL; Kaufman 1997). Patients with active suicidal intent were included in the study Baseline Fluoxetine + CBT n = 105 (%) Fluoxetine n = 103 (%) Thoughts 50 (47.6) 48 (46.6) Ideation 40 (38.1) 44 (42.7) Acts 13 (12.4) 21 (20.4) Medical lethality 3 (2.9) 4 (3.9) Self harm 30 (28.6) 23 (22.3) Baseline severity of depression: Children’s Depression Rating Scale‐Revised (CDRS‐R; Poznanski 1996). Mean t‐score (SD): Fluoxetine + CBT = 75.1 (6.7) Fluoxetine = 75.3 (6.7) Comorbidity included: Comorbidity Fluoxetine + CBT (n = 105) Fluoxetine (n = 103) Social Phobia 43 49 Obsessive compulsive disorder 42 37 Post‐traumatic stress disorder 42 36 Agorophobia 36 29 Separation anxiety disorder 31 28 Specific phobia 25 22 Conduct disorder 18 17 Panic disorder 21 14 Oppositional defiance disorder 17 13 Generalised anxiety disorder 19 13 Panic disorder (with agoraphobia) 20 13 ADHD 5 6 Bulemia Nervosa 8 4 Alcohol abuse 1 4 Transient tic disorder 2 3 Tourettes syndrome 2 2 Alcohol dependence 1 2 Encopresis 1 0 Enuresis 1 0 Dysthymia 1 0 Age: Range = 11 to 17 years Fluoxetine + CBT (median) = 14 Fluoxetine (median) = 14 Sex (M:F): Total: 54:154 Fluoxetine + CBT = 26:79 Fluoxetine = 28:75 Setting: Outpatient setting Excluded psychiatric diagnoses: Schizophrenia or bipolar disorder; global learning disability (formal testing not undertaken) Country: UK | |
| Interventions | Combination (Fluoxetine+CBT) N = 105 Name: CBT with core interventions including engagement and goal setting, emotional recognition, self monitoring, self reinforcement and activity scheduling, challenging negative thinking and cognitive restructuring, social problem‐solving and communication skills # sessions/length: 19 sessions over 28 weeks. (1 session per week for 12 weeks, 1 session per fortnight for 12 weeks, 1 final session at 28 weeks) Manualised (Y/N): Yes Individual or group: Individual Parent involvement: Encouraged at the end of each session by therapist Fidelity check: Yes. Audiotapes of the session were rated with a modified version of the cognitive therapy scale (Vallis 1986) Inter‐rater reliability k = 0.8 Delivered by: 4 Psychiatrists who either had previous CBT training or attended a 3‐day training course on CBT for depression, and 10 CBT therapists (mostly Psychologists) Name (class & type): SSRI (Fluoxetine). However, 26 participants were taking a different SSRI when admitted to the trial; 3 switched to fluoxetine and 11 changed from fluoxetine to another SSRI Dose (mg/day)/length: 10 mg daily for 1 week, increasing to 20 mg for 5 weeks. If no response, increase considered to 40 mg on alternate days for one week followed by 5 weeks of 40 mg. Option to increase dose to 60 mg on alternate days for 1 week followed by 60 mg daily for 5 weeks if participant did not respond by 12 weeks. Overall, there was a mean dose of 30 mg for both groups, and 2 patients received 60 mg Delivered by: psychiatrists in the context of ongoing clinical care. The content of contact was an explanation of depression and attention to recent family or peer group conflicts. Liaison with schools and other agencies undertaken when appropriate. Participants offered 9 outpatient sessions of usual care over 28 weeks, with the option of more if needed Medication Only N = 103 Medication details as above Y/N: Yes | |
| Outcomes | Clinician reported The Children’s Depression Rating Scale‐Revised (CDRS‐R; Poznanski 1996) Children’s Global Assessment Scale (C‐GAS; Shaffer 1983) Suicidality items from the Kiddie Schedule for Affective Disorders and Schizophrenia Present and Lifetime Version (K‐SADS‐PL; Kaufman 1997) Self reported The Mood and Feelings Questionnaire (MFQ; Wood 1995) Parent reported The Clinical Global Impression Improvement Scale (CGI‐I; Guy 1976) Additional Measures The Health of the Nation Outcome Scales for Children and Adolescents (HoNOSCA; Gowers 1999) The Clinical Global Impression Improvement Scale (CGI‐I; Guy 1976) | |
| Notes | Dropouts during treatment to any or at least 1 adverse reaction: 1 participant experienced a fit possibly related to SSRI and 1 had an allergic reaction (possibly secondary to medication) Suicide‐related outcome as an adverse event of treatment: 4 required admission for suicidality or self harm and were withdrawn from the study Authors only report median age | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Participants were randomised to SSRI alone or SSRI plus CBT by an equal allocation ratio using stochastic minimisation balancing for severity (Childrens Global Assessment Scale <40), centre, sex, concurrent comorbidity disorder, and age” pg. 2/8 (Under heading Assignment) |
| Allocation concealment (selection bias) | Low risk | “Research staff from the clinical sites enrolled patients, and an independent telephone randomisation centre allocated treatment” pg. 2 /8 (Under heading Assignment) |
| Blinding (performance bias and detection bias) | Low risk | “...research assistants blind to treatment assignment assessed outcome” pg. 4/8 (Under heading Outcomes) |
| Blinding (performance bias and detection bias) | High risk | No placebo or control psychotherapy was used. As such, participants would be aware that the medication was active and the therapy was CBT |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis: “Analysis was by intention to treat subject to the availability of the data” pg. 4/8 (Under heading Statistical Analysis) Number randomised: Fluoxetine + CBT: 105 Fluoxetine: 103 Total: 208 Number of dropouts during intervention: Fluoxetine + CBT: 11 Fluoxetine:6 Total: 17 Number dropouts in follow‐up: Fluoxetine + CBT: 7 Fluoxetine: 7 Total: 14 Number analysed post‐intervention: Fluoxetine + CBT: 105 Fluoxetine: 103 Total: 208 Number analysed follow‐up 1: Fluoxetine + CBT: 105 Fluoxetine: 103 Total: 208 Reasons for dropout in each group: 12 patients were formally withdrawn from the study for the following reasons: 4 required admission to hospital for suicidality or self harm, 5 failed to improve, 1 had a fit, 1 had an allergic reaction, 1 was prescribed paroxetine by a GP 18 families withdrew participants from the study: 6 were improving and did not want further treatment, 5 did not want more treatment, 2 wanted CBT, 2 did not want CBT, 1 wanted a female therapist, 1 was getting worse, 1 moved |
| Selective reporting (reporting bias) | Unclear risk | Authors reported data for all outcomes specified in their methods. Do not have access to trial protocol |
| Other bias | Low risk | |
| Methods | Duration: 8 weeks Follow‐up assessment points: Post‐intervention, 12 months Funded by: National Institute of Mental Health (NIMH) | |
| Participants | N = 63 Adolescent only (12 to 18 years) Depression diagnoses included: DSM‐III‐R Major Depressive Disorder (MDD). Participants also had to obtain a score of 35 or more on the CDRS‐R (Poznanski 1996) Baseline risk of suicide: Not measured Baseline severity of depression: Chidren’s Depression Rating Scale (CDRS‐R; Poznanski 1985). Mean score (SD): Imipramine + CBT = 46.8 (9.5) Placebo + CBT = 52.5 (10.8) Comorbidity included: All 63 subjects met criteria for at least 1 anxiety disorder based on either adolescent or parental interviews Age mean (SD): Total = 13.9 (3.6) Sex (M:F): 25:38 Setting: Unclear. Likely an outpatient setting based on information regarding medication monitoring throughout the trial. Excluded psychiatric diagnoses: ADHD, conduct disorder, bipolar disorder, eating disorder, alcohol or drug abuse on the Diagnostic Interview for Children and Adolescents‐Revised‐Adolescent Version (DICA‐R‐A) or Parent Version (DICA‐R‐P; Reich 1990), or both, mental retardation by history, bipolar affective disorder in first degree relative Country: USA | |
| Interventions | Combination (Imipramine + CBT) N = 31 Name: CBT. Based on school refusal treatment by Last 1998. Included the identification of negative thoughts surrounding school attendance and teaching adaptive coping strategies. # sessions/length: 8 (45 to 60 minutes) sessions over 8 weeks Manualised (Y/N): Yes Individual or group: Individual Parent involvement: Yes. Parents joined each session for 10 to 15 minutes at the end Fidelity check: No formal check. Weekly discussions with all therapists and principal investigators, and a fortnightly telephone consultation with an expert on CBT for school refusal Delivered by: 3 therapists (1 behaviorally trained Clinical Psychologist, 1 Doctoral level therapist and 1 Masters level therapist) Name (class & type): TCA (Imipramine) Dose (mg/day)/length: Dose based on body weight. A gradual increase every 3 to 5 days to 3 mg/kg per day by the end of week 2 Mean dose at week 3 was 184.6mg + 33.3 Delivered how: Weekly appointments monitoring side effects, and compliance were undertaken with a psychiatrist. Blood imipramine levels were monitored at 3 and 8 weeks Combination (Placebo medication + CBT) N = 32 Details as above (Y/N): Yes | |
| Outcomes | Clinician reported Children’s Depression Rating Scale‐Revised (CDRS‐R; Poznanski 1985) with a score of ≤ 35 Children’s Depression Rating Scale‐Revised (CDRS‐R; Poznanski 1985) Self reported The Beck Depression Inventory (BDI; Beck 1979) Additional Measures Anxiety Rating for Children‐Revised (ARC‐R; Bernstein 1996) The Revised Children’s Manifest Anxiety Scale (RCMAS; Reynolds 1978) Weekly school attendance rates | |
| Notes | Dropouts during treatment to any or at least 1 adverse reaction: 1 participant developed manic symptoms and 1 developed psychiatric symptoms and required hospitalisation Suicide‐related outcome as an adverse event of treatment: No Denominator and numerator for remission rates calculated from percentages reported in the publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “Random assignment to treatment was blocked on gender and whether subjects had a school vacation that lasted 5 or more days during the 8 week treatment period”. (Under heading Procedure) |
| Allocation concealment (selection bias) | Unclear risk | No information contained in paper to make a judgement |
| Blinding (performance bias and detection bias) | Low risk | “All project personnel...were blind to medication assignment”. (Under heading Procedure) |
| Blinding (performance bias and detection bias) | Low risk | "...imipramine pills and matching placebo" “To preserve the blind, increases and decreases were also suggested for randomly selected patients on placebo”. (Under heading Medication Management) |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis: “All randomized subjects were included in analyses based on intent to treat”. (Under heading Statistical Analyses) Number randomised: Imipramine + CBT: 31 Placebo + CBT: 32 Total: 63 Number of dropouts during intervention Imipramine + CBT: 7 Placebo + CBT: 9 Total: 16 Number analysed post‐intervention: Imipramine + CBT: 31 Placebo + CBT:32 Total: 63 Reasons for dropout in each group: 1 missed 22 does of medication, 1 missed 2 therapy appointments, 1 developed manic symptoms on study medication, 1 required hospitalisation for psychiatric symptoms, and 12 declined further participation |
| Selective reporting (reporting bias) | Unclear risk | Authors report data for all outcomes post‐intervention. Do not have access to trial protocol |
| Other bias | High risk | Authors note that placebo group significantly more symptomatic at baseline compared with imipramine group despite randomisation |
| Methods | Duration: 6 weeks Follow‐up assessment points: Post‐intervention, 12, 26 and 52 weeks Funded by: The Agency for Healthcare Research and Quality and the Garfield Memorial Fund | |
| Participants | N = 152 Adolescent only (12 to 18 years) Depression diagnoses included: DSM‐IV episode of major depression Baseline risk of suicide: 73.7% (112/152) of participants reported significant levels of suicidal behaviour; assessment tool not explicitly referenced Baseline severity of depression: Centre for Epidemiological Studies ‐ Depression Scale (CES‐D; Radloff 1977): TAU + CBT = 35.4 (11.8) TAU = 33.7 (9.3) Comorbidity included: Not reported Age mean (SD): Total = 15.30 (1.61) TAU + CBT = 15.29 (1.62) TAU = 15.32 (1.60) Sex (M:F): 34:118 TAU + CBT = 17:60 TAU = 17:58 Setting: Primary care health maintenance organization (HMO) Excluded psychiatric diagnoses: Schizophrenia or a significant developmental/intellectual disability Country: USA | |
| Interventions | TAU (SSRI) + CBT N = 77 Name: CBT employing cognitive restructuring, or behavioural training, or both. Participants able to choose which type to try first. After completion of first module (2 to 5 sessions), therapist and youth reviewed recovery and decided whether to proceed with the second module (sessions 6 to 9), focusing on skills training # sessions/length: Between 0 and 9, mean 5.3 sessions. Each session 1 hour. Weekly in frequency Manualised (Y/N): No information Individual or group: Individual Parent involvement: Clinicians organised separate parent meetings, however "parents' attendance was "sparse" Fidelity check: Yes. All sessions audio taped. 57 sessions selected at random and rated by a senior supervisor. 87.2% adherence to protocol Delivered by: Masters level Psychologists Name (class & type): SSRI (varied). All trial participants were able to receive any medications provided by either the HMO or outside providers Dose (mg/day)/length: Varied Delivered by: No information TAU (SSRI) N = 75 Details as above (Y/N): Yes | |
| Outcomes | Clinician reported Mood disorders module of the Schedule for Affective Disorders and Schizophrenia for School‐age Children‐Present and Lifetime version (K‐SADS‐PL; Kaufman 1997) and the Longitudinal Interview Follow‐Up Evaluation (Keller 1982). This was used to define remission i.e. those who had did not have a continuing or new mood disorder since the last interview according to the K‐SADS‐PL). It was unclear if DSM‐IV or ICD time criteria were employed Centre for Epidemiological Studies‐Depression Scale (CES‐D; Radloff 1977), cut‐off of ≤ 15 Children’s Global Adjustment Scale (C‐GAS; Shaffer 1983) Self reported Centre for Epidemiological Studies‐Depression Scale (CES‐D; Radloff 1977) Parent reported The Child Behaviour Checklist (CBCL; Achenbach 1978) Additional Measures Hamilton Rating Scale for Depression (HAM‐D; Hamilton 1960) Youth Self Report (YSR; Achenbach 1991) Internalising and Externalising subscales and an extracted depression subscale created to match DSM criteria for major depression (Clarke 1992) Social Adjustment Scale Self Report for Youth (Weissman 1980) Short Form‐12 (Ware 1998) | |
| Notes | Authors do not report reasons for dropout Dropouts during treatment to any or at least 1 adverse reaction: Not reported Suicide‐related outcome as an adverse event of treatment: Not reported Numbers who reached remission by interview were calculated by review authors using percentages based on depressive episodes (Table 3). Data from Table 3 were based on observed cases not ITT following advice from statistician | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Youths were randomized using a blocked procedure to minimize study arm imbalance”. pg. 889 |
| Allocation concealment (selection bias) | Unclear risk | No information contained in paper to make a judgement |
| Blinding (performance bias and detection bias) | Low risk | ”Blinded interviewers assessed each adolescent and a participating parent by telephone at baseline and at 6, 12, 26 and 52 weeks post‐randomization”. pg. 890 |
| Blinding (performance bias and detection bias) | High risk | No placebo or therapy control arm. As such, participants were aware if they were receiving CBT in the trial or not |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT analysis: "...all subjects were considered part of the study from the point of randomisation (an intent‐to‐treat design)" pg.890 “We examined continuous depression and functioning outcome measures using random effect regression analysis”. pg. 892 Number randomised: TAU + CBT: 77 TAU: 75 Total: 152 Number completing post‐intervention: (dropouts) TAU + CBT: 67 (10) TAU: 65 (10) Total: 132 (20) Number completing follow‐up 12 weeks: TAU + CBT: 61 (16) TAU: 61 (14) Total: 122 (30) Number completing follow‐up 26 weeks: TAU + CBT: 65 (12) TAU: 62 (13) Total: 127 (25) Number completing follow‐up 52 weeks: TAU + CBT: 56 (21) TAU: 58 (17) Total: 114 (38) *Data obtained from Fig 1. Summary of study procedures. Number analysed not clearly stated in paper Reasons for dropout in each group: Not reported |
| Selective reporting (reporting bias) | Unclear risk | Remission rates only reported at 52 weeks. Do not have access to trial protocol |
| Other bias | High risk | Authors note that telephone administration of self report measures may have created bias |
| Methods | Duration: 12 weeks Follow‐up assessment points: Post‐intervention (12 weeks) Funded by: National Institute on Alcohol Abuse and Alcoholism | |
| Participants | N = 50 Adolescent only (15 to 20 years) Depression diagnoses included: DSM‐IV diagnosis of major depressive disorder (MDD) Baseline risk of suicide: Not measured and suicidality not stated as an exclusion criteria Baseline severity of depression: Hamilton Rating Scale for Depression (HAM‐D; Hamilton 1960) Mean (SD): CBT + fluoxetine = 16.88 (7.09) CBT + placebo = 22.88 (8.79) Comorbidity included: All participants were required to have a DSM‐IV diagnosis of an alcohol use disorder (AUD) confirmed using the Substance Use Disorders Section of the Structured Clinical Interview for the DSM (SCID) Age mean: Not reported Sex (M:F): Total = 28:22 CBT + fluoxetine = 12:12 CBT + placebo = 16:10 Setting: Outpatient? Psychiatric diagnoses excluded: DSM‐IV diagnosis of bipolar disorder, schizoaffective disorder, or schizophrenia, persons with and substance abuse or dependence other than nicotine dependence or cannabis use and dependence, persons with a history of intravenous drug use, persons who had received antipsychotic or antidepressant medication within 1 month prior to baseline assessment also excluded Country: USA | |
| Interventions | Combination: Psychotherapy + Medication N = 24 Name (description): CBT for depressive disorder and the treatment of alcohol use disorder combined with Motivation Enhancement Therapy (MET) for the treatment of alcohol use disorder # sessions/length: 9 sessions over 12 weeks Manualised (Y/N): Yes Individual or group: Not reported Parent involvement: Not reported Fidelity check: No fidelity check reported Delivered by: Not reported Medication Name (class & type): SSRI; fluoxetine Dose (mg/day)/length: initiated at 10 mg, increased to 20 mg after week 2 until the end of the study, as 20 mg was target dose of the study Delivered how: Study physicians prescribed all medication Combination: Psychotherapy + Placebo N = 26 Delivered how: Pill placebo delivered in the same context as above | |
| Outcomes | Clinician reported Hamilton Rating Scale for Depression (HAM‐D; Hamilton 1960) Self reported Beck Depression Inventory (BDI; Beck 1988) Additional Measures Drinking behaviour measured using the Timeline Follow‐back Method (TLFB; Sobell 1988) | |
| Notes | Dropouts during treatment to any or at least 1 adverse reaction: 0 Suicide‐related outcome as an adverse event of treatment: 0 Suicidality was not measured with a formalised tool 3 dropouts during study from placebo group due to persistent depressive symptoms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patient randomisation was conducted by urn randomisation stratified by gender” pg. 906 (Treatment and assessment) |
| Allocation concealment (selection bias) | Low risk | “Active medication and matching placebo were prepared by the research pharmacy” pg. 906 (Treatment and assessment) |
| Blinding (performance bias and detection bias) | High risk | “The study was conducted in a double blind fashion, though one study physician remained non‐blinded in order to handle any problems which may have arisen” pg. 906 (Assessment and treatment) |
| Blinding (performance bias and detection bias) | Low risk | ”...participants were randomly assigned to receive fluoxetine or placebo administered in identical‐looking opaque capsules” pg. 906 (Assessment and treatment) |
| Incomplete outcome data (attrition bias) | Unclear risk | “Statistical analyses were completed on an intent‐to‐treat study group” pg. 907 (Statistical Analyses) Number randomised: CBT + fluoxetine: 24 CBT + placebo: 26 Number dropped out during intervention: CBT + fluoxetine: 0 CBT + placebo: 3 Number analysed post‐intervention: CBT + fluoxetine: 24 CBT + placebo: 26 |
| Selective reporting (reporting bias) | Unclear risk | Do not have access to trial protocol |
| Other bias | Low risk | Baseline imbalance of HAM‐D and BDI scores with fluoxetine group have significantly lower baseline depression scores |
| Methods | Duration:12 weeks Follow‐up assessment points: Post‐intervention Funded by: National Institute of Alcohol and Alcoholism (NIAAA) | |
| Participants | N = 10 Adolescent only (15 to 18 years) Depression diagnoses included: Not clearly stated. The Child Schedule for Affective Disorders and Schizophrenia (K‐SADS; Chambers 1985) was used to assess psychiatric disorders Baseline risk of suicide: Not measured Baseline severity of depression: measured using the Hamilton Rating Scale for Depression (HAM‐D; Hamilton 1960) Sertraline + CBT = 20.40 (5.55) Placebo + CBT = 20.80 (5.45) Comorbidity included: All participants presented with an alcohol use disorder Age mean (SD): Total = 16.6 (0.52) Sertraline + CBT = 16.4 (0.55) Placebo + CBT = 16.8 (0.45) Sex (M:F): 8:2 Sertraline + CBT = 4:1 Placebo + CBT = 4:1 Setting: Outpatient Excluded psychiatric diagnoses: Not reported Country: USA | |
| Interventions | Combination (Sertraline+CBT) N = 5 Name (description): CBT focusing on relapse prevention, coping skills, anger management, modelling and role playing # sessions/length: 12, average attendance was 8.2 sessions and 10.6 sessions for the placebo and sertraline groups respectively Manualised (Y/N): No Individual or group: Group Parent involvement: Not reported Fidelity check: Not reported Delivered by: A psychiatrist, on a weekly basis Name (class & type): SSRI (Sertraline) Dose (mg/day)/length: 25 mg/day, increased to 25 mg weekly, to a maximum dose of 100 mg in about 4 weeks Delivered by: A psychiatrist monitored side effects, made medication adjustments, and supplied participants with additional medication on a weekly basis Combination (Placebo medication + CBT) N = 5 Details as above (Y/N): Yes | |
| Outcomes | Self reported Outcome 4: Hamilton Rating Scale for Depression (HAM‐D; Hamilton 1960) Additional Measures The Time Line Follow Back (TLFB; Sobell 1988) assessed alcohol use | |
| Notes | Dropouts during treatment to any or at least 1 adverse reaction: No. Authors note that all the side effects of sertraline were transient and did not lead to any dropouts Suicide‐related outcome as an adverse event of treatment: No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Following the baseline assessments, subjects were randomized using a computer‐generated randomisation table into sertraline or placebo groups” pg. 462 |
| Allocation concealment (selection bias) | Low risk | All of the medication supplied by the study pharmacist were identical in appearance" pg. 462 |
| Blinding (performance bias and detection bias) | Unclear risk | “This study was a 12 week double blind, placebo‐controlled trial” pg. 462 |
| Blinding (performance bias and detection bias) | Low risk | Participants in both arms were blind to medication. Both received CBT “This study was a 12 week double blind, placebo‐controlled trial” pg. 462 |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT analysis: All subjects randomised were included in the final analysis. No information is reported regarding imputation method for missing data Number randomised: SSRI + CBT: 5 Placebo + CBT: 5 Total: 10 Number of dropouts during intervention:* SSRI + CBT: 2 Placebo + CBT: 0 Total: 2 Number analysed post‐intervention: SSRI + CBT: 5 Placebo + CBT:5 Total: 10 Treatment completion was defined a priori as 8 sessions |
| Selective reporting (reporting bias) | Unclear risk | All outcome data specified in methods was reported. Do not have access to trial protocol |
| Other bias | High risk | Small study sample, and no follow‐up |
| Methods | Duration: 8 weeks Follow‐up assessment points: postintervention; 4 weeks post intervention Funded by: Korean Game Culture Foundation | |
| Participants | N = 72 Adolescent only (13 to 18 years) Depression diagnoses included: Major Depressive Disorder diagnosed using The Korean Child Schedule for Affective Disorders and Schizophrenia (K‐SADS; Chambers 1985); score of 19 or more on the BDI Baseline risk of suicide: Not measured Baseline severity of depression: measured using the Beck Depression Inventory (Beck 1961) Buproion + CBT = 32.7 (SD 8.8) Bupropion = 33.3 (SD 8.7) Comorbidity included: Excluded other psychiatric disorders Age mean (SD): Total = 16 Bupropion + CBT = 16.2 (1.4) Bupropion = 15.9 ± 1.6 Sex (M:F): 72:0 Sertraline + CBT = 35:0 Placebo + CBT = 37:0 Setting: University clinic Excluded psychiatric diagnoses: all other psychiatric disorders excluded Country: South Korean | |
| Interventions | Medication (Bupropion) N = 37 Name (class & type): NDRI (Bupropion) Dose (mg/day)/length: 150 mg/day for first week, increased to 300 mg daily for 7 weeks Delivered by: A psychiatrist who meet with the participant on a weekly basis for 10 minutes to monitor progress with regard to online gaming Combination (Bupropion+CBT) N = 35 Name (description): CBT includes focus on negative consequences of online gaming and irrational beliefs about on‐line gamiing, problem solving and decision making, communication skills trianing, self control training, family therapy, planning for the future # sessions/length: 8 one‐hour sessions delivered weekly, no details provided regarding actual attendance rates Manualised (Y/N): No Individual or group: Group Parent involvement: One of the 8 CBT sessions was described as family therapy Fidelity check: No Delivered by: a multidisciplinary treatment team including a psychiatrist, nurse, psychologist, and social worker Name (class & type): NDRI (Bupropion) Dose (mg/day)/length: 150 mg/day for first week, increased to 300 mg daily for 7 weeks Delivered by: A psychiatrist who meet with the participant on a weekly basis for 10 minutes to monitor progress with regard to online gaming | |
| Outcomes | Self reported Beck Depression Inventory (Beck 1961) Additional Measures Young Internet Addiction Scale (YIAS) (Young 1996) Beck Anxiety Inventory (BAI) (Beck 1988). Modified‐School Problematic Behavior Scale (Baker 1984). Modified Student’s Life Satisfaction Scale (Huebner 1991) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding (performance bias and detection bias) | Unclear risk | No assessor rated outcomes |
| Blinding (performance bias and detection bias) | High risk | Participants not blinded (no placebo CBT condition) |
| Incomplete outcome data (attrition bias) | High risk | 10% missing data; observed case data used in analysis |
| Selective reporting (reporting bias) | Unclear risk | Authors report on all outcomes specified in their methods section. No access to trial protocol. |
| Other bias | Unclear risk | No rating of fidelity; very short follow‐up period |
| Methods | Duration: 6 weeks Follow‐up assessment points: Post‐intervention Funded by: Not specified | |
| Participants | N = 33 Child and adolescent (8 to 17 years) Depression diagnoses included: DSM‐IV Major Depression Baseline risk of suicide: Participants who were acutely suicidal were excluded form the study. No other specific suicide measurements were administered Baseline severity of depression: Not reported Comorbidity included: Not reported Age mean (SD): Total based on completed participants: 12.7 (2.88) Sex (M:F): 25:8 Setting: Outpatient Excluded psychiatric diagnoses: Schizophrenia, mental retardation and Gilles de la Tourette’s syndrome Country: USA | |
| Interventions | Combination (SNRI + Psychotherapy) N = 20 Name: Predominantly behavioural/cognitive in nature # sessions/length: One weekly session over 6 weeks Manualised (Y/N): Not reported Individual or group: Individual sessions of 60 minutes (45 minutes plus 15 minutes "collateral") Parent involvement: 15 minutes at the end of each session was "collateral" with parents and participants Fidelity check: Not reported Delivered by: Masters level therapists, trained in the procedural aspects of the study
Name (class & type): SNRI (Venlafaxine) Dose (mg/day)/length: Children (8 to 12 yrs) began at 12.5 mg q.d for 3 days, increasing to 12.5 mg b.i.d for 3 days, and further increased to 12.5 mg t.i.d for the remainder of the study. Adolescents (13‐17yrs) began at 25mg q.d. for 3 days, increased to 25mg b.i.d for 3 days and then 25mg t.i.d. for the remainder of the study Delivered how: Weekly clinic supplied medication/placebo and monitored vital signs and side effects Combination (Placebo+Psychotherapy) N = 20 Details as above (Y/N): Yes | |
| Outcomes | Clinician reported The Child Depression Rating Scale (CDRS; Poznanski 1979) Self reported Children’s Depression Inventory (CDI; Kovacs 1992) Parent reported The Child Behaviour Checklist (CBCL; Achenbach 1993) Additional Measures The Hamilton Rating Scale for Depression (Hamilton 1960) | |
| Notes | Age and gender calculated manually from Figure 1 (pg 151 of the Mandoki 1997 publication) Dropouts during treatment to any or at least 1 adverse reaction: One participant developed a manic episode, was hospitalised and subsequently put on lithium. Authors note in discussion that "There are specific side effects associated with venlafaxine treatment....However, these side effects were not severe enough to discontinue the medication" Suicide‐related outcome as an adverse event of treatment: None reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information contained in paper to make a judgement |
| Allocation concealment (selection bias) | Unclear risk | As authors quote study as being 'double blind' |
| Blinding (performance bias and detection bias) | Low risk | "After the 6‐week treatment, the study ended. The blind was broken...”. pg. 151 Under heading Procedures, Measurements, and Medication Dose |
| Blinding (performance bias and detection bias) | Low risk | “The patients were randomly assigned, in a double blind fashion, to either the venlafaxine and psychotherapy or the placebo and psychotherapy treatment group”. pg. 151 Under heading Procedures, Measurements, and Medication Dose |
| Incomplete outcome data (attrition bias) | High risk | ITT analysis: No. Figure 1 (pg 151 of the Mandoki 1997 publication) shows the age and sex composition of the final sample on which the statistical analysis was based”. Manual calculation shows the analysis was conducted only on participants completing the trial. pg. 150 Under heading Subjects Number randomised: SNRI + Psychotherapy: 20 Placebo + Psychotherapy: 20 Total: 40 Number of dropouts during intervention: SNRI + Psychotherapy: 4 Placebo + Psychotherapy: 3 Total: 7 Number analysed post‐intervention: SNRI + Psychotherapy: 16 Placebo + Psychotherapy: 17 Total: 33 Reasons for dropouts: 6 did not continue coming to the clinic by week 2 for unknown reasons, and 1 patient (in the venlafaxine group) developed a manic episode and was hospitalised |
| Selective reporting (reporting bias) | High risk | No numerical outcome data was reported in the article, all data was presented in graphs only. Do not have access to trial protocol |
| Other bias | Low risk | |
| Methods | Duration: 12 weeks Follow‐up assessment points: Post‐intervention, 6 months. Funded by: Beyond Blue, Premiers Youth Suicide Taskforce, Department of Human Services Victoria and Australian Rotary Health Research Fund. | |
| Participants | N = 73 Adolescents only (12 to 18 years) Depression diagnoses included: DSM‐IV major depressive disorder (MDD), dysthymic disorder (DD) and depressive disorder not otherwise specified (DDNOS) Baseline risk of suicide: Participants who were 'actively suicidal' were excluded from the study, however 'suicidally depressed teenagers (who did not require hospitalisation) were included. Measured using the Suicidal Ideation Questionnaire‐Junior High School Version (SIQ‐JR; Reynolds 1987) CBT = 26.05 (19.93) Sertraline = 29.42 (27.24) Sertraline + CBT = 30.64 (24.42) Participants exhibiting active suicidality that required acute hospital admission were excluded from the study Baseline severity of depression: CBT = 83.77 (13.8) Sertraline = 84.92 (11.20) Sertraline + CBT = 83.96 (15.01) Comorbidity included: 69% were diagnosed with at least 1 comorbid disorder, 22% were diagnosed with 2 or more Comorbid disorder (n) CBT Sertraline Sertraline + CBT Anxiety disorders 8 9 10 Dysthymic disorder 1 2 3 Conduct Disorder/ODD 2 3 1 Body dysmorphic disorder 1 0 0 Adjustment disorder with anxiety 0 1 0 Enuresis 1 0 0 Reading Disorder 0 1 0 Cannabis‐related disorder NOS 0 1 0 Parent‐child relational problem 5 6 8 Sibling relational problem 1 2 3 Age mean (SD): 15.3 (1.5) CBT= 15.0 Sertraline = 15.5 CBT + Sertraline = 15.3 Sex (M:F): 25:48 CBT = 7:15 Sertraline = 7:19 CBT + Sertraline = 11:14 Setting: 3 clinics collocated with public child and adolescent mental health services Excluded psychiatric diagnoses: Bipolar disorder, psychotic disorder, primary diagnosis of substance abuse disorder, severe psychiatric disturbance that required acute hospital admission, and intellectual disability of sufficient severity to preclude participation in the study Country: Australia | |
| Interventions | Psychotherapy (CBT) N = 22 Name: CBT course based in the Adolescent Coping with Depression Course (Clarke 1990). Modules included; goal setting, psycho education, affective education, self monitoring, relaxation training, social skills training, pleasant events scheduling, cognitive therapy and life goals planning # sessions/length: Twelve 50 minute sessions over 12 weeks. Three ‘booster’ sessions were also delivered over 3 months Manualised (Y/N): Yes Individual or group: Individual Parent involvement: Parents who chose to participate received concurrent CBT sessions, with 2 family sessions Fidelity check: No formal check. Clinicians received weekly to twice weekly supervision with an expert therapist. Peer supervision held weekly Delivered by: 7 registered psychologists, a supervised probationary psychologist, 2 general medical practitioners, and a social worker with experience in providing CBT for adolescent depression. Training provided by chief investigators Medication (Sertraline) N = 26 Name (class & type): SSRI (Sertraline) Dose (mg/day)/length: 25 mg/day for 1 week, increased to 50 mg/day at week 2 depending on response and adverse events. Maximum dose of 100 mg/day administered depending on clinical response and tolerability Delivered how: Review sessions occurred every 2 to 3 weeks to monitor adverse effects, and included education about depression but no CBT strategies Combination (Sertraline + CBT) N = 25 Details as above (Y/N): Yes | |
| Outcomes | Clinician reported The Schedule for Affective Disorders and Schizophrenia for School‐Age Children‐Lifetime Version (KSADS‐PL; Kaufman 1997) was used to assess for disorder or remission, which was based on DSM‐IV criteria for full remission (i.e. 8 weeks asymptomatic). The Global Assessment of Functioning Scale (GAF; APA 1994) Dropouts: Post‐intervention: CBT: 21/22 completed (1 dropout) Sertraline: 21/26 completed treatment (5 dropouts) Sertraline + CBT: 20/25 completed treatment (5 dropouts) 6 month follow‐up: CBT: 19/22 completed assessment (3 dropouts) Sertraline: 23/26 completed assessment (3 dropouts) Sertraline + CBT: 24/25 completed assessment (1 dropout) Self reported Reynolds Adolescent Depression Scale (RADS; Reynolds 1986) The Suicidal Ideation Questionnaire‐Junior High School Version (SIQ‐JR; Reynolds 1987) Parent reported The Child Behaviour Checklist (CBCL; Achenbach 1991) Additional Measures The Global Assessment of Relational Functioning Scale (APA 1994) Revised Children’s Manifest Anxiety Scale (RCMAS; Reynolds 1978) The Self Efficacy Questionnaire for Depressed Adolescents (SEQ‐DA; Tonge 2005) Family Assessment Device General Functioning Scale (Epstein 1983) | |
| Notes | Dropouts during treatment to any or at least 1 adverse reaction: 6% discontinued medication due to adverse affects. These effects included slurred speech and dizziness, feeling agitated and restless, and diarrhoea Suicide‐related outcome as an adverse event of treatment: 11.1% (n = 45) of participants taking sertraline either alone or with CBT reported suicidal ideation. 1 participant in the sertraline + CBT received an inpatient admission for several hours, however treatment according to protocol was subsequently continued | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “...subjects were randomly allocated by an independent statistician using a computer generated assignment to CBT, MED or COMB”. pg. 1154 |
| Allocation concealment (selection bias) | Low risk | ”...allocated by an independent statistician...Allocation for those eligible for the trial was concealed to all until after pre‐treatment assessment”. pg. 1154 |
| Blinding (performance bias and detection bias) | High risk | “Independent raters blind to treatment allocation were not used because of resource limitations but may have reduced the risk of experimenter bias in assessments”. pg. 1160 |
| Blinding (performance bias and detection bias) | High risk | Psychotherapy administered in both groups and no placebo control used for medication |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT analysis: “data were analysed using an intent‐to‐treat strategy to counter any possible overestimation of treatment outcomes, using the last observation carried forward method (Nelson 1996)”. pg. 1155 Number randomised: CBT: 22 Sertraline: 26 CBT + Sertraline: 25 Total: 73 Number of dropouts during intervention CBT: 1 Sertraline: 5 CBT + Sertraline: 5 Total: 11 Number dropouts in follow‐up: CBT: 3 Sertraline: 3 CBT + Sertraline: 1 Total:7 Number analysed post‐intervention: CBT: 22 Sertraline: 26 CBT + Sertraline: 25 Total: 73 Number analysed follow‐up 1: CBT: 22 Sertraline: 26 CBT + Sertraline: 25 Total: 73 Reasons for dropouts: CBT: At post‐intervention, 1 participant reported symptoms had improved. At 6‐month follow‐up, 2 refused to attend and 1 was unable to be located Sertraline: At post‐intervention, 2 participants reported symptoms had improved, 1 reported side effects, 1 dissatisfied with programme and 1 did not pursue treatment. At 6‐month follow‐up, 1 participant refused to attend, 1 was unable to be located and 1 'trial closure' CBT + sertraline: At post‐intervention: 2 reported side effects, 1 symptoms improved, 1 dissatisfied with programme, and 1 did not respond. At 6‐month follow‐up, 1 refused to attend |
| Selective reporting (reporting bias) | Unclear risk | Remission data not reported by group and functioning data not reported in a useable format. All other outcomes were reported. Do not have access to trial protocol |
| Other bias | Low risk | |
| Methods | Duration:16 weeks Follow‐up assessment points: Post‐intervention Funded by: National Institute on Drug Abuse, National Institutes of Health | |
| Participants | N = 126 Adolescent only (13 to 19 years) Depression diagnoses included: DSM‐IV current MDD episode Baseline risk of suicide:Primary measure of suicidality was question 13 on the Childhood Depression Rating Scale‐Revised (CDRS‐R; Poznanski 1996) Baseline suicidality data: Fluoxetine + CBT = 25/63 (39.7%) CBT + placebo = 24/63 (38.1%). N = 13 displayed severe suicidal ideation (> 5 on CDRS‐R Q13) “Adolescents with past, current or intermittent suicidal ideation (39% at baseline) were not excluded from study participation unless suicidal ideation were severe or they were otherwise considered by the study physician and according to baseline CDRS‐R ratings (question 13) to be at high risk for a suicide attempt during the trial” Baseline severity of depression: Children’s Depression Rating Scale‐Revised (CDRS‐R; Poznanski 1996) mean t‐score (SD): Fluoxetine + CBT: 73.74 (8.51) Placebo + CBT: 73.03 (7.70) Comorbidity included: All participants had at least 1 non‐tobacco Substance Use Disorder (SUD), and lifetime Conduct Disorder (CD) Age mean (SD): Total = 17.16 (1.66) Sex (M:F): Total = 85:41 Setting: Outpatient Excluded psychiatric disorders: Current or past diagnosis of a psychotic disorder or of bipolar disorder (type I or II) Country: USA | |
| Interventions | Combination (Fluoxetine + CBT) N = 63 Name: CBT approach using behavioural, cognitive behavioural and motivational enhancement techniques to help adolescents reduce their drug use. The programme contains 1 session specifically on depression, helping adolescents to identify, manage and regulate mood states that often trigger substance use. # sessions/length: 1 hour, 16 weekly sessions Manualised (Y/N): Yes Individual or group: Individual Parent involvement: Not specifically but could include up to 2 parent sessions Fidelity check: Yes. All sessions videotaped and self rated by therapists. 32 videotapes randomly selected and independently rated for adherence and fidelity. “...neither therapist fell below present fidelity/adherence standards during any point of the study” Delivered by: Study therapists (MD) who were trained and certified by one of the manuals developers. The developer provided ongoing supervision and quality monitoring Name (class and type): SSRI (Fluoxetine) Dose (mg/day)/length: 20 mg fixed daily dose Delivered how: Monitoring of adverse effects and medication adherence was undertaken by research nurses, and occurred either immediately before or after the weekly CBT session Combination (Placebo + CBT) Details as above (Y/N): Yes | |
| Outcomes | Clinician reported Remission of depression defined as as post‐intervention Children’s Depression Rating Scale‐Revised (CDRS‐R; Poznanski 1996) score of ≤ 28 Children’s Depression Rating Scale‐Revised (CDRS‐R; Poznanski 1996) The Clinical Global Impression Improvement rating (CGI‐I; Guy 1976) Self reported Question 13 on the Children’s Depression Rating Scale (CDRS‐R; Poznanski 1996) Additional Measures Self reported number of non‐tobacco drugs used in the past 30 days Urine samples for substance use Conduct Disorder: Number of self reported DSM‐IV symptoms in the past 30 days | |
| Notes | Group means for age and gender not reported Dropouts during treatment to any or at least 1 adverse reaction: Authors list 6 as ‘lost to follow‐up’ and 2 to ‘withdrew consent’ but do not disclose if this was due to an adverse reaction Suicide‐related outcome as an adverse event of treatment: 5 participants (4 in the fluoxetine + CBT group and 1 in the Placebo + CBT group were evaluated in an emergency department or hospitalised for concerns of worsening suicidality during the study. Standard error and sample size was used to calculate standard deviations for group means | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “A non‐blinded research pharmacist assigned eligible participants to receive 20 mg of fluoxetine hydrochloride or matching placebo using a small block (6) randomisation scheme of 20 blocks to achieve balance in the treatment assignment”. pg. 1027 |
| Allocation concealment (selection bias) | Low risk | “Active medication and matching placebo were prepared by the research pharmacy at the University of Colorado at Denver and Health Sciences Centre and then provided to clinical research staff in pre‐randomized and pre‐blinded medication bottles”. pg. 1027 |
| Blinding (performance bias and detection bias) | Low risk | “Research staff....remained blinded to medication status throughout the trial”. pg.1027 |
| Blinding (performance bias and detection bias) | Low risk | : “.....participants remained blinded to medication status throughout the trial”. pg.1027 |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis: “All analyses were intent‐to‐treat (including all randomized study participants)”. pg. 1028. Impulation method used: “Analyses of dichotomous and continuous primary outcome measures over time used generalized estimating equation (GEE) and likelihood based methods, respectively. Both allow for estimates of changes in repeated measures in the presence of missing data, assuming those data were missing at random”. pg. 1028 Number randomised: Combination (Fluoxetine + CBT): 63 Combination (Placebo + CBT): 63 Total: 126 Number of dropouts during intervention: Combination (Fluoxetine+CBT): 11 Combination (Placebo + CBT):9 Total:20 Number analysed post‐intervention: Combination (Fluoxetine + CBT): 63 Combination (Placebo + CBT): 63 Total:126 Reasons for dropouts: Fluoxetine + CBT: 4 participants went to jail/detention, 3 went to residential treatment at facility and were unable to continue the study, 3 were lost to follow‐up and 1 moved out of area. Placebo + CBT: 1 participant went to jail/detention, 3 were lost to follow‐up, 3 moved out of area and 2 withdrew consent |
| Selective reporting (reporting bias) | Unclear risk | No group data on suicide outcomes reported. All other outcomes specified in methods reported. Do not have access to trial protocol |
| Other bias | Low risk | |
| Methods | Duration: 12 weeks acute treatment, 6 weeks continuation treatment and 18 weeks maintenance treatment Follow‐up assessment points: Post‐intervention (12 weeks), 18 weeks (after continuation), 36 weeks (after maintenance) Funded by: National Institution of Mental Health to Duke University Medical Centre | |
| Participants | N = 439 Adolescent only (12 to 17 years) Depression diagnoses included: DSM‐IV Major Depressive Disorder (MDD) and a score of 45 or more on the CDRS‐R (Poznanski 1996) Baseline risk of suicide: *data obtained from Table 2, 2004 paper Measured using the Suicidal ideation Questionnaire‐Junior High School Version (SIQ‐JR; Reynolds 1987) Adjusted mean (SD): CBT: 21.91 (16.28) Fluoxetine: 21.81 (15.68) Fluoxetine + CBT: 27.33 (18.51) Placebo: 24.20 (16.46) Analysed according to a cut‐off score of ≤ 31 CBT: 27/107 (25.2%) Fluoxetine: 28/107 (26.2%) Fluoxetine + CBT: 42/106 (39.6%) Participants excluded if deemed ‘high risk’ because of a suicide attempt requiring medical attention within 6 months. Also excluded on the basis of having a clear intent or active plan to attempt suicide, or suicidal ideation accompanied by a disorganised family unable to guarantee adequate safety monitoring Baseline severity of depression: *data obtained from Table 1, 2004 paper, t‐scores presented Children’s Depression Rating Score (CDRS‐R; Poznanski 1996): CBT: 75.37 (6.32) Fluoxetine: 74.73 (6.74) Fluoxetine + CBT: 75.67 (6.53) Placebo: 76.14 (6.11)
Comorbidity included: Comorbidity (%) CBT Fluoxetine Fluoxetine+CBT Placebo Any psychiatric comorbidity 58.18 43.12 55.66 51.35 Dysthymia 15.45 5.5 10.28 10.71 Anxiety 32.43 23.85 28.4 25.23 Disruptive behaviour 24.32 22.94 21.50 25.00 Obsessive compulsive/tic 1.80 1.83 3.74 3.57 Substance use 0.90 2.75 2.80 0 Attention‐deficit/hyperactivity 12.61 11.93 13.08 16.96 Taking medications 3.60 2.75 3.74 8.93 Age mean (SD): Total = 14.6 (1.54) CBT = 14.62 (1.50) Fluoxetine = 14.50 (1.57) CBT + Fluoxetine = 14.6 (1.48) Placebo = 14.51 (1.62) Sex (M:F): 200:239 CBT= 50:61 Fluoxetine = 50:59 CBT + Fluoxetine = 47:60 Placebo = 53:59 Setting: Outpatient Excluded psychiatric disorders: Current or past diagnosis of bipolar disorder, severe conduct disorder, current substance abuse or dependence, pervasive developmental disorder(s), thought disorder or psychiatric disorders requiring out of protocol treatments Country: USA | |
| Interventions | Psychotherapy (CBT) N = 111 Name: CBT modules included psycho education about depression and it’s causes, goal‐setting, mood monitoring, increasing pleasant activities, social problem‐solving, and cognitive restructuring # sessions/length: Fifteen 1 hour sessions during stage 1, 6 additional sessions for partial responders and bi‐weekly sessions for full responders in stage 2, and 3 sessions (1 every 6 weeks) in stage 3 Manualised (Y/N): Yes Individual or group: Individual Parent involvement: 1 to 3 conjoint parent and adolescent sessions took place Fidelity check: Not reported Delivered by: Not reported Medication (Fluoxetine) N = 109 Name (class and type): Fluoxetine (SSRI) Dose (mg/day)/length: 10 mg/day and increased up to 40 mg/day by week 8. At week 12, dose raised to 50 to 60mg/day for ‘partial responders’ and ‘full responders’ remained on same fluoxetine dose Delivered how: Monitoring and status and medication effects occurred during 20 to 30 minute visits. Clinician also offered general encouragement about the effectiveness of pharmacotherapy for MDD Combination (Fluoxetine+CBT) N = 107 Details as above (Y/N): Yes Placebo N = 112 | |
| Outcomes | Clinician reported Schedule for Affective Disorder and Schizophrenia for School‐Age Children‐Present and Lifetime Version (K‐SADS‐P‐L; Kaufman 1997) This was used to define remission i.e. those who had did not have a continuing or new mood disorder since the last interview according to the K‐SADS‐PL).It was unclear if DSM‐IV or ICD time criteria were employed Children’s Depression Rating Scale‐Revised (CDRS‐R; Poznanski 1996) Self reported Reynolds Adolescent Depression Scale (RADS; Reynolds 1986). Suicidal ideation Questionnaire‐Junior High School Version (SIQ‐JR; Reynolds 1987) Additional Measures Clinical Global Impression Improvement (CGI‐I; Guy 1976) Child and Adolescent Impact Assessment (Angold 1998) Columbia University classification scheme of the US Food and Drug Administration analyses of antidepressant‐associated suicidal events | |
| Notes | Dropouts during treatment to any or at least 1 adverse reaction: Suicide‐related outcome as an adverse event of treatment: 24 (5.5%) of participants experienced a suicide‐related adverse event Total number (%): *data obtained from 2006 paper CBT: 5 (4.5) Fluoxetine: 10 (9.2) CBT + Fluoxetine: 5 (4.7) Placebo: 3 (2.7) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Eligible participants were randomly assigned...using a computerized stratified randomisation, a 1:1:1:1 treatment allocation ratio, permuted blocking (first block size = 4, with subsequent random block sizes of 4 and 8) within each striatum, and site and sex stratification variables”. pg. 808 |
| Allocation concealment (selection bias) | Low risk | “Participants were randomly assigned...at the coordinating centre”. pg. 449 |
| Blinding (performance bias and detection bias) | Low risk | “TADS used 2 primary measures of depression status assessed...by an independent evaluator blind to condition”. pg. 448 |
| Blinding (performance bias and detection bias) | High risk | “Participants and all study staff remained masked in the ‘pills only’ condition (fluoxetine therapy and placebo) until the end of stage 1 (week 12). Patients and treatment providers in the combination and CBT conditions were aware of treatment assignment”. pg. 1133 |
| Incomplete outcome data (attrition bias) | Unclear risk | “The primary analyses of remission rates...were conducted using an “intention to treat” (ITT) approach in which the analysis included all participants randomized to treatment regardless of protocol adherence and/or treatment completion”. pg. Under heading Data Analysis, 2009 Imputation method: LOCF Number randomised: CBT: 111 Fluoxetine: 109 Fluoxetine + CBT: 107 Placebo: 112 Total: 439 Number of dropouts during intervention CBT: 41 Fluoxetine: 38 Fluoxetine + CBT: 23 Placebo: 14 Total: 116 Number of dropouts in follow‐up (18 weeks): CBT: 21 Fluoxetine: 37 Fluoxetine + CBT: 15 Placebo: 8 Total: 81 Number of dropouts in follow‐up (36 weeks): CBT: 25 Fluoxetine: 21 Fluoxetine+CBT:23 Placebo: 15 Total: 84 Number analysed post‐intervention: CBT: 111 Fluoxetine: 109 Fluoxetine + CBT:107 Placebo: 112 Total: 439 Number analysed follow‐up 1 (18 weeks): CBT: 111 Fluoxetine: 109 Fluoxetine + CBT:107 Total: 327 Number analysed follow‐up 2 (36 weeks): CBT: 111 Fluoxetine: 109 Fluoxetine + CBT: 107 Total: 327 For active treatment arms: 84/327 exited the study because of loss of follow‐up or withdrawal of consent (n = 21 for CBT + fluoxetine, n = 32 for fluoxetine, n = 31 for CBT). 96/327 discontinued treatment before week 36 due to premature termination or non‐response at the end of stage 1 (n = 25 for CBT + fluoxetine, n = 39 for fluoxetine, n = 32 for CBT), and this discontinuation was decided by the study physician For placebo: 13/112 participants were terminated prematurely from the study by week 12 due to clinical worsening |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol located |
| Other bias | High risk | Combination therapy group had an excess of suicidal ideation at baseline relative to fluoxetine or CBT |
| Methods | Duration: 6 months Follow‐up assessment points: Post‐intervention (24 weeks) Funded by: National Institute of Mental Health (NIMH) | |
| Participants | N = 124 Adolescent only (12 to 18 years) Depression diagnoses included: DSM‐IV Major Depressive Disorder (MDD), Dysthymic Disorder (DD) or Depressive Disorder not otherwise specified (DD‐NOS). Participants also had to obtain a score of 36 or more on the CDRS‐R (Poznanski 1996) Baseline risk of suicide: Participants were only eligible for participation if they had made a suicide attempt in the last 90 days. Beck Scale for Suicidal Ideation (SSI; Beck et al 1979). Mean (SD): Total = 6.3 (7.7) CBT‐SP = 5.0 (6.0) SSRI = 3.9 (6.0) SSRI+CBT‐SP = 6.9 (8.2) Baseline severity of depression: 96% met criteria for MDD and 10.5% had DD and DD. Children’s Depression Rating Scale‐Revised (CDRS‐R; Poznanski 1996). Mean (SD): Total = 50.4 (12.6) CBT‐SP = 46.9 (14.7) SSRI = 43.4 (11.1) SSRI+CBT‐SP= 52.1 (12.0) Comorbidity included: Comorbidity (%) CBT‐SP SSRI SSRI+CBT‐SP Anxiety 23.5 28.6 63.4 ADHD 11.8 14.3 23.7 ODD/CD 0.0 35.7 15.1 Age mean (SD): Total = 15.7 (1.5) CBT‐SP = 15.7 (1.5) SSRI = 15.6 (1.4) SSRI + CBT‐SP = 15.7 (1.6) Sex (M:F): 28:96 CBT‐SP = 1:16 SSRI = 1:13 SSRI + CBT‐SP = 26:67 Setting: Academic sites Excluded psychiatric diagnoses: Substance dependence, bipolar disorder, psychosis and pervasive developmental disorders (PDD) Country: USA | |
| Interventions | Psychotherapy (CBT‐SP) N = 17 Name: CBT + Suicide prevention (CBT‐SP). Modules included; chain analysis of the suicide attempt, safety planning, formulation of the participants cognitive, behavioural, affective and contextual problems, behavioural activation, cognitive restructuring, problem‐solving, and relapse prevention # sessions/length: Up to 22 sessions, length not specified Manualised (Y/N): Yes Individual or group: Individual Parent involvement: Parent‐youth sessions were included Fidelity check: No formal check. Weekly telephone conferences were held to review cases Delivered by: Trained psychotherapists under the supervision of senior experts Medication (SSRI) N = 14 Name (class and type): SSRI. Step 1: Monotherapy with an SSRI. Step 2: In the case of non‐response changed to a different SSRI. Stage 3: Medication changed to an alternative class (venlafaxine, duloxetine, mirtazapine, or bupropion) with option of augmenting with lithium or another SSRI Dose (mg/day)/length: Not specified Delivered how: By psychopharmacologists Combination (SSRI + CBT‐SP) N = 93 Details as above (Y/N): Yes | |
| Outcomes | Clinician reported Children’s Depression Rating Scale‐Revised (CDRS‐R; Poznanski 1996) Children’s Global Assessment Scale (C‐GAS; Shaffer 1983) Self reported Beck Depression Inventory (BDI; Beck 1988) Beck Scale for Suicidal Ideation (SSI; Beck 1979a) Recurrence of a suicidal event Additional Measures The Montgomery‐Asberg Depression Rating Scale (MADRS; Montgomery 1979) The Multidimensional Anxiety Scale for Children (MASC; March 1997) The Clinical Global Impressions‐Severity (CGI‐S) and Improvement scales (CGI‐I; Guy 1976) | |
| Notes | Dropouts during treatment to any or at least 1 adverse reaction: Suicide‐related outcome as an adverse event of treatment: 19.5% of participants experienced a suicide event and 12% made a suicide attempt. 1 participant died of suicide 20 days after completing the 24 week SSRI + CBT ‐ SP treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “The study started as a randomized controlled trial; however; after approximately 9 months of low enrolment despite intensive recruitment efforts, the design was changed so that patients and their families could accept randomisation or choose which treatment to receive”. pg. 998 |
| Allocation concealment (selection bias) | High risk | As participants chose their treatment condition, allocation concealment is not applicable |
| Blinding (performance bias and detection bias) | Low risk | “Independent evaluators were trained to ensure interrater reliability and remained blind to patient treatment assignment”. pg. 999 |
| Blinding (performance bias and detection bias) | High risk | “Most (n = 104) chose their treatment rather than being randomized”. pg. 1000 |
| Incomplete outcome data (attrition bias) | Unclear risk | “The data were analysed with an intent‐to‐treat approach”. pg. 999 Imputation method: LOCF Number enrolled (included non‐randomised participants): CBT‐SI: 17 SSRI: 14 SSRI + CBT‐SP: 93 Total: 124 Number of dropouts during intervention CBT‐SI: 6 SSRI: 6 SSRI + CBT‐SP: 26 Total: 36 Number analysed post‐intervention: CBT‐SI: 17 SSRI: 14 SSRI+ CBT‐SP: 93 Total: 124 Reasons for dropout: 2 participants reported suicidal intent with inability to commit to safety plan, 5 showed lack of adherence to treatment, 9 had a need for different treatments and services and 23 withdrew consent or failed to return for visits for unspecified reasons |
| Selective reporting (reporting bias) | High risk | Only total and combination treatment outcomes reported. Do not have access to trial protocol |
| Other bias | High risk | The SSRI + CBT ‐ SP group had higher levels of depression severity at baseline compared with the SSRI or CBT alone group. The SSRI + CBT ‐ SP group also had a higher prevalence of comorbid anxiety and more functional impairment than the other 2 groups Only 20/124 participants were randomised, the remaining 104/124 chose their treatment option |
ADHD: Attention Deficit Hyperactvity Disorder; b.i.d: twice daily; CDI: Children's Depression Inventory; CDRS‐R; Childrens Depression Rating Scale‐Revised; DSM‐III: Diagnostic and Statistical Manual of Mental Disorders, Third Edition; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; HMO: Health Maintenance Organisation; ICD: Internation Classification of Diseases; ITT: Intention to Treat; NHS: National Health Service; NOS: Not Otherwise Specified; ODD; Oppositional Definant Disorder; q.i.d: four times daily; SD: Standard Deviation; SSRI: Selective Serotonin REuptake Inhibitor; TAU: Treatment As Usual; t.i.d: three times daily.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Antidepressant versus placebo only, no psychological intervention | |
| Mean age of participants was 21 (too old for inclusion in this review) | |
| Randomised cross‐over trial, which is an exclusion criteria | |
| Trial did not include a suitable comparison condition | |
| Medical algorithm | |
| Relapse prevention study | |
| Trial did not include a suitable comparison condition | |
| Not an RCT (parents chose the treatment condition) | |
| Trial did not include a suitable comparison condition. | |
| Relapse prevention study, plus trial did not include a suitable comparison condition (participants in any arm could receive fluoxetine) | |
| Trial did not include a suitable comparison condition | |
| Participants outside the age range for inclusion in this review, plus it was a treatment resistant trial (sertraline in addition to CBT vs CBT alone in young people who have failed to respond to acute treatment) | |
| Trial did not include a suitable comparison condition | |
| Mean age of participants was 29 (too old for inclusion in this review) | |
| Trial did not include a suitable comparison condition | |
| Treatment of resistant depression | |
| Antidepressant versus placebo only, no psychological intervention | |
| Participants did not have a primary diagnosis of depressive disorder | |
| Participants did not have a primary diagnosis of depressive disorder |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | NCT01740726 |
| Methods | Randomized, treatment efficacy study Parallel assignment, single blind (outcomes assessor) |
| Participants |
|
| Interventions | Behavioral Activation: 18 weeks of 1‐hour individual therapy focused on increasing rewarding behaviors, (BA intervention includes monthly booster sessions offered throughout 6‐month follow up) Drug Therapy: Fluoxetine ‐ initial 10mg dose titrated as necessary to 40mg daily (therapists will be available between sessions and throughout the follow‐up interval to manage clinical concerns or emergencies) |
| Outcomes | Children's Depression Rating Scale ‐ Revised (CDRS‐R) Beck Depression Inventory, 2nd Edition (BDI‐II) (self‐report depressive symptom severity) |
| Starting date | 2013 |
| Contact information | |
| Notes |
| Trial name or title | YoDA‐C |
| Methods | Randomised controlled treatment trial Parallel assignment, double blind |
| Participants |
Exclusion criteria
|
| Interventions | Antidepressant fluoxetine hydrochloride or a placebo tablet will be administered in conjunction with CBT over a period of 12 weeks. The starting dose of fluoxetine or placebo will be 1 capsule or 20mg, administered orally. The dose may be increased any time after the first four weeks to a maximum dose of 2 capsules or 40 mg. CBT is delivered weekly in 50 minute sessions; where possible clinicians will rescheduled missed appointments. CBT is being delivered according to a newly devised manual that is modular and flexible in its approach and targets the the age group specifically. Each session is being recorded and random sessions will be selected for coding for fidelity. |
| Outcomes | Primary outcome: change in the Montgomery‐Asberg Depression Rating Scale (MADRS) at week 12 Quick Inventory of Depression Symptomatology–Self Report (QIDS) CGI Scale severity score CGI Scale improvement score Patient Global Impression improvement score Social and Occupational Functioning Scale Social Adjustment Scale–Self‐Report Quality of Life Enjoyment and Satisfaction Questionnaire–Short Form Suicidal Ideation Questionnaire Columbia Suicide Severity Rating Scale |
| Starting date | 2013 |
| Contact information | |
| Notes |
| Trial name or title | NCT01802437 |
| Methods | Randomized, single blind, defficacy study, |
| Participants |
|
| Interventions | Interpersonal psychotherapy ‐ adolescent randomized to an increase in therapy (4 extra therapy sessions) Fluoxetine ‐ adolescents randomized to an increase in therapy (4 extra therapy sessions) (Adolescents who receive pharmacotherapy will be prescribed fluoxetine for 12 weeks. The dosage schedule will be 10 mg per day for the first week and 20 mg per day for the following 5 weeks. If no treatment response is observed by week 6, the dosage can be increased to 40 mg per day. Pharmacotherapy sessions will be scheduled weekly for the first 4 weeks and every other week thereafter) |
| Outcomes | KSADS at week 16 and 32 BDI‐II at weeks 4/8/12/16/24/32 HRSD at weeks 4/8/12/16/24/32 CSSR‐S at weeks 4/8/12/16/24/32 |
| Starting date | 2010 |
| Contact information | |
| Notes |
| Trial name or title | NCT02017535 |
| Methods | Randomized, single blind, continuation study of NCT01802437 (Gunlicks‐Stoessel 2013 a) |
| Participants |
|
| Interventions | Interpersonal Psychotherapy ‐ adolescents randomized to an increase in therapy (4 extra therapy sessions) Fluoxetine ‐ adolescents randomized to an increase in therapy (4 extra therapy sessions) (Adolescents who receive pharmacotherapy will be prescribed fluoxetine for 12 weeks. The dosage schedule will be 10 mg per day for the first week and 20 mg per day for the following 5 weeks. If no treatment response is observed by week 6, the dosage can be increased to 40 mg per day. Pharmacotherapy sessions will be scheduled weekly for the first 4 weeks and every other week thereafter) |
| Outcomes | KSADS at week 16 and 32 BDI‐II at weeks 4/8/12/16/24/32 HRSD at weeks 4/8/12/16/24/32 CSSR‐S at weeks 4/8/12/16/24/32 |
| Starting date | 2012 |
| Contact information | |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Remission by clinical interview (post‐intervention) ITT Show forest plot | 2 | 268 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.28, 1.35] |
| Analysis 1.1  Comparison 1 Psychological therapy versus antidepressant medication, Outcome 1 Remission by clinical interview (post‐intervention) ITT. | ||||
| 2 Remission by clinical interview (post‐intervention) OC Show forest plot | 2 | 220 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.27, 0.98] |
| Analysis 1.2 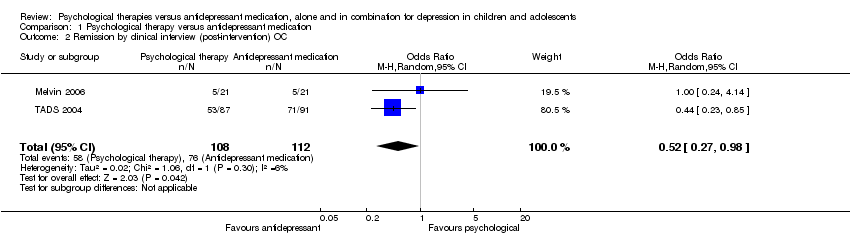 Comparison 1 Psychological therapy versus antidepressant medication, Outcome 2 Remission by clinical interview (post‐intervention) OC. | ||||
| 3 Remission by clinical interview (six to nine months follow‐up) ITT Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Psychological therapy versus antidepressant medication, Outcome 3 Remission by clinical interview (six to nine months follow‐up) ITT. | ||||
| 4 Remission by clinical interview (six to nine months follow‐up) OC Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.4 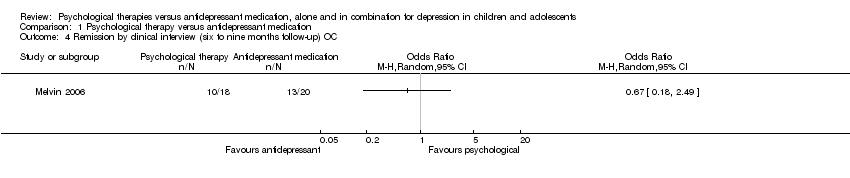 Comparison 1 Psychological therapy versus antidepressant medication, Outcome 4 Remission by clinical interview (six to nine months follow‐up) OC. | ||||
| 5 Dropouts (post‐intervention) Show forest plot | 2 | 271 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.11, 3.28] |
| Analysis 1.5  Comparison 1 Psychological therapy versus antidepressant medication, Outcome 5 Dropouts (post‐intervention). | ||||
| 6 Dropouts (six to nine months follow‐up) Show forest plot | 2 | 223 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.63, 2.19] |
| Analysis 1.6 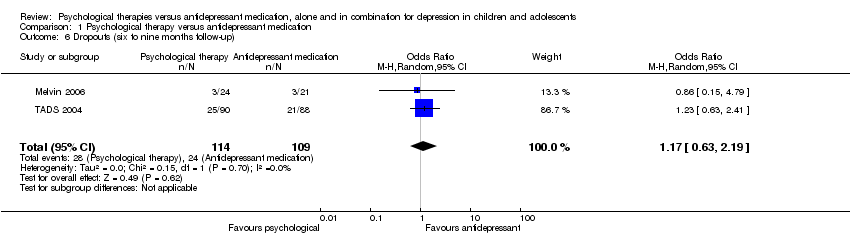 Comparison 1 Psychological therapy versus antidepressant medication, Outcome 6 Dropouts (six to nine months follow‐up). | ||||
| 7 Suicidal ideation (post‐intervention) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.7 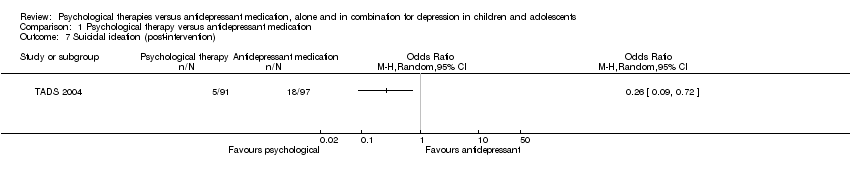 Comparison 1 Psychological therapy versus antidepressant medication, Outcome 7 Suicidal ideation (post‐intervention). | ||||
| 8 Suicidal ideation (six to nine months follow‐up) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.8  Comparison 1 Psychological therapy versus antidepressant medication, Outcome 8 Suicidal ideation (six to nine months follow‐up). | ||||
| 9 Suicidal ideation (post‐intervention) Show forest plot | 2 | 268 | Mean Difference (IV, Random, 95% CI) | ‐3.12 [‐5.91, ‐0.33] |
| Analysis 1.9 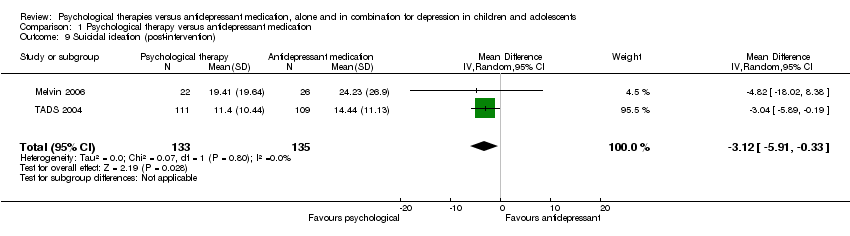 Comparison 1 Psychological therapy versus antidepressant medication, Outcome 9 Suicidal ideation (post‐intervention). | ||||
| 10 Suicidal ideation (six to nine months follow‐up) Show forest plot | 2 | 268 | Mean Difference (IV, Random, 95% CI) | ‐2.89 [‐5.49, ‐0.28] |
| Analysis 1.10  Comparison 1 Psychological therapy versus antidepressant medication, Outcome 10 Suicidal ideation (six to nine months follow‐up). | ||||
| 11 Suicidal ideation (12 months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.11 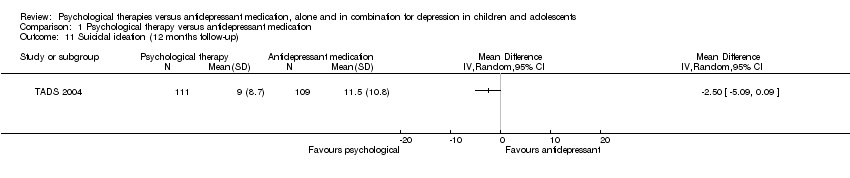 Comparison 1 Psychological therapy versus antidepressant medication, Outcome 11 Suicidal ideation (12 months follow‐up). | ||||
| 12 Remission by cut‐off (post‐intervention) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.12  Comparison 1 Psychological therapy versus antidepressant medication, Outcome 12 Remission by cut‐off (post‐intervention). | ||||
| 13 Remission by cut‐off (six to nine months follow‐up) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.13  Comparison 1 Psychological therapy versus antidepressant medication, Outcome 13 Remission by cut‐off (six to nine months follow‐up). | ||||
| 14 Remission by cut‐off (12 months follow‐up) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.14 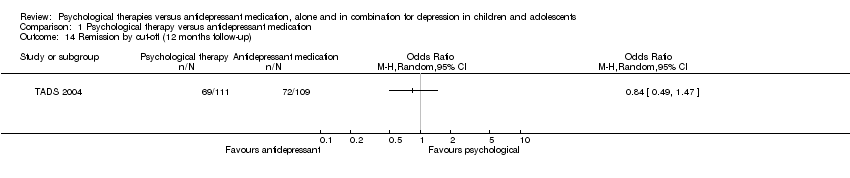 Comparison 1 Psychological therapy versus antidepressant medication, Outcome 14 Remission by cut‐off (12 months follow‐up). | ||||
| 15 Depression symptoms clinician rated (CDRS‐R) (post‐intervention) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.15 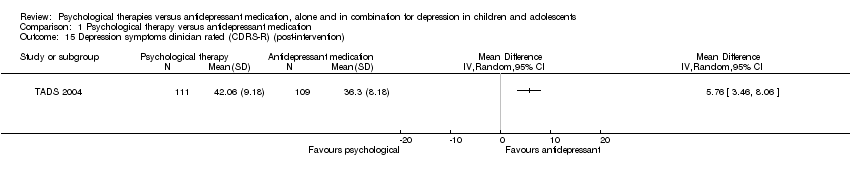 Comparison 1 Psychological therapy versus antidepressant medication, Outcome 15 Depression symptoms clinician rated (CDRS‐R) (post‐intervention). | ||||
| 16 Depression symptoms clinician rated (CDRS‐R) (six to nine months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.16  Comparison 1 Psychological therapy versus antidepressant medication, Outcome 16 Depression symptoms clinician rated (CDRS‐R) (six to nine months follow‐up). | ||||
| 17 Depression symptoms clinician rated (CDRS‐R) (12 months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.17  Comparison 1 Psychological therapy versus antidepressant medication, Outcome 17 Depression symptoms clinician rated (CDRS‐R) (12 months follow‐up). | ||||
| 18 Depression symptoms self rated (post‐intervention) Show forest plot | 2 | 255 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.69, 1.01] |
| Analysis 1.18  Comparison 1 Psychological therapy versus antidepressant medication, Outcome 18 Depression symptoms self rated (post‐intervention). | ||||
| 19 Depression symptoms self rated (six to nine months follow‐up) Show forest plot | 2 | 268 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.51, 0.42] |
| Analysis 1.19  Comparison 1 Psychological therapy versus antidepressant medication, Outcome 19 Depression symptoms self rated (six to nine months follow‐up). | ||||
| 20 Depression symptoms self rated (12 months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.20 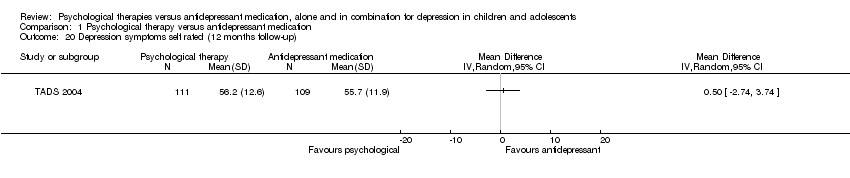 Comparison 1 Psychological therapy versus antidepressant medication, Outcome 20 Depression symptoms self rated (12 months follow‐up). | ||||
| 21 Functioning (post‐intervention) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.21 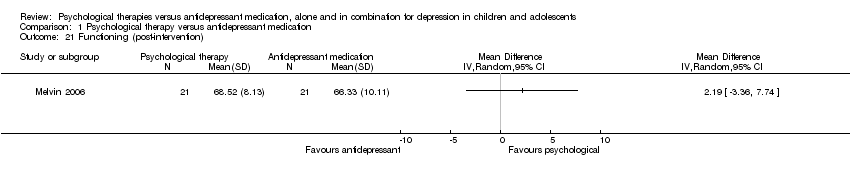 Comparison 1 Psychological therapy versus antidepressant medication, Outcome 21 Functioning (post‐intervention). | ||||
| 22 Functioning (six to nine months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.22 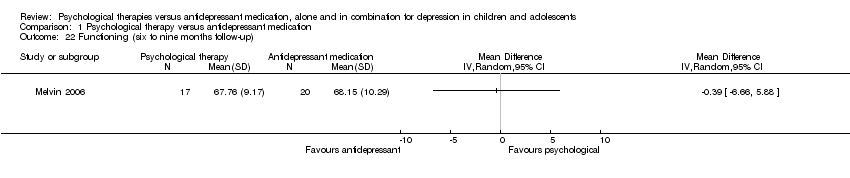 Comparison 1 Psychological therapy versus antidepressant medication, Outcome 22 Functioning (six to nine months follow‐up). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Remission by clinical interview (post‐intervention) ITT Show forest plot | 3 | 419 | Odds Ratio (M‐H, Random, 95% CI) | 1.50 [0.99, 2.27] |
| Analysis 2.1 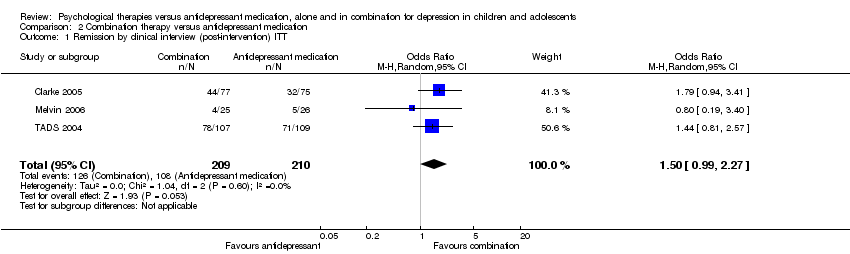 Comparison 2 Combination therapy versus antidepressant medication, Outcome 1 Remission by clinical interview (post‐intervention) ITT. | ||||
| 2 Remission by clinical interview (post‐intervention) OC Show forest plot | 3 | 378 | Odds Ratio (M‐H, Random, 95% CI) | 1.56 [0.98, 2.47] |
| Analysis 2.2  Comparison 2 Combination therapy versus antidepressant medication, Outcome 2 Remission by clinical interview (post‐intervention) OC. | ||||
| 3 Remission by clinical interview (six to nine months follow‐up) ITT Show forest plot | 2 | 203 | Odds Ratio (M‐H, Random, 95% CI) | 1.93 [0.93, 4.00] |
| Analysis 2.3  Comparison 2 Combination therapy versus antidepressant medication, Outcome 3 Remission by clinical interview (six to nine months follow‐up) ITT. | ||||
| 4 Remission by clinical interview (six to nine months follow‐up) OC Show forest plot | 2 | 193 | Odds Ratio (M‐H, Random, 95% CI) | 1.94 [0.88, 4.27] |
| Analysis 2.4  Comparison 2 Combination therapy versus antidepressant medication, Outcome 4 Remission by clinical interview (six to nine months follow‐up) OC. | ||||
| 5 Remission by clinical interview (12 months follow‐up) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 Combination therapy versus antidepressant medication, Outcome 5 Remission by clinical interview (12 months follow‐up). | ||||
| 6 Dropouts (post‐intervention) Show forest plot | 5 | 699 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.51, 1.39] |
| Analysis 2.6  Comparison 2 Combination therapy versus antidepressant medication, Outcome 6 Dropouts (post‐intervention). | ||||
| 7 Dropouts (six to nine months follow‐up) Show forest plot | 3 | 420 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.54, 1.64] |
| Analysis 2.7 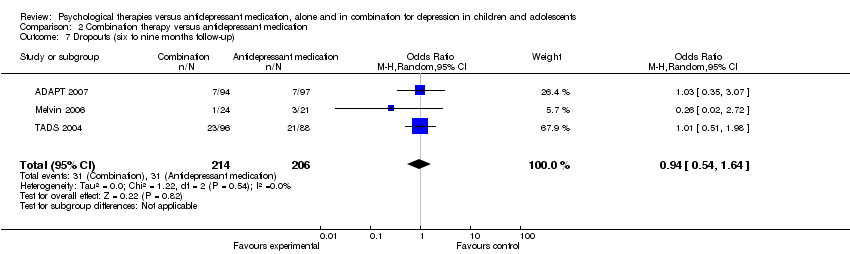 Comparison 2 Combination therapy versus antidepressant medication, Outcome 7 Dropouts (six to nine months follow‐up). | ||||
| 8 Dropouts (12 months follow‐up) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.8  Comparison 2 Combination therapy versus antidepressant medication, Outcome 8 Dropouts (12 months follow‐up). | ||||
| 9 Suicidal ideation (post‐intervention) Show forest plot | 2 | 388 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.26, 2.16] |
| Analysis 2.9  Comparison 2 Combination therapy versus antidepressant medication, Outcome 9 Suicidal ideation (post‐intervention). | ||||
| 10 Suicidal ideation (six to nine months follow‐up) Show forest plot | 2 | 344 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.06, 4.58] |
| Analysis 2.10  Comparison 2 Combination therapy versus antidepressant medication, Outcome 10 Suicidal ideation (six to nine months follow‐up). | ||||
| 11 Suicidal ideation (12 months follow‐up) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.11  Comparison 2 Combination therapy versus antidepressant medication, Outcome 11 Suicidal ideation (12 months follow‐up). | ||||
| 12 Suicidal ideation (post‐intervention) Show forest plot | 2 | 267 | Mean Difference (IV, Random, 95% CI) | ‐2.57 [‐5.53, 0.40] |
| Analysis 2.12 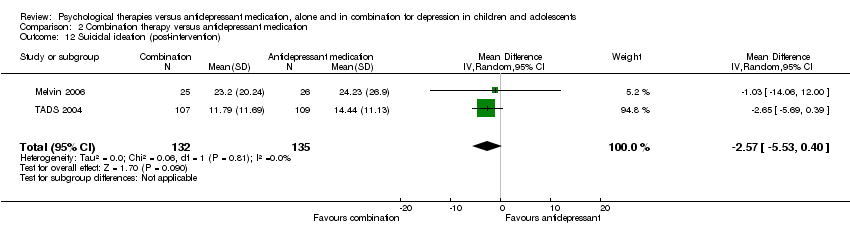 Comparison 2 Combination therapy versus antidepressant medication, Outcome 12 Suicidal ideation (post‐intervention). | ||||
| 13 Suicidal ideation (six to nine months follow‐up) Show forest plot | 2 | 267 | Mean Difference (IV, Random, 95% CI) | ‐1.89 [‐4.50, 0.72] |
| Analysis 2.13 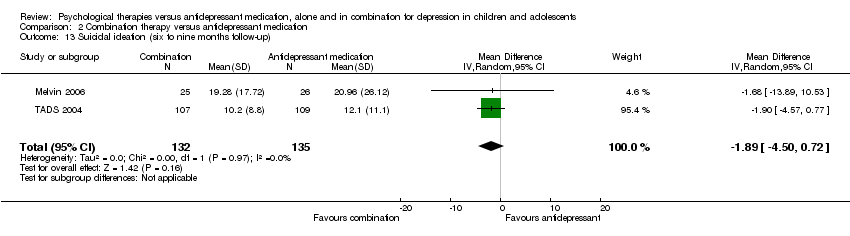 Comparison 2 Combination therapy versus antidepressant medication, Outcome 13 Suicidal ideation (six to nine months follow‐up). | ||||
| 14 Suicidal ideation (12 months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.14  Comparison 2 Combination therapy versus antidepressant medication, Outcome 14 Suicidal ideation (12 months follow‐up). | ||||
| 15 Remission by cut‐off (post‐intervention) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.15 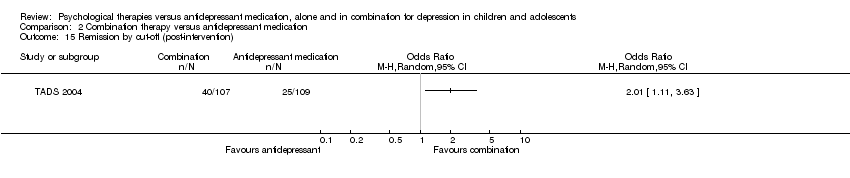 Comparison 2 Combination therapy versus antidepressant medication, Outcome 15 Remission by cut‐off (post‐intervention). | ||||
| 16 Remission by cut‐off (six to nine months follow‐up) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.16 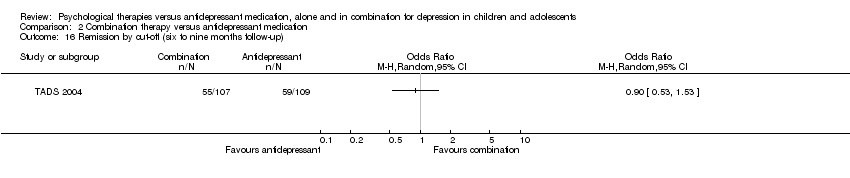 Comparison 2 Combination therapy versus antidepressant medication, Outcome 16 Remission by cut‐off (six to nine months follow‐up). | ||||
| 17 Remission by cut‐off (12 months follow‐up) Show forest plot | 2 | 319 | Odds Ratio (M‐H, Random, 95% CI) | 1.45 [0.60, 3.52] |
| Analysis 2.17 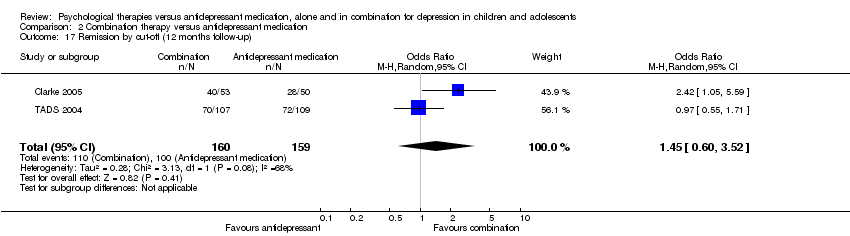 Comparison 2 Combination therapy versus antidepressant medication, Outcome 17 Remission by cut‐off (12 months follow‐up). | ||||
| 18 Depression symptoms clinician rated (CDRS‐R) (post‐intervention) Show forest plot | 2 | 415 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐4.95, 4.41] |
| Analysis 2.18  Comparison 2 Combination therapy versus antidepressant medication, Outcome 18 Depression symptoms clinician rated (CDRS‐R) (post‐intervention). | ||||
| 19 Depression symptoms clinician rated (CDRS‐R) (six to nine months follow‐up) Show forest plot | 2 | 408 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐2.26, 1.72] |
| Analysis 2.19 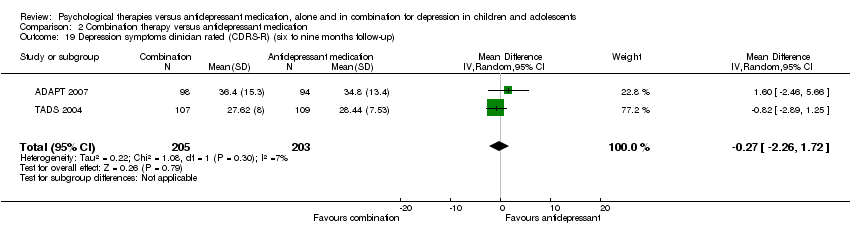 Comparison 2 Combination therapy versus antidepressant medication, Outcome 19 Depression symptoms clinician rated (CDRS‐R) (six to nine months follow‐up). | ||||
| 20 Depression symptoms clinician rated (CDRS‐R) (12 months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.20  Comparison 2 Combination therapy versus antidepressant medication, Outcome 20 Depression symptoms clinician rated (CDRS‐R) (12 months follow‐up). | ||||
| 21 Depression symptoms self rated (post‐intervention) Show forest plot | 5 | 683 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.36, 0.09] |
| Analysis 2.21 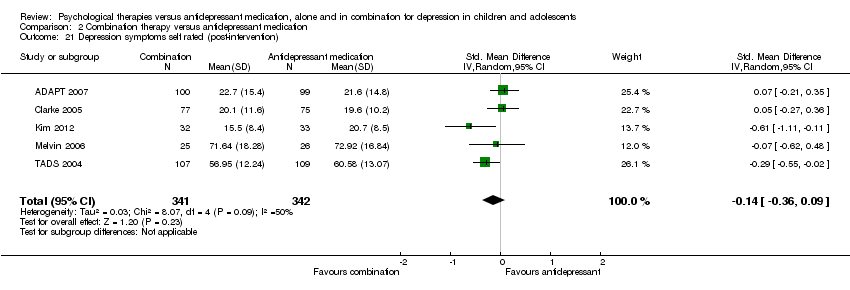 Comparison 2 Combination therapy versus antidepressant medication, Outcome 21 Depression symptoms self rated (post‐intervention). | ||||
| 22 Depression symptoms self rated (six to nine months follow‐up) Show forest plot | 4 | 610 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.28, 0.17] |
| Analysis 2.22  Comparison 2 Combination therapy versus antidepressant medication, Outcome 22 Depression symptoms self rated (six to nine months follow‐up). | ||||
| 23 Depression symptoms self rated (12 months follow‐up) Show forest plot | 2 | 368 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.46, ‐0.05] |
| Analysis 2.23 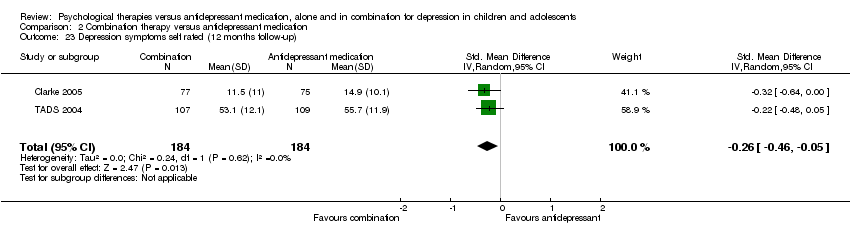 Comparison 2 Combination therapy versus antidepressant medication, Outcome 23 Depression symptoms self rated (12 months follow‐up). | ||||
| 24 Functioning (post‐intervention) Show forest plot | 3 | 396 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.11, 0.28] |
| Analysis 2.24  Comparison 2 Combination therapy versus antidepressant medication, Outcome 24 Functioning (post‐intervention). | ||||
| 25 Functioning (six to nine months follow‐up) Show forest plot | 3 | 385 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.12, 0.28] |
| Analysis 2.25 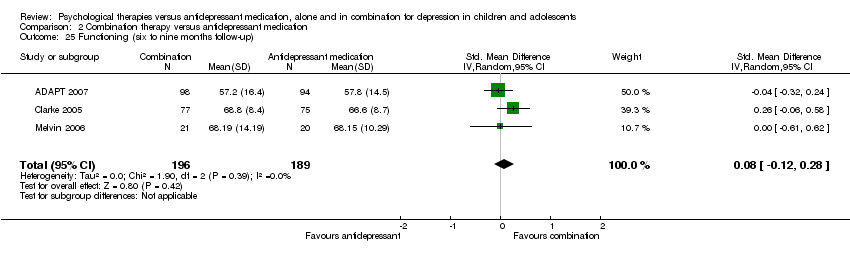 Comparison 2 Combination therapy versus antidepressant medication, Outcome 25 Functioning (six to nine months follow‐up). | ||||
| 26 Functioning (12 months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.26  Comparison 2 Combination therapy versus antidepressant medication, Outcome 26 Functioning (12 months follow‐up). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Remission by clinical interview (post‐intervention) ITT Show forest plot | 2 | 265 | Odds Ratio (M‐H, Random, 95% CI) | 1.61 [0.38, 6.90] |
| Analysis 3.1  Comparison 3 Combination therapy versus psychological therapy, Outcome 1 Remission by clinical interview (post‐intervention) ITT. | ||||
| 2 Remission by clinical interview (post‐intervention) OC Show forest plot | 2 | 222 | Odds Ratio (M‐H, Random, 95% CI) | 1.82 [0.38, 8.68] |
| Analysis 3.2 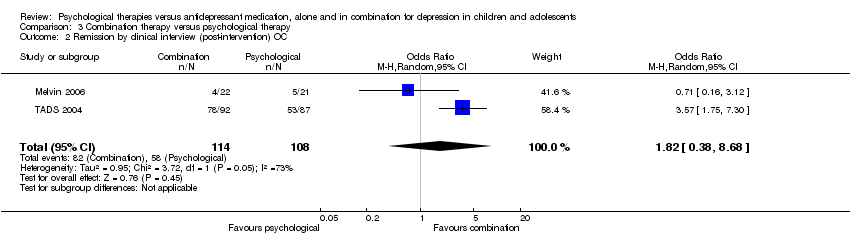 Comparison 3 Combination therapy versus psychological therapy, Outcome 2 Remission by clinical interview (post‐intervention) OC. | ||||
| 3 Remission by clinical interview (six to nine months follow‐up) ITT Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3 Combination therapy versus psychological therapy, Outcome 3 Remission by clinical interview (six to nine months follow‐up) ITT. | ||||
| 4 Remission by clinical interview (six to nine months follow‐up) OC Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 3.4 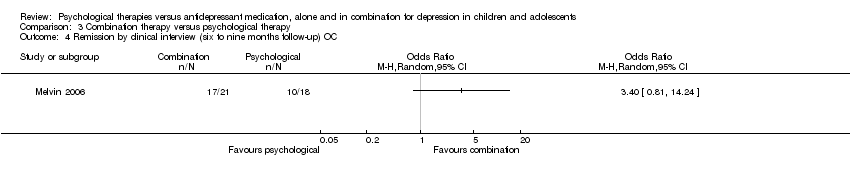 Comparison 3 Combination therapy versus psychological therapy, Outcome 4 Remission by clinical interview (six to nine months follow‐up) OC. | ||||
| 5 Dropouts (post‐intervention) Show forest plot | 2 | 265 | Odds Ratio (M‐H, Random, 95% CI) | 1.23 [0.12, 12.71] |
| Analysis 3.5  Comparison 3 Combination therapy versus psychological therapy, Outcome 5 Dropouts (post‐intervention). | ||||
| 6 Dropouts (six to nine months follow‐up) Show forest plot | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.40, 1.42] |
| Analysis 3.6 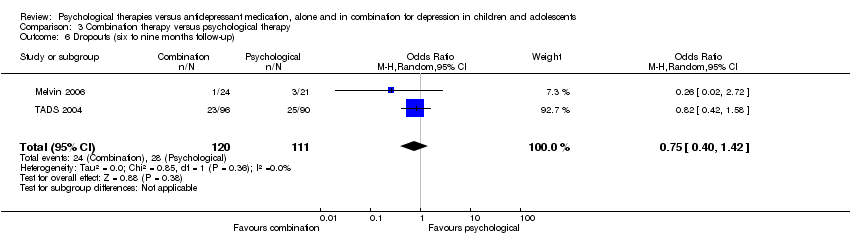 Comparison 3 Combination therapy versus psychological therapy, Outcome 6 Dropouts (six to nine months follow‐up). | ||||
| 7 Suicidal ideation (post‐intervention) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 3.7  Comparison 3 Combination therapy versus psychological therapy, Outcome 7 Suicidal ideation (post‐intervention). | ||||
| 8 Suicidal ideation (six to nine months follow‐up) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 3.8 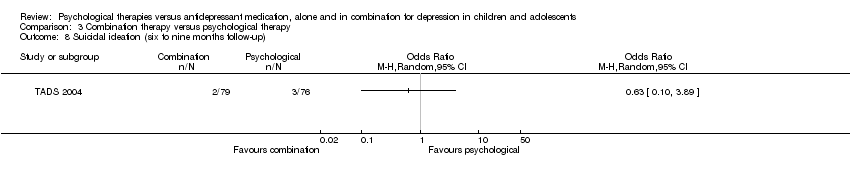 Comparison 3 Combination therapy versus psychological therapy, Outcome 8 Suicidal ideation (six to nine months follow‐up). | ||||
| 9 Suicidal ideation (post‐intervention) Show forest plot | 2 | 265 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐2.25, 3.45] |
| Analysis 3.9  Comparison 3 Combination therapy versus psychological therapy, Outcome 9 Suicidal ideation (post‐intervention). | ||||
| 10 Suicidal ideation (six to nine months follow‐up) Show forest plot | 2 | 265 | Mean Difference (IV, Random, 95% CI) | 1.78 [‐2.29, 5.85] |
| Analysis 3.10 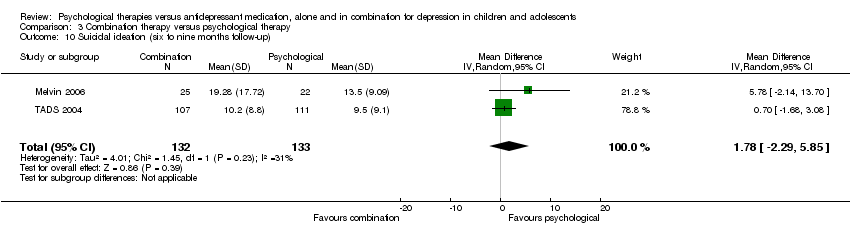 Comparison 3 Combination therapy versus psychological therapy, Outcome 10 Suicidal ideation (six to nine months follow‐up). | ||||
| 11 Suicidal ideation (12 months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.11  Comparison 3 Combination therapy versus psychological therapy, Outcome 11 Suicidal ideation (12 months follow‐up). | ||||
| 12 Remission by cut‐off (post‐intervention) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 3.12 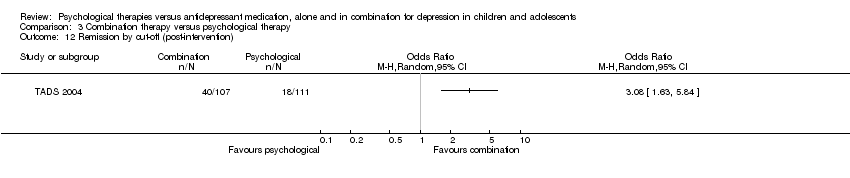 Comparison 3 Combination therapy versus psychological therapy, Outcome 12 Remission by cut‐off (post‐intervention). | ||||
| 13 Remission by cut‐off (six to nine months follow‐up) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 3.13  Comparison 3 Combination therapy versus psychological therapy, Outcome 13 Remission by cut‐off (six to nine months follow‐up). | ||||
| 14 Remission by cut‐off (12 months follow‐up) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 3.14 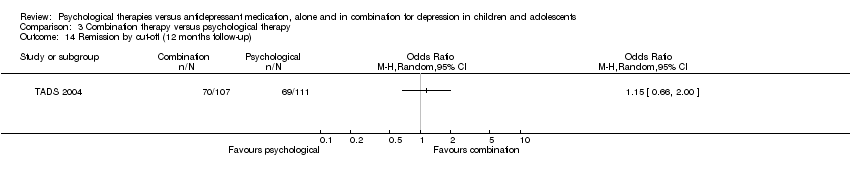 Comparison 3 Combination therapy versus psychological therapy, Outcome 14 Remission by cut‐off (12 months follow‐up). | ||||
| 15 Depression symptoms clinician rated (CDRS‐R) (post‐intervention) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.15 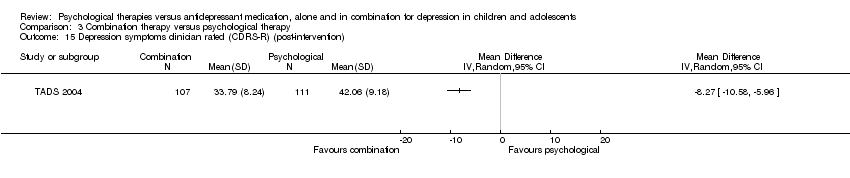 Comparison 3 Combination therapy versus psychological therapy, Outcome 15 Depression symptoms clinician rated (CDRS‐R) (post‐intervention). | ||||
| 16 Depression symptoms clinician rated (CDRS‐R) (six to nine months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.16  Comparison 3 Combination therapy versus psychological therapy, Outcome 16 Depression symptoms clinician rated (CDRS‐R) (six to nine months follow‐up). | ||||
| 17 Depression symptoms clinician rated (CDRS‐R) (12 months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.17 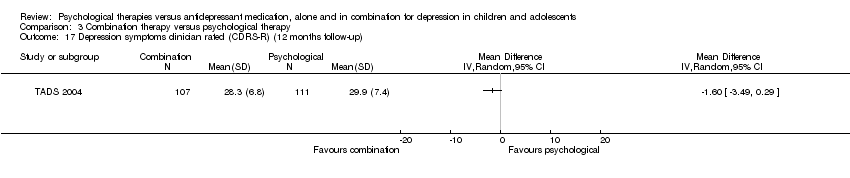 Comparison 3 Combination therapy versus psychological therapy, Outcome 17 Depression symptoms clinician rated (CDRS‐R) (12 months follow‐up). | ||||
| 18 Depression symptoms self rated (post‐intervention) Show forest plot | 2 | 265 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐1.41, 0.84] |
| Analysis 3.18 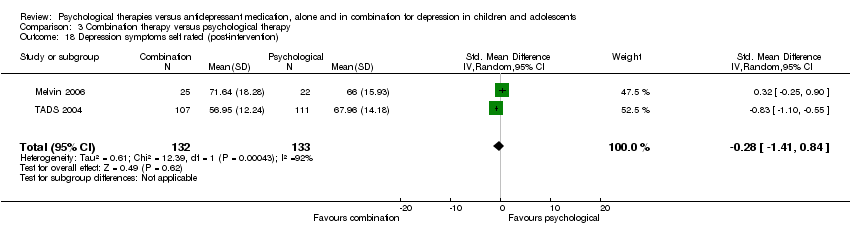 Comparison 3 Combination therapy versus psychological therapy, Outcome 18 Depression symptoms self rated (post‐intervention). | ||||
| 19 Depression symptoms self rated (six to nine months follow‐up) Show forest plot | 2 | 265 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.63, 0.31] |
| Analysis 3.19  Comparison 3 Combination therapy versus psychological therapy, Outcome 19 Depression symptoms self rated (six to nine months follow‐up). | ||||
| 20 Depression symptoms self rated (12 months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.20 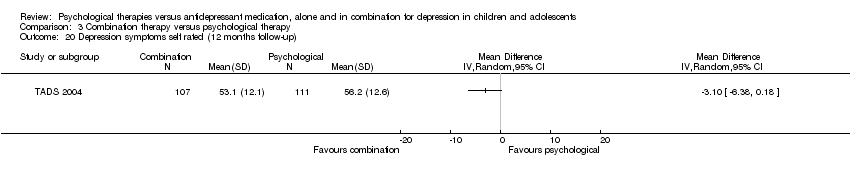 Comparison 3 Combination therapy versus psychological therapy, Outcome 20 Depression symptoms self rated (12 months follow‐up). | ||||
| 21 Functioning (post‐intervention) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.21  Comparison 3 Combination therapy versus psychological therapy, Outcome 21 Functioning (post‐intervention). | ||||
| 22 Functioning (six to nine months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.22 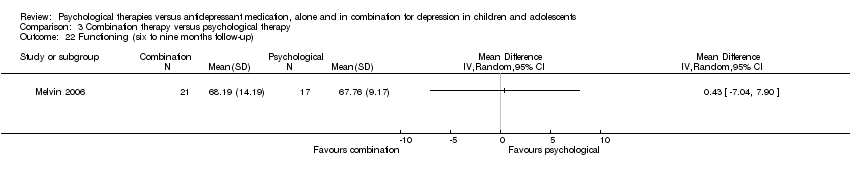 Comparison 3 Combination therapy versus psychological therapy, Outcome 22 Functioning (six to nine months follow‐up). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts (post‐intervention) Show forest plot | 4 | 249 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.42, 2.28] |
| Analysis 4.1  Comparison 4 Combination therapy versus psychological therapy plus placebo, Outcome 1 Dropouts (post‐intervention). | ||||
| 2 Suicidal ideation (post‐intervention) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.2  Comparison 4 Combination therapy versus psychological therapy plus placebo, Outcome 2 Suicidal ideation (post‐intervention). | ||||
| 3 Remission by cut‐off (post‐intervention) Show forest plot | 2 | 173 | Odds Ratio (M‐H, Random, 95% CI) | 2.15 [1.15, 4.02] |
| Analysis 4.3  Comparison 4 Combination therapy versus psychological therapy plus placebo, Outcome 3 Remission by cut‐off (post‐intervention). | ||||
| 4 Remission by cut‐off (12 months follow‐up) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 4.4  Comparison 4 Combination therapy versus psychological therapy plus placebo, Outcome 4 Remission by cut‐off (12 months follow‐up). | ||||
| 5 Depression symptoms clinician rated (CDRS‐R) (post‐intervention) Show forest plot | 3 | 239 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.78, ‐0.26] |
| Analysis 4.5  Comparison 4 Combination therapy versus psychological therapy plus placebo, Outcome 5 Depression symptoms clinician rated (CDRS‐R) (post‐intervention). | ||||
| 6 Depression symptoms self rated (post‐intervention) Show forest plot | 3 | 123 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.70, 0.02] |
| Analysis 4.6 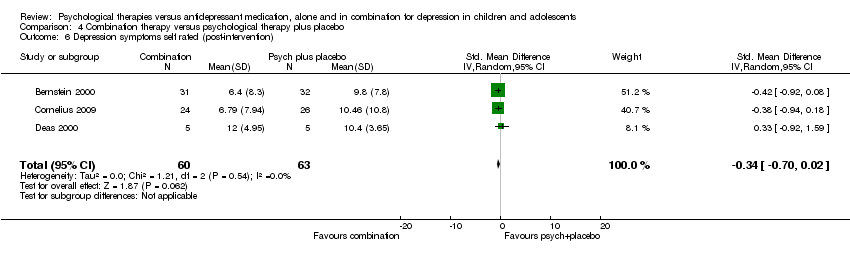 Comparison 4 Combination therapy versus psychological therapy plus placebo, Outcome 6 Depression symptoms self rated (post‐intervention). | ||||

PRISMA flow diagram: CCDANCTR update search June 2014
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Comparison 1 Psychological therapy versus antidepressant medication, Outcome 1 Remission by clinical interview (post‐intervention) ITT.
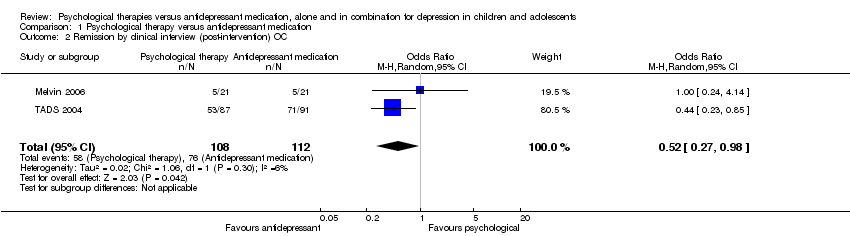
Comparison 1 Psychological therapy versus antidepressant medication, Outcome 2 Remission by clinical interview (post‐intervention) OC.

Comparison 1 Psychological therapy versus antidepressant medication, Outcome 3 Remission by clinical interview (six to nine months follow‐up) ITT.

Comparison 1 Psychological therapy versus antidepressant medication, Outcome 4 Remission by clinical interview (six to nine months follow‐up) OC.
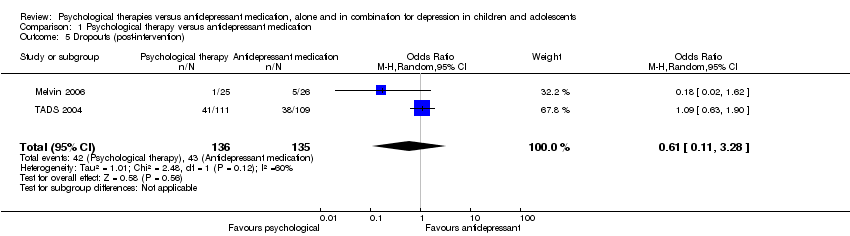
Comparison 1 Psychological therapy versus antidepressant medication, Outcome 5 Dropouts (post‐intervention).

Comparison 1 Psychological therapy versus antidepressant medication, Outcome 6 Dropouts (six to nine months follow‐up).

Comparison 1 Psychological therapy versus antidepressant medication, Outcome 7 Suicidal ideation (post‐intervention).

Comparison 1 Psychological therapy versus antidepressant medication, Outcome 8 Suicidal ideation (six to nine months follow‐up).

Comparison 1 Psychological therapy versus antidepressant medication, Outcome 9 Suicidal ideation (post‐intervention).
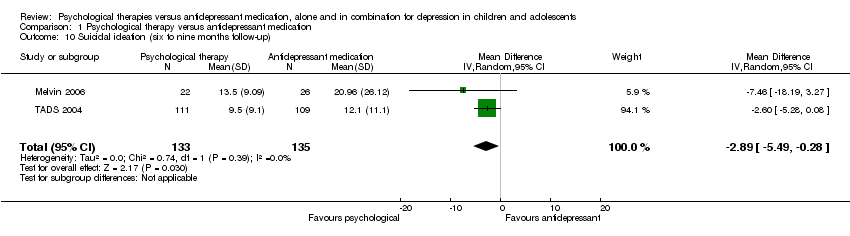
Comparison 1 Psychological therapy versus antidepressant medication, Outcome 10 Suicidal ideation (six to nine months follow‐up).
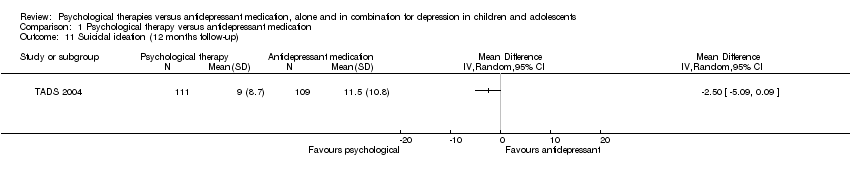
Comparison 1 Psychological therapy versus antidepressant medication, Outcome 11 Suicidal ideation (12 months follow‐up).

Comparison 1 Psychological therapy versus antidepressant medication, Outcome 12 Remission by cut‐off (post‐intervention).

Comparison 1 Psychological therapy versus antidepressant medication, Outcome 13 Remission by cut‐off (six to nine months follow‐up).

Comparison 1 Psychological therapy versus antidepressant medication, Outcome 14 Remission by cut‐off (12 months follow‐up).

Comparison 1 Psychological therapy versus antidepressant medication, Outcome 15 Depression symptoms clinician rated (CDRS‐R) (post‐intervention).
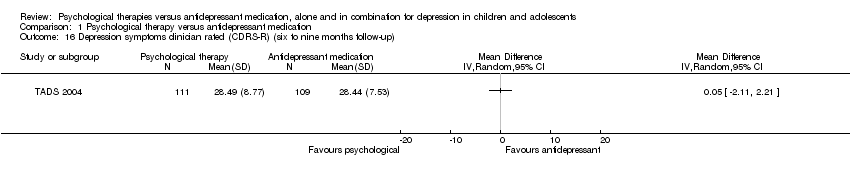
Comparison 1 Psychological therapy versus antidepressant medication, Outcome 16 Depression symptoms clinician rated (CDRS‐R) (six to nine months follow‐up).
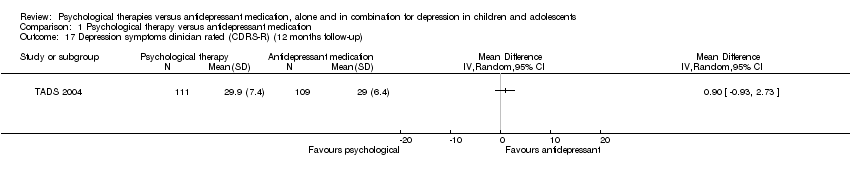
Comparison 1 Psychological therapy versus antidepressant medication, Outcome 17 Depression symptoms clinician rated (CDRS‐R) (12 months follow‐up).

Comparison 1 Psychological therapy versus antidepressant medication, Outcome 18 Depression symptoms self rated (post‐intervention).
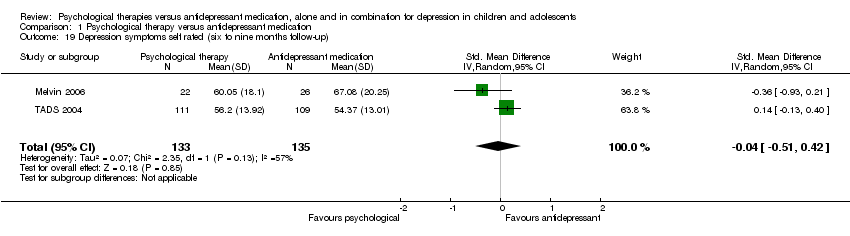
Comparison 1 Psychological therapy versus antidepressant medication, Outcome 19 Depression symptoms self rated (six to nine months follow‐up).

Comparison 1 Psychological therapy versus antidepressant medication, Outcome 20 Depression symptoms self rated (12 months follow‐up).

Comparison 1 Psychological therapy versus antidepressant medication, Outcome 21 Functioning (post‐intervention).
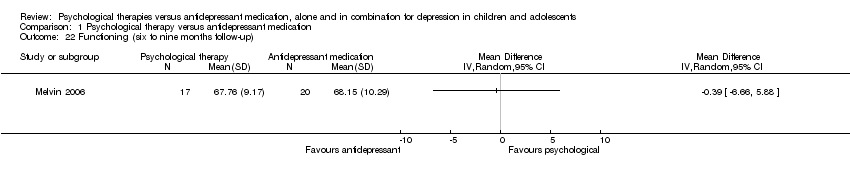
Comparison 1 Psychological therapy versus antidepressant medication, Outcome 22 Functioning (six to nine months follow‐up).
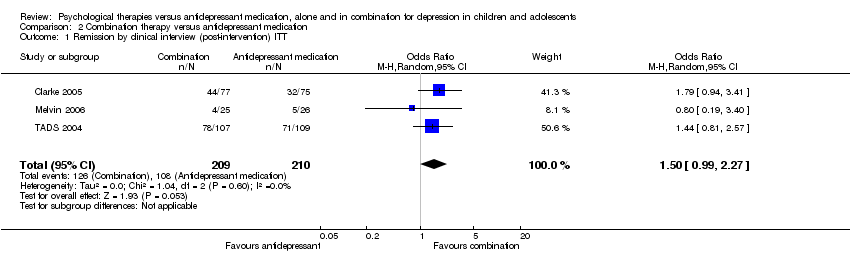
Comparison 2 Combination therapy versus antidepressant medication, Outcome 1 Remission by clinical interview (post‐intervention) ITT.
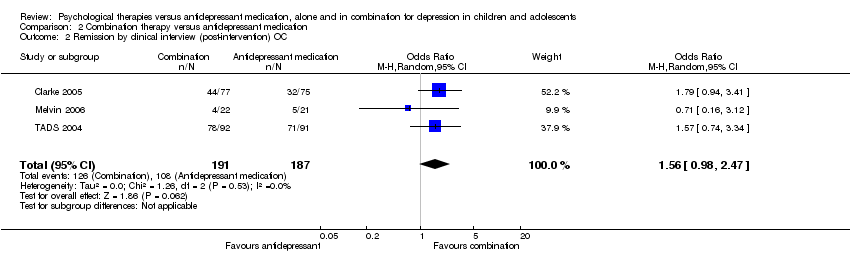
Comparison 2 Combination therapy versus antidepressant medication, Outcome 2 Remission by clinical interview (post‐intervention) OC.

Comparison 2 Combination therapy versus antidepressant medication, Outcome 3 Remission by clinical interview (six to nine months follow‐up) ITT.
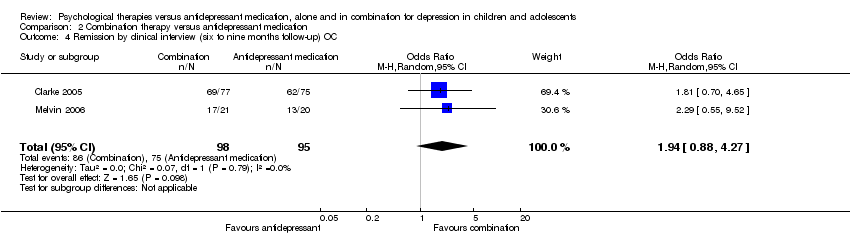
Comparison 2 Combination therapy versus antidepressant medication, Outcome 4 Remission by clinical interview (six to nine months follow‐up) OC.
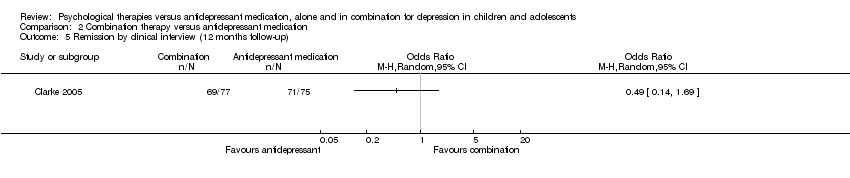
Comparison 2 Combination therapy versus antidepressant medication, Outcome 5 Remission by clinical interview (12 months follow‐up).

Comparison 2 Combination therapy versus antidepressant medication, Outcome 6 Dropouts (post‐intervention).

Comparison 2 Combination therapy versus antidepressant medication, Outcome 7 Dropouts (six to nine months follow‐up).

Comparison 2 Combination therapy versus antidepressant medication, Outcome 8 Dropouts (12 months follow‐up).
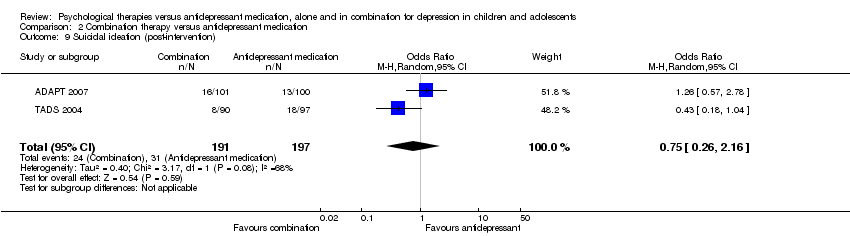
Comparison 2 Combination therapy versus antidepressant medication, Outcome 9 Suicidal ideation (post‐intervention).

Comparison 2 Combination therapy versus antidepressant medication, Outcome 10 Suicidal ideation (six to nine months follow‐up).

Comparison 2 Combination therapy versus antidepressant medication, Outcome 11 Suicidal ideation (12 months follow‐up).
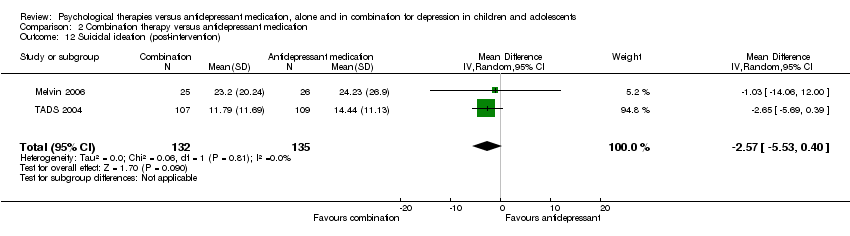
Comparison 2 Combination therapy versus antidepressant medication, Outcome 12 Suicidal ideation (post‐intervention).

Comparison 2 Combination therapy versus antidepressant medication, Outcome 13 Suicidal ideation (six to nine months follow‐up).
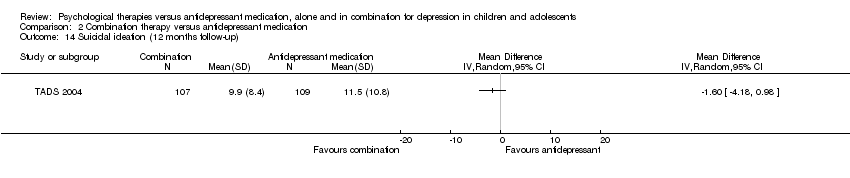
Comparison 2 Combination therapy versus antidepressant medication, Outcome 14 Suicidal ideation (12 months follow‐up).

Comparison 2 Combination therapy versus antidepressant medication, Outcome 15 Remission by cut‐off (post‐intervention).
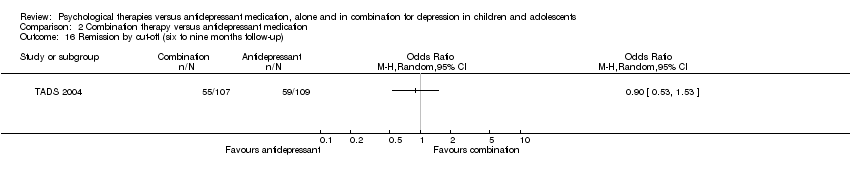
Comparison 2 Combination therapy versus antidepressant medication, Outcome 16 Remission by cut‐off (six to nine months follow‐up).

Comparison 2 Combination therapy versus antidepressant medication, Outcome 17 Remission by cut‐off (12 months follow‐up).
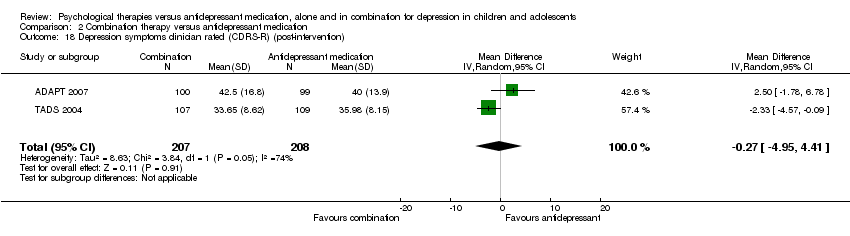
Comparison 2 Combination therapy versus antidepressant medication, Outcome 18 Depression symptoms clinician rated (CDRS‐R) (post‐intervention).
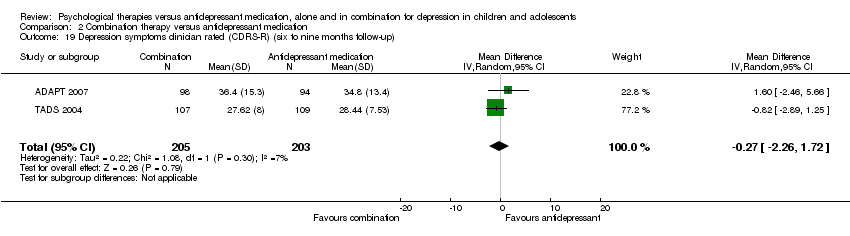
Comparison 2 Combination therapy versus antidepressant medication, Outcome 19 Depression symptoms clinician rated (CDRS‐R) (six to nine months follow‐up).

Comparison 2 Combination therapy versus antidepressant medication, Outcome 20 Depression symptoms clinician rated (CDRS‐R) (12 months follow‐up).

Comparison 2 Combination therapy versus antidepressant medication, Outcome 21 Depression symptoms self rated (post‐intervention).
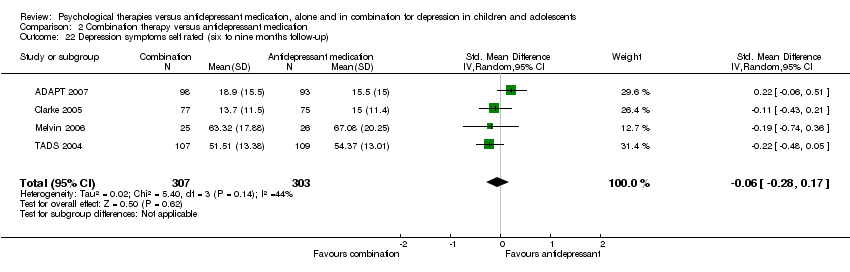
Comparison 2 Combination therapy versus antidepressant medication, Outcome 22 Depression symptoms self rated (six to nine months follow‐up).

Comparison 2 Combination therapy versus antidepressant medication, Outcome 23 Depression symptoms self rated (12 months follow‐up).

Comparison 2 Combination therapy versus antidepressant medication, Outcome 24 Functioning (post‐intervention).

Comparison 2 Combination therapy versus antidepressant medication, Outcome 25 Functioning (six to nine months follow‐up).

Comparison 2 Combination therapy versus antidepressant medication, Outcome 26 Functioning (12 months follow‐up).
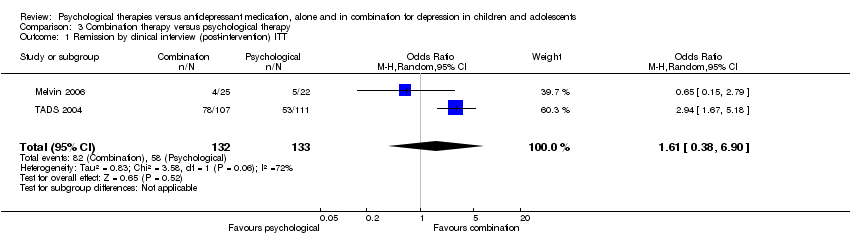
Comparison 3 Combination therapy versus psychological therapy, Outcome 1 Remission by clinical interview (post‐intervention) ITT.
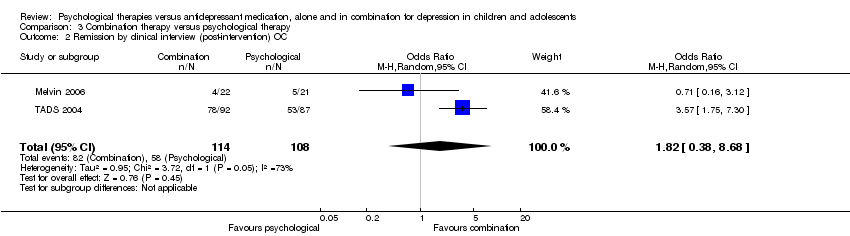
Comparison 3 Combination therapy versus psychological therapy, Outcome 2 Remission by clinical interview (post‐intervention) OC.

Comparison 3 Combination therapy versus psychological therapy, Outcome 3 Remission by clinical interview (six to nine months follow‐up) ITT.

Comparison 3 Combination therapy versus psychological therapy, Outcome 4 Remission by clinical interview (six to nine months follow‐up) OC.
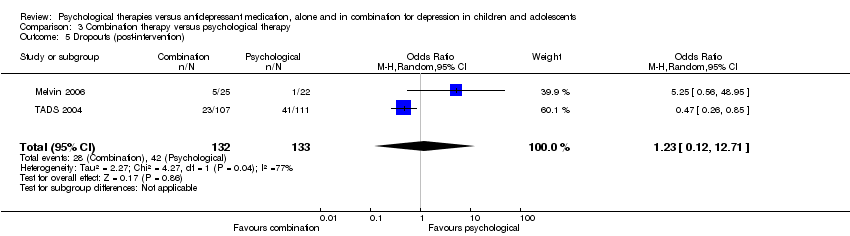
Comparison 3 Combination therapy versus psychological therapy, Outcome 5 Dropouts (post‐intervention).

Comparison 3 Combination therapy versus psychological therapy, Outcome 6 Dropouts (six to nine months follow‐up).

Comparison 3 Combination therapy versus psychological therapy, Outcome 7 Suicidal ideation (post‐intervention).

Comparison 3 Combination therapy versus psychological therapy, Outcome 8 Suicidal ideation (six to nine months follow‐up).

Comparison 3 Combination therapy versus psychological therapy, Outcome 9 Suicidal ideation (post‐intervention).

Comparison 3 Combination therapy versus psychological therapy, Outcome 10 Suicidal ideation (six to nine months follow‐up).

Comparison 3 Combination therapy versus psychological therapy, Outcome 11 Suicidal ideation (12 months follow‐up).

Comparison 3 Combination therapy versus psychological therapy, Outcome 12 Remission by cut‐off (post‐intervention).

Comparison 3 Combination therapy versus psychological therapy, Outcome 13 Remission by cut‐off (six to nine months follow‐up).
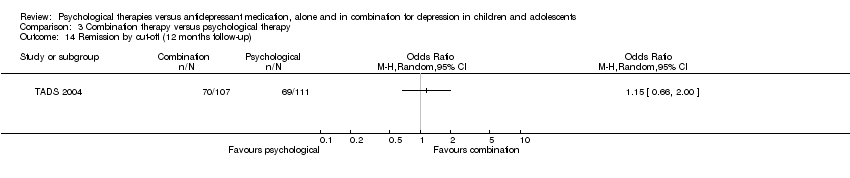
Comparison 3 Combination therapy versus psychological therapy, Outcome 14 Remission by cut‐off (12 months follow‐up).

Comparison 3 Combination therapy versus psychological therapy, Outcome 15 Depression symptoms clinician rated (CDRS‐R) (post‐intervention).
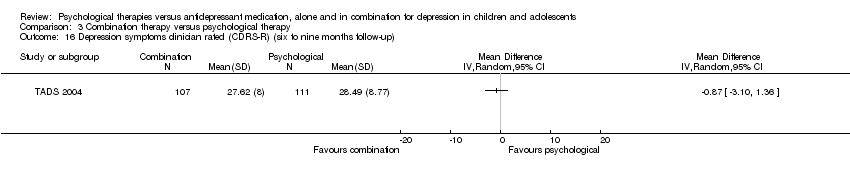
Comparison 3 Combination therapy versus psychological therapy, Outcome 16 Depression symptoms clinician rated (CDRS‐R) (six to nine months follow‐up).

Comparison 3 Combination therapy versus psychological therapy, Outcome 17 Depression symptoms clinician rated (CDRS‐R) (12 months follow‐up).
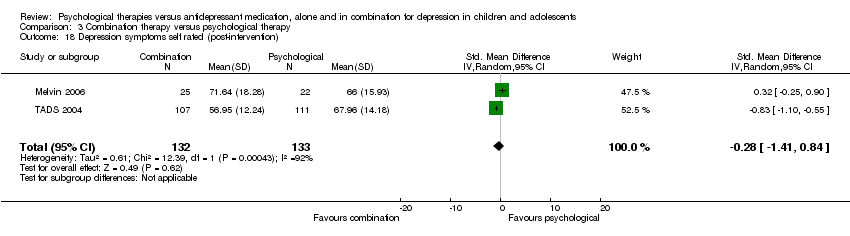
Comparison 3 Combination therapy versus psychological therapy, Outcome 18 Depression symptoms self rated (post‐intervention).
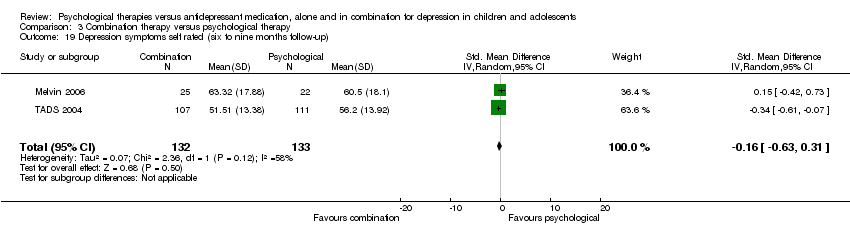
Comparison 3 Combination therapy versus psychological therapy, Outcome 19 Depression symptoms self rated (six to nine months follow‐up).

Comparison 3 Combination therapy versus psychological therapy, Outcome 20 Depression symptoms self rated (12 months follow‐up).

Comparison 3 Combination therapy versus psychological therapy, Outcome 21 Functioning (post‐intervention).

Comparison 3 Combination therapy versus psychological therapy, Outcome 22 Functioning (six to nine months follow‐up).

Comparison 4 Combination therapy versus psychological therapy plus placebo, Outcome 1 Dropouts (post‐intervention).

Comparison 4 Combination therapy versus psychological therapy plus placebo, Outcome 2 Suicidal ideation (post‐intervention).

Comparison 4 Combination therapy versus psychological therapy plus placebo, Outcome 3 Remission by cut‐off (post‐intervention).
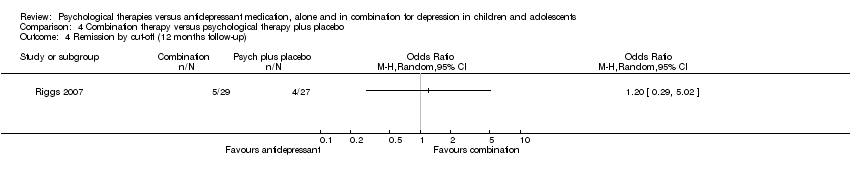
Comparison 4 Combination therapy versus psychological therapy plus placebo, Outcome 4 Remission by cut‐off (12 months follow‐up).

Comparison 4 Combination therapy versus psychological therapy plus placebo, Outcome 5 Depression symptoms clinician rated (CDRS‐R) (post‐intervention).
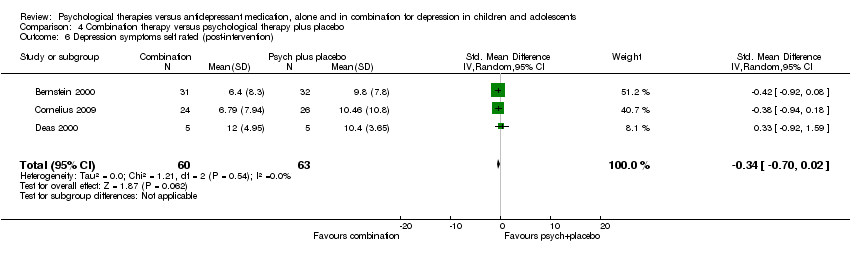
Comparison 4 Combination therapy versus psychological therapy plus placebo, Outcome 6 Depression symptoms self rated (post‐intervention).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Remission by clinical interview (post‐intervention) ITT Show forest plot | 2 | 268 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.28, 1.35] |
| 2 Remission by clinical interview (post‐intervention) OC Show forest plot | 2 | 220 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.27, 0.98] |
| 3 Remission by clinical interview (six to nine months follow‐up) ITT Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Remission by clinical interview (six to nine months follow‐up) OC Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Dropouts (post‐intervention) Show forest plot | 2 | 271 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.11, 3.28] |
| 6 Dropouts (six to nine months follow‐up) Show forest plot | 2 | 223 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.63, 2.19] |
| 7 Suicidal ideation (post‐intervention) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8 Suicidal ideation (six to nine months follow‐up) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9 Suicidal ideation (post‐intervention) Show forest plot | 2 | 268 | Mean Difference (IV, Random, 95% CI) | ‐3.12 [‐5.91, ‐0.33] |
| 10 Suicidal ideation (six to nine months follow‐up) Show forest plot | 2 | 268 | Mean Difference (IV, Random, 95% CI) | ‐2.89 [‐5.49, ‐0.28] |
| 11 Suicidal ideation (12 months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12 Remission by cut‐off (post‐intervention) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 13 Remission by cut‐off (six to nine months follow‐up) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 14 Remission by cut‐off (12 months follow‐up) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 15 Depression symptoms clinician rated (CDRS‐R) (post‐intervention) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 16 Depression symptoms clinician rated (CDRS‐R) (six to nine months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 17 Depression symptoms clinician rated (CDRS‐R) (12 months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 18 Depression symptoms self rated (post‐intervention) Show forest plot | 2 | 255 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.69, 1.01] |
| 19 Depression symptoms self rated (six to nine months follow‐up) Show forest plot | 2 | 268 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.51, 0.42] |
| 20 Depression symptoms self rated (12 months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 21 Functioning (post‐intervention) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 22 Functioning (six to nine months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Remission by clinical interview (post‐intervention) ITT Show forest plot | 3 | 419 | Odds Ratio (M‐H, Random, 95% CI) | 1.50 [0.99, 2.27] |
| 2 Remission by clinical interview (post‐intervention) OC Show forest plot | 3 | 378 | Odds Ratio (M‐H, Random, 95% CI) | 1.56 [0.98, 2.47] |
| 3 Remission by clinical interview (six to nine months follow‐up) ITT Show forest plot | 2 | 203 | Odds Ratio (M‐H, Random, 95% CI) | 1.93 [0.93, 4.00] |
| 4 Remission by clinical interview (six to nine months follow‐up) OC Show forest plot | 2 | 193 | Odds Ratio (M‐H, Random, 95% CI) | 1.94 [0.88, 4.27] |
| 5 Remission by clinical interview (12 months follow‐up) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Dropouts (post‐intervention) Show forest plot | 5 | 699 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.51, 1.39] |
| 7 Dropouts (six to nine months follow‐up) Show forest plot | 3 | 420 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.54, 1.64] |
| 8 Dropouts (12 months follow‐up) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9 Suicidal ideation (post‐intervention) Show forest plot | 2 | 388 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.26, 2.16] |
| 10 Suicidal ideation (six to nine months follow‐up) Show forest plot | 2 | 344 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.06, 4.58] |
| 11 Suicidal ideation (12 months follow‐up) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 12 Suicidal ideation (post‐intervention) Show forest plot | 2 | 267 | Mean Difference (IV, Random, 95% CI) | ‐2.57 [‐5.53, 0.40] |
| 13 Suicidal ideation (six to nine months follow‐up) Show forest plot | 2 | 267 | Mean Difference (IV, Random, 95% CI) | ‐1.89 [‐4.50, 0.72] |
| 14 Suicidal ideation (12 months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15 Remission by cut‐off (post‐intervention) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 16 Remission by cut‐off (six to nine months follow‐up) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 17 Remission by cut‐off (12 months follow‐up) Show forest plot | 2 | 319 | Odds Ratio (M‐H, Random, 95% CI) | 1.45 [0.60, 3.52] |
| 18 Depression symptoms clinician rated (CDRS‐R) (post‐intervention) Show forest plot | 2 | 415 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐4.95, 4.41] |
| 19 Depression symptoms clinician rated (CDRS‐R) (six to nine months follow‐up) Show forest plot | 2 | 408 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐2.26, 1.72] |
| 20 Depression symptoms clinician rated (CDRS‐R) (12 months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 21 Depression symptoms self rated (post‐intervention) Show forest plot | 5 | 683 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.36, 0.09] |
| 22 Depression symptoms self rated (six to nine months follow‐up) Show forest plot | 4 | 610 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.28, 0.17] |
| 23 Depression symptoms self rated (12 months follow‐up) Show forest plot | 2 | 368 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.46, ‐0.05] |
| 24 Functioning (post‐intervention) Show forest plot | 3 | 396 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.11, 0.28] |
| 25 Functioning (six to nine months follow‐up) Show forest plot | 3 | 385 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.12, 0.28] |
| 26 Functioning (12 months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Remission by clinical interview (post‐intervention) ITT Show forest plot | 2 | 265 | Odds Ratio (M‐H, Random, 95% CI) | 1.61 [0.38, 6.90] |
| 2 Remission by clinical interview (post‐intervention) OC Show forest plot | 2 | 222 | Odds Ratio (M‐H, Random, 95% CI) | 1.82 [0.38, 8.68] |
| 3 Remission by clinical interview (six to nine months follow‐up) ITT Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Remission by clinical interview (six to nine months follow‐up) OC Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Dropouts (post‐intervention) Show forest plot | 2 | 265 | Odds Ratio (M‐H, Random, 95% CI) | 1.23 [0.12, 12.71] |
| 6 Dropouts (six to nine months follow‐up) Show forest plot | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.40, 1.42] |
| 7 Suicidal ideation (post‐intervention) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8 Suicidal ideation (six to nine months follow‐up) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9 Suicidal ideation (post‐intervention) Show forest plot | 2 | 265 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐2.25, 3.45] |
| 10 Suicidal ideation (six to nine months follow‐up) Show forest plot | 2 | 265 | Mean Difference (IV, Random, 95% CI) | 1.78 [‐2.29, 5.85] |
| 11 Suicidal ideation (12 months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12 Remission by cut‐off (post‐intervention) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 13 Remission by cut‐off (six to nine months follow‐up) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 14 Remission by cut‐off (12 months follow‐up) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 15 Depression symptoms clinician rated (CDRS‐R) (post‐intervention) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 16 Depression symptoms clinician rated (CDRS‐R) (six to nine months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 17 Depression symptoms clinician rated (CDRS‐R) (12 months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 18 Depression symptoms self rated (post‐intervention) Show forest plot | 2 | 265 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐1.41, 0.84] |
| 19 Depression symptoms self rated (six to nine months follow‐up) Show forest plot | 2 | 265 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.63, 0.31] |
| 20 Depression symptoms self rated (12 months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 21 Functioning (post‐intervention) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 22 Functioning (six to nine months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts (post‐intervention) Show forest plot | 4 | 249 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.42, 2.28] |
| 2 Suicidal ideation (post‐intervention) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Remission by cut‐off (post‐intervention) Show forest plot | 2 | 173 | Odds Ratio (M‐H, Random, 95% CI) | 2.15 [1.15, 4.02] |
| 4 Remission by cut‐off (12 months follow‐up) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Depression symptoms clinician rated (CDRS‐R) (post‐intervention) Show forest plot | 3 | 239 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.78, ‐0.26] |
| 6 Depression symptoms self rated (post‐intervention) Show forest plot | 3 | 123 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.70, 0.02] |

